This study aimed to suggest an initial pediatric vancomycin dose regimen through population pharmacokinetic-pharmacodynamic modeling. A population pharmacokinetic approach was used to analyze vancomycin concentration-time data from a large pediatric cohort.
KEYWORDS: pharmacodynamics, pharmacokinetics, population pharmacokinetics
ABSTRACT
This study aimed to suggest an initial pediatric vancomycin dose regimen through population pharmacokinetic-pharmacodynamic modeling. A population pharmacokinetic approach was used to analyze vancomycin concentration-time data from a large pediatric cohort. Pharmacokinetic target attainment for patients with bloodstream isolates was compared with clinical outcome using logistic regression and classification and regression trees. Change in serum creatinine during treatment was used as an indicator of acute nephrotoxicity. Probability of acute kidney injury (50% increase from baseline) or kidney failure (75% increase from baseline) was evaluated using logistic regression. An initial dosing regimen was derived, personalized by age, weight, and serum creatinine, using stochastic simulations. Data from 785 hospitalized pediatric patients (1 day to 21 years of age) with suspected Gram-positive infections were collected. Estimated (relative standard error) typical clearance, volume of distribution 1, intercompartmental clearance, and volume of distribution 2 were (standardized to 70 kg) 4.84 (2.38) liters/h, 39.9 (8.15) liters, 3.85 (17.3) liters/h, and 37.8 (10.2) liters, respectively. While cumulative vancomycin exposure correlated positively with the development of nephrotoxicity (713 patients), no clear relationship between vancomycin area under the plasma concentration-time curve and efficacy was found (102 patients). Predicted probability of acute kidney injury and kidney failure with the optimized dosing regimen at day 5 was 10 to 15% and 5 to 10%, increasing by approximately 50% on day 7 and roughly 100% on day 10 across all age groups. This study presents the first data-driven pediatric dose selection to date accounting for nephrotoxicity, and it indicates that cumulative vancomycin exposure best describes risk of acute kidney injury and acute kidney failure.
TEXT
Vancomycin is a glycopeptide antibiotic effective against Gram-positive bacteria, and it plays a crucial role in the treatment of serious and resistant infections in both adults and children (1). Previous studies have reported vancomycin pharmacokinetics (PK) in children and proposed model-based dose optimizations (2–11). Dose selection in these studies adopted a 24-h steady-state area under the plasma concentration-time curve (AUC) (12)/MIC value of greater than 400 mg·h/liter. However, this PK-pharmacodynamic (PKPD) endpoint was adopted from adults without further evaluation in children and without taking adverse effects, such as nephrotoxicity, into account. The overall aim of this study was to revisit the pediatric initial vancomycin dosing regimen, and with this in mind, there were four main aims.
The first aim was to study vancomycin population PK in a large cohort. Several previous studies have sought to describe pediatric vancomycin PK, reporting clearance (median [range], 4.52 [1.00 to 1.57] liters/h) and steady-state volume of distribution (median [range], 37.8 [31.0 to 119] liters) (2–8). Most of these studies used small sample sizes or focused on pediatric subpopulations, such as neonates, meaning parameter comparisons between studies is challenging, not least because important covariates, such as age and weight, are often not parameterized in a standard way (13). Moreover, vancomycin distribution often requires two and, in some cases, three disposition compartments (14), although most pediatric vancomycin PK papers have previously reported a one-compartment model (2–7). Vancomycin is mainly bound to albumin in the blood (15), with protein binding ranging between 50% and 55%, resulting in free vancomycin exposure at only half the equivalent of total exposure (14).
The second aim was to identify the target concentration for efficacy in pediatrics. The vancomycin AUC/MIC value was found to be more predictive of efficacy than time above MIC with methicillin-resistant Staphylococcus aureus sepsis or with methicillin-resistant Staphylococcus aureus infection of the lower respiratory tract (16, 17). Adult patients with a target AUC/MIC value of ≥400 mg·h/liter appeared to have a lower risk of treatment failure (16–18), and as free antibiotic concentrations drive the antibacterial effects, AUCfree/MIC of ≥200 mg·h/liter has consequently been reported as the target efficacy threshold (14).
The third aim was to identify predictors of nephrotoxicity. Nephrotoxicity indices have been defined for pediatric patients (19), and risk factors for nephrotoxicity (20), such as vancomycin loading dose, duration of vancomycin therapy, concomitant therapy, and demographic features, have been defined for adult patients (21). Vancomycin trough levels and vancomycin AUC have been defined in both adults and children (22), resulting in a toxicity threshold AUC of 700 mg·h/liter for the adult population and 800 mg·h/liter for the pediatric population, rendering a therapeutic window of 400 to 800 mg·h/liter (22, 23).
The fourth aim was to further refine initial dosing recommendations, taking into account the findings from the modeling described above. Therapeutic drug monitoring (TDM) is often used to ensure that vancomycin concentrations fall within the therapeutic window (2), but optimizing the starting dose may limit the need for dose adjustments. Traditionally vancomycin trough concentrations (Ctrough) have been preferred for TDM, although Bayesian forecasting is now more readily available, so AUC is becoming the preferred endpoint (3). It is therefore crucial that efficacy and toxicity thresholds are adequately identified in pediatric patients.
RESULTS
Population pharmacokinetics.
A total of 616 patients, contributing two or more vancomycin plasma samples, as well as age, body weight, creatinine, and dosing (intravenous infusion for 1 h) data, were used to build the PK model and were labeled training data (Table 1). Data from 169 patients, contributing only one vancomycin plasma sample, as well as age, body weight, creatinine, and dosing information (intravenous infusion for 1 h) data, were used for external validation of the population PK model and were labeled test data (Table 1). Patients were only included if matching records in the TDM system were taken no later than 48 h after a dose. Sample times reported before 1.5 h (during or immediately after the infusion) after a dose were considered reporting errors (likely time of sample being left for the porter recorded rather than actual sampling time). These samples were subsequently considered trough values. This yielded vancomycin plasma concentration samples between 1.5 and 48 h after dosing.
TABLE 1.
Summary of pharmacokinetic analysis data
| Parameter | Value [mean (range)] for: |
|
|---|---|---|
| Training data | Test data | |
| Study size (n) | 616 | 169 |
| Sample size (n) | 4,137 | 169 |
| Samples per patient (n) | 7 (2–50) | 1 (1–1) |
| Treatment length (days) | 8 (0–83) | 3 (0–15.2) |
| First episodea (n) | 9 (0–83) | 3 (0–15.2) |
| Consecutive episodesa (n) | 7 (0–43.7) | 2 (0–3.6) |
| Age (mo) | 61 (0.03–255) | 63 (0.08–204) |
| 0–1 month (n) | 39 | 9 |
| 1 mo to 2 yr (n) | 195 | 59 |
| 2–12 yr (n) | 314 | 75 |
| >12 yr (n) | 68 | 26 |
| Body weight (kg) | 19 (0.742–95) | 20 (1.18–107) |
| Creatinine (μmol/liter) | 39 (5–892) | 35 (8–291) |
| Creatinine samples per patient (n) | 14 (1–118) | 3 (1–13) |
A treatment episode was defined as consecutive doses no longer than 48 h apart, and 0 represents patients only having received one dose of vancomycin. CoNS, coagulase-negative Staphylococcus.
A two-compartment disposition model performed substantially better (P < 0.001) than a one-compartment model. Interindividual variability (IIV) on clearance (CL) and central volume (VC) displayed reasonably high shrinkage (24) (32% and 41%, respectively), but epsilon shrinkage was low at 8%. Body weight as a continuous covariate on volume and clearance using allometric size scaling, a sigmoidal postmenstrual age maturation function, and age-corrected creatinine as a continuous covariate on clearance were all included a priori. A body weight power of 0.632 on elimination clearance provided a better fit to the data than a power of 0.75 (ΔOFV, −29.3 [OFV is an objective function value defined as −2 × log likelihood] ). Backward exclusion of bodyweight as a continuous covariate on volume and clearance parameters (P < 0.001), postmenstrual age as a maturation function on CL (P < 0.001), and creatinine on elimination CL (P < 0.001) resulted in significant worsening of the model fit and so was retained (see Fig. S1 in the supplemental material).
The model adequately described the vancomycin concentration-time data with a mean prediction error on the test data of 0.96 mg/liter (Fig. 1, Table 2, and Fig. S2 and S3). The final model was reestimated on a data set where the corrected time samples (originally reported before 1.5 h) were omitted as sensitivity analysis, and this yielded similar parameter estimates for CL (−1.03%), postnatal age half-maximum organ maturation (PMA50) (−5.78%), Hill coefficient (36.9%), and exponent on creatinine function (power creatinine) (−2.31%), which was important as dose optimizations focus on AUC.
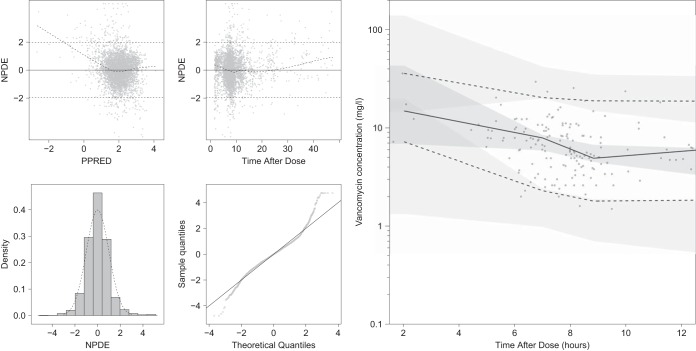
FIG 1.
(Left) Simulation-based goodness-of-fit plots on the training data, including normalized prediction distributed error (NPDE) versus population predictions (PPRED: on natural logarithm scale), NPDE versus time after dose, density distribution of NPDE, and a qq-plot for NPDE. (Right) visual predictive check of 2,000 simulated concentration-time profiles using the final model for the test data. Points represent the observations, black lines represent the 2.5th, 50th, and 97.5th percentiles, and the shaded areas represent the 95% confidence intervals of the corresponding predicted vancomycin concentration percentiles. The x axis of the visual predictive check was constrained between 1.5 and 12 h, leaving 14 scattered samples between 12 and 48 h not shown.
TABLE 2.
Summary of population pharmacokinetic parameter estimatesa
| Parameter | Fixed effect (RSE) | IIV (RSE) |
|---|---|---|
| CL (liters/h) | 4.84 (2.38) | 50.4 (11.8) |
| PMA50 | 50.2 (3.34) | |
| Hill coefficient | 3.52 (14.8) | |
| Power creatinine | −0.692 (5.28) | |
| VC (liters) | 39.9 (8.15) | 232 (17.2) |
| Q (liters/h) | 3.85 (17.3) | |
| VP (liters) | 37.8 (10.2) | |
| RUV | 0.243 (4.85) | |
| ηCL-ηVC | 0.535 (11.2) |
CL, elimination clearance; VC, central compartment distribution volume; Q, intercompartmental clearance; VP, peripheral compartment distribution volume; PMA50, postnatal age half-maximum organ maturation; power creatinine, exponent on creatinine function; RUV, additive residual variability on log-transformed data; ηCL-ηVC, correlation between variability on clearance and central compartment distribution volume. Clearance and volume parameters were centralized around a 70-kg patient using 0.632, 0.75, and 1 as power functions for CL, Q, and the distribution volumes (VC and VP), respectively. IIV, interindividual variability (); RSE, relative standard errors derived from 962 (out of 1,000) converged nonparametric bootstraps in NONMEM as 100 × (SD/means).
Efficacy.
Among the included patients, 102 had Gram-positive bloodstream isolates for which the MIC was measured (Table 3). Coagulase-negative Staphylococcus infections, which are largely a result of line infections (25) or contamination, accounted for 80, rendering a limited number of true Gram-positive bloodstream infections (Table 3).
TABLE 3.
PKPD analysis of efficacy
| Organism | Study size (n) | Died (n) | Recurrence (n) | Microbiological failure (n) | MIC [mg/liter; mean (range)] | AUC/MIC [mg/liter; mean (range)] |
|---|---|---|---|---|---|---|
| All | 102 | 3 | 6 | 7 | 2 (0.12–4) | 320 (50–2,755) |
| CoNS | 80 | 3 | 5 | 7 | 2 (0.5–4) | 260 (50–846) |
| Micrococcus luteus | 1 | 0 | 0 | 0 | 0.12 | 2755 |
| Unknown | 10 | 0 | 1 | 0 | 1 (0.5–2) | 348 (127–620) |
| S. aureus | 9 | 0 | 0 | 0 | 2 (0.5–2) | 369 (125–1,007) |
| Viridans streptococci | 2 | 0 | 0 | 0 | 1 (0.5–1) | 1155 (364–1,947) |
In patients with a bloodstream organism, treatment failure was defined as at least one of the following criteria being met: (i) deceased within 30 days of vancomycin treatment initiation, (ii) recurrent infection between 48 h and 60 days following vancomycin treatment discontinuation, and (iii) microbiologically confirmed growth 7 days after the initiation of therapy but before treatment completion (16). Treatment outcome was classified as successful if none of the above criteria were met. Neither trough concentration/MIC nor AUC/MIC ratios correlated with probability of treatment failure in a multivariate generalized logistic regression model or in a classification and regression tree analysis (Table 4 and Fig. S4).
TABLE 4.
Summary of PKPD analysis of efficacy
| Parameter | AUC/MIC |
Ctrough/MIC |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | z value | P value | Estimate | SE | z value | P value | |
| Intercept | −4.18 | 2.8 | −1.49 | 0.135 | −1.88 | 0.826 | −2.27 | 0.0231b |
| Log(AUC/MIC) | 0.487 | 0.498 | 0.977 | 0.329 | ||||
| Log(Ctrough/MIC) | 0.296 | 0.331 | 0.894 | 0.371 | ||||
| Group | ||||||||
| Micrococcus luteus | −18.5 | 6520 | −0.00284 | 0.998 | −18 | 6520 | −0.00277 | 0.998 |
| Unknowna | −1.14 | 1.16 | −0.982 | 0.326 | −1.08 | 1.14 | −0.941 | 0.347 |
| S. aureus | −17.3 | 2140 | −0.00806 | 0.994 | −17.1 | 2120 | −0.00803 | 0.994 |
| Viridans streptococci | −18.8 | 4490 | −0.00418 | 0.997 | −18.4 | 4580 | −0.00401 | 0.997 |
| Creatinine | 0.000607 | 0.0147 | 0.0413 | 0.967 | 0.00116 | 0.0148 | 0.0786 | 0.937 |
| Age | 0.0151 | 0.0127 | 1.19 | 0.234 | 0.0145 | 0.0128 | 1.14 | 0.256 |
| Body weight | −0.0384 | 0.0683 | −0.562 | 0.574 | −0.0406 | 0.0679 | −0.599 | 0.549 |
| Age/body weight ratio | −0.0000234 | 0.000376 | −0.0624 | 0.95 | −0.00000403 | 0.000375 | −0.0107 | 0.991 |
Unidentified bacterial species.
P < 0.05.
Nephrotoxicity.
A total of 713 patients, contributing PK, baseline demographics, nephrotoxicity data, and concomitant medication data, were included for the characterization of predictors for nephrotoxicity (Table 5). Urine output data were not available; hence, nephrotoxicity severity was defined based on the change in creatinine criteria in the pediatric risk, injury, failure, loss, end-stage renal disease (pRIFLE) score (19). The two outcome classes, acute kidney injury and acute kidney failure, corresponded to a 50% and 75% increase, respectively, in plasma creatinine compared to baseline values.
TABLE 5.
PKPD analysis of nephrotoxicity
| Parametera | All data | Normal kidney function | Kidney injury | Kidney failure |
|---|---|---|---|---|
| Study size (n) | 713 | 618 | 41 | 54 |
| Aminoglycosides [n (%)] | 405 (56.8) | 336 (54.4) | 30 (73.2) | 39 (72.2) |
| Diuretics [n (%)] | 219 (30.7) | 172 (27.8) | 18 (43.9) | 29 (53.7) |
| NSAIDs [n (%)] | 166 (23.3) | 149 (24.1) | 8 (19.5) | 9 (16.7) |
| Cyclosporine [n (%)] | 112 (15.7) | 78 (12.6) | 11 (26.8) | 23 (42.6) |
| Colistin [n (%)] | 6 (0.842) | 5 (0.809) | 1 (2.44) | 0 (0) |
| No dose change (n) | 381 | 346 | 16 | 19 |
| One dose change (n) | 200 | 170 | 12 | 18 |
| Fraction increased dose at first dose | 0.732 | 0.75 | 0.72 | 0.6 |
| Two dose changes (n) | 102 | 80 | 11 | 11 |
| Fraction increased dose at second dose | 0.682 | 0.676 | 0.672 | 0.706 |
| Three dose changes (n) | 21 | 17 | 1 | 3 |
| Fraction increased dose at third dose | 0.8 | 0.773 | 1 | 0.833 |
| Four dose changes (n) | 8 | 5 | 0 | 3 |
| Fraction increased dose at fourth dose | 0.667 | 0.6 | 1 | |
| Five dose changes (n) | 1 | 0 | 1 | 0 |
| Fraction increased dose at fifth dose | 1 | 1 |
Fraction increase dose: the upward number of dose changes over the total number of dose changes.
The multivariable logistic regression model (Tables 6 and 7) demonstrated an increase in the probability of acute kidney injury or kidney failure with increasing cumulative area under the plasma concentration-time curve up to 8 h after the last dose (AUCCUM) [increase of estimate ± standard errors, 1.17 ± 0.178 per unit increase in log(AUCCUM); P < 0.001]. Similarly, an increase in the probability of acute kidney failure with increasing AUCCUM [increase of 1.32 ± 0.237 per unit increase in log(AUCCUM); P < 0.001] and concomitant therapy with cyclosporine (increase of 0.739 ± 0.358 per unit increase in creatinine; P < 0.05) was observed. Baseline plasma creatinine displayed a nonsignificant trend (P < 0.1) of increased probability of acute kidney injury and acute kidney failure (Table 7). Approximately half of the patients had their dose changed during the first week of the treatment, with most of the modifications being dose increases (Table 5). Consequently, unlike AUCCUM, 24-h AUC was not a significant predictor for nephrotoxicity in the multivariable logistic regression (Tables 6 and 7).
TABLE 6.
Summary of PKPD analysis of nephrotoxicity using AUC
| Parameter | AUC and kidney injury or failure |
AUC and kidney failure |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | z value | P value | Estimate | SE | z value | P value | |
| Intercept | −0.333 | 1.84 | −0.18 | 0.857 | −1.48 | 2.33 | −0.639 | 0.523 |
| Log(AUC) | −0.186 | 0.342 | −0.544 | 0.586 | 0.0277 | 0.428 | 0.0647 | 0.948 |
| Aminoglycosides | 0.372 | 0.263 | 1.41 | 0.158 | 0.17 | 0.341 | 0.498 | 0.618 |
| Diuretic | 0.704 | 0.244 | 2.89 | 0.0039b | 0.808 | 0.313 | 2.58 | 0.00995b |
| NSAIDs | −0.0129 | 0.313 | −0.0412 | 0.967 | 0.0201 | 0.42 | 0.0479 | 0.962 |
| Cyclosporine | 1.17 | 0.274 | 4.26 | 0.0000209b | 1.44 | 0.341 | 4.23 | 0.0000234b |
| Colistin | 0.203 | 1.13 | 0.179 | 0.858 | −14 | 955 | −0.0147 | 0.988 |
| Creatinine | −0.051 | 0.0203 | −2.52 | 0.0118a | −0.0811 | 0.0276 | −2.94 | 0.0033b |
| Age | −0.00523 | 0.00419 | −1.25 | 0.212 | −0.0117 | 0.00504 | −2.32 | 0.0203a |
| Creatinine/age ratio | 0.000281 | 0.000122 | 2.31 | 0.0211a | 0.000484 | 0.000156 | 3.1 | 0.00192b |
P < 0.05.
P < 0.01.
TABLE 7.
Summary of PKPD analysis of nephrotoxicity using AUCCUM
| Parameter | AUCCUM and kidney injury or failure |
AUCCUM and kidney failure |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | z value | P value | Estimate | SE | z value | P value | |
| Intercept | −11.5 | 1.42 | −8.12 | 4.68E−16b | −13.4 | 1.92 | −6.96 | 3.29E−12b |
| Log(AUCCUM) | 1.17 | 0.178 | 6.55 | 5.71E−11b | 1.32 | 0.237 | 5.57 | 0.000000026b |
| Aminoglycosides | 0.276 | 0.276 | 1 | 0.317 | 0.0584 | 0.358 | 0.163 | 0.87 |
| Diuretic | 0.179 | 0.258 | 0.696 | 0.486 | 0.216 | 0.328 | 0.657 | 0.511 |
| NSAIDs | 0.0274 | 0.327 | 0.0839 | 0.933 | 0.104 | 0.435 | 0.239 | 0.811 |
| Cyclosporine | 0.549 | 0.291 | 1.88 | 0.0596 | 0.739 | 0.358 | 2.06 | 0.0391a |
| Colistin | 1.12 | 1.16 | 0.962 | 0.336 | −13 | 923 | −0.0141 | 0.989 |
| Creatinine | 0.00591 | 0.00321 | 1.84 | 0.0655 | 0.00643 | 0.00361 | 1.78 | 0.0751 |
| Age | −0.00369 | 0.00256 | −1.44 | 0.15 | −0.00556 | 0.00332 | −1.68 | 0.0938 |
| Creatinine/age ratio | −0.000000257 | 0.0000283 | −0.00911 | 0.993 | 0.00000702 | 0.0000303 | 0.232 | 0.817 |
P < 0.05.
P < 0.01.
The predictive performance of the multivariable logistic regression models for acute kidney injury and acute kidney failure, with only statistically significant predictors included, was further evaluated. The models were trained on 70% of the patients, which were selected at random. The models were subsequently tested on the other 30% of the patients, and area under the receiver operating characteristic (ROC) curve was used as a diagnostic tool. If the area under the ROC curve was ≥0.6, the logistic regression model was refitted on the full data set (26). AUCCUM was included a priori in the predictive logistic regression models for acute renal injury (Table S1), with cyclosporine concomitant therapy on top for acute renal failure (Table S2), and displayed areas under the ROC curve of 0.640 and 0.643. The predictive logistic regression model for acute renal injury on full data had an area under the ROC curve of 0.676 (Table S3), and the predictive logistic regression model for acute renal failure on full data had an area under the ROC curve of 0.685 (Table S4).
Dose optimization.
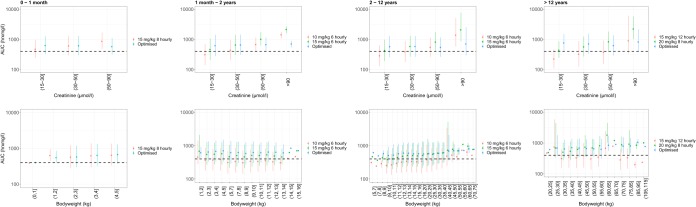
Current pediatric initial vancomycin dosing regimens, 15 mg/kg of body weight every 8 h (q8h) for children 0 to 1 month, 10 to 15 mg/kg q6h for children 1 month to 11 years, and 15 to 20 mg/kg q8h to q12h for children 12 years and above (1), were revisited using the developed population pharmacokinetic model and 2,000 stochastic simulations. As the efficacy analysis in this study did not yield target levels in the pediatric patient population, a vancomycin AUC target attainment of ≥400 was adopted from an adult (16–18). Most patients achieved target attainment when the current standard dosing regimens were stratified by bodyweight, although a clear positive correlation with increased plasma creatinine was apparent; hence, patients in the lower creatinine band displayed a lower vancomycin AUC (Fig. 2). Further stratification of the vancomycin dosing regimen by baseline plasma creatinine yielded target attainment for most of the patients when stratified by bodyweight and baseline plasma creatinine (Table 8 and Fig. 2). Estimated glomerular filtration rate (eGFR) for each baseline plasma creatinine and age group, using the Schwartz formula (27), indicated that the proposed dosing regimen remained untested for pediatric patients with kidney failure, with all eGFR estimates above 20 ml/min (Table 8).
FIG 2.
Vancomycin trough concentrations versus creatinine levels (top row) and bodyweight (bottom row) after the standard dosing regimen (green and red) and optimized dosing regimen (blue). Results were stratified for age group (by column). The dashed black horizontal lines represent the target exposure (i.e., 400 h·mg/liter). Dots represent the mean median values from 2,000 simulations, and the error bars represent the mean 5th and 95th percentiles. The parentheses symbol on the x axes indicate equal to and larger than, and the bracket symbol indicates smaller than.
TABLE 8.
Overview of refined pediatric initial dosing regimen
| Creatinine band (μmol/liter) | 0–1 mo |
1 mo to 2 yr |
2–12 yr |
>12 yr |
||||
|---|---|---|---|---|---|---|---|---|
| eGFR (ml/min) | Dosage, q8h (mg/kg) | eGFR (ml/min) | Dosage, q6h (mg/kg) | eGFR (ml/min) | Dosage, q6h (mg/kg) | eGFR (ml/min) | Dosage, q8h (mg/kg) | |
| (15–30] | 84.2 | 20 | 123 | 20 | 197 | 20 | 272 | 35 |
| (30–50] | 47.4 | 15 | 69.4 | 15 | 111 | 15 | 153 | 25 |
| (50–90] | 27.1 | 10 | 39.7 | 10 | 63.2 | 10 | 87.5 | 15 |
| >90 | 21.1 | 10a | 30.9 | 5 | 49.2 | 5 | 68.0 | 7.5 |
Not supported by data, as no patients were available in this category. eGFR, estimated glomerular filtration rate, (k × height)/plasma creatinine.
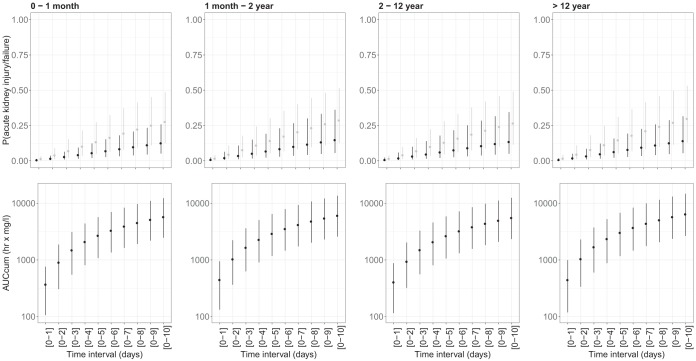
Subsequently, correlations between vancomycin AUCCUM and probability of acute kidney injury or acute kidney failure, with the optimized initial vancomycin dosing regimen, was studied using the predictive multivariable logistic regression models for acute kidney injury and acute kidney failure and 500 stochastic simulations. The predicted probability of acute kidney injury and kidney failure at day 5 for children 0 to 1 month old was 13.3% (4.95% to 27.3%) and 5.55% (2.00% to 12.3%), increasing to 19.4% (7.88% to 37.2%) and 8.36% (3.21% to 18.0%) on day 7 and 27.7% (12.4% to 48.5%) and 12.6% (5.13% to 25.8%) on day 10 (Fig. 3). A similar probability of acute kidney injury and kidney failure was predicted at day 5 for children 1 month to 2 years of age at 14.2% (2.29% to 30.2%) and 6.78% (2.27% to 19.1%), increasing to 20.4% (8.20% to 40.2%) and 10.0% (3.54% to 26.7%) on day 7 and to 28.7% (12.6% to 51.5%) and 14.8% (5.52% to 36.2%) on day 10 (Fig. 3). For children 2 to 11 years old, predicted probability of acute kidney injury and kidney failure was similar, with 12.9% (4.69% to 28.0%) and 6.10% (2.02% to 17.9%) after 5 days of treatment, increasing to 18.7% (7.33% to 37.8%) and 9.07% (3.16% to 25.2%) on day 7 and 26.6% (11.4% to 49.2%) and 13.5% (4.95% to 34.5%) on day 10 (Fig. 3). Day 5 predictions for probability of acute kidney injury and acute kidney failure in the eldest children of 12 years and above were in a similar range at 14.6% (5.24% to 31.1%) and 6.33% (2.15% to 15.8%), increasing to 21.1% (8.29% to 41.6%) and 9.47% (3.42% to 22.6%) on day 7 and 29.8% (12.9% to 53.1%) and 14.1% (5.44% to 31.6%) on day 10 (Fig. 3). For comparison, approximately 6.38% and 3.55% of the patients in the data had observed acute kidney injury or acute renal failure at day 5, and approximately 13.0% and 4.63% of the patients in the data had observed acute kidney injury and acute renal failure at day 10.
FIG 3.
Probability of nephrotoxicity (gray, acute kidney injury; black, acute kidney failure) (top row) and vancomycin exposure (bottom row) for treatment with the optimized dosing regimen for different durations. Results were stratified for age group (by column). Dots represent the mean median values from 500 simulations, and the error bars represent the mean 5th and 95th percentiles.
DISCUSSION
This study provides a comprehensive evaluation of vancomycin PKPD in a pediatric population with, to our knowledge, the largest sample size to date. Our major finding is that AUCCUM is associated with risk of nephrotoxicity. The optimized dosing regimen resulted in a predicted 10 to 15% and 5 to 10% probability of acute kidney injury and kidney failure at day 5, increasing by approximately 50% on day 7 and roughly 100% on day 10 across all age groups (Fig. 3).
Population pharmacokinetics.
In general, vancomycin population PK characteristics were in agreement with those previously published. For example, creatinine levels, relative to the mean age-adjusted creatinine levels for the individual patient, displayed a negative exponential correlation with vancomycin elimination clearance similar to that of other renally cleared drugs, such as gentamicin (28). Unlike in most other pediatric vancomycin PK studies, where mostly a one-compartment disposition model was identified, a two-compartment disposition model was identified in this pediatric patient population due to sampling taken 1 h postinfusion for a part of our data set (Fig. 1, Table 2, and Fig. S2 and S3).
A 0.632 body weight power on CL was evaluated in addition to the conventional 0.75 power, as vancomycin is eliminated renally. The 0.632 power provided a superior model fit (ΔOFV, −29.3) over the 0.75 power, which could be explained by renal maturation and, therefore, drug elimination (29). The power function on intercompartmental clearance was fixed to 0.75 and to 1 for distribution volumes based on tissue blood flow and proportional growth between body size, respectively.
Efficacy.
Neither vancomycin trough concentrations nor AUC correlated with treatment failure in this pediatric patient population with a variety of bloodstream infections (Table 4 and Fig. S3), although several studies in adults with methicillin-resistant Staphylococcus aureus bloodstream infections concluded that the PKPD endpoint of an AUC/MIC value of ≥400 mg·h/liter was clinically relevant (16–18). A plausible explanation for this discrepancy is the large number of coagulase-negative Staphylococcus infections (Table 3). Coagulase-negative Staphylococcus infections are likely the result of line infections (25) or contamination and cause limited morbidity. For the remaining 22 Gram-positive bloodstream infections, there was insufficient statistical power to refute AUC target attainment of ≥400 mg·h/liter (16–18). The fact that only 22 of 785 patients had confirmed Gram-positive isolates on blood culture highlights the lack of infections at the study center, possibly due to good infection control procedures, and shows how difficult running prospective pediatric antimicrobial clinical trials is when so few patients have identifiable infections.
Nephrotoxicity.
Validation of renal toxicity biomarkers in children is lacking, which directly stipulates the limitation of the nephrotoxic results presented in this investigation. The most commonly studied renal biomarkers have limited validity, e.g., urinary and serum neutrophil gelatinase-associated lipocalin (NGAL; this biomarker rises with creatinine but also with white cell count), procalcitonin, and C-reactive protein, so its use is limited in the setting of infection/acute inflammation. Normal ranges are also lacking, e.g., Smertka et al. found similar NGAL levels in babies with and without renal impairment (30).
Acute kidney injury and acute kidney failure corresponded to a 50% and 75% increase in plasma creatinine compared to baseline values in this study. Even though such increases in creatinine as a percentage of baseline levels may be high, creatinine levels may be still in the range, further complicating the interpretation of the biomarker.
Nonetheless, duration of treatment turned out to be an important risk factor for renal disease, underwritten by a significant correlation between acute kidney injury or acute kidney failure and AUCCUM (Tables 6 and 7). The predictive performance of AUCCUM aligns with the previously described delay in nephrotoxicity, which has been found to occur late in the first week of vancomycin therapy (31). Creatinine levels should therefore be monitored carefully in patients on vancomycin to facilitate early detection and intervention. Moreover, vancomycin treatment for more than 7 days should be carefully considered and weighted against the probability of acute kidney injury and acute kidney failure. It should be noted here that our simulations show the probability of nephrotoxicity without dose adjustment.
While Zasowski et al. recently suggested a toxicity threshold AUC of 700 mg·h/liter and thereby a therapeutic window of 400 to 700 mg·h/liter (23), no significant correlation between acute kidney injury or acute kidney failure and AUC could be identified in our data (Table 6). The fact that almost half of the patients in this study had their doses changed during the first 7 days of treatment, with the majority of the dose changes being dose increases (Table 5), may be responsible. This may highlight that the use of 24-h AUC as a predictive variable for kidney injury and kidney failure is inappropriate in a clinical setting.
Dose optimization.
Vancomycin dosing recommendations have changed since the data collection period (32), with 15 mg/kg q8h for children 0 to 1 month of age, 10 to 15 mg/kg q6h for children 1 month to 11 years, and 15 to 20 mg/kg q8h to q12h for children 12 years and above as the most recent dosing recommendations (1). Using currently recommended vancomycin dosing, we stratified the milligram per kilogram doses by baseline creatinine level (Table 8). Even after stratifying milligram per kilogram dose by age and creatinine, it is clear that TDM will continue to be required, since target attainment is low in some categories (Fig. 2). From a nephrotoxicity perspective TDM continues to be required, hence vancomycin TDM is focused on avoiding renal failure. At day 5, approximately 6.38% and 3.55% of the patients had observable acute kidney injury or acute renal failure, and at day 10, approximately 13.0% and 4.63% of the patients had observable acute kidney injury and acute renal failure. This was substantially lower than results from simulations shown in Fig. 3, highlighting the impact of TDM as the virtual patient population remained on the initial dosing regimen for the entire duration of the simulated treatment, whereas the real observed patients underwent TDM-guided dose reduction.
Although trough levels of 10 to 15 mg/liter and 15 to 20 mg/liter have been recommended (32, 33), we chose to target AUC of >400 h·mg/liter, a target that is now becoming a preferred PK endpoint with increasing availability of Bayesian TDM software. It should be noted that this target has not been evaluated in children, and our attempt at modeling efficacy was hampered by the limited number of patients in our study having non-coagulase-negative Staphylococcus Gram-positive bloodstream infections.
Although we present one of the largest pediatric data sets, our study did have some limitations. A relatively high between-patient variability and shrinkage on elimination clearance (Table 2) emphasize the need for caution with regard to the interpretation of dose optimization results and the need for a confirmatory, prospective clinical study. Our center does not have a maternity unit; hence, only a small fraction of our patients were neonates (Table 1), most of whom were admitted for surgery. Moreover, eGFR might be most relevant for vancomycin dosing personalization, although patient heights were not routinely recorded, making individual-level eGFR calculations impossible (27). Instead, the baseline plasma creatinine band was used to further stratify initial pediatric vancomycin dosing, and indicative eGFR values were reported for each age and baseline creatinine group (Table 8). The use of eGFR for personalized initial pediatric vancomycin dosing has to be further evaluated in prospective clinical studies. Furthermore, while our PK model could be used for extrapolation to the preterm neonatal population, given our maturation parameters (Table 2) being similar to those of previous studies (28), in-depth evaluation of PD and nephrotoxicity in neonatal patients is required.
The current pediatric dosing regimen for vancomycin adequately accounts for changes in body weight, although variability could be substantially reduced by taking creatinine levels into account. Combining creatinine, age, and body weight can reduce the risk of toxicity by reduced variability in target attainment, although TDM continues to be required in order to ensure vancomycin exposure is adequate. This work indicates that pediatric target attainment from an efficacy perspective tends to be adequately reached, although monitoring of kidney function remains important in view of the increased probability of acute kidney injury or acute kidney failure with prolonged vancomycin treatment.
MATERIALS AND METHODS
Experimental design.
This study was a retrospective analysis of pediatric patients treated with vancomycin at a large tertiary pediatric hospital (Great Ormond Street Hospital) in London, United Kingdom. Deidentified data were extracted from electronic health records with ethical approval without the requirement for written informed consent (17/LO/0008). Patients included in the study were hospitalized between 2010 and 2016 and contributed vancomycin drug level, dosing (intravenous infusion for 1 h), and demographic data. For a selection of patients, MIC was available for bloodstream isolates, and these patients were included in the PKPD efficacy study.
Vancomycin assay.
Vancomycin quantification in plasma was undertaken at the Department of Medical Microbiology of the Great Ormond Street Hospital, London, United Kingdom, using Indiko Plus (a CE marked assay). Indiko Plus is fully atomized and uses a quantitative microsphere system immunoassay. The assay is based on the competition between drug in the sample and drug coated onto a microparticle for antibody binding sites and the rate of absorbance change, measured photometrically. The lower and upper limits of detection were 2.0 and 100 μg/ml.
Plasma creatinine assay.
Plasma creatinine was measured using an enzymatic creatinine method on a Vitros 5600 clinical chemistry autoanalyzer (Ortho Clinical Diagnostics, High Wycombe, UK). The assay is traceable to a gas chromatography isotopic dilution mass spectroscopy method and National Institute of Standards and Technology (NIST) SRM 914 creatinine standard reference material. The coefficient of variance for the assay was 2.1% at 76 μmol/liter and 2.5% at 479 μmol/liter. The limit of quantification is 4 μmol/liter.
MIC determination.
The MICs of vancomycin were determined by E-strips (manufactured by Oxoid) and Mueller-Hinton agar. The laboratory has maintained full accreditation with CPA and now UKAS, Ltd., under standard ISO 15189:2012-Medical Laboratories.
Data analysis.
(i) Population pharmacokinetics. Vancomycin concentration-time data transformed into their natural logarithm was modeled using a first-order conditional estimation method with interaction in NONMEM, v.7.3.0, with a gfortran compiler on a Windows 10 operating system. The supporting software packages PsN, v.4.2.0 (https://uupharmacometrics.github.io/PsN/), and R, v.3.2.3 (https://www.r-project.org/), were used for model building.
One- and two-compartment disposition models were tested in combination with bodyweight as the continuous covariate for clearance and volume parameters, with allometric scaling standardized to a 70-kg individual included a priori (34). A sigmoidal maturation factor based on postmenstrual age (PMA) (34) was estimated, and the effect of deviation from age-standardized serum creatinine was also tested using a power model (35, 36). Hierarchical models, developed using the model-building data, were evaluated and compared using normalized prediction distributed error (NPDE) and the objective function (−2× log likelihood) (37). IIV was calculated as , and relative standard errors were derived from nonparametric bootstraps in NONMEM (n = 1,000) as 100 × (standard deviation/mean). The best-performing model was subsequently externally evaluated using a visual predictive check (37) (nsimulations = 2,000) on the test data, and the mean prediction error (MPE) was calculated () with the dependent variable (DV) and individual predictions (IPRED).
(ii) Efficacy. Steady-state vancomycin AUC and trough concentrations after three doses of vancomycin were derived using empirical Bayes estimates (EBE)-parameter estimates for all patients in the training data set and test data set who also contributed MIC data. Logistic regression (P < 0.05) was used to identify the impact of vancomycin trough concentration, AUC, creatinine levels, body weight, and postnatal age on treatment efficacy, and breakpoints were identified using classification and regression tree analysis (P < 0.05) (38). Besides a full classification and regression tree analysis for treatment efficacy, another classification and regression tree analysis was performed. AUC/MIC was excluded in the latter analysis to identify break points relevant for clinical use when Bayesian forecasting software is not available.
(iii) Nephrotoxicity. Under the assumption that cumulative drug exposure is important for nephrotoxicity development, vancomycin AUCCUM during the first treatment episode was derived using EBE-parameter estimates for all patients in the training data set and test data set, where treatment episode was defined as a period of continuous vancomycin treatment of 48 h or longer without disruption. Logistic regression (P < 0.05) was conducted to identify the impact of AUCCUM, baseline creatinine, postnatal age, and concomitant therapy with aminoglycosides, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), cyclosporine, and colistin on acute kidney injury (50% increase from baseline) and acute kidney failure (75% increase from baseline) (19). A predictive logistic regression model (P < 0.05) was developed using the variables that were significantly associated with acute kidney injury or acute kidney failure.
(iv) Dose optimizations. Dose optimizations were carried out aiming to optimize target attainment, with an AUC of >400 h·mg/liter in a virtual patient population (n = 750), comprising demographics from patients in the training and test data sets with baseline creatinine levels of ≥15 μmol/liter. Vancomycin AUC after 15 mg/kg q8h for children 0 to 1 month of age, 10 to 15 mg/kg q6h for children 1 month to 11 years, and 15 to 20 mg/kg q8h to q12h for children 12 years and above (1) first were simulated (n = 2,000) to elucidate the impact of body weight and creatinine in four distinct age ranges, i.e., 0 to 1 month (n = 47), 1 month to 2 years (n = 231), 2 to 12 years (n = 380), and older than 12 years (n = 92).
Subsequently, dosages were refined based on the baseline plasma creatinine band to ensure adequate exposure throughout the virtual patient population. The probability on an AUC of >400 mg·h/liter was compared between the standard and optimized treatments using 2,000 stochastic simulations of the identical virtual patient population characteristics, as used for exploratory purposes. Corresponding eGFR for each of the age and baseline plasma creatinine level groups was calculated using the Schwartz formula, (k × height)/plasma creatinine, with 0.413 for k, height in centimeters, and plasma creatinine in milligrams per deciliter (27). Average height for age, derived from WHO tables, was 51.9, 76.1, 121, and 168 cm for children 0 to 1 month of age, 1 month to 2 years, 2 to 12 years, and >12 years, respectively (39, 40). Median plasma creatinine was 0.255, 0.452, 0.792, and 1.02 mg/dl for the (15 to 30], (30 to 50], (50 to 90), and >90 μmol/liter baseline creatinine band, respectively (in creatinine band notation, a parenthesis indicates equal to or larger than the value, and a bracket indicates less than the value). Dose optimizations for patients with creatinine levels of <10 μmol/liter and >100 μmol/liter were considered unreliable and therefore should be interpreted with caution.
Predictive generalized linear models for acute kidney injury or acute kidney failure and AUCCUM, as well as cyclosporine concomitant therapy, were used to evaluate the nephrotoxicity risk profile during 10 days of treatment with the optimized vancomycin dosing schedule in a virtual patient population having baseline creatinine levels of ≥15 μmol/liter and for which concomitant therapy data were available (n = 680).
Supplementary Material
ACKNOWLEDGMENTS
No specific funding was received for this study. F.K. (MR/P014534/1) and J.F.S. (MR/M008665/1) have conducted the research as part of their Medical Research Council fellowships. The Medical Research Council had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Support at the institutional level came from the National Institute for Health Research Biomedical Research Center at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. L.F.H. is supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King’s College Hospital NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. We have no competing interests to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00067-19.
REFERENCES
- 1.BNF. 2018. BNF for children 2018-2019. BMJ Group, London, United Kingdom. [Google Scholar]
- 2.Anderson BJ, Allegaert K, Van den Anker JN, Cossey V, Holford NH. 2007. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol 63:75–84. doi: 10.1111/j.1365-2125.2006.02725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilhaumou R, Marsot A, Dupouey J, Galambrun C, Boulamery A, Coze C, Simon N, Andre N. 2016. Pediatric patients with solid or hematological tumor disease: vancomycin population pharmacokinetics and dosage optimization. Ther Drug Monit 38:559–566. doi: 10.1097/FTD.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 4.Lanke S, Yu T, Rower JE, Balch AH, Korgenski EK, Sherwin CM. 2017. AUC-guided vancomycin dosing in adolescent patients with suspected sepsis. J Clin Pharmacol 57:77–84. doi: 10.1002/jcph.782. [DOI] [PubMed] [Google Scholar]
- 5.Lo YL, van Hasselt JG, Heng SC, Lim CT, Lee TC, Charles BG. 2010. Population pharmacokinetics of vancomycin in premature Malaysian neonates: identification of predictors for dosing determination. Antimicrob Agents Chemother 54:2626–2632. doi: 10.1128/AAC.01370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockmann C, Sherwin CM, Zobell JT, Lubsch L, Young DC, Olson J, Noyes BE, Ampofo K, Spigarelli MG. 2013. Population pharmacokinetics of intermittent vancomycin in children with cystic fibrosis. Pharmacotherapy 33:1288–1296. doi: 10.1002/phar.1320. [DOI] [PubMed] [Google Scholar]
- 7.Zhao W, Zhang D, Fakhoury M, Fahd M, Duquesne F, Storme T, Baruchel A, Jacqz-Aigrain E. 2014. Population pharmacokinetics and dosing optimization of vancomycin in children with malignant hematological disease. Antimicrob Agents Chemother 58:3191–3199. doi: 10.1128/AAC.02564-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song L, He CY, Yin NG, Liu F, Jia YT, Liu Y. 2017. A population pharmacokinetic model for individualized dosage regimens of vancomycin in Chinese neonates and young infants. Oncotarget 8:105211–105221. doi: 10.18632/oncotarget.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frymoyer A, Stockmann C, Hersh AL, Goswami S, Keizer RJ. 27 December 2017. Individualized empiric vancomycin dosing in neonates using a model-based approach. J Pediatric Infect Dis Soc doi: 10.1093/jpids/pix109. [DOI] [PubMed] [Google Scholar]
- 10.Stockmann C, Hersh AL, Roberts JK, Bhongsatiern J, Korgenski EK, Spigarelli MG, Sherwin CM, Frymoyer A. 2015. Predictive performance of a vancomycin population pharmacokinetic model in neonates. Infect Dis Ther 4:187–198. doi: 10.1007/s40121-015-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le J, Bradley JS, Murray W, Romanowski GL, Tran TT, Nguyen N, Cho S, Natale S, Bui I, Tran TM, Capparelli EV. 2013. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J 32:e155–e163. doi: 10.1097/INF.0b013e318286378e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother 55:601–607. doi: 10.1093/jac/dki079. [DOI] [PubMed] [Google Scholar]
- 13.Germovsek E, Barker CIS, Sharland M, Standing JF. 2019. Pharmacokinetic-pharmacodynamic modeling in pediatric drug development, and the importance of standardized scaling of clearance. Clin Pharmacokinet 58:39–52. doi: 10.1007/s40262-018-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 15.De Cock PA, Desmet S, De Jaeger A, Biarent D, Dhont E, Herck I, Vens D, Colman S, Stove V, Commeyne S, Vande Walle J, De Paepe P. 2017. Impact of vancomycin protein binding on target attainment in critically ill children: back to the drawing board? J Antimicrob Chemother 72:801–804. doi: 10.1093/jac/dkw495. [DOI] [PubMed] [Google Scholar]
- 16.Lodise TP, Drusano GL, Zasowski E, Dihmess A, Lazariu V, Cosler L, McNutt LA. 2014. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: how much is enough? Clin Infect Dis 59:666–675. doi: 10.1093/cid/ciu398. [DOI] [PubMed] [Google Scholar]
- 17.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 18.Britt NS, Patel N, Horvat RT, Steed ME. 2016. Vancomycin 24-hour area under the curve/minimum bactericidal concentration ratio as a novel predictor of mortality in methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 60:3070–3075. doi: 10.1128/AAC.02714-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soler YA, Nieves-Plaza M, Prieto M, García-De Jesús R, Suárez-Rivera M. 2013. Pediatric risk, injury, failure, loss, end-stage renal disease score identifies acute kidney injury and predicts mortality in critically ill children: a prospective study. Pediatr Crit Care Med 14:e189–e195. doi: 10.1097/PCC.0b013e3182745675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative Workgroup. 2004. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippone EJ, Kraft WK, Farber JL. 2017. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 102:459–469. doi: 10.1002/cpt.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le J, Ny P, Capparelli E, Lane J, Ngu B, Muus R, Romanowski G, Vo T, Bradley J. 2015. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc 4:e109–e116. doi: 10.1093/jpids/piu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zasowski EJ, Murray KP, Trinh TD, Finch NA, Pogue JM, Mynatt RP, Rybak MJ. 2018. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother 62:e01684-17. doi: 10.1128/AAC.01684-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savic RM, Karlsson MO. 2009. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J 11:558–569. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worth LJ, Daley AJ, Spelman T, Bull AL, Brett JA, Richards MJ. 2018. Central and peripheral line-associated bloodstream infections in Australian neonatal and pediatric intensive care units: findings from a comprehensive Victorian surveillance network, 2008-2016. J Hosp Infect 99:55–61. doi: 10.1016/j.jhin.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Müller M, Siegert S. 2017. pROC: display and analyze ROC curves, v1.10.0. https://cran.r-project.org/web/packages/pROC/index.html.
- 27.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. 2009. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Germovsek E, Kent A, Metsvaht T, Lutsar I, Klein N, Turner MA, Sharland M, Nielsen EI, Heath PT, Standing JF. 2016. Development and evaluation of a gentamicin pharmacokinetic model that facilitates opportunistic gentamicin therapeutic drug monitoring in neonates and infants. Antimicrob Agents Chemother 60:4869–4877. doi: 10.1128/AAC.00577-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, Chatelut E, Grubb A, Veal GJ, Keir MJ, Holford NH. 2009. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 24:67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 30.Smertka M, Wroblewska J, Suchojad A, Majcherczyk M, Jadamus-Niebroj D, Owsianka-Podlesny T, Brzozowska A, Maruniak-Chudek I. 2014. Serum and urinary NGAL in septic newborns. Biomed Res Int 2014:717318. doi: 10.1155/2014/717318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. 2011. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr 158:422–426. doi: 10.1016/j.jpeds.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 33.Marsot A, Boulamery A, Bruguerolle B, Simon N. 2012. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet 51:1–13. doi: 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Germovsek E, Barker CI, Sharland M, Standing JF. 2017. Scaling clearance in pediatric pharmacokinetics: all models are wrong, which are useful? Br J Clin Pharmacol 83:777–790. doi: 10.1111/bcp.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson AM, Hill N, Perisoglou M, Whelan J, Karlsson MO, Standing JF. 2011. A population pharmacokinetic/pharmacodynamic model of methotrexate and mucositis scores in osteosarcoma. Ther Drug Monit 33:711–718. doi: 10.1097/FTD.0b013e31823615e1. [DOI] [PubMed] [Google Scholar]
- 36.Hennig S, Standing JF, Staatz CE, Thomson AH. 2013. Population pharmacokinetics of tobramycin in patients with and without cystic fibrosis. Clin Pharmacokinet 52:289–301. doi: 10.1007/s40262-013-0036-y. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen THT, Mouksassi M-S, Holford N, Al-Huniti N, Freedman I, Hooker AC, John J, Karlsson MO, Mold DR, Pérez Ruixo JJ, Plan EL, Savic R, van Hasselt JGC, Weber B, Zhou C, Comets E, Mentré F, Model Evaluation Group of the International Society of Pharmacometrics Best Practice Committee. 2017. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol 6:87–109. doi: 10.1002/psp4.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Therneau T, Atkinson B, Ripley B. 2017. rpart: recursive partitioning and regression trees, v4.1-11 https://CRAN.R-project.org/package=rpart.
- 39.Anonymous. 2009. WHO child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children's Fund, Geneva. WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 40.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. 2007. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.