Critically ill patients are frequently treated with empirical antibiotic therapy, including vancomycin and β-lactams. Recent evidence suggests an increased risk of acute kidney injury (AKI) in patients who received a combination of vancomycin and piperacillin-tazobactam (VPT) compared with patients who received vancomycin alone or vancomycin in combination with cefepime (VC) or meropenem (VM), but most studies were conducted predominately in the non-critically ill population.
KEYWORDS: acute kidney injury, cefepime, meropenem, piperacillin-tazobactam, vancomycin
ABSTRACT
Critically ill patients are frequently treated with empirical antibiotic therapy, including vancomycin and β-lactams. Recent evidence suggests an increased risk of acute kidney injury (AKI) in patients who received a combination of vancomycin and piperacillin-tazobactam (VPT) compared with patients who received vancomycin alone or vancomycin in combination with cefepime (VC) or meropenem (VM), but most studies were conducted predominately in the non-critically ill population. A retrospective cohort study that included 2,492 patients was conducted in the intensive care units of a large university hospital with the primary outcome being the development of any AKI. The rates of any AKI, as defined by the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, were 39.3% for VPT patients, 24.2% for VC patients, and 23.5% for VM patients (P < 0.0001 for both comparisons). Similarly, the incidences of stage 2 and stage 3 AKI were also significantly higher for VPT patients than for the patients in the other groups. The rates of stage 2 and stage 3 AKI, respectively, were 15% and 6.6% for VPT patients, 5.8% and 1.8% for VC patients, and 6.6% and 1.3% for VM patients (P < 0.0001 for both comparisons). In multivariate analysis, the use of vancomycin in combination with piperacillin-tazobactam was found to be an independent predictor of AKI (odds ratio [OR], 2.161; 95% confidence interval [CI], 1.620 to 2.883). In conclusion, critically ill patients receiving the combination of VPT had the highest incidence of AKI compared to critically ill patients receiving either VC or VM.
TEXT
Critically ill patients commonly present with sepsis and with hospital-acquired infections often requiring empirical antimicrobial therapy comprised of vancomycin in combination with an antipseudomonal beta-lactam (1, 2). These patients are also exposed to a multitude of factors, such as hemodynamic shock and nephrotoxins, which put them at high risk for acute kidney injury (AKI), an outcome associated with increased morbidity and mortality (3–8).
The current literature shows an association between the combination of vancomycin and piperacillin-tazobactam (VPT) and an increased risk of AKI, although the mechanism of this association has not been well characterized (9–25). The increased incidence of AKI with VPT has been observed in comparisons with patients who received vancomycin alone, vancomycin in combination with cefepime (VC), and vancomycin in combination with meropenem (VM) in both the adult and pediatric patient populations (9–25). Available literature supports this association in non-critically ill patient populations, but limited studies in the critically ill population have failed to demonstrate an increased risk of AKI with VPT (14, 24, 26–29).
Other areas of clinical interest and need (which have not fully been previously assessed) include patients receiving vancomycin in combination with a carbapenem and patients with baseline renal dysfunction. The objective of the study was to determine the incidence of AKI in critically ill patients receiving vancomycin in combination with piperacillin-tazobactam, cefepime, or meropenem.
RESULTS
Baseline characteristics.
A total of 4,165 patients were identified as eligible for evaluation (see Fig. S1 in the supplemental material). The major reasons for exclusion were receipt of multiple beta-lactams (1,021 patients) and receipt of dialysis prior to admission (268 patients) or within the first 48 h of admission (217 patients). After all exclusion criteria were applied, 2,492 patients were included in the analysis (1,734 in the VC arm, 366 in the VPT arm, and 392 in the VM arm).
Table 1 summarizes the baseline characteristics used for the comparisons between groups. Overall baseline characteristics were fairly well matched. Notable exceptions included intensive care unit (ICU) location, with VPT use being more common in the surgical/burn/trauma ICU (SICU) and VC and VM use being more common in the medical ICU (MICU). More patients in the VPT group had sepsis, systolic blood pressure of <90 mm Hg, and vasopressor use at baseline than in the VC group. Patients in the VM group had higher modified acute physiology and chronic health evaluation II (APACHE II) scores and a higher Charlson comorbidity index value. Initial vancomycin doses were similar between groups, although they were higher in the VPT group than in the group of patients receiving VM. Patients receiving VPT had a higher initial vancomycin trough than the VC group, although this difference was clinically negligible. Antibiotic durations were similar for both comparisons.
TABLE 1.
Baseline characteristicsa
| Patient baseline characteristic | Value(s) |
|||||
|---|---|---|---|---|---|---|
| VPT (n = 366) |
VC (n= 1,734) |
P | VPT (n = 366) |
VM (n = 392) |
P | |
| Age ± SD | 56.7 ± 16.8 | 58.6 ± 17.4 | 0.072 | 56.7 ± 16.8 | 58.1 ± 15.5 | 0.250 |
| No. (%) of males | 189 (51.6) | 938 (54.1) | 0.392 | 189 (51.6) | 221 (56.4) | 0.191 |
| Body weight, kg ± SD | 87.9 ± 32.5 | 82.1 ± 87.9 | 0.002 | 87.9 ± 32.5 | 83.6 ± 32.5 | 0.055 |
| ICU location (%) | <0.0001 | <0.0001 | ||||
| MICU | 105 (28.7) | 829 (47.8) | 105 (28.7) | 249 (63.5) | ||
| SICU | 248 (67.8) | 523 (30.2) | 248 (67.8) | 104 (26.5) | ||
| NNICU | 13 (3.6) | 382 (22.0) | 13 (3.6) | 39 (9.9) | ||
| APACHE II [IQR] | 16.02 [12.02–20.01] | 16.01 [12.02–20.01] | 0.855 | 16.02 [12.02–20.01] | 17.01 [13.02–21.01] | 0.043 |
| Charlson score [IQR] | 3 [1–5] | 3 [1–6] | 0.141 | 3 [1–5] | 3 [2–6] | 0.005 |
| No. (%) with sepsis | 205 (56.0) | 688 (39.7) | <0.0001 | 205 (56.0) | 213 (54.3) | 0.643 |
| No. (%) with mechanical ventilation | 189 (51.6) | 913 (52.7) | 0.724 | 189 (51.6) | 203 (51.8) | 0.968 |
| No. (%) with SBP < 90 mm Hg | 271 (74.0) | 1097 (63.3) | <0.0001 | 271 (74.0) | 285 (72.7) | 0.677 |
| No. (%) with vasopressor use | 187 (51.1) | 783 (45.2) | 0.038 | 187 (51.1) | 218 (55.6) | 0.213 |
| No. (%) with nephrotoxin exposure | ||||||
| No. of concomitant nephrotoxins ≥ 1 | 278 (76.0) | 1,353 (78.0) | 0.387 | 278 (76.0) | 324 (82.7) | 0.023 |
| No. of concomitant nephrotoxins ≥ 2 | 152 (41.5) | 741 (42.7) | 0.672 | 152 (41.5) | 179 (45.7) | 0.252 |
| No. of concomitant nephrotoxins ≥ 3 | 63 (17.2) | 289 (16.7) | 0.799 | 63 (17.2) | 73 (18.6) | 0.613 |
| Baseline WBC [IQR] | 15.5 [11.2–21.4] | 12.8 [8.9–18.3] | <0.0001 | 15.5 [11.2–21.4] | 14.3 [9.4–20.3] | 0.747 |
| Baseline CrCl [IQR] | 65.5 [46.8–94.0] | 69.0 [47.0–90.0] | 0.902 | 65.5 [46.8–94.0] | 65.0 [46.0–88.0] | 0.185 |
| No. (%) with CHF | 67 (18.3) | 457 (26.4) | 0.001 | 67 (18.3) | 101 (25.8) | 0.013 |
| No. (%) with COPD | 123 (33.6) | 623 (35.9) | 0.399 | 123 (33.6) | 128 (32.7) | 0.780 |
| No. (%) with diabetes | 86 (23.5) | 341 (19.7) | 0.098 | 86 (23.5) | 80 (20.4) | 0.304 |
| No. (%) with severe hepatitis | 21 (5.7) | 73 (4.2) | 0.199 | 21 (5.7) | 41 (10.5) | 0.018 |
| Initial vancomycin dose (mg) per 24 h [IQR] | 2,000 [1,750–3,000] | 2,000 [1,500–3,000] | 0.087 | 2,000 [1,750–3,000] | 2,000 [1,500–2,500] | 0.002 |
| Initial vancomycin trough (μg/ml) [IQR] | 12.0 [8.1–18.0] | 11.6 [7.9–17] | 0.034 | 12.0 [8.1–18.0] | 12.0 [8.2–18.1] | 0.804 |
| No. of days of antibiotic therapy [IQR] | 4.0 [3.0–5.0] | 3.0 [3.0–5.0] | 0.643 | 4.0 [3.0–5.0] | 3.0 [2.0–5.75] | 0.236 |
P values are shown for comparisons made separately between VPT and VC and between VPT and VM. Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; IQR, interquartile range; MICU, medical intensive care unit; NNICU, neurosurgical/neurological intensive care unit; SBP, systolic blood pressure; SICU, surgical/burn/trauma intensive care unit; VC, vancomycin plus cefepime; VM, vancomycin plus meropenem; VPT, vancomycin plus piperacillin-tazobactam; WBC, white blood cell count.
Primary outcome.
The incidence of AKI was significantly higher in patients receiving the combination of VPT in both comparisons (39.3% for patient receiving VPT, 24.2% for patients receiving VC, and 23.5% for patients receiving VM; P < 0.0001 for both comparisons). Unadjusted odds ratios for any AKI were higher in patients receiving VPT compared with VC (OR, 2.04; 95% confidence interval [CI], 1.61 to 2.58) and were higher in patients receiving VPT compared with VM (OR, 2.12; 95% CI, 1.61 to 2.90). Similarly, the incidence of stage 2 and stage 3 AKI was also significantly higher for patients receiving the combination of VPT versus VC and VM (Table 2).
TABLE 2.
Outcomes associated with the combination of vancomycin and beta-lactamsa
| Patient characteristic or outcome |
Value(s) |
|||||
|---|---|---|---|---|---|---|
| VPT (n = 366) |
VC (n= 1,734) |
Pb | VPT (n = 366) |
VM (n = 392) |
Pb | |
| No. (%) with any AKI | 144 (39.3) | 419 (24.2) | <0.0001 | 144 (39.3) | 92 (23.5) | <0.0001 |
| No. (%) with stage 2 AKI | 55 (15.0) | 101 (5.8) | <0.0001 | 55 (15.0) | 26 (6.6) | <0.0001 |
| No. (%) with stage 3 AKI | 24 (6.6) | 31 (1.8) | <0.0001 | 24 (6.6) | 5 (1.3) | <0.0001 |
| No. (%) with RRT | 4 (1.1) | 21 (1.2) | 1.000 | 4 (1.1) | 6 (1.5) | 0.754 |
| Day of AKI occurrence | 3.2 ± 2.6 | 2.6 ± 2.5 | 0.030 | 3.2 ± 2.6 | 2.2 ± 2.1 | 0.003 |
| ICU LOS, h [IQR] | 82.5 [46.74–151.0] | 94.0 [46.0–214.5] | <0.0001 | 82.5 [46.74–151.0] | 94.0 [53.0–210.75] | 0.001 |
| ICU LOS – AKI subgroup LOS | 93 [51–168] | 107 [58–223] | 0.013 | 93 [51–168] | 110 [60–220] | 0.058 |
| Hospital LOS, days [IQR] | 9.0 [6.0–17.0] | 9.0 [5.0–16.0] | 0.558 | 9.0 [6.0–17.0] | 10.0 [6–17.75] | 0.236 |
| No. (%) with hospital mortality | 42 (11.5) | 265 (15.3) | 0.061 | 42 (11.5) | 67 (17.1) | 0.028 |
| No. (%) with hospital mortality –no. (%) in AKI subgroup | 25 (17.4) | 111 (26.4) | 0.018 | 25 (17.4) | 26 (28.3) | 0.057 |
Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; OR, odds ratio; RRT, renal replacement therapy; VC, vancomycin plus cefepime; VM, vancomycin plus meropenem; VPT, vancomycin plus piperacillin-tazobactam.
P values are shown for comparisons made separately between VPT and VC (OR [95% CI], 2.04 [1.61 to 2.58]) and between VPT and VM (OR, 2.12 [1.61 to 2.90]). Unadjusted bivariate odds ratio values represent the primary outcome for any AKI. Comparisons were made separately between VPT and VC and between VPT and VM.
Secondary outcomes.
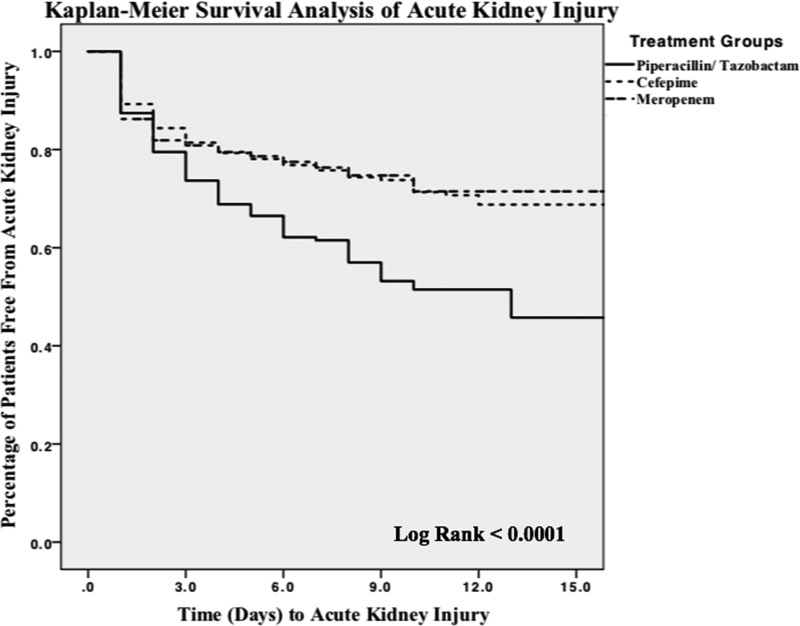
The median time to the occurrence of AKI was significantly longer in patients receiving VPT (3.2 days) than in patients receiving VC (2.6 days), p = 0.03, and VM (2.2 days), p = 0.003. Survival analysis of the time to AKI (Fig. 1) demonstrated a significantly increased risk of AKI in the VPT group.
FIG 1.
Kaplan-Meier survival analysis for acute kidney injury.
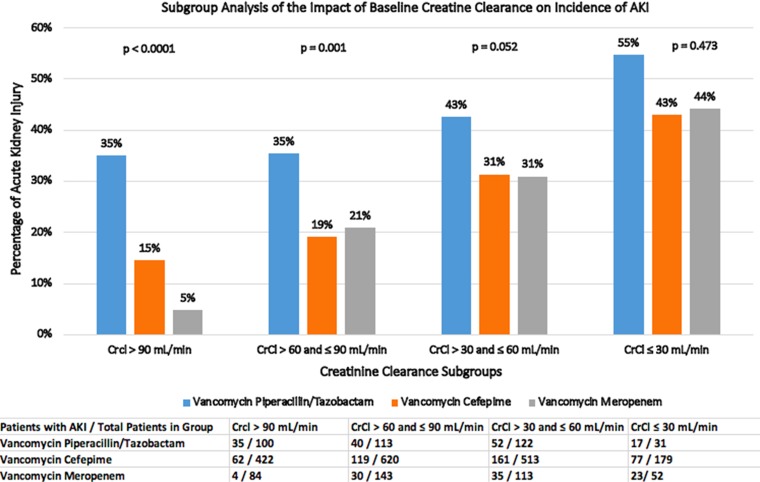
AKI rates based on the four quartiles of baseline creatinine clearance (CrCl) showed that patients in both quartiles of CrCl values of >90 as well as ≤90 and >60 ml/min with VPT receipt were found to have significantly increased AKI rates compared with VC or VM receipt (Fig. 2). A subgroup analysis separating patients into three groups based on the initial vancomycin trough concentration found an increased incidence of AKI with progressively higher initial vancomycin troughs in all three combination groups (Fig. S2). Patients with initial vancomycin trough concentrations of ≤20 or >15 μg/ml and >20 μg/ml had higher rate of AKI than those with concentrations of ≤15 μg/ml (P < 0.0001 in both analyses).
FIG 2.
Acute kidney injury rates as a function of baseline creatinine clearance.
The overall ICU length of stay (LOS) was significantly greater in both the VC and VM groups than in the VPT group (Table 2). Among survivors, those who developed an AKI during the admission had a longer ICU LOS (145 versus 162 h, P = 0.037) and hospital LOS (13.9 versus 15.3 days, P = 0.011) than those who did not develop AKI. Overall hospital mortality rates were similar in the patients receiving VPT and those receiving VC; however, they were significantly higher in patients receiving VM than in those receiving VPT (Table 2). In all patients, hospital mortality rates were highest in the MICU versus the neurosurgical/neurology ICU and the SICU (17.3% versus 14.7% and 12%, respectively, P = 0.0037).
Multivariate analysis.
Any-stage AKI occurred in 655 (26.2%) patients. In the bivariate analysis of patients who did and did not have an AKI, the following factors were found to be statistically significant: modified APACHE II score, use of one or more concomitant nephrotoxic agents, higher initial vancomycin trough concentration, baseline CrCl value of ≤60 ml/min, and VPT (see Table S1 in the supplemental material).
In the multivariate regression analysis, the following independent variables were significantly associated with AKI: VPT, baseline CrCl ≤ 60 ml/min, vasopressor use, initial vancomycin trough concentration of >20 mg/dl, and baseline Charlson comorbidity index (Table 3).
TABLE 3.
Multivariate analysis of independent predictors of acute kidney injury in the combined populationa
| Covariate | Odds ratio | 95% CI |
|---|---|---|
| Vancomycin plus piperacillin-tazobactam | 2.161 | 1.620–2.883 |
| Baseline CrCl ≤ 60 ml/min | 2.220 | 1.791–2.751 |
| Vasopressor use | 1.877 | 1.512–2.329 |
| Initial vancomycin trough > 20 μg | 1.680 | 1.281–2.205 |
| Charlson comorbidity index | 1.051 | 1.012–1.091 |
Variables included in model: use of piperacillin-tazobactam, cefepime, or meropenem; baseline creatinine clearance below 60 ml/min; use of one or more vasopressor agent; admission to SICU; Charlson comorbidity index; initial vancomycin trough value of >20. Backwards logistic regression was used for multivariate analysis (Hosmer and Lemeshow test value = 0.569).
DISCUSSION
Our results demonstrate that critically ill patients receiving the combination of VPT have an increased risk of AKI compared to those receiving the combination of VC or VM. Our results suggest that the receipt of VPT is associated with an approximately 15% increase for any AKI, 10% increase for stage 2 AKI, and 5% increase for stage 3 AKI. Additionally, in multivariate analysis of the entire cohort, the combination of vancomycin and piperacillin-tazobactam had the highest odds ratio for AKI in this critically ill population.
The mechanism by which VPT induces AKI has not been well characterized. It has been thought that vancomycin may cause direct proximal tubular toxicity and/or result in the formation of obstructive tubular casts (30, 31). The concomitant use of VPT has been hypothesized to potentiate AKI via acute interstitial nephritis or decreased secretion of creatinine and vancomycin, but further investigation is warranted to better elucidate the mechanism (32–34).
Previous studies that identified the combination of VPT as a risk factor for AKI have focused predominantly in a non-critically ill patient population (9–25), with only three studies evaluating this interaction solely in the adult critically ill patient population (14, 24, 29). Those studies did not identify a difference in nephrotoxicity between populations of patients receiving combinations of vancomycin and various beta-lactams. Unfortunately, two of the studies were underpowered for the critically ill patient population (n = 122 and 333), with many competing risk factors for AKI (14, 29). One of the studies evaluated the brief (<72 h) use of combination therapy in critically ill patients (24). Recent meta-analyses were also unable to adequately assess the interaction of VPT in the critically ill patient population due to a lack of adequate data (26–28).
Our study had several strengths. We believe that our study was able to address previous study limitations through the use of strict enrollment criteria, large patient enrollment for analysis, and the evaluation of at least 48 h of combination therapy. The previous finding that the use of short (<72-h) courses of combination therapy in critically ill patients does not confer a risk of AKI (29) may also be supported by our analysis, as our Kaplan-Meier curve data suggest that the observed increase in risk of AKI occurs at or after 72 h of therapy.
Our study included the largest patient population with baseline renal dysfunction, defined as CrCl ≤60 ml/min. Although patients with baseline renal dysfunction are assumed to be at an increased risk of AKI, patients with baseline renal dysfunction have previously been excluded from analysis. Our analysis suggests that patients with a baseline CrCl level of ≤30 ml/min have the highest incidence of AKI with VPT, with an incidence rate of 55%. This outcome, we believe, is of particular clinical importance due to the increasing prevalence of chronic kidney disease in the general population and the risks of chronic kidney disease progressing to end-stage renal disease in critical illness (35, 36).
Our study also specifically examined patients who received the combination of VM as a separate comparison group. Only two previous studies have adequately studied the combination of VM as a separate analysis, with the other previous analyses assuming the rates of AKI to be similar to those seen with VC (22, 24). Our results are consistent with a recent large analysis showing higher rates of AKI with VPT than with VM (22). We believe that our results provide additional data supporting the use of VM but recommend caution with respect to the extrapolation of these results to other carbapenems, such as ertapenem or imipenem/cilastatin, without further investigation. The findings of the use of VM resulting in a reduced rate of AKI compared to VPT and of a similar incidence of AKI compared to VC can be of significant clinical importance when possible alternatives to the combination of VPT are needed.
Our overall results are consistent with previous studies in predominantly non-critically ill patients, albeit with a higher baseline AKI incidence rate due to the critically ill patient population being evaluated. The results of our study are also consistent with previous analyses examining AKI survival (21). Our Kaplan-Meier survival analysis suggests a clear separation in the rates of survival of AKI at day 3 that continues and increases with each additional day of therapy. Although our median time to AKI was significantly shorter with VC and VM than with VPT, this was likely due to confounding factors such as other causes of AKI and the sicker patient populations in the VC and VM groups.
Additionally, our vancomycin trough analysis suggests that the incidence of AKI continued to be high in patients receiving VPT, despite low initial vancomycin trough levels. This finding supports the possibility that the discordant literature on vancomycin nephrotoxicity may be due to the lack of reporting or control for concomitant antipseudomonal agents (21). The increased rates of AKI with higher initial vancomycin trough concentrations are also consistent with what has been previously reported (37).
Finally, the similar incidences of AKI in patients receiving VC and VM provide additional validation that the increased risk associated with VPT is not a result of confounding variables. In addition, the finding of increased risk of AKI with VPT in both comparison groups and the finding in multivariate analysis of the entire cohort represent strong validation for this association.
Our study had limitations that should be noted. First, our definition of AKI did not include urine output assessment, as we were unable to adequately and consistently assess this information retrospectively. Previous analysis of AKI with and without urine output assessment has shown a higher rate of diagnosis of AKI with urine output assessment (38); thus, our results may represent an underestimation of the risk. While the data may represent underestimations, the majority of previous analyses of this interaction have used a definition without urine output assessment; thus, our results are consistent with existing VPT literature (9–25).
Second, the possibility of selection bias due to differing indications and the differences in the critically ill patient populations cannot be ruled out; however, each subgroup of patient populations evaluated showed no difference in AKI rates based on ICU location and any ICU location difference in bivariate analysis was accounted for in the multivariate analysis without a significant finding. In addition, mortality rates were the highest in the MICU population, indicating a potentially sicker population even with lower VPT use.
Third, this was a single-center retrospective cohort analysis that included a disproportionate number of patients receiving the combination of VC. This was due to cefepime being the primary antipseudomonal beta-lactam agent at our institution. The decision to include all patients who met inclusion and exclusion criteria was made to decrease the likelihood of chance findings in our analysis. Additionally, our institutional practice utilizes intermittent dosing of antibiotics; thus, our report does not provide any additional data to support the theory that prolonged or continuous infusions would result in reduced risk of AKI (39).
Fourth, although we controlled for confounding variables in our study, several notable confounders need further clarification. The number of nephrotoxic agents received by each patient included the cumulative number of agents that met our definition; however, we recognize that individual agents are likely to have differing degrees of nephrotoxicity. We were also unable to collect information on contrast dye administration; thus, it is not accounted for in our analysis. Additionally, our study included a large number of patients with sepsis and also encompassed a study period that included a change in the Surviving Sepsis Campaign Guidelines. This may have impacted fluid management in the patient being treated with sepsis and may have resulted in differences in management at differing points in time. However, our institutional order set for sepsis and fluid management has been previously published (40) and remained unchanged during this time period, in addition to our use of dynamic fluid assessment techniques to assess fluid responsiveness.
Finally, we were unable to demonstrate consistent differences in patient-centered outcomes with respect to ICU LOS and overall morality between comparison groups. Despite this lack of consistent differences, which can be affected by numerous factors, including bed availability, the patients in each group with an AKI were consistently shown to have a higher in-hospital mortality rate, ICU LOS, and overall hospital LOS than those in the overall group. This association again highlights the importance of minimizing AKI risk factors for critically ill patients.
Conclusion.
Patients experiencing critical illness have multiple risks for AKI which have previously been shown to have effects on morbidity, mortality, and ICU LOS (3–8). In this study of critically ill patients, VPT was associated with an increased incidence of AKI compared separately with VC and with VM. Multivariate analysis of the entire cohort found the combination of VPT to be the strongest predictor of AKI. This analysis also found patients receiving VPT with a baseline CrCl rate of ≤30 ml/min to be at the highest risk of AKI. In critically ill patients requiring empirical antimicrobial coverage with vancomycin and an antipseudomonal agent, clinicians should give strong consideration to institutional antimicrobial resistance patterns, likely causative pathogens, and patient risk of AKI and should consider the use of agents other than the combination of vancomycin and piperacillin-tazobactam.
MATERIALS AND METHODS
Study settings and design.
This was a retrospective cohort study that was conducted at Barnes-Jewish Hospital, a tertiary referral medical center in St. Louis, MO, with 1,300 adult beds and 155 intensive care unit (ICU) beds. The institutional review boards of Washington University and St. Louis College of Pharmacy approved the initiation and design of this study prior to the start of data collection.
Patient population.
Patients admitted between 1 August 2014 and 1 August 2017 were evaluated. Patients were eligible to be included in the study if they were ≥18 years of age, were admitted to the medical ICU (MICU), surgical/burn/trauma ICU (SICU), or neurosurgical/neurology ICU (NNICU), and had received the combination of VPT, VC, or VM for ≥48 h with each agent initiated within 24 h of initiation of the other. Additionally, the patient was required to have a baseline serum creatinine (SCr) value during the evaluated hospitalization prior to or at initiation of antibiotic administration.
Patients were excluded if they had a history of dialysis at any point prior to admission, if dialysis was initiated within 48 h of antibiotic initiation, if the patient had a history of cystic fibrosis or had been started on vancomycin plus a beta-lactam agent and the beta-lactam agent had then been changed to another beta-lactam during the same ICU admission. The first hospital admission and the first ICU encounter were used for patients with repeat hospital and ICU admissions. All other admissions were excluded, and only the first administration of combination therapy during the hospitalization was assessed.
Study outcomes.
The primary outcome was the development of any AKI, as determined on the basis of the Kidney Disease: Improving Global Outcomes (KDIGO) definition (41), during combination antibiotic therapy and within 72 h after completion of combination antibiotic therapy. Secondary outcomes included time to the development of AKI, the need for subsequent dialysis, AKI rates compared with vancomycin troughs, AKI rates compared with baseline creatinine clearance (CrCl), ICU and hospital length of stay (LOS), hospital mortality, and evaluation of overall risk factors for AKI.
Data collection and definitions.
Data abstracted from medical records included baseline demographics and comorbid conditions, through the Charlson comorbidity index (42). A modified APACHE II scoring that excluded the Glasgow coma score (43) was collected along with diagnoses of sepsis and septic shock, use of vasopressor agents, use of mechanical ventilation, and initial white blood cell count.
Definitions of AKI were based on the respective SCr cutoffs for KDIGO definition stage 1 (an increase in SCr by 1.5× baseline or an increase of ≥0.3mg/dl from baseline), stage 2 (increase in SCr by 2× baseline), and stage 3 (increase in SCr by 3× baseline), without the inclusion of urine output, as these data were not readily available for collection.
Initial vancomycin trough levels, if obtained, were evaluated. Subsequent vancomycin doses were collaboratively adjusted by physicians and pharmacists to target a vancomycin trough similar to those in national guideline recommendations (44). Our institution utilizes intermittent dosing of vancomycin and beta-lactam antimicrobial agents. Data corresponding to the use of additional nephrotoxic agents were collected and categorized, with nephrotoxic medications in this study consisting of aminoglycosides, amphotericin, vasopressors, acyclovir, cidofovir, ganciclovir, foscarnet, chloramphenicol, colistin, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors/angiotensin receptor blocking agents, diuretics, and calcineurin inhibitors.
Statistical analysis.
For the primary outcome of AKI development defined by the KDIGO definition, we estimated that the VPT group would have an increase of at least 10% in AKI compared between groups, as previously reported (14, 45). We determined a sample size based on an estimated baseline AKI rate of 25% in our ICUs, from which we estimated we would need at least 328 patients per group to achieve a statistical power of 80% using an α of 0.05.
The reference group chosen for statistical comparisons was the VPT group. For categorical data, the Pearson χ2 test or Fisher’s exact test was used, as appropriate. For continuous data, the Student's t test and Wilcoxon rank sum tests were used, as appropriate. An odds ratio with 95% confidence intervals was utilized for the primary outcome of any AKI development. For the secondary outcome of time to development of AKI, comparisons were made with log rank tests for survival analysis from any AKI and plotted on a Kaplan-Meier curve.
A prespecified, a priori multivariate analysis was conducted to evaluate risk factors of AKI by separating the entire patient cohort into two groups based on the development of AKI. Using backwards logistic regression analysis to control for confounding baseline characteristics, any characteristic corresponding to results of a comparison between groups with a P value of <0.2 and tested for possible collinearity was included in the model. Statistical analysis was performed by us using SPSS 22 software (SPSS Inc., Chicago, IL, USA), and all two-sided P values of <0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nicholas Hampton (PharmD) for his contributions in assisting with data collection.
The work performed by M.K. was supported by the Barnes-Jewish Hospital Foundation. No funding was utilized in the completion of this study and/or preparation of the manuscript.
All of us have no conflicts of interest to report.
A.M.B., J.N.L., C.M., M.K., S.M., and P.J. all made substantial contributions to the conception or design of the work and to the acquisition, analysis, and/or interpretation of data for the work; drafted and/or revised the work critically for important intellectual content; provided final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02658-18.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson S, Singer M, Thompson S, Townsend B, Van der Poll T, Vincent J-L, Wiersinga W, Zimmerman J, Dellinger R. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 2.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital acquired and ventilator associated pneumonia: 2016 clinical practice guidelines by the Infectious Disease Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. 2005. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 4.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. 2015. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 5.Kellum JA, Murugan R. 2016. Effects of non-severe acute kidney injury on clinical outcomes in critically ill patients. Crit Care 20:159. doi: 10.1186/s13054-016-1295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sileanu FE, Murugan R, Lucko N, Clermont G, Kane-Gill SL, Handler SM, Kellum JA. 2015. AKI in low-risk versus high-risk patients in intensive care. Clin J Am Soc Nephrol 10:187–196. doi: 10.2215/CJN.03200314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perinel S, Vincent F, Lautrette A, Dellamonica J, Mariat C, Zeni F, Cohen Y, Tardy B, Souweine B, Darmon M. 2015. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med 43:e269–e275. doi: 10.1097/CCM.0000000000001077. [DOI] [PubMed] [Google Scholar]
- 8.Wang HE, Muntner P, Chertow GM, Warnock DG. 2012. Acute kidney injury and mortality in hospitalized patients. Am J Nephrol 35:349–355. doi: 10.1159/000337487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess LD, Drew RH. 2014. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin/tazobactam. Pharmacotherapy 34:670–676. doi: 10.1002/phar.1442. [DOI] [PubMed] [Google Scholar]
- 10.Davies SW, Efird JT, Guidry CA, Dietch ZC, Willis RN, Shah PM, Sawyer RG. 2016. Top guns: the “Maverick” and “Goose” of empiric therapy. Surg Infect (Larchmt) 17:38–47. doi: 10.1089/sur.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fodero KE, Horey AL, Krajewski MP, Ruh CA, Sellick JA Jr, Mergenhagen KA. 2016. Impact of an antimicrobial stewardship program on patient safety in veterans prescribed vancomycin. Clin Ther 38:494–502. doi: 10.1016/j.clinthera.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Gao S, Li J, Li Z. 2015. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized children with or without concomitant piperacillin-tazobactam. Fudan Univ J Med Sci 42:743–748. [Google Scholar]
- 13.Gomes DM, Smotherman C, Birch A, Dupree L, Della Vecchia BJ, Kraemer DF, Jankowski CA. 2014. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy 34:662–669. doi: 10.1002/phar.1428. [DOI] [PubMed] [Google Scholar]
- 14.Hammond DA, Smith MN, Painter JT, Meena NK, Lusardi K. 2016. Concomitant vancomycin and piperacillin-tazobactam does not increase acute kidney injury in critically ill patients. Pharmacotherapy 36:463–471. [DOI] [PubMed] [Google Scholar]
- 15.Knoderer CA, Gritzman AL, Nichols KR, Wilson AC. 2015. Late-occurring vancomycin-associated acute kidney injury in children receiving prolonged therapy. Ann Pharmacother 49:1113–1119. doi: 10.1177/1060028015594190. [DOI] [PubMed] [Google Scholar]
- 16.Kim T, Kandiah S, Patel M, Rab S, Wong J, Xue W, Easley K, Anderson AM. 2015. Risk factors for kidney injury during vancomycin and piperacillin/tazobactam administration, including increased odds of injury with combination therapy. BMC Res Notes 8:579. doi: 10.1186/s13104-015-1518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meaney CJ, Hynicka LM, Tsoukleris MG. 2014. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy 34:653–661. doi: 10.1002/phar.1423. [DOI] [PubMed] [Google Scholar]
- 18.Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. 2014. Acute renal failure associated with vancomycin and β-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect 20:O384–O389. doi: 10.1111/1469-0691.12410. [DOI] [PubMed] [Google Scholar]
- 19.McQueen KE, Clark DW. 2016. Does combination therapy with vancomycin and piperacillin-tazobactam increase the risk of nephrotoxicity versus vancomycin alone in pediatric patients? J Pediatr Pharmacol Ther 21:332–338. doi: 10.5863/1551-6776-21.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullins BP, Kramer CJ, Bartel BJ, Catlin JS, Gilder RE. 2018. Comparison of the nephrotoxicity of vancomycin in combination with cefepime, meropenem, or piperacillin/tazobactam: a prospective multicenter study. Ann Pharmacother 52:639–644. doi: 10.1177/1060028018757497. [DOI] [PubMed] [Google Scholar]
- 21.Navalkele B, Pogue JM, Karino S, Nishan B, Salim M, Solanki S, Pervaiz A, Tashtoush N, Shaikh H, Koppula S, Koons J, Hussain T, Perry W, Evans R, Martin ET, Mynatt RP, Murray KP, Rybak MJ, Kaye KS. 2017. Risk of acute kidney injury in patients on concomitant vancomycin and piperacillin-tazobactam compared to those on vancomycin and cefepime. Clin Infect Dis 64:116–123. doi: 10.1093/cid/ciw709. [DOI] [PubMed] [Google Scholar]
- 22.Rutter WC, Burgess DS. 26 June 2018. Incidence of acute kidney injury among patients treated with piperacillin/tazobactam or meropenem in combination with vancomycin. Antimicrob Agents Chemother doi: 10.1128/ACC.00264-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peyko V, Smalley S, Cohen H. 2017. Prospective comparison of acute kidney injury during treatment with the combination of piperacillin-tazobactam and vancomycin versus the combination of cefepime or meropenem and vancomycin. J Pharm Pract 30:209–213. doi: 10.1177/0897190016628960. [DOI] [PubMed] [Google Scholar]
- 24.Schreier DJ, Kashani KB, Sakhuja A, Mara KC, Tootooni MS, Personett HA, Nelson S, Rule AD, Steckelberg JM, Tande AJ, Barreto EF. 24 August 2018. Incidence of acute kidney injury among critically ill patients with brief empiric use of anti-pseudomonal beta-lactams with vancomycin. Clin Infect Dis doi: 10.1093/cid/ciy724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton JD, Mynatt RP, Kaye KS, Murray KP, Rybak MJ, Pogue JM. 2015. Nephrotoxicity comparison of two commercially available generic vancomycin products. Antimicrob Agents Chemother 59:5470–5474. doi: 10.1128/AAC.00388-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. 2018. Vancomycin plus piperacillin/tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med 46:12–20. doi: 10.1097/CCM.0000000000002769. [DOI] [PubMed] [Google Scholar]
- 27.Hammond DA, Smith MN, Li C, Hayes SM, Lusardi K, Bookstaver PB. 2017. Systematic review and metaanalysis of acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam. Clin Infect Dis 64:666–674. doi: 10.1093/cid/ciw811. [DOI] [PubMed] [Google Scholar]
- 28.Giuliano CA, Patel CR, Kale-Pradhan PB. 2016. Is the combination of piperacillin/tazobactam and vancomycin associated with development of acute kidney injury? A meta-analysis. Pharmacotherapy 36:1217–1228. doi: 10.1002/phar.1851. [DOI] [PubMed] [Google Scholar]
- 29.Buckley MS, Hartsock NC, Berry AJ, Bikin DS, Richards EC, Yerondopoulos MJ, Kobic E, Wicks LM, Hammond DA. 2018. Comparison of acute kidney injury risk associated with vancomycin and concomitant piperacillin/tazobactam or cefepime in the intensive care unit. J Crit Care 48:32–38. doi: 10.1016/j.jcrc.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Bamgbola O. 2016. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab 7:136–147. doi: 10.1177/2042018816638223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luque Y, Louis K, Jouanneau C, Placier S, Esteve E, Bazin D, Rondeau E, Letavernier E, Wolfromm A, Gosset C, Boueilh A, Burbach M, Frère P, Verpont M-C, Vandermeersch S, Langui D, Daudon M, Frochot V, Mesnard L. 2017. Vancomycin-associated cast nephropathy. J Am Soc Nephrol 28:1723–1728. doi: 10.1681/ASN.2016080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pill M, O’Neill C, Chapman M, Singh A. 1997. Suspected acute interstitial nephritis induced by piperacillin-tazobactam. Pharmacotherapy 17:166–169. [PubMed] [Google Scholar]
- 33.Jensen J-US, Hein L, Lundgren B, Bestle MH, Mohr T, Andersen MH, Thornberg KJ, Løken J, Steensen M, Fox Z, Tousi H, Søe-Jensen P, Lauritsen AØ, Strange DG, Reiter N, Thormar K, Fjeldborg PC, Larsen KM, Drenck N-E, Johansen ME, Nielsen LR, Østergaard C, Kjær J, Grarup J, Lundgren JD. 2012. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open 2:e000635. doi: 10.1136/bmjopen-2011-000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komuro M, Maeda T, Kakuo H, Matsushita H, Shimada J. 1994. Inhibition of the renal excretion of tazobactam by piperacillin. J Antimicrob Chemother 34:555–564. doi: 10.1093/jac/34.4.555. [DOI] [PubMed] [Google Scholar]
- 35.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY. 2016. Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 165:473–481. doi: 10.7326/M16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimes-Stigare C, Frumento P, Bottai M, Mårtensson J, Martling CR, Bell M. 2015. Long term mortality and risk factors for development of end-stage renal disease in critically ill patients with and without chronic kidney disease. Crit Care 19:383. doi: 10.1186/s13054-015-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Hal SJ, Paterson DL, Lodise TP. 2013. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 57:734–744. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koeze J, Keus F, Dieperink W, van der Horst IC, Zijlstra JG, van Meurs M. 2017. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol 18:70. doi: 10.1186/s12882-017-0487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watkins R, Deresinski S. 2017. Increasing evidence of the nephrotoxicity of piperacillin/tazobactam and vancomycin combination therapy – what is the clinician to do? Clin Infect Dis 65:2137–2143. doi: 10.1093/cid/cix675. [DOI] [PubMed] [Google Scholar]
- 40.Thiel S, Asghar M, Micek S, Reichley R, Doherty J, Kollef M. 2009. Hospital wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med 37:819–824. doi: 10.1097/CCM.0b013e318196206b. [DOI] [PubMed] [Google Scholar]
- 41.Kidney Disease: Improving Global Outcomes (KDIGO). 2012. Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. doi: 10.1038/kisup.2011.38. [DOI] [Google Scholar]
- 42.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 43.Knaus VA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health System Pharmacists, the Infectious Disease Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 45.Bellomo R, Ronco C, Mehta RL, Asfar P, Boisramé-Helms J, Darmon M, Diehl J-L, Duranteau J, Hoste EAJ, Olivier J-B, Legrand M, Lerolle N, Malbrain MLNG, Mårtensson J, Oudemans-van Straaten HM, Parienti J-J, Payen D, Perinel S, Peters E, Pickkers P, Rondeau E, Schetz M, Vinsonneau C, Wendon J, Zhang L, Laterre P-F. 2017. Acute kidney injury in the ICU: from injury to recovery: reports from the 5th Paris International Conference. Ann Intensive Care 7:49. doi: 10.1186/s13613-017-0260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.