Isoniazid (INH) is a first-line antituberculosis drug. The incidence of adverse reactions accompanied by inflammation in the liver during drug administration to tuberculosis patients is high and severely affects clinical treatment.
KEYWORDS: ERS, apoptosis, autophagy, inflammatory state, isoniazid, liver injury, zebrafish
ABSTRACT
Isoniazid (INH) is a first-line antituberculosis drug. The incidence of adverse reactions accompanied by inflammation in the liver during drug administration to tuberculosis patients is high and severely affects clinical treatment. To better understand the mechanism of hepatotoxicity induced by INH under the inflammatory state, we compared the differences in levels of hepatotoxicity from INH between normal zebrafish and zebrafish in an inflammatory state to elucidate the hepatotoxic mechanism using different endpoints such as mortality, malformation, inflammatory effects, liver morphology, histological changes, transaminase analysis, and expression levels of certain genes. The results showed that the toxic effect of INH in zebrafish in an inflammatory state was more obvious than that in normal zebrafish, that liver size was significantly decreased as measured by liver fatty acid binding protein (LFABP) reporter fluorescence and intensity, and that alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were significantly increased. Hematoxylin and eosin (HE) staining and electron microscopy showed that hepatocyte injury was more obvious in the inflammatory state. In the inflammatory state, INH significantly increased the expression levels of endoplasmic reticulum stress (ERS)-related factors (GRP78, ATF6, PERK, IRE1, XBP1s, GRP94, and CHOP), autophagy-related factors (beclin 1, LC3, Atg3, and Atg12), and apoptosis-related factors (caspase-3, caspase-8, caspase-9, Bax, p53, and Cyt) in larvae. Correlational analyses indicated that the transcription levels of the inflammatory factors interleukin-1b (IL-1b), tumor necrosis factor beta (TNF-β), cyclooxygenase 2 (COX-2), and TNF-ɑ were strongly positively correlated with ALT and AST. Furthermore, the ERS inhibitor sodium 4-phenylbutyrate (4-PBA) could ameliorate the hepatotoxicity of INH-lipopolysaccharide (LPS) in zebrafish larvae. These results indicated that INH hepatotoxicity was enhanced in the inflammatory state. ERS and its mediated autophagy and apoptosis pathways might be involved in INH-induced liver injury promoted by inflammation.
TEXT
Tuberculosis is a chronic infectious disease caused by Mycobacterium tuberculosis infection. The Global Tuberculosis Report 2017 released by the World Health Organization (WHO) indicated that approximately 10.40 million new cases of tuberculosis occurred globally in 2016 and that approximately 1.70 million people died of tuberculosis (1). Isoniazid (INH) is a first-line antituberculosis drug that is irreplaceable in standard antituberculosis therapy and plays an important role in tuberculosis chemotherapy (2). However, the issue of INH hepatotoxicity severely affects its use in clinical treatment (3).
Clinical studies have shown that when tuberculosis patients also have hepatitis, their possibility of developing hepatotoxicity increases after being administered INH (4). Hepatocyte injury after administration of INH to patients with chronic hepatitis B viral (HBV) infection was shown to be more severe than that in patients without HBV infection. In addition, when HBV infection is more severe, the incidence of INH hepatotoxicity is higher, and the onset time is earlier (5). Approximately 20% of patients with INH hepatotoxicity are also reported to have inflammation (6). Inflammation can reduce the body’s threshold for drug-induced toxicity as well as shorten the treatment window; thus, drugs within the safe-dose range will also produce toxicity (7). Therefore, development of INH hepatotoxicity is thought to be correlated with inflammatory reactions. While receiving this medication, tuberculosis patients may experience liver injury caused by increased sensitivity to INH hepatotoxicity enhanced by the accompanied inflammation.
Animal models of mild inflammation established using nontoxic doses of the endotoxin lipopolysaccharide (LPS) can be used to evaluate the hepatotoxicity of drugs in models of the inflammatory state (8, 9). LPS is a cell wall component of Gram-negative bacteria and is an important inducer of inflammatory reactions in the body. After LPS enters the blood circulation, it can activate various inflammatory cells, inducing them to release inflammatory mediators that cause inflammatory reactions (10). Hassan and colleagues’ study showed that INH hepatotoxicity in the inflammatory state might be associated with the inhibition of CYP2E1 activity and a reduction in the metabolic rate of INH, thus resulting in bile acid metabolic disorders (11). Currently, few studies have been conducted on INH hepatotoxicity in the inflammatory state, and the mechanism of this hepatotoxicity is unclear. Therefore, targeted liver protection therapy cannot be performed, and INH use will easily aggravate liver burdens and cause severe consequences.
Zebrafish (Danio rerio) and humans share highly conserved structures and functions of immunity- and liver-associated genes (12, 13). Results from drug hepatotoxicity evaluations using both zebrafish and mammals are highly reproducible (14). Zebrafish embryos are transparent, and their development can be visualized; therefore, in vivo imaging technology enables direct observations of the toxic effects of drugs on their organs in real time (15). Our group successfully established a transgenic (Tg) zebrafish line, Tg(lfabp:EGFP; lyz:DsRED2), with enhanced green fluorescence protein (EGFP)-labeled hepatocytes and red fluorescence-labeled inflammatory cells. This line can be used to rapidly detect inflammatory reactions in the body and dynamic changes in the liver simultaneously and is a more convenient animal model for studying drug hepatotoxicity in the inflammatory state.
In this study, we used low-dose LPS pretreatment to induce inflammatory reactions in zebrafish larvae to establish a drug hepatotoxicity evaluation model based on an inflammatory state. The effect of INH on hepatotoxicity in zebrafish in an inflammatory state was investigated to confirm INH hepatotoxicity enhancement in an inflammatory state. Real-time PCR was performed to study the effect of INH on changes in the expression levels of key genes in the endoplasmic reticulum stress (ERS), autophagy, and apoptosis pathways in zebrafish in an inflammatory state and elucidate the molecular regulatory mechanism of INH-induced hepatotoxicity in an inflammatory state to provide theoretical bases for rational clinical use of INH and to monitor and treat adverse reactions.
RESULTS
Establishment of the endotoxin-induced mild inflammatory model in zebrafish.
The experimental results indicated that 0.025 mg/ml LPS can induce significant inflammatory cell aggregation but had no toxic effects on gross and liver morphology in zebrafish larvae (see Fig. S1 to S3 in the supplemental material). Therefore, an LPS dose of 0.025 mg/ml was selected to establish an endotoxin-induced mild inflammation model to study INH hepatotoxicity in zebrafish in an inflammatory state.
Effect of INH on the mortality and morphology of zebrafish in an inflammatory state.
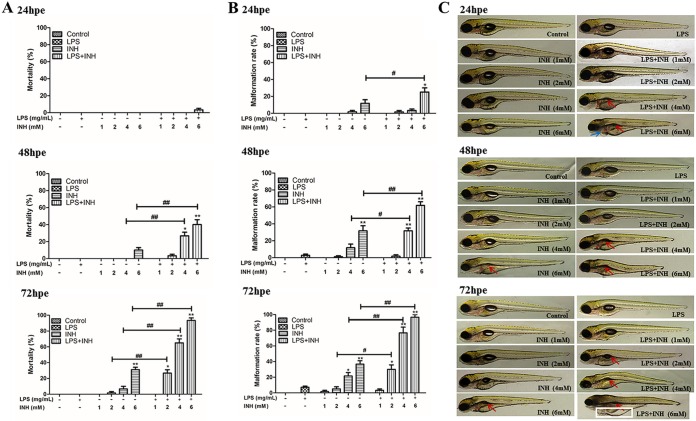
The mortality and malformation rates of all groups dosed with LPS and INH (LPS-INH) were significantly higher than those in the INH-alone group (Fig. 1A and B). Compared with that in the normal state, the toxic effect of INH was enhanced in zebrafish in an inflammatory state. For example, at 72 h postexposure, the swim bladders were missing in the 6 mM INH group. In the LPS–6 mM INH group, the extent of zebrafish malformation was aggravated, with severe edema in the bodies and abdomens, missing swim bladders, tail malformation, undeveloped fish lips and fins, and delayed somitogenesis (Fig. 1C).
FIG 1.
Effect of INH exposure at 24, 48, and 72 h postexposure (hpe) on the cumulative mortality rate (A), malformation rate (B), and morphological changes (C) of normal zebrafish and zebrafish in an inflammatory state. In panel C, red arrows indicate reduced or missing swim bladders, blue arrows indicate pericardium edema, and white squares indicate severe cardiac and abdominal edema. *, P < 0.05; **, P < 0.01 (both, versus results for the control); #, P < 0.05; ##, P < 0.01 (both, versus results in the absence of LPS).
Effect of INH on hepatotoxicity in zebrafish in an inflammatory state.
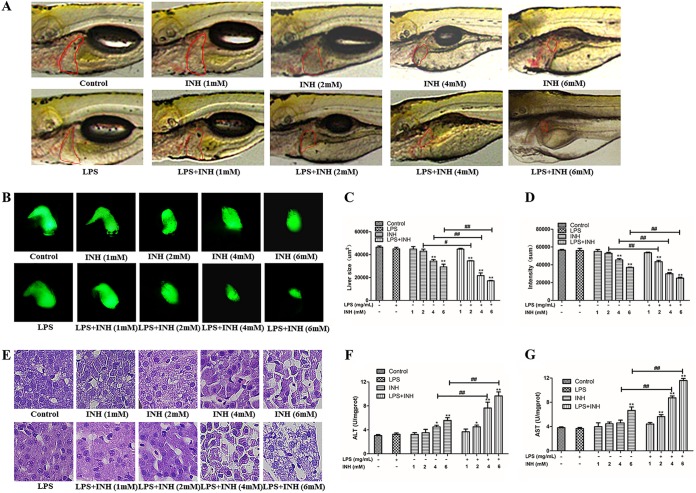
Morphological changes in zebrafish livers were observed under bright-field and fluorescence images for all groups after 72 h of INH exposure (Fig. 2A and B). As shown in Fig. 2A, the zebrafish that received the vehicle control and LPS (0.025 mg/ml) alone exhibited clear liver tissue, while zebrafish liver lost transparency and became dark or brown after INH treatment. Liver degeneration (Fig. 2A), reduced liver size as measured by liver fatty acid binding protein (LFABP) reporter fluorescence and intensity (Fig. 2C and D), and increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels (Fig. 2F and G) in the LPS-INH group with same INH dose in the inflammatory state were more obvious than those in the INH-alone group.
FIG 2.
Effect of 72 h of INH exposure on zebrafish hepatotoxicity. (A) Liver morphology from zebrafish larvae. Liver degeneration is indicated by a red circle. (B) Green fluorescence-labeled liver morphology. (C) Change in liver size. (D) Change in liver fluorescence intensity. (E) Morphology of liver pathological sections (HE staining). (F and G) ALT and AST levels. *, P < 0.05; **, P < 0.01 (both, versus results for the control); #, P < 0.05; ##, P < 0.01 (both, versus results in the absence of LPS).
Hematoxylin and eosin (HE) staining results showed that hepatocytes in the control group were orderly arranged, with tight cell-cell junctions and centered nuclei with regular round shapes. Some cell nuclei in the groups treated with 4 mM INH and 6 mM INH were atrophied and malformed. Injured hepatocytes showed more severe nuclear atrophy malformation, dissolution of cytoplasm, and vacuolization in the control group than in the LPS–4 mM INH and LPS–6 mM INH groups (Fig. 2E). These results indicated that INH hepatotoxicity was increased in the inflammatory state compared with that in the normal state.
Effect of INH on liver ultrastructure in zebrafish.
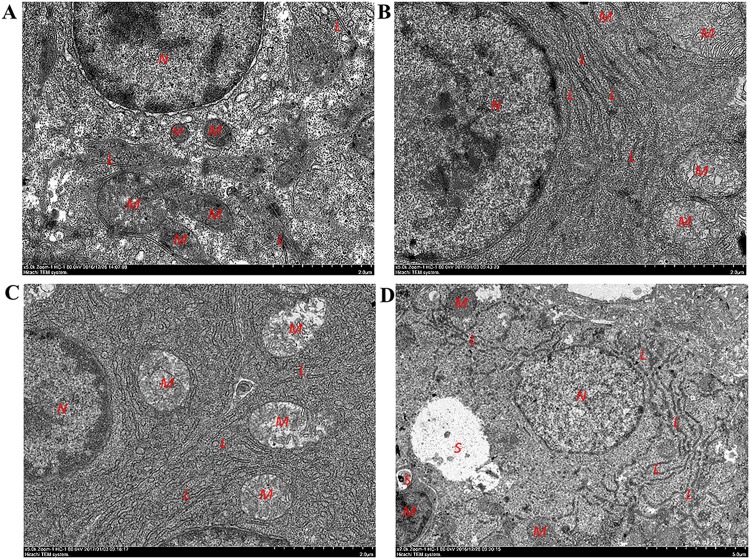
Transmission electron microscopy results showed that the cell membrane and organelle structure in the zebrafish livers in the blank control group remained intact. Other than cristal swelling in individual mitochondria, no other lesions were observed in the groups treated with 0.025 mg/ml LPS and 4 mM INH alone. In the group that received 0.025 mg/ml LPS–4 mM INH, some hepatocytic organelles were dissolved, the contour was unclear, the ER was expanded, swollen, and fractured, and autophagosomes were present (Fig. 3). These results suggested that INH hepatotoxicity was enhanced in zebrafish larvae under the inflammatory state and that the enhancement may have been associated with ERS and autophagy.
FIG 3.
Effect of 72 h of INH exposure on zebrafish hepatocytic ultrastructure. Images represent the following treatment conditions: control (A), 0.025 mg/ml LPS (B), 4 mM INH (C), 0.025 mg/ml LPS–4 mM INH (D). L, rough endoplasmic reticulum; N, cell nuclei; M, mitochondria; S, autophagosomes.
In vivo real-time tracking of inflammatory cell migration and aggregation and changes in ROS fluorescence levels in zebrafish.
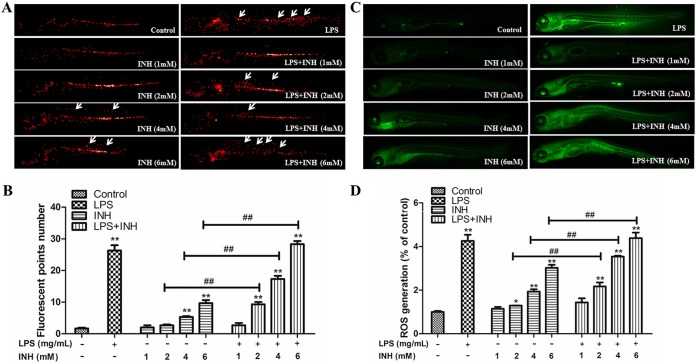
Inflammatory cell migration and enrichment levels in zebrafish in the LPS (0.025 mg/ml) and LPS-INH (2, 4, and 6 mM) groups were significantly higher than those in the blank control group. These results indicated that larvae in the LPS exposure group exhibited inflammatory reactions (Fig. 4A and B). Reactive oxygen species (ROS) levels in the LPS-INH (2, 4, and 6 mM) groups were significantly higher than those in the INH-alone (2, 4, and 6 mM) groups (Fig. 4C and D), indicating that INH caused ROS levels to increase in zebrafish in the inflammatory state.
FIG 4.
Effect of INH on inflammatory cell aggregation and reactive oxygen species (ROS) levels in zebrafish. (A) In vivo real-time tracking of the inflammatory cell migration and aggregation behavior in transgenic zebrafish. The migrated and aggregated inflammatory cells are indicated by white arrows. (B) Migrated and aggregated inflammatory cell numbers in the zebrafish trunks. (C) ROS fluorescence staining in zebrafish. (D) ROS fluorescence levels in zebrafish. *, P < 0.05; **, P < 0.01 (both, versus results for the control); ##, P < 0.01 (versus results in the absence of LPS).
Disruption of gene expression in zebrafish after INH-LPS cotreatment.
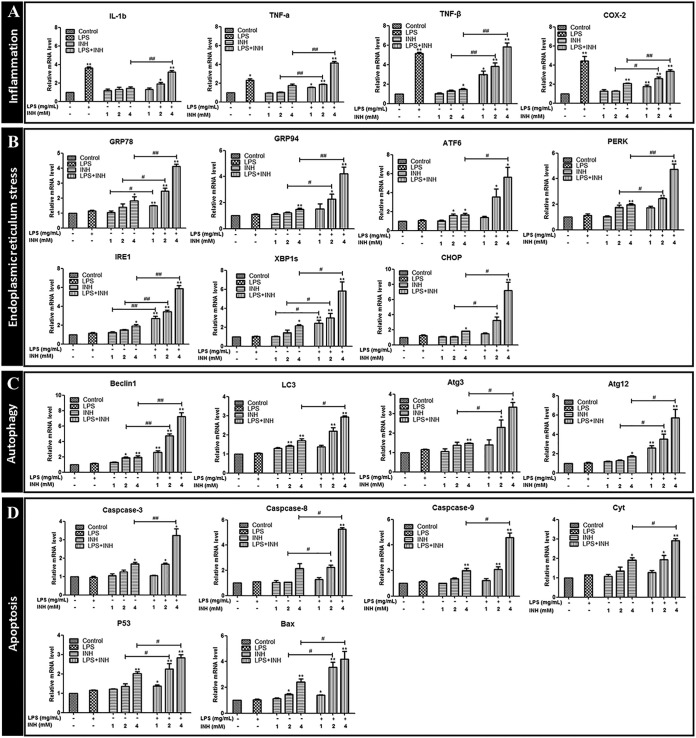
(i) Effect of INH on inflammation-related gene expression. Expression levels of interleukin-1b (IL-1b), tumor necrosis factor alpha (TNF-ɑ), tumor necrosis factor beta (TNF-β), and cyclooxygenase 2 (COX-2) genes in the LPS group, LPS–2 mM INH group, and LPS–4 mM INH group were significantly increased compared with levels in the blank control group. TNF-ɑ, TNF-β, and COX-2 transcription levels in the LPS– 2 mM INH group were significantly increased compared with those in the 2 mM INH group. IL-1b, TNF-ɑ, TNF-β, and COX-2 transcription levels in the LPS–4 mM INH group were significantly increased compared with those in the 4 mM INH group (Fig. 5A).
FIG 5.
(A to D) Expression of inflammatory-, ER stress-, autophagy-, and apoptosis-related gene signaling pathways following INH exposure. *, P < 0.05; **, P < 0.01 (both, versus results for the control); #, P < 0.05; ##, P < 0.01 (both, versus results in the absence of LPS).
(ii) Effect of INH on ERS-related gene expression. Compared with levels in the 1 mM INH group, glucose-regulated protein 78 (GRP78), inositol-requiring kinase 1 (IRE1), and splice X-box binding protein 1 (XBP1s) transcription levels were significantly upregulated in the LPS–1 mM INH group. Compared with those in the 2 mM INH group, GRP78, GRP94, PKR-like ER kinase (PERK), IRE1, XBP1s, and CHOP transcription levels were significantly upregulated in the LPS–2 mM INH group. Compared with those in the 4 mM INH group, the GRP78, GRP94, activating transcription factor 6 ATF6, PERK, IRE1, XBP1s, and CHOP transcription levels were significantly upregulated in the LPS–4 mM INH group (Fig. 5B).
(iii) Effect of INH on autophagy-related gene expression. Beclin 1, Atg3, and Atg12 transcription levels in the LPS–2 mM INH group were significantly upregulated compared with those in the 2 mM INH group. Transcription levels of beclin 1, LC3, Atg3, and Atg12 in the LPS–4 mM INH group were significantly upregulated compared with those in the 4 mM INH group. In comparison to levels in zebrafish larvae in the normal state, INH caused significantly increased levels of autophagy-related gene expression in larvae in the inflammatory state (Fig. 5C).
(iv) Effect of INH on apoptosis-related gene expression. Compared with levels in the 2 mM INH group, transcription levels of caspase-8, p53, and Bax were significantly upregulated in the LPS–2 mM INH group. Caspase-3, caspase-8, caspase-9, Bax, p53, and Cyt transcription levels in the LPS–4 mM INH group were significantly higher than those in the 4 mM INH group (Fig. 5D).
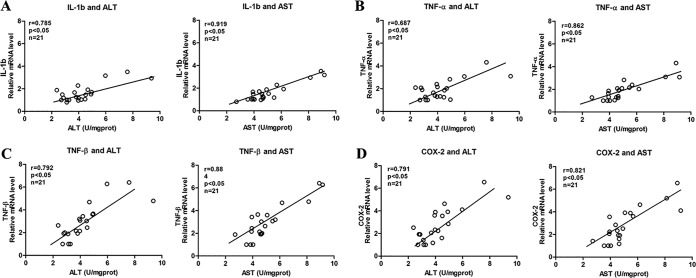
Correlational analysis between inflammatory factors and hepatotoxicity indicators (ALT and AST) in zebrafish.
In the blank control group, INH (1, 2, and 4 mM) groups, and LPS-INH (1, 2, and 4 mM) groups, three sample batches were collected, yielding 21 samples. Each batch had 30 zebrafish pieces. The Pearson correlation coefficient was used to analyze the correlation between mRNA levels in the genes of the inflammatory cytokines IL-1b, TNF-ɑ, TNF-β, and COX-2 and the levels of ALT and AST (Fig. 6). Correlational analysis results indicated that inflammatory cytokines IL-1b, TNF-ɑ, TNF-β, and COX-2 were strongly positively correlated with ALT (0.6 < r < 0.8, P < 0.05) and with AST (0.8 < r < 1.0, P < 0.05). These results indicated that inflammatory reactions correlated with INH hepatotoxicity, and when inflammation was more severe, the INH hepatotoxicity was stronger.
FIG 6.
(A to D) Correlational analyses between mRNA levels in genes of the inflammatory cytokines IL-1b, TNF-ɑ, TNF-β, and COX-2 and those of ALT and AST in zebrafish.
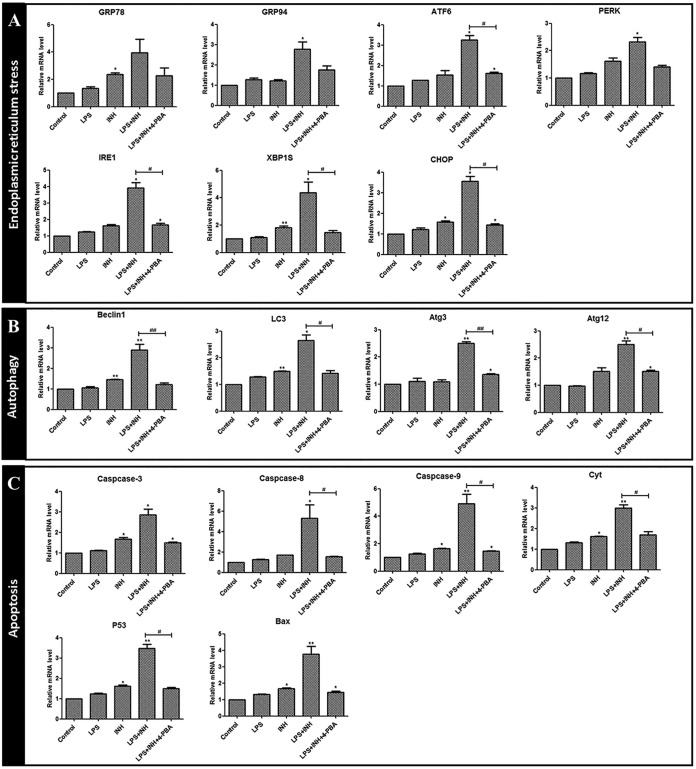
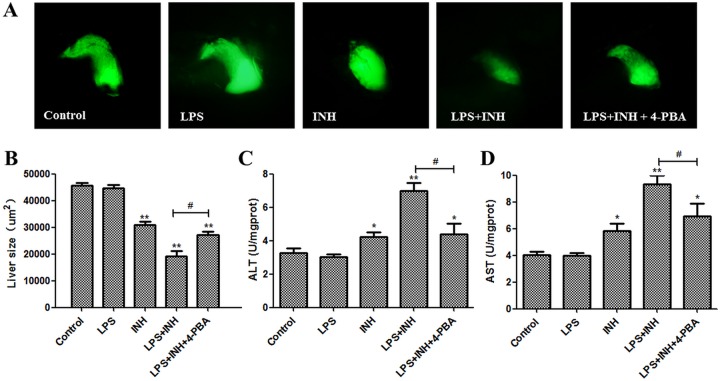
4-PBA ameliorates the hepatotoxicity induced by INH-LPS.
To confirm whether blocking ERS signaling could reverse the liver injury induced by INH-LPS, we used the ERS inhibitor sodium 4-phenylbutyrate (4-PBA) to test whether it could ameliorate the toxic effects of INH-LPS in zebrafish larvae. Our results showed that ERS-related genes levels, including those of ATF6, IRE1, XBP1s, and CHOP, were increased after LPS-INH treatment, and this increase was reversed in the presence of 4-PBA. Furthermore, 4-PBA diminished the autophagy and apoptosis-related gene expression levels, such as those of beclin 1, LC3, Atg3, Atg12, caspase-9, p53, and Cyt (Fig. 7). 4-PBA alleviated the liver atrophy caused by LPS-INH (Fig. 8A and B). In addition, 4-PBA hampered the increase of ALT and AST induced by LPS-INH (Fig. 8C and D). Hence, we proved that the activation of ERS could aggravate the hepatotoxicity induced by LPS-INH treatment.
FIG 7.
(A to C) The effect of 4-PBA on ERS-, autophagy-, and apoptosis-related gene expression levels after INH-LPS cotreatment. *, P < 0.05; **, P < 0.01 (both, versus results for the control); #, P < 0.05; ##, P < 0.01 (both, versus results in the cotreatment group).
FIG 8.
4-PBA ameliorates LPS-INH-induced hepatotoxicity in zebrafish larvae. (A) Green fluorescence-labeled liver morphology. (B) Change in liver size. (C and D) ALT and AST levels. *, P < 0.05; **, P < 0.01 (both, versus results for the control); #, P < 0.05 (versus results in the INH-LPS cotreatment group).
DISCUSSION
Our group established a transgenic line of zebrafish, Tg(lfabp:EGFP; lyz:DsRED2), to track dynamic real-time in vivo inflammatory and liver injury effects simultaneously. Larvae were pretreated with 0.025 mg/ml LPS to establish a zebrafish model of mild inflammation to compare the differences in INH hepatotoxicity levels between the normal and inflammatory states. The results showed that the liver size as measured by LFABP reporter fluorescence and intensity caused by the same INH dose was significantly decreased in zebrafish in the inflammatory state compared with that in normal zebrafish, while the ALT and AST levels were significantly elevated. Pathological sections showed that compared with the normal state, hepatocyte injury caused by INH was more severe in the inflammatory state, the hepatocytes showed nuclear atrophy and dissolution, and cell junctions were sparse. These results indicated that INH hepatotoxicity was enhanced in zebrafish in the inflammatory state.
The reactive oxygen species detection results showed that INH more significantly increased ROS levels in zebrafish in the inflammatory state than in normal zebrafish, suggesting that oxidative stress induced by INH was enhanced in zebrafish in the inflammatory state. Electron microscopy results showed that INH aggravated ER injury in hepatocytes of zebrafish in the inflammatory state and that hepatocytes exhibited autophagosomes, suggesting that INH might cause ERS and autophagy in zebrafish in the inflammatory state. Therefore, we further investigated the effect of INH on changes in the expression levels of key genes in the ERS and autophagy pathways in zebrafish in the inflammatory state. ERS is a cellular defense reaction to stress in the body. An appropriate ERS-induced unfolded protein response (UPR) can upregulate the protein folding mechanism to attenuate stress and protect cells (16). However, strong or long-duration stress reactions will result in functional and metabolic disorders and cell and tissue injury (17). Many studies have indicated that ERS development is closely associated with liver diseases, such as nonalcoholic fatty liver disease, viral hepatitis, liver cancer, and drug-induced liver injury. The ERS process requires the involvement of three ER transmembrane proteins: inositol-requiring kinase 1 (IRE1), activating transcription factor 6 (ATF6), and PKR-like ER kinase (PERK). When the ER is homeostatic, N termini of IRE1, ATF6, and PERK bind to glucose-regulated protein (GRP) molecular chaperones (e.g., GRP78 and GRP94) to maintain a nonactive state. During ERS, unfolded or misfolded proteins will cause GRP to dissociate from receptor molecules. After activation, IRE1, ATF6, and PERK induce the transduction of downstream signals and expression of related genes through the IRE1/XBP1, ATF6, and PERK/eIF2α pathways to cause cell injury (18). We found that INH caused significantly higher mRNA expression levels of the ERS marker molecules GRP78 and GRP94 in zebrafish in the inflammatory state than in normal zebrafish. In addition, we detected whether the three signaling pathways in the UPR, IRE1-XBP1, ATF6, and PERK-eIF2α-ATF4-GADD34, were activated. The results showed that the mRNA levels of IRE1, XBP1s, ATF6, and PERK in the LPS-INH group showed dose-dependent upregulation compared with those in the INH-alone group, indicating that ERS participated in enhancing INH hepatotoxicity caused by the inflammatory state.
Different ER stressors can activate autophagy in cells by activating different UPR signaling pathways (19). Autophagy is also a cellular defense mechanism against dangerous stimuli (20). However, when ERS is too strong or too long, the UPR will excessively activate autophagy to promote cell death and cause injury in the body (21). Upstream factors such as beclin 1 participate in autophagy initiation and nucleation, and downstream factors, such as Atg3, Atg5, Atg7, and LC3 (Atg8), promote autophagy membrane elongation and closure (22). LC3 is a marker protein on the autophagosomal membrane. LC3 modification and Atg12 binding play important roles in autophagosomal formation (23). The results from this study showed that after exposure to the same INH dose, the mRNA expression levels of the autophagy-related factors beclin 1, LC3, Atg3, and Atg12 in zebrafish in the combined LPS and INH group were significantly higher than those in the INH-alone group, suggesting that INH might cause autophagy and cell injury in inflammatory zebrafish.
Recent studies showed that autophagy and apoptosis may have a common regulatory mechanism and occur at the same time (22). Excessive ERS can induce cell apoptosis by initiating the CHOP pathway (24). CHOP is a marker protein in ERS-induced apoptosis. CHOP expression levels are very low under normal conditions but significantly increase during ERS. High CHOP expression causes upregulated Bax protein expression in the cytoplasm and induces the mitochondria to release Cyt to activate caspase-9 and caspase-3 and induce apoptosis (25, 26). As a transcription factor, p53 regulates the expression of many proapoptosis target genes to exert its proapoptosis function (27). Bax is the most important proapoptosis protein. After Bax is activated by apoptosis signals, Bax forms more homodimers to promote apoptosis (28). This study showed that the apoptosis factors, caspase-3, caspase-8, caspase-9, Bax, Cyt, and p53, were significantly higher in the LPS–4 mM INH group than in the 4 mM INH group. The increased hepatocyte apoptosis caused by INH in the inflammatory state was thought to be the reason for the increased INH hepatotoxicity in zebrafish in the inflammatory state.
To further investigate the effect of inflammation on INH hepatotoxicity, we used an in vivo imaging system to monitor the migratory and aggregation levels of red fluorescence-labeled inflammatory cells. We also used real-time PCR to detect changes in inflammation-related gene expression (IL-1b, TNF-ɑ, TNF-β, and COX-2). In addition, correlations were analyzed between the expression levels of inflammation-related genes (IL-1b, TNF-ɑ, TNF-β, and COX-2) and the classic hepatotoxicity indicators (ALT and AST). The results showed that LPS pretreatment caused increased migratory and aggregation levels of inflammatory cells in larvae and significantly increased inflammation-related gene transcription levels (IL-1b, TNF-ɑ, TNF-β, and COX-2). In addition, the transcription levels of the inflammatory factors IL-1b, TNF-β, COX-2, and TNF-ɑ were strongly positively correlated with ALT and AST in the inflammatory state. These results indicated that the level of inflammation was closely associated with the level of hepatocyte injury. Further research is needed to find out how inflammatory reactions participate in the hepatic injury process caused by INH.
4-PBA inhibits ERS by reducing the accumulated misfolded proteins in the ER (29). In this study, 4-PBA ameliorated LPS-INH-induced hepatotoxicity in zebrafish larvae, indicating the key role of ERS in INH hepatotoxicity caused by the inflammatory state. 4-PBA also reduced the autophagy and apoptosis-related gene expression levels after LPS-INH treatment, which may be due to the inhibition of the ER stress-mediated autophagy and apoptosis pathways (19, 30). However, the role and mutual regulation mechanism of ERS, autophagy, and apoptosis pathways in INH-induced liver injury promoted by inflammation still need to be further studied.
In summary, this study showed that inflammation caused enhanced INH hepatotoxicity. INH aggravated ER injury in the livers of zebrafish in the inflammatory state. Hepatocytes showed many autophagosomes, and the expression levels of ERS-related factors GRP78, ATF6, PERK, IRE1, XBP1s, GRP94, and CHOP, autophagy-related factors beclin 1, LC3, Atg3, and Atg12, and apoptosis-related factors caspase-3, caspase-8, caspase-9, Bax, p53, and Cyt significantly increased. Therefore, ERS and its mediated autophagy and apoptosis pathways may promote INH hepatotoxicity by inflammation. These study results may provide theoretical bases for rational clinical INH use and for monitoring and treating adverse reactions.
MATERIALS AND METHODS
Chemicals.
INH (CAS number 54-85-3), sodium 4-phenylbutyrate (4-PBA; CAS number 1716-12-7), and LPS (catalog no. L2880 [Sigma]; derived from Escherichia coli serotype 055:B5) were obtained from Sigma (St. Louis, MO, USA). INH and LPS were prepared in the required concentrations using zebrafish embryo culture water. The formula for the zebrafish embryo culture water was 5 mM NaCl, 0.17 mM KCl, 0.4 mM CaCl2, and 0.16 mM MgSO4. All other chemicals and reagents used in this study were of analytical grade.
Zebrafish.
Wild-type AB, Tg(lfabp:EGFP), and Tg(lyz:DsRED2) fish strains were obtained from the Key Laboratory for Drug Screening Technology of Shandong Academy of Sciences. The details about the generation of Tg(lfabp:EGFP) and Tg(lyz:DsRED2) fish strains were described previously (31, 32). The Tg(lfabp:EGFP; lyz:DsRED2) doubly transgenic line with green fluorescence-labeled hepatocytes and red fluorescence-labeled inflammatory cells was generated by mating F1 Tg(lfabp:EGFP) fish with Tg(lyz:DsRED2) fish. Female and male adult zebrafish were separately housed at 28°C under a 14-h/10-h light/dark cycle. Healthy and sexually matured zebrafish were used for mating at a male-to-female ratio of 1:1 or 2:1. The barrier was removed at 8:30 a.m. the following morning, and zebrafish embryos were obtained from 9:00 to 10:00 a.m. Embryos were washed three times and disinfected using 0.1% methylene blue. Embryos were transferred into zebrafish embryo culture water and cultured at 28°C with light control. All experiments were carried out in compliance with the standard ethical guidelines and under the control of the Biology Institute, Qilu University of Technology committee.
Establishment of an endotoxin-induced mild inflammatory model in zebrafish.
The Tg(lfabp:EGFP; lyz:DsRED2) zebrafish at 3 days postfertilization were used as study subjects. The blank control group (embryo culture water) and LPS-treated groups (doses of 0.025, 0.05, and 0.1 mg/ml) were set up. Drug exposure was performed by dissolving the drug in the culture water for three consecutive days, and the culture water was replaced daily. After 24, 48, and 72 h of exposure, mortality, morphological development, inflammatory effects, and liver injury were detected to confirm the proper LPS dose for model establishment.
Effect of INH on hepatotoxicity in inflammatory zebrafish.
After the Tg(lfabp:EGFP; lyz:DsRED2) zebrafish embryos developed for 3 days postfertilization, zebrafish larvae with normal appearances were selected and transferred into six-well plates with 30 larvae per well. The blank control group (embryo culture water) and the groups treated with 0.025 mg/ml LPS, 1 mM INH, 2 mM INH, 4 mM INH, 6 mM INH, LPS–1 mM INH, LPS–2 mM INH, LPS–4 mM INH, and LPS–6 mM INH were determined based on the preexperimental results. Each concentration group was set in 3 replicate wells. Embryos were then hatched in a light incubator at a constant temperature (28°C). The fish were exposed to the drugs for three consecutive days, and the culture water was replaced daily.
Effect of 4-PBA on INH-LPS induced hepatotoxicity.
To determine whether the activation of ERS participated in the hepatotoxicity induced by INH-LPS cotreatment, we examined the impact of the ERS inhibitor 4-PBA on INH-LPS hepatotoxicity. Healthy and normally developing Tg(lfabp:EGFP; lyz:DsRED2) zebrafish embryos were selected at 72 h postfertilization and randomly divided into five groups. The control group (embryo culture water) and groups treated with 0.025 mg/ml LPS, 4 mM INH, LPS–4 mM INH, and LPS–4 mM INH–0.05 mM 4-PBA were set up with three duplicate wells for each group. Subsequently, the zebrafish were incubated at a constant temperature (28°C) for three consecutive days.
Observation of zebrafish larval morphology.
The numbers of dead and malformed zebrafish at 24, 48 and 72 h postexposure were recorded to calculate the mortality and malformation rates. Larvae were anesthetized using 0.16% tricaine, and zebrafish were immobilized on their sides on slides using 3% methylcellulose. Morphological changes in the zebrafish were observed and photographed under a stereomicroscope (SZX16; Olympus, Tokyo, Japan), and the results were recorded.
In vivo real-time tracking of the migratory and aggregation behaviors of inflammatory cells in transgenic zebrafish.
Larvae from all groups were anesthetized at 24, 48, and 72 h postexposure using 0.16% tricaine. Zebrafish were immobilized on their sides on slides using 3% methylcellulose. Inflammatory cell migration and aggregation were observed and photographed under a fluorescence microscope (SZX16; Olympus, Tokyo, Japan), and the inflammatory cells that migrated to the trunk were counted.
Observation of zebrafish liver morphology.
After the larvae were anesthetized at 24, 48, and 72 h postexposure, zebrafish were immobilized on their sides using methylcellulose and photographed.
The fluorescence and bright-field images were taken for each larva using a stereomicroscope (SZX16; Olympus, Tokyo, Japan). For each larva, the bright-field image was measured for liver degeneration, and the fluorescence image was measured for liver size. Liver size as measured by LFABP reporter fluorescence and intensity was calculated using Image Pro Plus software (Media Cybernetics, Bethesda, MD, USA).
Measurement of liver transaminase levels in zebrafish.
At 72 h postexposure, 150 zebrafish were collected per group to prepare zebrafish tissue homogenates in cold saline. The homogenates were centrifuged at 2,500 rpm at 4°C for 10 min, and the supernatants were collected. The concentrations of total protein in each supernatant were assayed using the Bradford method (33). Alanine transaminase (ALT) and aspartate transaminase (AST) levels were determined using spectrophotometric diagnostic kits according to the manufacturer’s protocols (Nanjing Jiancheng Biotechnology Institute, Nanjing, China). The ALT and AST activities were determined at 510 nm and are expressed as the number of units per milligram of protein.
Detection of zebrafish liver histopathology.
At 72 h postexposure, larvae from each group were fixed in 4% paraformaldehyde, and liver histopathological changes were observed by hematoxylin and eosin (HE) staining. Some larvae were fixed in 5% glutaraldehyde, and ultrastructural changes in the liver tissues were observed under a transmission electron microscope.
Determination of ROS levels in the zebrafish.
Reactive oxygen species (ROS) levels in the zebrafish were determined using the 2ʹ,7ʹ-dichlorofluorescein diacetate (DCFH-DA) reactive oxygen detection probe. This experiment used normally developed wild-type AB zebrafish at 3 days postfertilization. The blank control group (embryo culture water) and the groups treated with 0.025 mg/ml LPS, 1 mM INH, 2 mM INH, 4 mM INH, 6 mM INH, LPS–1 mM INH, LPS–2 mM INH, LPS–4 mM INH, and LPS–6 mM INH were set up. Each concentration group had three replicate wells. The groups were exposed to the drugs for three consecutive days, and the culture water was replaced daily. At 72 h postexposure, the ROS fluorescence probe (DCFH-DA; 10 mM stock solution diluted to 10 μM working solution with culture water) was added to larvae from each group and stained at 28°C in the dark for 1 h. Larvae were washed with embryo culture water until the solution became clear. The results were photographed under a fluorescence microscope (SZX16; Olympus, Tokyo, Japan). Images were processed and analyzed using ImageJ software.
Determination of gene expression level changes using quantitative real-time PCR.
Hepatocytes were isolated from Tg(lfabp:EGFP; lyz:DsRED2) zebrafish larvae at 72 h postexposure based on EGFP expression. To enrich the liver cells, the central parts of zebrafish larvae (after removal of the head and tail regions) were used for fluorescence-activated cell sorting (FACS) using a cell sorter (BD FACSAria, part 643245; BD Biosciences). The liver-enriched central parts were dissociated into single cells using a 40-μm-pore-size mesh (BD Falcon, part 352340; BD Biosciences) and enzymatically digested with 0.05% trypsin (T1426; Sigma), as previously described (34).
Total RNA was extracted using a NanoMag Animal and Fish RNA Isolation kit (Shannuo Scientific Company, Tianjin, China). The RNA concentration and purity were determined using UV absorbance. RNA samples from each group were reverse transcribed to obtain cDNA. The expression levels of inflammation-, ERS-, autophagy-, and apoptosis pathway-related genes were detected using a Bio-Rad CFX96 real-time system (Bio-Rad, CA, USA). The quantitative real-time PCR amplification reaction conditions were 30 s at 95°C followed by 40 cycles of 5 s at 95°C and 10 s at 60°C. A melting curve analysis was performed for each reaction with a 65 to 95°C ramp. Fluorescence signals were collected after the annealing cycles. β-Actin was used as the internal control for data analysis. Relative quantitative analyses were performed on the results. PCR primers for target genes and the internal control gene β-actin were synthesized by Nanjing SunShine Biotechnology. Gene primer designs are shown in Table S1 in the supplemental material.
Data analyses.
Data were processed using SPSS, version 16.0, software. All experimental data were expressed as the means ± standard errors (SE). Statistical differences were analyzed using analysis of variance (ANOVA). Between-group comparisons were performed using Tukey’s test. A P value of < 0.05 indicated a significant difference, and a P values of < 0.01 indicated a very significant difference.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant no. 81703624) and the Natural Science Foundation of Shandong Province (grant nos. ZR2015YL010 and ZR2016YL009).
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Y.Z., J.C., Z.J., L.Z., and K.L. participated in the research design; Y.Z., Z.J., Q.X., and R.W. carried out the experiments; Y.Z., X.W., and X.C. performed the data analysis; Y.Z. and C.D.H. wrote the manuscript. All authors read and approved the submitted version.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01639-18.
REFERENCES
- 1.World Health Organization. 2017. Global tuberculosis report 2017. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Swindells S. 2018. New and noteworthy in tuberculosis diagnostics and treatment. Top Antivir Med 26:58–61. [PMC free article] [PubMed] [Google Scholar]
- 3.Hassan HM, Guo HL, Yousef BA, Luyong Z, Zhenzhou J. 2015. Hepatotoxicity mechanisms of isoniazid: A mini-review. J Appl Toxicol 35:1427–1432. doi: 10.1002/jat.3175. [DOI] [PubMed] [Google Scholar]
- 4.Hassan HM, Guo H, Yousef BA, Ping-Ping D, Zhang L, Jiang Z. 2017. Dexamethasone pretreatment alleviates isoniazid/lipopolysaccharide hepatotoxicity: inhibition of inflammatory and oxidative stress. Front Pharmacol 8:133. doi: 10.3389/fphar.2017.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang LH, Geng WK, Zhang J, Lin DW, Dong F, Zhou LS, Dong YR, Zhang XW. 2009. Study on liver damage caused by anti-TB drug intermittent treatment on patients with HBV-TB co-infection. Zhonghua Liu Xing Bing Xue za Zhi 30:286–289. (In Chinese.) [PubMed] [Google Scholar]
- 6.Tafazoli S, Mashregi M, O'Brien PJ. 2008. Role of hydrazine in isoniazid-induced hepatotoxicity in a hepatocyte inflammation model. Toxicol Appl Pharmacol 229:94–101. doi: 10.1016/j.taap.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Yee SB, Kinser S, Hill DA, Barton CC, Hotchkiss JA, Harkema JR, Ganey PE, Roth RA. 2000. Synergistic hepatotoxicity from coexposure to bacterial endotoxin and the pyrrolizidine alkaloid monocrotaline. Toxicol Appl Pharmacol 166:173–185. doi: 10.1006/taap.2000.8968. [DOI] [PubMed] [Google Scholar]
- 8.Li CY, Tu C, Gao D, Wang RL, Zhang HZ, Niu M, Li RY, Zhang CE, Li RS, Xiao XH, Yang MH, Wang JB. 2016. Metabolomic study on idiosyncratic liver injury induced by different extracts of polygonum multiflorum in rats integrated with pattern recognition and enriched pathways analysis. Front Pharmacol 7:483. doi: 10.3389/fphar.2016.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulsen KL, Olivero-Verbel J, Beggs KM, Ganey PE, Roth RA. 2014. Trovafloxacin enhances lipopolysaccharide-stimulated production of tumor necrosis factor-alpha by macrophages: role of the DNA damage response. J Pharmacol Exp Ther 350:164–170. doi: 10.1124/jpet.114.214189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang-Sheng C, Jie A, Jian-Jun L, Lan H, Zeng-Bao X, Chang-Qing L. 2017. Piperine attenuates lipopolysaccharide (LPS)-induced inflammatory responses in BV2 microglia. Int Immunopharmacol 42:44–48. doi: 10.1016/j.intimp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Hassan HM, Guo H, Yousef BA, Guerram M, Hamdi AM, Zhang L, Jiang Z. 2016. Role of inflammatory and oxidative stress, cytochrome P450 2E1, and bile acid disturbance in rat liver injury induced by isoniazid and lipopolysaccharide cotreatment. Antimicrob Agents Chemother 60:5285–5293. doi: 10.1128/AAC.00854-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gioacchini G, Giorgini E, Olivotto I, Maradonna F, Merrifield DL, Carnevali O. 2014. The influence of probiotics on zebrafish Danio rerio innate immunity and hepatic stress. Zebrafish 11:98–106. doi: 10.1089/zeb.2013.0932. [DOI] [PubMed] [Google Scholar]
- 13.Zheng X, Dai W, Chen X, Wang K, Zhang W, Liu L, Hou J. 2015. Caffeine reduces hepatic lipid accumulation through regulation of lipogenesis and ER stress in zebrafish larvae. J Biomed Sci 22:105. doi: 10.1186/s12929-015-0206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Han L, He Q, Chen W, Sun C, Wang X, Chen X, Wang R, Hsiao CD, Liu K. 2017. A rapid assessment for predicting drug-induced hepatotoxicity using zebrafish. J Pharmacol Toxicol Methods 84:102–110. doi: 10.1016/j.vascn.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Liu K, Hassan HM, Guo H, Ding P, Han L, He Q, Chen W, Hsiao CD, Zhang L, Jiang Z. 2016. Liver fatty acid binding protein deficiency provokes oxidative stress, inflammation, and apoptosis-mediated hepatotoxicity induced by pyrazinamide in zebrafish larvae. Antimicrob Agents Chemother 60:7347–7356. doi: 10.1128/AAC.01693-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu J, Zhang X, Chen P, Zhang Y. 2016. Endoplasmic reticulum stress is involved in 2,4-dichlorophenol-induced hepatotoxicity. J Toxicol Sci 41:745–756. doi: 10.2131/jts.41.745. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Zhu F, Jiang J, Sun C, Zhong Q, Shen M, Wang X, Tian R, Shi C, Xu M, Peng F, Guo X, Hu J, Ye D, Wang M, Qin R. 2016. Simultaneous inhibition of the ubiquitin-proteasome system and autophagy enhances apoptosis induced by ER stress aggravators in human pancreatic cancer cells. Autophagy 12:1521–1537. doi: 10.1080/15548627.2016.1191722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iurlaro R, Munoz-Pinedo C. 2016. Cell death induced by endoplasmic reticulum stress. FEBS J 283:2640–2652. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- 19.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. 2006. Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha S, Panigrahi DP, Patil S, Bhutia SK. 2018. Autophagy in health and disease: a comprehensive review. Biomed Pharmacother 104:485–495. doi: 10.1016/j.biopha.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Rubiolo JA, Lopez-Alonso H, Martinez P, Millan A, Cagide E, Vieytes MR, Vega FV, Botana LM. 2013. Yessotoxin induces ER-stress followed by autophagic cell death in glioma cells mediated by mTOR and BNIP3. Cell Signal 26:419–432. doi: 10.1016/j.cellsig.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Song S, Tan J, Miao Y, Li M, Zhang Q. 2017. Crosstalk of autophagy and apoptosis: involvement of the dual role of autophagy under ER stress. J Cell Physiol 232:2977–2984. doi: 10.1002/jcp.25785. [DOI] [PubMed] [Google Scholar]
- 23.Parzych KR, Klionsky DJ. 2014. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabas I, Ron D. 2011. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun X, Liao W, Wang J, Wang P, Gao H, Wang M, Xu C, Zhong Y, Ding Y. 2016. CSTMP induces apoptosis and mitochondrial dysfunction in human myeloma RPMI8226 cells via CHOP-dependent endoplasmic reticulum stress. Biomed Pharmacother 83:776–784. doi: 10.1016/j.biopha.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Qiu Y, Yang L, Peng L, Xia Z, Hou LN, Fang C, Qi H, Chen HZ. 2011. Desipramine induces apoptosis in rat glioma cells via endoplasmic reticulum stress-dependent CHOP pathway. J Neurooncol 101:41–48. doi: 10.1007/s11060-010-0237-2. [DOI] [PubMed] [Google Scholar]
- 27.Ha M, Wei L, Guan X, Li L, Liu C. 2016. p53-dependent apoptosis contributes to di-(2-ethylhexyl) phthalate-induced hepatotoxicity. Environ Pollut 208:416–425. doi: 10.1016/j.envpol.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Elmore S. 2007. Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, Ask K. 2015. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol 61:45–52. doi: 10.1016/j.biocel.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. 2004. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Her GM, Chiang CC, Chen WY, Wu JL. 2003. In vivo studies of liver-type fatty acid binding protein (L-FABP) gene expression in liver of transgenic zebrafish (Danio rerio). FEBS Lett 538:125–133. doi: 10.1016/S0014-5793(03)00157-1. [DOI] [PubMed] [Google Scholar]
- 32.Hall C, Flores MV, Storm T, Crosier K, Crosier P. 2007. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol 7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruger NJ. 1994. The Bradford method for protein quantitation. Methods Mol Biol 32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- 34.Manoli M, Driever W. 2012. Fluorescence-activated cell sorting (FACS) of fluorescently tagged cells from zebrafish larvae for RNA isolation. Cold Spring Harbor Protocols 8:pdb.prot069633. doi: 10.1101/pdb.prot069633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.