The gut is a hot spot for transfer of antibiotic resistance genes from ingested exogenous bacteria to the indigenous microbiota. The objective of this study was to determine the fate of two nearly identical blaCMY-2-harboring plasmids introduced into the human fecal microbiota by two Escherichia coli strains isolated from a human and from poultry meat.
KEYWORDS: CoMiniGut model, Escherichia coli, IncI1, cephalosporin
ABSTRACT
The gut is a hot spot for transfer of antibiotic resistance genes from ingested exogenous bacteria to the indigenous microbiota. The objective of this study was to determine the fate of two nearly identical blaCMY-2-harboring plasmids introduced into the human fecal microbiota by two Escherichia coli strains isolated from a human and from poultry meat. The chromosome and the CMY-2-encoding plasmid of both strains were labeled with distinct fluorescent markers (mCherry and green fluorescent protein [GFP]), allowing fluorescence-activated cell sorting (FACS)-based tracking of the strain and the resident bacteria that have acquired its plasmid. Each strain was introduced into an established in vitro gut model (CoMiniGut) inoculated with individual feces from ten healthy volunteers. Fecal samples collected 2, 6, and 24 h after strain inoculation were analyzed by FACS and plate counts. Although the human strain survived better than the poultry meat strain, both strains transferred their plasmids to the fecal microbiota at concentrations as low as 102 CFU/ml. Strain survival and plasmid transfer varied significantly depending on inoculum concentration and individual fecal microbiota. Identification of transconjugants by 16S rRNA gene sequencing and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) revealed that the plasmids were predominantly acquired by Enterobacteriaceae species, such as E. coli and Hafnia alvei. Our experimental data demonstrate that exogenous E. coli of human or animal origin can readily transfer CMY-2-encoding IncI1 plasmids to the human fecal microbiota. Small amounts of the exogenous strain are sufficient to ensure plasmid transfer if the strain is able to survive the gastric environment.
INTRODUCTION
The spread of β-lactamase-encoding plasmids conferring resistance to broad-spectrum cephalosporins is of particular concern due to the clinical importance of these antibiotics in human health care (1, 2). One of the β-lactamases most commonly reported in poultry and other animal reservoirs is CMY-2 (3–5). Various studies suggest that CMY-2-encoding plasmids of poultry origin may be transferred from animal to human bacteria via consumption of contaminated poultry meat, as indicated by the detection of almost identical plasmids in Escherichia coli strains from humans, poultry, and poultry meat (5–8). E. coli is an integral part of the commensal gut microbiota in both animals and humans but is also a common cause of opportunistic infections. Thus, acquisition of exogenous CMY-2-encoding plasmids introduced into the gut microbiota by bacteria from food and other sources can potentially lead to E. coli infections that cannot be treated with broad-spectrum cephalosporins.
The objective of this study was to determine the fate of two nearly identical CMY-2-encoding plasmids introduced into the human fecal microbiota by exogenous E. coli of human (C20-GM) and poultry meat (1061-1-GM) origin. Strain survival and plasmid transfer were studied over a period of 24 h using an established in vitro gut model called the CoMiniGut, which was inoculated with individual feces from 10 human volunteers. In addition to standard phenotypic counts, donor and transconjugant cells were counted and sorted by fluorescence-activated cell sorting (FACS), allowing evaluation of plasmid host range and transfer dynamics in the nonculturable fraction of the fecal microbiota.
RESULTS
Preliminary experiments on strain inoculum.
Five different concentrations of the human urinary tract infection (UTI) strain C20-GM were tested in the in vitro gut model CoMiniGut to determine the strain inoculum to be used in the following experiments. These experiments were performed under oxic and anoxic conditions using three randomly selected fecal samples (A, E, and O). FACS analysis after 24 h showed persistence of the exogenous strain in all three fecal samples (Fig. S1). Pearson correlation coefficients revealed a strong positive correlation between the inoculum concentration and the number of C20-GM cells under anoxic conditions (P values = 0.007, <0.0001, and 0.003 for samples A, E, and O, respectively) (Fig. 1a). Such correlations were also statistically significant under oxic conditions for sample A (P value = 0.008) but not for samples E and O (Fig. 1b).
FIG 1.
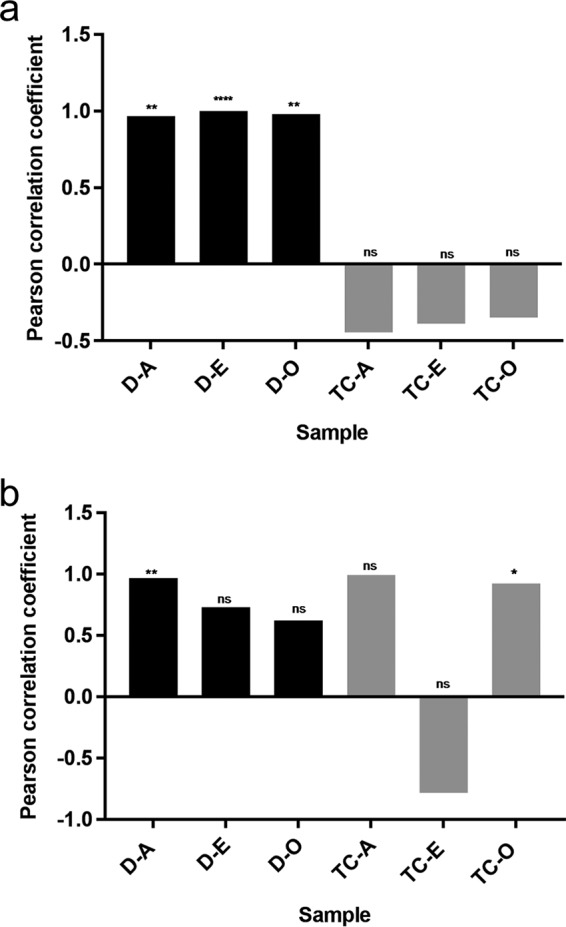
Pearson correlation coefficient (y axis) between the inoculum concentration and the number of donor cells (D) (black bars), transconjugants (TC) (gray bars) in fecal samples A, E, and O under (a) anoxic (An) and (b) oxic (O) conditions. ns, nonsignificant; *, P < 0.05; **, P < 0.005; ****, P < 0.0001.
Transconjugants were detected in all tested conditions (except in fecal samples A and E) using either a very low (10 CFU/ml) or very high (108 CFU/ml) inoculum under oxic conditions (Fig. S2). Under anoxic conditions, the Pearson correlation coefficient indicated moderate negative correlations between inoculum concentration and numbers of transconjugants from all samples (Fig. 1a). This pattern was not observed under oxic conditions, where a negative correlation was only observed for sample E and was not statistically significant (Fig. 1b).
The lowest inoculum at which both donors and transconjugants were detected in all samples was 102 CFU/ml in both oxic and anoxic conditions. Based on these results, we chose to use this inoculum concentration in the following experiments.
Experiments on strain survival and plasmid transfer.
CoMiniGut cultures of 10 fecal samples from healthy volunteers were inoculated separately with the poultry meat strain (1061-1-GM) and the human strain (C20-GM) and incubated under anoxic conditions. Samples were collected at 2, 6, and 24 h after strain inoculation and analyzed by FACS to quantify donor (red fluorescence) and transconjugant (green fluorescence) cells.
After 2 h, both strains were detected in all samples (range, 33 to 227 for 1061-1-GM and 45 to 231 for C20-GM), and transconjugants were detected in all but one sample for 1061-1-GM (range, 0 to 68) and in all but two samples for C20-GM (range, 0 to 28) (Fig. 2a).
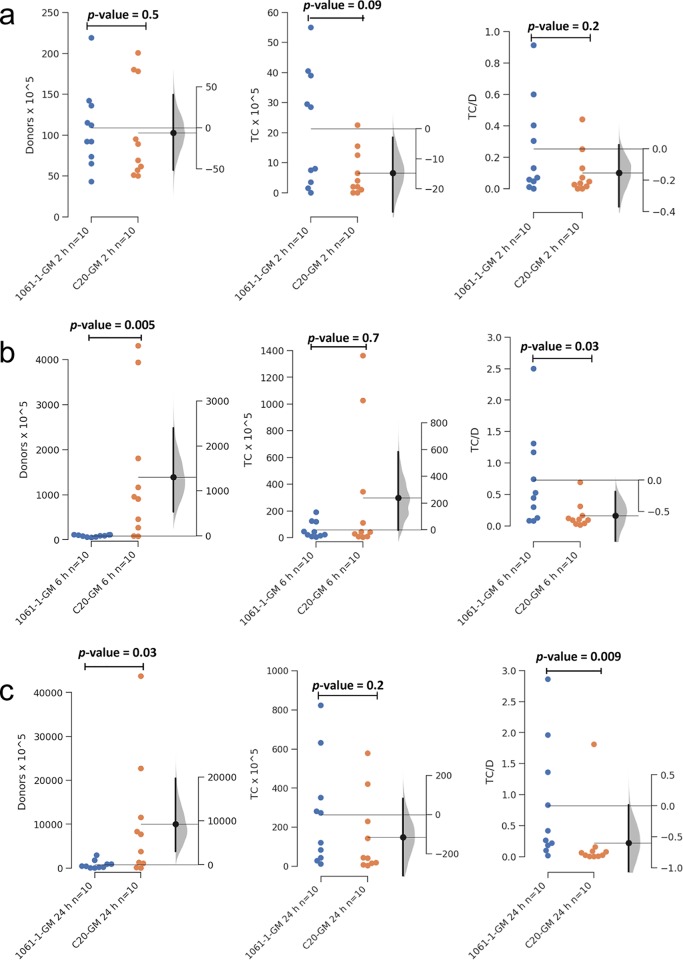
FIG 2.
Gardner-Altman 2-group mean-difference plot showing the difference between donors (D) transconjugants (TC) and transconjugant/donor (TC/D) ratios at (a) 2 h, (b) 6 h, and (c) 24 h for the poultry meat strain 1061-1-GM (blue) and the human strain C20-GM (orange) in CoMiniGut cultures. The left axis shows the number of donors detected by FACS. On the right axis, the filled curve indicates the complete Δ distribution given the observed data. The human strain C20-GM survives better than the poultry strain 1061-1-GM; however, more transconjugants were detected from the poultry meat strain than the human UTI strain. The low and high bias-corrected and accelerated bootstrap interval values are shown as a density plot on the right side. The confidence interval of the mean differences at 95% is illustrated by the thick black line. Significance was determined by the Mann-Whitney U test.
After 6 h, donor numbers were significantly lower for 1061-1-GM (range, 23 to 178) than for C20-GM (range, 52 to 5,572) (P value = 0.005). Even though the number of transconjugants did not significantly differ between the two strains (range, 1 to 242 for 1061-1-GM and 2 to 1,772 for C20-GM), the transconjugant/donor ratio was significantly higher for 1061-1-GM (range, 0.1 to 3.46) than for C20-GM (range, 0.0005 to 0.45) (P value = 0.03) (Fig. 2b). After 24 h, the numbers of 1061-1-GM (range 31 to 3,744) were still significantly lower than those for C20-GM (range, 19 to 46,310) (P value = 0.03). At this time point, transconjugants were detected in all samples, without significant differences between the two strains (range, 8 to 846 and 1 to 661, respectively), but the transconjugant/donor ratio proved to be significantly higher for 1061-1-GM (range 0.007 to 3.6) than for C20-GM (range, 0.00002 to 1.9) (P value= 0.009) (Fig. 2c). Altogether, the different survival dynamics displayed by the two strains in human feces did not affect their ability to transfer IncI1 CMY-2-encoding plasmids to the indigenous microbiota.
Influence of the initial Enterobacteriaceae concentration on survival of exogenous E. coli.
The numbers of the two exogenous E. coli strains measured by FACS at the three different time points were compared to the initial Enterobacteriaceae counts (Table S1) to determine if the strain’s survival was influenced by the concentration of indigenous Enterobacteriaceae in the recipient fecal sample. In general, there was a negative correlation between counts of preexisting Enterobacteriaceae and survival of both exogenous strains, although such a negative correlation was statistically significant only after 24 h (P values = 0.03 for 1061-1-GM and 0.04 for C20-GM) (Fig. 3a). When limited to strain 1061-1-GM, the Pearson correlation coefficient showed a significant negative correlation between counts of preexisting Enterobacteriaceae and numbers of transconjugants detected by FACS after 24 h (P value= 0.04) (Fig. 3b).
FIG 3.
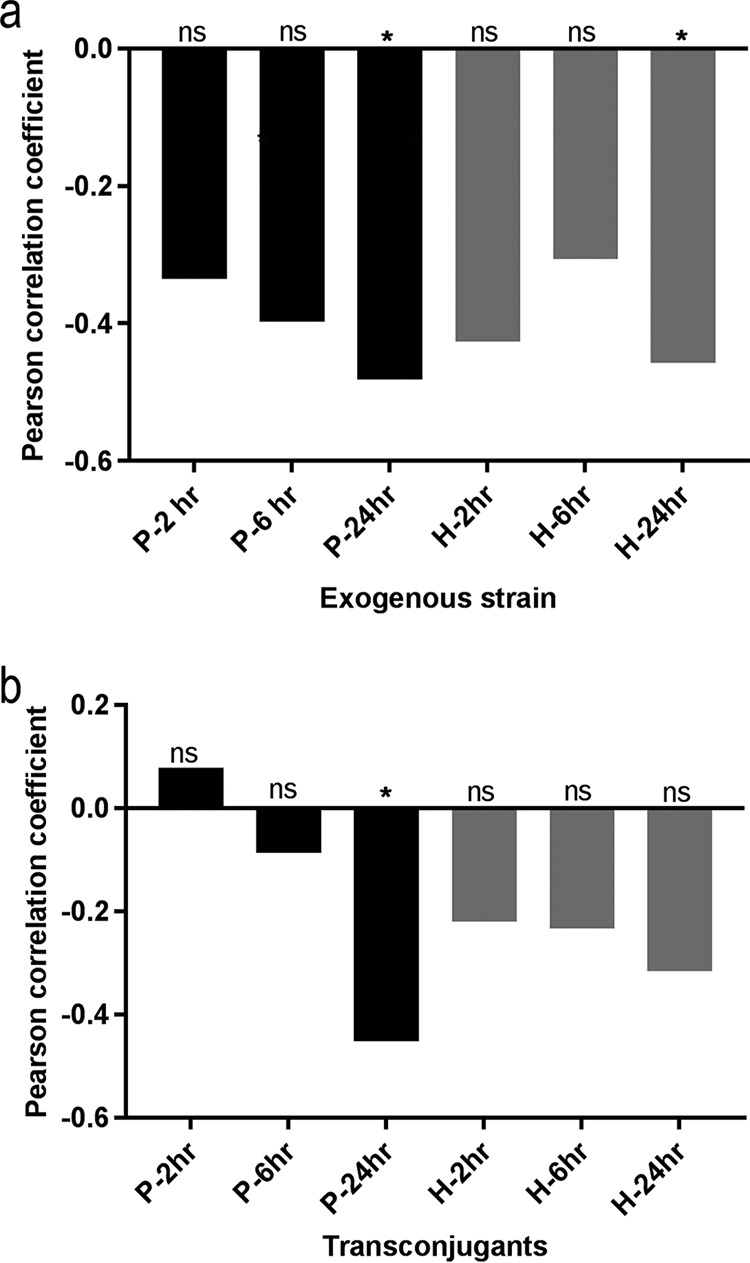
Pearson correlation coefficient (y axis) indicating the relationship between initial Enterobacteriaceae counts in the fecal samples and (a) numbers of the exogenous strain poultry strain (black bars) and human strain (gray bars) or (b) transconjugants that acquired their plasmids over time. After 24 h, the numbers of the two exogenous strains negatively correlated with the counts of preexisting Enterobacteriaceae in the original fecal sample (a). A significant negative correlation was also seen between counts of preexisting Enterobacteriaceae and the numbers of transconjugants that received the plasmid from poultry strain after 24 h (b). ns, nonsignificant; *, P < 0.05.
Bacterial community composition of different fecal samples.
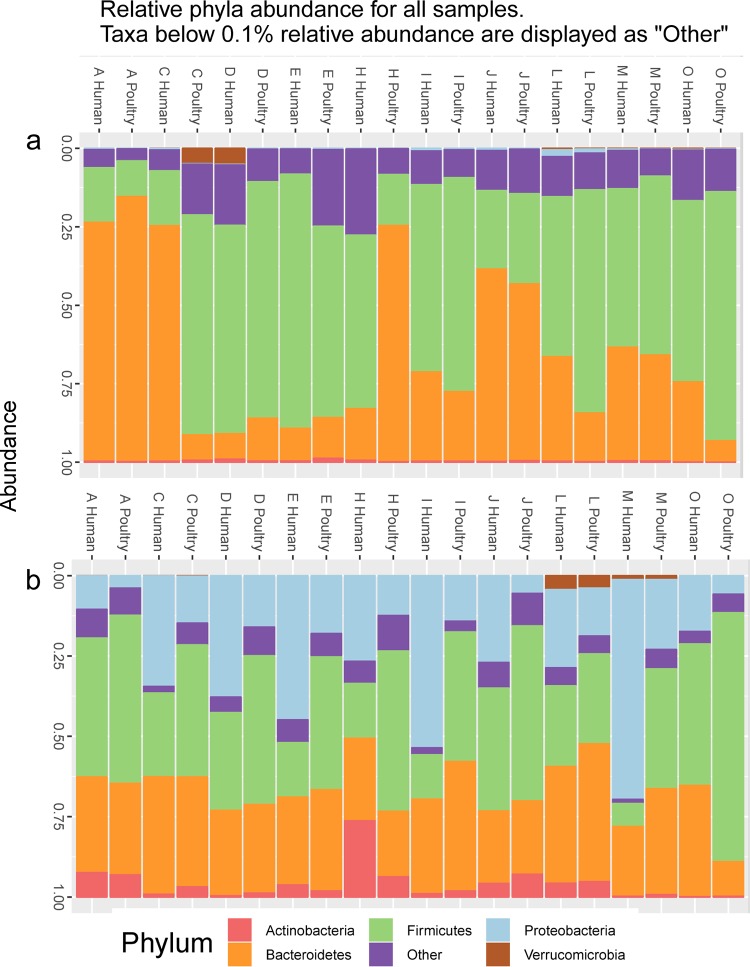
Bacterial community composition was determined by 16S rRNA gene amplicon sequencing. This analysis was performed in the 10 fecal sample stocks, as well as 24 h after the samples were inoculated with the exogenous strains in CoMiniGut, including all biological replicates (n = 30 per strain). The initial bacterial community composition varied between fecal samples, with either Firmicutes or Bacteroidetes being the dominant phylum (Fig. 4a). The abundance of Proteobacteria increased in all samples during CoMiniGut culture, most likely due to the experimental conditions favoring fast-growing bacteria, but the magnitude of this increase varied markedly between samples (Fig. 4b).
FIG 4.
Relative abundance at phylum level in 10 fecal samples (A to O) before (a) and 24 h after (b) inoculation of the two exogenous strains of human and poultry origin into the corresponding CoMiniGut culture. The figure shows that the abundance of Proteobacteria increased after inoculation of the exogenous strains, although with marked differences between individual fecal samples.
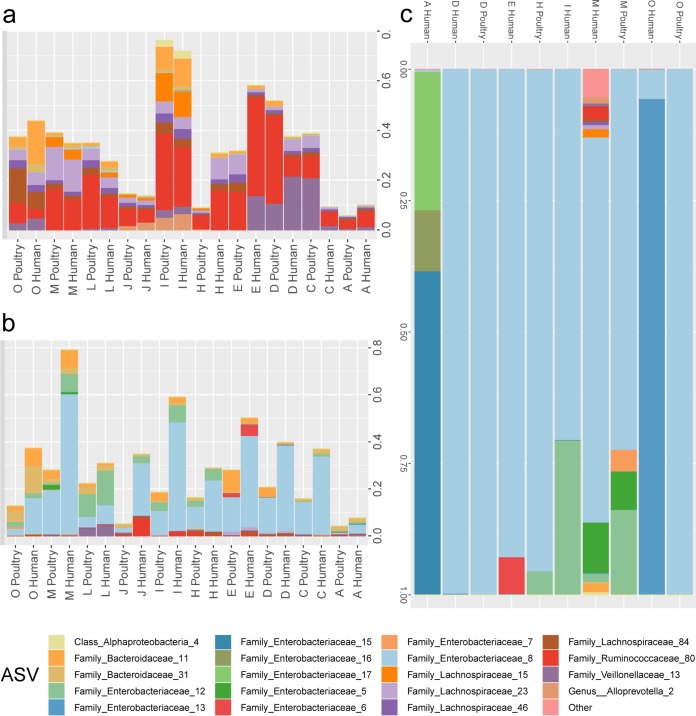
The abundance of relative amplicon sequence variants (ASVs) found in sorted transconjugants was compared to abundance of those ASVs in the fecal samples and CoMiniGut samples. The most-recovered ASVs in sorted transconjugants were not common in the initial fecal community and were only moderately enriched after 24 h of incubation in the CoMiniGut, yet the plasmid was acquired predominantly by specific ASVs from Enterobacteriaceae species (Fig. 5).
FIG 5.
Relative ASV abundance as a function of fecal donor and strain source; only ASVs detected in the sorted transconjugants from CoMiniGut culture are shown. ASVs with <0.05% relative abundance are grouped in “other.” (a) Fecal sample, (b) CoMiniGut samples, (c) sorted transconjugants. The key shows the lowest taxonomic rank (family/class/genus) that could be confidently attributed to each amplicon sequence variant using Bayesian classification.
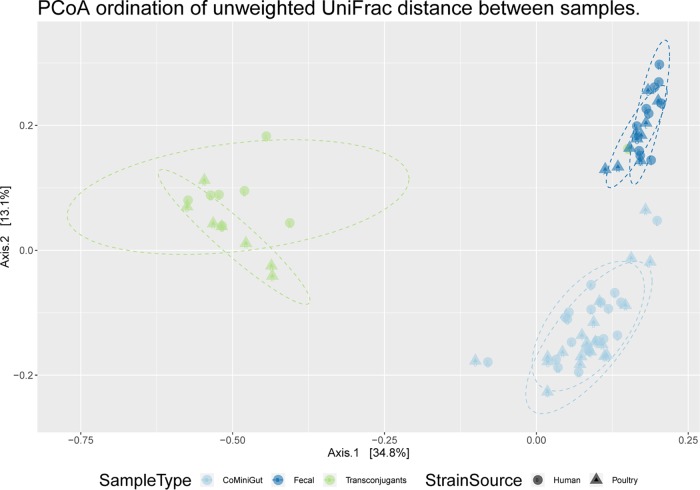
Principal-coordinate analysis (PCoA) of the unweighted UniFrac distance matrix based on ASV counts for all samples showed that the bacterial communities from the initial fecal sample, after 24-h CoMiniGut incubation, and from sorted transconjugants formed tight clusters and that this grouping was significant (P value < 0.001) based on permutational multivariate analysis of variance of the UniFrac distance matrix (Fig. 6).
FIG 6.
Unweighted UniFrac-based principal-coordinate analysis (PCoA) showing the clustering of bacterial communities according to the sample type and strain source. The strain source is human donor assay (circles) or poultry donor assay (triangles). The sample types are CoMiniGut cultures after 24 h (light blue), fecal samples before inoculation (dark blue), and sorted transconjugants from both assays (green). Each dot represents a sample.
Bacterial recipients of plasmids.
The diversity of transconjugants was investigated by 16S rRNA gene amplicon sequencing of the green cells isolated by FACS (gate P7). This was done on samples in which the transconjugant population was at least 0.1% of the 100,000 bacteria that were analyzed by FACS (D, E, H, M, and O for 1061-1-GM, and A, D, E, I, M, and O for C20-GM). The most abundant ASVs belonged to the Enterobacteriaceae, with multiple sequence variants detected in all samples, except for sample D after inoculation of both strains and sample O after inoculation of 1061-1-GM. The transconjugants detected in sample M after inoculation of C20-GM were more diverse than those in other samples and included those belonging to the Gram-negative Bacteroidaceae genus Alloprevotella and the Gram-positive families Lachnospiraceae and Ruminococcaeae (Fig. 6).
Additionally, non-red cells (n = 106) were sorted from all CoMiniGut cultures after 24 h and plated on blood agar plates supplemented with kanamycin and cefotaxime for isolation of presumptive transconjugants. Isolates where the presence of the donor plasmid was confirmed by PCR targeting the regions upstream and downstream of the green fluorescent protein (GFP) cassette and by confocal microscopy for green fluorescence were identified to the species level by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). Following inoculation of 1061-1-GM, transconjugants were isolated from samples D (n = 24), E (n = 15), H (n = 8), and M (n = 46), and all transconjugant isolates were identified as E. coli. After inoculation of C20-GM, transconjugants were detected in samples D (n = 22), E (n = 10), H (n = 16), M (n = 30), and O (n = 12), and all transconjugant isolates were E. coli, with the exception of transconjugants from sample O that were identified as Hafnia alvei, another member of the Enterobacteriaceae family.
DISCUSSION
We investigated horizontal gene transfer of the GFPmut3-tagged IncI1/pST12 CMY-2-encoding plasmids from exogenous E. coli of human and poultry origin to the fecal bacterial communities from 10 human donors. GFPmut3-expressing transconjugant cells were isolated by FACS, allowing transconjugant detection and identification in spite of their relatively low abundance in the CoMiniGut model. This model was used to simulate the colon environment and mimic the effect of ingesting exogenous CMY-2-producing E. coli from contaminated food or by the fecal-oral route. Our results indicate that CMY-2-encoding IncI1 plasmids can readily transfer to the indigenous fecal microbiota at inoculum concentrations as low as 100 CFU (Fig. S1 and S2). This finding highlights the possibility that low numbers of exogenous strains are sufficient to transfer blaCMY-2 to the resident gut microbiota, provided that the strains are able to survive the gastric environment of the stomach and reach the colon.
Gut colonization by exogenous strains is not a prerequisite for plasmid transfer, as indicated by the early detection of transconjugants shortly (2 h) after strain inoculation in 8 of the 10 fecal samples tested. Accordingly, even a brief transit of exogenous E. coli through the colon may lead to acquisition of CMY-2-encoding IncI1 plasmids by the indigenous microbiota. This is important from a public health point of view because once the plasmid has transferred to a resident recipient, the resulting transconjugant can itself act as donor.
The number of donors detected by FACS after 6 h and 24 h in the fecal microbiota from human volunteers indicated that the human strain survived better than the strain isolated from poultry meat (Fig. 2 and Fig. S3). Based on multilocus sequence typing (MLST), both strains belonged to sequence types (ST) (ST155 and ST10 for the human and poultry strain, respectively) frequently detected among E. coli isolates from food, animals, and humans worldwide (9, 10). Even though the general composition of the fecal microbiota is similar between humans and other vertebrates, the poultry fecal microbiota significantly differs from human fecal microbiota (11). Perhaps the strain from human UTI was more adapted than the poultry meat strain to survive within human fecal microbiota. However, this observation cannot be generalized since only single strains of human and poultry origin were tested.
The numbers of transconjugants detected from the poultry meat strain were higher than those from the human UTI strain. The transconjugant/donor ratio was also higher for the poultry meat strain because of the high numbers of transconjugants and lower numbers of donors than those in the human strain for all samples except one, sample D (Fig. 2 and Fig. S3). The plasmid transfer efficiency in human fecal microbiota was likely similar for both strains. Indeed, in the in vitro experiments with lab strain, both strains had a transfer efficiency of 10−5 transconjugants per donor cell (12). The plasmid transfer thus was not dependent on the concentration of the exogenous strain but on transconjugant survival and secondary transfer. Relatively high conjugation frequencies, in the range of 10−2 to 10−6 transconjugants/recipient, have been previously reported for IncI1 plasmids (13), which are highly prevalent in Enterobacteriaceae (14).
Several studies have documented in vivo plasmid transfer from a donor of animal origin to a human recipient strain (15–18), but in all these experiments, the in vivo models were fed with high numbers of donor and recipient strains (107 to 109 CFU). Such high numbers of E. coli are unlikely to be ingested via food in real life. A previous study conducted in Belgium reported 7% and 3% likelihoods of humans being exposed to 10 CFU or 100 CFU extended-spectrum β-lactamase (ESBL)-producing E. coli from poultry meat, respectively (19). Evers et al. showed only a 6.9% chance of humans being exposed to 1 CFU of bacteria through consumption of poultry meat (20). Thus, the inoculum size of 102 CFU/ml (500 CFU) used in our study is more realistic, considering the information on human exposure to this type of bacteria and the expected reduction of the initial inoculum present on poultry meat due to washing and/or cooking, as well as the low pH in the stomach (pH = 2), which acts as a natural barrier to ingested microbes.
The FACS-sorted transconjugants were predominantly identified as members of Enterobacteriaceae, which is consistent with the narrow host range of IncI1 plasmids (14) (Fig. 5). Various anaerobic phyla seemed to acquire the IncI1 plasmid from the human UTI strain in sample M. However, these presumptive anaerobic transconjugants were not verified by cultivation, as the agar plates were only incubated under aerobic conditions. The presence of IncI1 plasmids in species other than members of the Enterobacteriaceae has not been shown before, but most of the previous studies relied on culture-based detection of transconjugants and did not investigate the fate of these plasmids in complex bacterial communities, such as those residing in human feces.
Correlation coefficients between the initial Enterobacteriaceae population and donor survival along with plasmid transfer indicated a moderate negative correlation for both 1061-1-GM and C20-GM (Fig. 3). E. coli bacteria are less efficient at establishing themselves in microbiomes when there are higher numbers of Enterobacteriaceae already present in the population, possibly because they compete for the same ecological niche.
As the experimental setup was limited to 24 h, it is impossible to determine whether the magnitude of plasmid transfer and the number of bacterial taxa involved would have increased if the experiment was continued for a longer period. It should be noted that our experimental setup cannot differentiate between primary transconjugants that have obtained the plasmid from the exogenous donor strain and those that have acquired the plasmid from primary transconjugants acting as donors. In addition, our approach cannot distinguish between horizontal and vertical transfer, since the transconjugants detected in our experiment may well represent the offspring of transconjugants transmitting the acquired plasmid vertically. Consequently, the observed variations in numbers of transconjugants do not necessary directly reflect the plasmid transfer frequencies, which are generally estimated within one or two bacterial generation times. Another limitation of the study is the antibiotic concentration used for the culture-based detection of transconjugants, which was selected based on breakpoints specific for Enterobacteriaceae. Thus, transconjugants belonging to other bacterial families could fail to grow at these antibiotic concentrations because resistance genes are usually poorly expressed in distantly related heterologous hosts (21).
ASV sequences identified in transconjugants, belonging predominantly to Enterobacteriaceae, were present at very low abundances in the initial fecal sample. Such Enterobacteriaceae populations increased after 24 h of incubation in the CoMiniGut, but the transfer of plasmids primarily to Enterobacteriaceae also points toward the narrow host range of IncI1 plasmids (Fig. 5). The enrichment of Proteobacteria in CoMiniGut cultures was mainly due to the experimental conditions (24 h culture), because they are among the fastest-growing bacteria.
We conclude that foodborne and fecal-oral transmission are a possible route for transfer of antibiotic resistance IncI1 CMY-2-encoding plasmids carried by exogenous E. coli, provided that the host strain survives cooking and stomach pH, even if in small numbers. To further assess this risk, in vivo quantitative studies are needed to evaluate the effect of the stomach environment on concentrations of E. coli strains transiting through the gut.
MATERIALS AND METHODS
Strains and media.
The two genetically modified human and poultry meat E. coli strains used in this study (C20-GM and 1061-1-GM, respectively) were constructed and validated previously (12). The strains were typed as ST155 (C20-GM) and ST10 (1061-1-GM) by MLST and harbor blaCMY-2 on IncI1 plasmids of sequence type 12 (pST12) sharing 99% nucleotide identity over 97% of the length of the plasmid (European Nucleotide Archive accession number PRJEB9625) (5). The strains were genetically modified by inserting an mCherry fluorescent marker (red) into the pseudogene ybeM on the chromosome and a GFP fluorescent marker (green) into a noncoding region on the IncI1 plasmid (12).
Media used were Luria Bertani broth (LB-B), Luria Bertani agar (LB-A), MacConkey agar, 5% blood agar (Oxoid, Ltd., Roskilde, Denmark), and complex colon medium prepared according to Macfarlane et al. (22). Antibiotics were used at the following concentrations throughout the work unless mentioned otherwise: 1 mg/liter of cefotaxime and 50 mg/liter of kanamycin.
Phosphate-buffered saline (PBS) 1 M (pH 7) was prepared as follows (g/liter): NaCl (8); KCl (0.2); Na2HPO4 · 2H2O (1.44); KH2PO4 (0.24) in distilled H2O. PBS 0.1 M (pH 5.6) was prepared from the PBS 1 M stock NaCl 0.9% solution (g/liter) in distilled H20. All chemicals were obtained from the company Sigma-Aldrich (Søborg, Denmark) unless otherwise stated. All solutions were autoclaved before using.
Fecal samples.
Fecal samples were collected from 10 healthy human volunteers not exposed to antibiotics during the last 6 months. Their ages ranged from 5 to 68 years. Ethical permission for collection of these samples was waived by the Danish National Committee on Health Research Ethics. The samples were delivered to the laboratory immediately after collection and kept at −20°C until processing. All samples were processed within 24 h after they were received. Feces were weighed and an equal amount (wt/vol) of 20% glycerol/0.1 M PBS solution was added prior to homogenization in a stomacher for 2 × 60 sec. The resulting fecal suspensions were labeled and frozen in cryotubes at −80°C. Immediately before the start of the experiment, each suspension was thawed and diluted 1:5 with 0.1 M PBS at pH 5.6 (working stock).
CoMiniGut experiments.
CoMiniGut is an in vitro system that simulates the colon passage of the human gut (23). The CoMiniGut has five vessels running in parallel. Each vessel, which has a total 5-ml volume comprising of media, fecal sample, and donor strain, was inoculated with 10% (vol/vol) of fecal sample in the complex colon medium. Over 24 h, the pH increased from 5.7 to 6.0 in the first 8 h to simulate the proximal colon. Then, in the following 8 h, it increased to 6.5 to represent the transverse colon, and it finally reached 6.9 in the last 8 h to simulate the distal colon environment. Preliminary experiments were performed using the human strain C20-GM to determine the strain inoculum concentration. These experiments were performed in oxic and anoxic conditions. Briefly, three fecal samples (A, E, and O) were randomly selected and challenged with C20-GM to reach five different concentrations of C20-GM (108, 106, 104, 102, and 10 CFU/ml) in each CoMiniGut vessel.
The lowest inoculum concentration for which the donor was detected 24 h after inoculation was selected for the final experiment, in which all the 10 fecal samples were independently challenged with C20-GM and 1061-1-GM under anoxic conditions. The experimental design was set up to mimic the colon environment of human gut. A volume of 300 μl was collected from each vessel 2, 6, and 24 h after strain inoculation in the CoMiniGut. All experiments were run in two biological replicates. The average value from biological replicates was used for further analysis.
Cell collection and multiple-gated FACS of transconjugants.
All samples from the CoMiniGut experiments were analyzed by an FACSAria IIIu flow cytometer (Becton, Dickinson Biosciences, San Jose, CA). Samples from anoxic cultures were diluted 100-fold in 1 M PBS (pH 7) and exposed to oxygen by shaking at 110 rpm at 4°C for up to 3 h. This allowed the fluorescent proteins to mature properly before FACS analysis (24). The settings used were the same as described by Anjum et al. (12). All samples were diluted in 0.9% NaCl to ∼2,000 counting events · s−1 before FACS to ensure optimal detection of donors and sorting of transconjugants. Control laboratory strains expressing only mCherry or GFP or without any fluorescent marker were used to design gates for analysis with FACS. Six gates were defined in bivariate plots to sort for detection of donors and for transconjugants. On the side scatter-A versus forward scatter-A plot, a gate for only particles of bacterial size was selected. On the PE-Texas Red-A versus side scatter-A plot, a gate was set that covered all red fluorescent particles, and on the duplicate plot, the same gate was set to detect and sort non-red fluorescent particles. On the fluorescein isothiocyanate (FITC)-A versus side scatter-A plot, a gate was set that covered all green fluorescent particles. As the particles from fecal sample and media autofluoresced, thus interfering with the gates selected for detection of mCherry and GFPmut3, a more stringent gate (P7) was selected for sorting, as follows. On the FITC-A versus side scatter-A plot, a gate was set up based on GFP-expressing control lab strain in complex colon media that covered transconjugants with the highest GFP expression. An additional non-red gate on the PE-Texas Red-A versus FITC-A plot ensured exclusion of small autofluorescent particles from fecal sample, media, or leaking donors to sort out only transconjugants. This may have resulted in loss of sorting of some transconjugants that did not have a high GFP expression, but it ensured that the cells sorted were indeed the correct transconjugants. The threshold for detection was set at 100,000 counting events, and therefore the numbers of donors and transconjugants from FACS analysis are given out of 105 cells analyzed in FACS.
For each sample sorted, a minimum of 15,000 and a maximum of 30,000 transconjugants were sorted. The cutoff for sorting of transconjugants was set so that it was performed only for the samples from one time point, in which the numbers of transconjugants detected were at least 0.1% of the whole population in gate P7. Sorted cells were collected in 5-ml sterile polystyrene round-bottomed Falcon tubes with 0.5 ml of 0.9% NaCl solution.
Sorting was also performed for isolation of 106 cells that were not red for all samples from both biological replicates. This fraction was plated on blood agar plates containing cefotaxime and kanamycin and incubated in anoxic conditions at 37°C overnight. All colonies were observed with confocal microscopy to detect green and red fluorescence. The green colonies were subjected to PCR targeting the region on the plasmid where the GFPmut3 cassette was inserted using the primers Fwd pC20/1061-1 confirm and Rev pC20/1061-1 confirm (12). All PCR-positive colonies were identified to species level by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (bioMérieux, France).
Sequence-based analysis of fecal microbiota.
Microbial community profiling was performed on fecal samples prior to CoMiniGut experiments, after 24 h CoMiniGut incubation, and on the FACS-sorted transconjugants (from gate P7) by 16S rRNA marker gene amplicon sequencing.
DNA from the original fecal sample and from the 24-h CoMiniGut culture was extracted using a DNeasy PowerSoil kit (Qiagen, Denmark) according to the manufacturer’s instructions. DNA was used for amplicon high-throughput sequencing of the 16S rRNA gene using a MiSeq benchtop sequencer (Illumina, CA). Amplicon libraries were obtained after a PCR targeting the hypervariable V3 region of the 16S rRNA gene.
Amplicon libraries for transconjugant analysis were performed by PCR of the cell pellets using the GenePurgeDirect (Nimagen) direct PCR kit. Sorted cells were transferred to 1.5-ml Eppendorf tubes and centrifuged at 10,000 × g for 30 min to collect cell pellets. The supernatant was carefully removed, and the cell pellet was suspended in 20 μl of GenePurgeDirect lysis matrix. The cell lysis mixture slurries were then transferred to 0.2-ml amplification tubes. Cell lysis was performed in the thermal cycler using the manufacturer’s instructions.
PCRs were performed with 5 μl of lysis mixture using primers targeting bacterial and archaeal 16S rRNA gene V3 regions with overhanging adapters compatible with the Nextera index kit (Illumina), rNXt_388_F: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGACWCCTACGGGWGGCAGCAG-3′ and NXt_518_R: 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGATTACCGCGGCTGCTGG-3′ (adapters in bold). The PCRs and library preparations were conducted as described previously (25). All individual sample libraries were then pooled in equimolar proportion and sequenced using a MiSeq v2 sequencing kit, producing 2 × 250-bp paired-end reads, on an Illumina MiSeq benchtop sequencer following the manufacturer’s guidelines.
Amplicon sequences were analyzed using the phyloseq R package (26) and the additional R packages vegan and ggplot2 (27, 28). Unweighted UniFrac distances were computed using phyloseq implementation of Fast UniFrac (29). Raw amplicon reads were denoised and clustered in ASV using DADA2 (30) implementation in QIIME2. Each unique sequence is classified against the SILVA NR99 rel. 132 SSU database (31) using the q2-feature-classifier naive Bayes classifier (32) at the lowest taxonomic rank up to the genus level, with a confidence threshold of 0.7. Each ASV sequence present above a cumulative abundance of 0.05% of reads in transconjugants samples was further identified manually using manual BLAST searches (Table S2).
Culture-based analysis of fecal samples.
For the culture-based analysis of species diversity within Enterobacteriaceae, 100 μl from the working stock solution of each fecal sample was spread on MacConkey agar (MAC) plates with or without cefotaxime. At least one colony per morphology observed on MAC plates was analyzed by MALDI-TOF.
Statistical methods.
The Pearson correlation coefficient was calculated, using Microsoft Excel software, to assess the relationships of inoculum concentrations with number of donors and transconjugants and transconjugant/donor ratio. The cutoff for negative correlation was set at r ≥ −0.25 and for positive correlation at r ≤ 0.25.
The Gardner-Altman 2-group mean-difference plots were drawn using the web application Estimation Statistics (http://www.estimationstats.com/#/), which is based on data analysis using Bootstrap-coupled ESTimation (DABEST) (33). Statistical significance was set at P < 0.05. The reference group in each analysis was assigned to the 1061-1-GM strain, and the experimental group was the C20-GM strain.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the University of Copenhagen Research Center for Control of Antibiotic Resistance (UC-Care, http://www.uc-care.ku.dk) and by the Center for Research in Pig Production and Health (CPH Pig, http://cphpig.ku.dk). The work by J.S.M., J.N., and S.J.S. was funded by the Joint Programming Initiative on Antimicrobial Resistance and the Danish Innovation Foundation (JPIAMR CoFund 2016, DARWIN project 7044-00004B) and the Lundbeckfonden (SHARE, grant R250-2017-1392).
We declare that we have no conflicts of interest.
This article does not contain any studies with human participants or animals that were performed by any of the authors. Ethical permission for collection of fecal samples from human volunteers was waived by the Danish National Committee on Health Research Ethics.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02528-18.
REFERENCES
- 1.European Centre for Disease Prevention and Control (ECDC), European Food Safety Authority (EFSA), European Medicines Agency (EMA). 2017. ECDC/EFSA/EMA first joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J 15:4872. doi: 10.2903/j.efsa.2017.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathers AJ, Peirano G, Pitout JDD. 2015. Escherichia coli ST131: the quintessential example of an international multiresistant high-risk clone. Adv Appl Microbiol 19:109–154. doi: 10.1016/bs.aambs.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Dierikx CM, van der Goot JA, Smith HE, Kant A, Mevius D. 2013. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: a descriptive study. PLos One 8:e79005. doi: 10.1371/journal.pone.0079005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bortolaia V, Hansen KH, Nielsen CA, Fritsche TR, Guardabassi L. 2014. High diversity of plasmids harbouring blaCMY-2 among clinical Escherichia coli isolates from humans and companion animals in the upper Midwestern USA. J Antimicrob Chemother 69:1492–1496. doi: 10.1093/jac/dku011. [DOI] [PubMed] [Google Scholar]
- 5.Hansen KH, Bortolaia V, Nielsen CA, Nielsen JB, Schonning K, Agerso Y, Guardabassi L. 2016. Host-specific patterns of genetic diversity among IncI1-I gamma and IncK plasmids encoding CMY-2 beta-lactamase in Escherichia coli isolates from humans, poultry meat, poultry, and dogs in Denmark. Appl Environ Microbiol 82:4705–4714. doi: 10.1128/AEM.00495-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Börjesson S, Jernberg C, Brolund A, Edquist P, Finn M, Landén A, Olsson-Liljequist B, Tegmark Wisell K, Bengtsson B, Englund S. 2013. Characterization of plasmid-mediated ampc-producing E. coli from Swedish broilers and association with human clinical isolates. Clin Microbiol Infect 19:E309–E311. doi: 10.1111/1469-0691.12192. [DOI] [PubMed] [Google Scholar]
- 7.de Been M, Lanza VF, de Toro M, Scharringa J, Dohmen W, Du Y, Hu J, Lei Y, Li N, Tooming-Klunderud A, Heederik DJJ, Fluit AC, Bonten MJM, Willems RJL, de la Cruz F, van Schaik W. 2014. Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet 10:e1004776. doi: 10.1371/journal.pgen.1004776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg ES, Wester AL, Ahrenfeldt J, Mo SS, Slettemeås JS, Steinbakk M, Samuelsen Ø, Grude N, Simonsen GS, Løhr IH, Jørgensen SB, Tofteland S, Lund O, Dahle UR, Sunde M. 2017. Norwegian patients and retail chicken meat share cephalosporin-resistant Escherichia coli and IncK/blaCMY-2 resistance plasmids. Clin Microbiol Infect 23:407.e9–407.e15. doi: 10.1016/j.cmi.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Manges AR, Johnson JR. 2012. Food-borne origins of Escherichia coli causing extraintestinal infections. Clin Infect Dis 55:712–719. doi: 10.1093/cid/cis502. [DOI] [PubMed] [Google Scholar]
- 10.Skurnik D, Clermont O, Guillard T, Launay A, Danilchanka O, Pons S, Diancourt L, Lebreton F, Kadlec K, Roux D, Jiang D, Dion S, Aschard H, Denamur M, Cywes-Bentley C, Schwarz S, Tenaillon O, Andremont A, Picard B, Mekalanos J, Brisse S, Denamur E. 2016. Emergence of antimicrobial-resistant Escherichia coli of animal origin spreading in humans. Mol Biol Evol 33:898–914. doi: 10.1093/molbev/msv280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JE, Lee S, Sung J, Ko G. 2011. Analysis of human and animal fecal microbiota for microbial source tracking. ISME J 5:362–365. doi: 10.1038/ismej.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anjum M, Madsen JS, Espinosa-Gongora C, Jana B, Wiese M, Nielsen DS, Sørensen SJ, Moodley A, Bortolaia V, Guardabassi L. 2018. A culture-independent method for studying transfer of IncI1 plasmids from wild-type Escherichia coli in complex microbial communities. J Microbiol Methods 152:18–26. doi: 10.1016/j.mimet.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Mo SS, Sunde M, Ilag HK, Langsrud S, Heir E. 2017. Transfer potential of plasmids conferring extended-spectrum-cephalosporin resistance in Escherichia coli from poultry. Appl Environ Microbiol 83:e00654-17. doi: 10.1128/AEM.00654-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moubareck C, Bourgeois N, Courvalin P, Doucet-Populaire F. 2003. Multiple antibiotic resistance gene transfer from animal to human Enterococci in the digestive tract of gnotobiotic mice. Antimicrob Agents Chemother 47:2993–2996. doi: 10.1128/AAC.47.9.2993-2996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trobos M, Lester CH, Olsen JE, Frimodt-Møller N, Hammerum AM. 2009. Natural transfer of sulphonamide and ampicillin resistance between Escherichia coli residing in the human intestine. J Antimicrob Chemother 63:80–86. doi: 10.1093/jac/dkn437. [DOI] [PubMed] [Google Scholar]
- 17.Faure S, Perrin-Guyomard A, Delmas JM, Chatre P, Laurentie M. 2010. Transfer of plasmid-mediated CTX-M-9 from Salmonella enterica serotype virchow to Enterobacteriaceae in human flora-associated rats treated with cefixime. Antimicrob Agents Chemother 54:164–169. doi: 10.1128/AAC.00310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparo M, Urbizu L, Solana MV, Pourcel G, Delpech G, Confalonieri A, Ceci M, Sánchez Bruni SF. 2012. High-level resistance to gentamicin: genetic transfer between Enterococcus faecalis isolated from food of animal origin and human microbiota. Lett Appl Microbiol 54:119–125. doi: 10.1111/j.1472-765X.2011.03182.x. [DOI] [PubMed] [Google Scholar]
- 19.Depoorter P, Persoons D, Uyttendaele M, Butaye P, De Zutter L, Dierick K, Herman L, Imberechts H, Van Huffel X, Dewulf J. 2012. Assessment of human exposure to 3rd generation cephalosporin resistant E. coli (CREC) through consumption of broiler meat in Belgium. Int J Food Microbiol 159:30–38. doi: 10.1016/j.ijfoodmicro.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Evers EG, Pielaat A, Smid JH, Van Duijkeren E, Vennemann FBC, Wijnands LM, Chardon JE. 2017. Comparative exposure assessment of ESBL-producing Escherichia coli through meat consumption. PLoS One 12:e0169589. doi: 10.1371/journal.pone.0169589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glick BR. 1995. Metabolic load and heterologous gene expression. Biotechnol Adv 13:247–261. doi: 10.1016/0734-9750(95)00004-A. [DOI] [PubMed] [Google Scholar]
- 22.Macfarlane GT, Macfarlane S, Gibson GR. 1998. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb Ecol 35:180–187. doi: 10.1007/s002489900072. [DOI] [PubMed] [Google Scholar]
- 23.Wiese M, Khakimov B, Nielsen S, Sørensen H, van den Berg F, Nielsen DS. 2018. CoMiniGut—a small volume in vitro colon model for the screening of gut microbial fermentation processes. PeerJ 6:e4268. doi: 10.7717/peerj.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinilla-Redondo R, Riber L, Sørensen SJ. 2018. Fluorescence recovery allows the implementation of a fluorescence reporter gene platform applicable for the detection and quantification of horizontal gene transfer in anoxic environments. Appl Environ Microbiol 84:e02507-17. doi: 10.1128/AEM.02507-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krych Ł, Kot W, Bendtsen KMB, Hansen AK, Vogensen FK, Nielsen DS. 2018. Have you tried spermine? A rapid and cost-effective method to eliminate dextran sodium sulfate inhibition of PCR and RT-PCR. J Microbiol Methods 144:1–7. doi: 10.1016/j.mimet.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 26.McMurdie PJ, Holmes S. 2013. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson L. 2011. ggplot2: Elegant Graphics for Data Analysis by Wickham, H. Biometrics 67:678–679. doi: 10.1111/j.1541-0420.2011.01616.x. [DOI] [Google Scholar]
- 28.Oksanen AJ, Blanchet FG, Friendly M, Kindt R, Legendre P, Mcglinn D, Minchin PR, Hara RBO, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. 2018. Package ‘vegan’ (version 2.4-0). https://cran.r-project.org/package=vegan.
- 29.Hamady M, Lozupone C, Knight R. 2010. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J 4:17–27. doi: 10.1038/ismej.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, Huttley GA, Caporaso JG. 2018. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90. doi: 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A. 2018. Moving beyond P values: everyday data analysis with estimation plots. bioRxiv 10.1101/377978. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.