In Enterobacter cloacae complex (ECC), the overproduction of the chromosome-encoded cephalosporinase (cAmpC) associated with decreased outer membrane permeability may result in carbapenem resistance. In this study, we have characterized ACT-28, a cAmpC with weak carbapenemase activity, from a single Enterobacter kobei lineage.
KEYWORDS: AmpC, carbapenem hydrolysis, cephalosporinase, dissemination
ABSTRACT
In Enterobacter cloacae complex (ECC), the overproduction of the chromosome-encoded cephalosporinase (cAmpC) associated with decreased outer membrane permeability may result in carbapenem resistance. In this study, we have characterized ACT-28, a cAmpC with weak carbapenemase activity, from a single Enterobacter kobei lineage. ECC clinical isolates were characterized by whole-genome sequencing (WGS), susceptibility testing, and MIC, and carbapenemase activity was monitored using diverse carbapenem hydrolysis methods. ACT-28 steady-state kinetic parameters were determined. Among 1,039 non-carbapenemase-producing ECC isolates with decreased susceptibility to carbapenems received in 2016-2017 at the French National Reference Center for antibiotic resistance, only 8 had a positive carbapenemase detection test (Carba NP). These eight ECC isolates were resistant to broad-spectrum cephalosporins due to AmpC derepression, showed decreased susceptibility to carbapenems, and were categorized as carbapenemase-producing Enterobacteriaceae (CPE) according to several carbapenemase detection assays. WGS identified a single clone of E. kobei ST125 expressing only its cAmpC, ACT-28. The blaACT-28 gene was expressed in a wild-type and in a porin-deficient Escherichia coli background and compared to the blaACT-1 gene. Detection of carbapenemase activity was positive only for E. coli expressing the blaACT-28 gene. Kinetic parameters of purified ACT-28 revealed a slightly increased imipenem hydrolysis compared to that of ACT-1. In silico porin analysis revealed the presence of a peculiar OmpC-like protein specific to E. kobei ST125 that could impair carbapenem influx into the periplasm and thus enhance carbapenem-resistance caused by ACT-28. We described a widespread lineage of E. kobei ST125 producing ACT-28, with weak carbapenemase activity that can lead to false-positive detection by several biochemical and phenotypic diagnostic tests.
INTRODUCTION
Enterobacter cloacae complex (ECC) organisms are highly adapted to the hospital environment and are mostly responsible for hospital-acquired infections such as intravenous-catheter-related bacteremia, urinary tract infections (UTIs), and pulmonary infections (1). The ECC shows a genomic heterogeneity with 13 clusters based on their hsp60 sequences (2) and currently comprises seven different species (3): Enterobacter asburiae, E. cloacae, Enterobacter hormaechei, Enterobacter kobei, Enterobacter ludwigii, Enterobacter mori, and Enterobacter xiangfangensis.
Enterobacter spp. naturally exhibit resistance to aminopenicillins, first- and second-generation cephalosporins, by the production of an inducible chromosomally encoded Ambler class C β-lactamase (cAmpC) characterized by its ability to hydrolyze cephalosporins without being inhibited by clavulanic acid or tazobactam (4). In Enterobacter spp., resistance to broad-spectrum cephalosporins can occur through acquisition of an extended-spectrum β-lactamase (ESBL) or by chromosomal mutations, mostly in ampD or in ampR, that lead to cAmpC derepression (5). Resistance to carbapenems in Enterobacter spp. can arise by acquisition of carbapenemases (mostly KPC, NDM, VIM, IMP, or OXA-48-like). However, alteration or loss of nonspecific porins, leading to outer membrane permeability defect, associated with overproduction of the natural AmpC and/or production of an ESBL is the most common mechanism (6).
cAmpC β-lactamases, through different genetic elements, may be mobilized on plasmids that may be acquired by Enterobacteriaceae that are not expected to produce an AmpC β-lactamase (7). Some of these plasmid-encoded AmpC (pAmpC) β-lactamases displayed a slight carbapenemase activity, such as CMY-10, CMY-2, and, to a lesser extent, ACT-1, as revealed by kinetic studies (8, 9). To date, no cAmpC has been proved to hydrolyze carbapenems in Enterobacteriaceae.
Here we describe a single E. kobei lineage capable of hydrolyzing imipenem, only due to the expression of a novel cAmpC β-lactamase called ACT-28. Whole-genome sequencing (WGS), cloning experiments, specific activity determination, and enzymatic purification were performed to characterize this clone and this enzyme. Comparative genomic approaches revealed that E. kobei producing ACT-28 formed a distinct lineage.
RESULTS AND DISCUSSION
Eight ECC isolates with AmpC derepressed and carbapenemase activity.
From January 2016 to December 2017, 1,465 ECC isolates with decreased susceptibility to carbapenems were received at the French National Reference Center for Antibiotic Resistance (F-NRC) for expertise. A total of 426 produced a carbapenemase of the OXA-48-like, KPC, NDM, and/or VIM type (data not shown), and among the 1,039 that were negative for these carbapenemases, only 8 isolates had a positive Carba NP test. These isolates were resistant or intermediate to penicillins, β-lactamase inhibitor-penicillin combinations, expanded-spectrum cephalosporins, and aztreonam but remained susceptible to cefepime, imipenem, and meropenem (Table 1). No synergy image was observed on disk diffusion antibiogram between of expanded-spectrum cephalosporins and clavulanic acid disks, suggesting AmpC overexpression rather than ESBL acquisition. This was confirmed by determining specific activity with cephalothin as a substrate. The activity was 2,000 to 5,000 times higher than the activity of the wild-type E. cloacae CIP79.33 used as a reference (see Table S2 in the supplemental material). This confirmed the derepressed status of the AmpC in the eight ECC isolates. Furthermore, there was no associated resistance to other antimicrobial agents, such as aminoglycosides or fluoroquinolones (data not shown).
TABLE 1.
MICs of β-lactams for E. kobei ST125 and transformantsa
| Drug | MIC (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| E. kobei (ACT-28 chr) | E. coli TOP10 (pTOPO-ACT-28) | E. coli TOP10 (pTOPO-ACT-1) | E. coli TOP10 | E. coli HB4-(pTOPO-ACT-28) | E. coli HB4-(pTOPO-ACT-1) | E. coli HB4 | |
| Ticarcillin | >256 | >256 | >256 | 2 | >256 | >256 | 4 |
| Cefoxitin | >256 | >256 | >256 | 2 | >256 | >256 | 256 |
| Cefotaxime | >32 | >32 | >32 | 0.06 | >32 | >32 | 0.38 |
| Ceftazidime | >256 | >256 | >256 | 0.12 | >256 | >256 | 4 |
| Cefepime | 0.5 | 0.25 | 0.25 | 0.06 | 6 | 2 | 0.75 |
| Ertapenem | 0.5 | 0.125 | 0.125 | 0.003 | >32 | >32 | 1 |
| Meropenem | 0.25 | 0.047 | 0.06 | 0.016 | >32 | >32 | 0.25 |
| Imipenem (Etest) | 0.75 | 0.5 | 0.5 | 0.25 | >32 | 12 | 0.25 |
| Imipenem (BMD) | ND | ND | ND | ND | >128 | 64 | ND |
BMD, broth microdilution; ND, not determined.
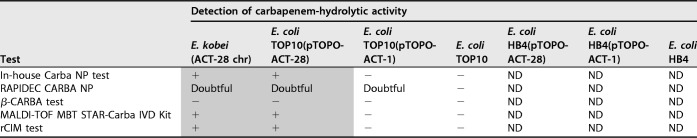
Disk diffusion susceptibility testing revealed zone inhibition diameters for ertapenem at 20 mm and imipenem at 26 mm, which were below the epidemiological cutoffs (ECOFFs) fixed by EUCAST (<25 for ertapenem and <28 mm for imipenem). Accordingly, several complementary tests were performed to detect the presence of a carbapenemase (Table 2). Five tests based on carbapenem hydrolysis were performed. Three of them gave a positive result: the Carba NP test (10), the MBT STAR-Carba IVD kit, a commercially available matrix-assisted laser desorption ionization–time of flight (MALDI-TOF)-based technique (11), and the rapid CIM (rCIM) (12), while the RAPIDEC CARBA NP was doubtful and the β-CARBA test remained negative (Table 2). Complementary tests based on the detection of the main 5 carbapenemases either by immunochromatographic assays (RESIST-4 O.K.N.V. kit [CORIS BioConcept] or NG-CARBA 5 test [NG Biotech]) or by molecular tests (in-house PCR or Xpert Carba-R test [Cepheid]) provided negative results (13–16). Antimicrobial susceptibility testing was also performed on Mueller-Hinton agar supplemented with cloxacillin. On this medium, the susceptibility of carbapenems and broad-spectrum cephalosporins was fully restored, suggesting that the observed phenotype might have been due to the overexpression of the cephalosporinase. To determine whether a pAmpC was present in these isolates, plasmid extractions and subsequent electroporation into Escherichia coli Top10 were performed. Despite repeated attempts, no plasmid could be evidenced by electrophoresis on a 0.7% agarose gel and no transfer of cephalosporin resistance could be evidenced.
TABLE 2.
Results of carbapenemase detection testsa
+, positive result; −, negative result; ND, not determined.
Characterization of ACT-28.
Whole-genome sequencing (WGS) was performed to identify the resistome of the eight ECC isolates and to investigate the molecular determinant of the imipenem hydrolysis. Genome annotation indicated that the unique β-lactamase-encoding gene was blaACT-28, a natural variant of the gene encoding cAmpC of ECC (GenBank accession number NG_048614). In addition, no antimicrobial resistance genes were found using Resfinder server, except a chromosomal allele of fosA (91% of nucleotide identity with fosA2) known to confer resistance to fosfomycin. Of note, the fosA gene is known to be widespread in ECC organisms, with a distribution in 82.4% in their genomes (17).
To determine whether the ACT-28 cephalosporinase possesses a carbapenemase activity, cloning experiments were performed. To date, 54 ACT variants have been described, and among them, no single amino acid mutant of ACT-28 has been found (18). Thus, we decided to compare the activity of ACT-28 to that of the well-characterized ACT-1, a pAmpC recovered from ertapenem-resistant Klebsiella pneumoniae with porin deficiency (8, 19).
To compare the β-lactam hydrolytic properties of ACT-28 and ACT-1, the two corresponding genes were cloned into pTOPO plasmid under the control of Plac promoter and expressed in E. coli TOP10. As expected, expression of the blaACT-1 and blaACT-28 genes conferred resistance to penicillins and to expanded-spectrum cephalosporins (Table 1). Surprisingly, despite the same MICs for imipenem (0.5 mg/liter) with both blaACT-1- and blaACT-28-expressing E. coli TOP10 clones, carbapenemase activities monitored by the Carba NP test, MALDI-TOF technique (MBT STAR-Carba IVD kit) and the rCIM test were positive only for blaACT-28-expressing clones (Table 2). To characterize more precisely whether ACT-28 possesses specific carbapenem hydrolytic properties, a steady-state kinetic parameters were determined. As expected, benzylpenicillin and cephalothin were excellent substrates for ACT-28 and ACT-1, whereas these two enzymes exhibited a lower catalytic activity toward cefotaxime and ceftazidime (Table 3) (5). Regarding imipenem, ACT-28 showed a 2-fold increase in imipenem affinity (Km at 1.9 ± 0.4 μM) compared to ACT-1 (4.6 ± 0.4 μM) (P < 0.01, Student t test) (Table 3). The catalytic efficiency of ACT-28 (13.3 ± 2.0 mM−1/s−1) was 1.5 times higher than that of ACT-1 (8.8 ± 0.02 mM−1/s−1). This significant difference (P < 0.05, Student t test) in catalytic efficiency toward imipenem was confirmed on several independent measurements.
TABLE 3.
Kinetic parameter values for ACT-28 and ACT-1a
| Drug |
Km (μM) |
kcat (s−1) |
kcat/Km (mM−1/s−1) |
|||
|---|---|---|---|---|---|---|
| ACT-28 | ACT-1 | ACT-28 | ACT-1 | ACT-28 | ACT-1 | |
| Penicillin G | 36 | 45 | 70 | 49 | 1,944 | 1,090 |
| Cephalothin | 138 | 110 | 384 | 325 | 2,783 | 2,955 |
| Cefotaxime | 3.8 | 4.3 | 0.07 | 0.05 | 18 | 12 |
| Ceftazidime | 306 | 32 | 0.10 | 0.03 | 0.33 | 0.94 |
| Imipenem | 1.9 | 4.6 | 0.025 | 0.041 | 13.3 | 8.8 |
Measurements were done in triplicates, and the error rates were below 10%.
Identification of a single E. kobei ST125 subgroup.
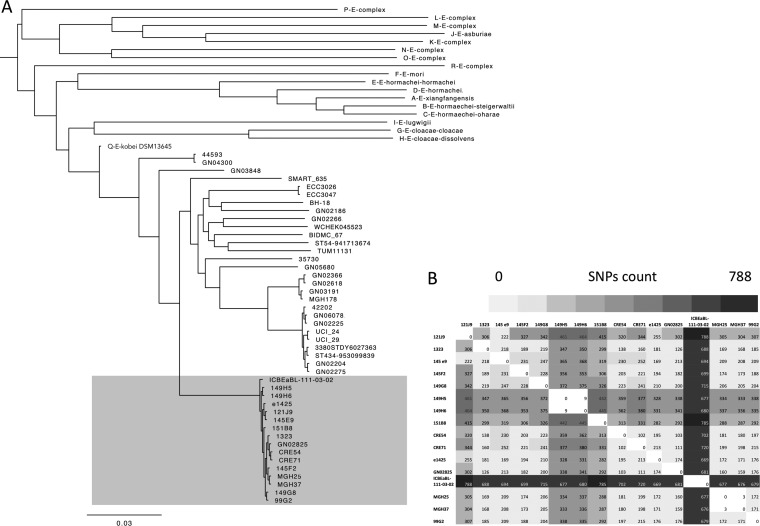
To date, MALDI-TOF mass spectrometry (MS)-based identification and biochemical tests (e.g., API galleries) are not able to distinguish the different species within the ECC (2, 20, 21). As proposed by Hoffman et al. (2), the construction of the hsp60-based maximum likelihood tree revealed that all ACT-28 producers clustered within hsp60 cluster II, i.e., E. kobei with 100% identity (data not shown). Recently, Chavda et al. extended the number of clusters in the ECC to 18 phylogenomic groups (A to R) by analyzing core single nucleotide polymorphisms (SNPs) in 390 whole genomes (3). Using the WGS data, the phylogenomic analysis confirmed that the 8 genomes of the ACT-28 producers were distributed within the Q-like group comprising E. kobei (Fig. 1A). Average nucleotide identity (ANI) between ECC 99G2 as a representative and E. kobei DSM 13645 as a reference was 99.21% (22).
FIG 1.
ECC ACT-28 producers belong to E. kobei. (A) Phylogeny based on SNP calling among the core genomes of ECC isolates. All ACT-28 producers (n = 16) belonged to Chavda’s phylogenomic group Q (23), as well as other E. kobei genomes publicly available online (n = 29). Among E. kobei isolates, ACT-28 producers form a single subgroup we propose to name E. kobei subsp. bicestrii, indicated by shading. (B) Heat map representing SNP counts among the core genomes of ACT-28-producing isolates: 8 strains were addressed to the F-NRC and 8 genomes were downloaded from the NCBI database (1323, e1425, MGH25, MGH37, ICBEaBL-111-03-02, CRE54, CRE71, and GN02825). Analysis was performed using CSI Phylogeny (27).
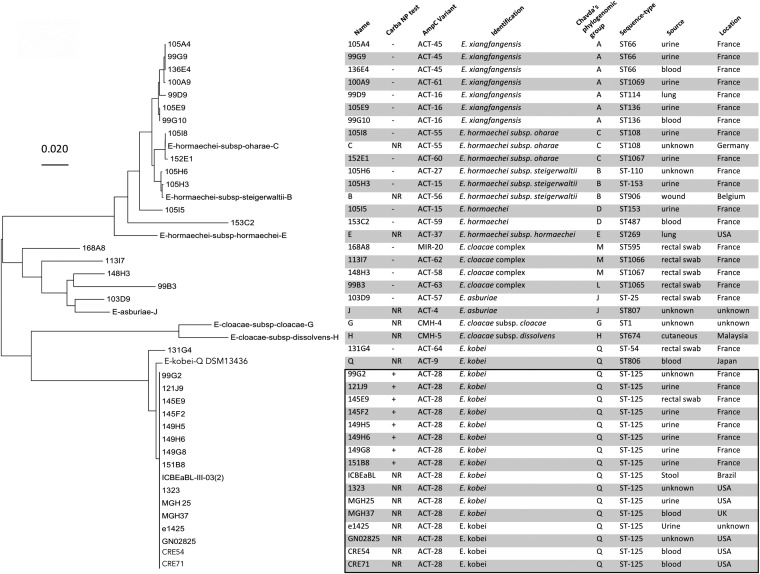
Multilocus sequence typing (MLST) analysis revealed that all ACT-28 producers belonged to sequence type 125 (ST125). This ST is not particularly known as a high-risk clone in the hospital environment (23). To evaluate the genetic diversity among non-carbapenemase ECC isolates with decreased susceptibility to carbapenems and negative by the Carba NP test, we analyzed the genomes of 19 randomly chosen ECC isolates addressed to the F-NRC between 2016 and 2017. Most of them were identified as E. hormaechei and E. xiangfangensis (Fig. 2). We observed a diversity of natural cAmpC and sequence types, but none was of ST125 (Fig. 2).
FIG 2.
Phylogenetic analysis of AmpC β-lactamases of ECC isolates addressed to the French National Reference Center for carbapenemase detection. This unrooted tree was constructed based on ampC genes using the maximum likelihood method with the Tamura-Nei model using the MEGA program (MEGA 7.0) The tree is drawn to scale, with branch lengths representing the evolutionary distances. ACT-28-producing ECC isolates from the NCBI database (1323, e1425, MGH25, MGH37, ICBEaBL-111-03-02, CRE54, CRE71, and GN02825) and ampC of ECC genomes of Chavda’s phylogenomic groups B, C, E, J, G, H, and Q were added to the analysis (23). NR, not realized.
Phylogenetic analysis of the 19 natural cAmpCs revealed that the AmpC of the isolate 131G4 was closely related to ACT-28 (Fig. 2). Indeed, AmpC of isolate 131G4, ACT-64 newly described here, shared 97% amino acid identity with ACT-28 (371/381). Of note isolate, 131G4 was identified as E. kobei, but the Carba NP test was negative, suggesting that ACT-64 lacked detectable carbapenemase activity with this test. Residues conserved between ACT-1 and ACT-64 but mutated in ACT-28 might be involved in the increased carbapenemase activity of ACT-28. Alignment of ACT-28, ACT-64, and ACT-1 revealed the presence of unique residues in ACT-28—His157, Ala166, Ile229 (Ω loop), Ile247 (Ω loop), and Leu254—that could be responsible for its carbapenemase activity (Fig. 3).
FIG 3.
Amino acid alignment of ACT-28, ACT-1, and ACT-64. To highlight the differences, amino acids in common with ACT-28 have been replaced by dashes. Amino acids conserved within serine β-lactamases are in bold. The two loops (Ω and R2) are shaded. Helices H-9, H-10, and H-11 are boxed.
ACT-28-producing E. kobei ST125 forms a distinct subspecies of E. kobei.
All ACT-28-producing isolates addressed to the F-RNC were isolated from patients with no known travel history. To determine whether E. kobei ST125 was frequently observed around the world, we searched for other genomes in the NCBI database. To date, 8 genomes with their shotgun contigs are deposited in the GenBank database and are from different countries (United States, Brazil, and United Kingdom), suggesting that E. kobei ST125 organisms are present at least in three distantly located geographical areas (Fig. 2). These 8 isolates were all ACT-28 producers.
To evaluate the genetic diversity between the 16 ACT-28-producing E. kobei ST125 isolates, phylogeny based on SNP calling in the core genome was performed using CSI Phylogeny (24). These strains differed by 102 to 788 SNPs (Fig. 1B). Only 3 SNPs were detected between MGH25 and MGH37 and 9 SNPs between 149H5 and 149H6, suggesting that in both cases, isolates came from related patients. Of note, the 149H5 and 149H6 ACT-28-producing E. kobei strains were isolated from two patients hospitalized in the same ward, suggesting a cross-transmission. ACT-28 producers were mostly identified as an agent of UTI (n = 8) and of bloodstream infection (n = 3). Phylogenomic comparison with E. kobei genomes available on the NCBI database (n = 24) revealed that ACT-28-producing isolates formed a single lineage (Fig. 1A and Fig. 2). A total of 11,720 to 13,617 SNPs were detected between E. kobei ST125 and other E. kobei isolates (Table S1). Thus, we propose to define ACT-28 producers as a single subspecies that we name Enterobacter kobei subsp. bicestrii.
ACT-28 in strains with decreased outer membrane permeability.
Previous studies indicated that AmpC β-lactamases could weakly hydrolyze some substrates (e.g., carbapenems) despite a low kcat as long as they possess concomitantly a high affinity (low Km) for the substrate (5, 25). The concentration of β-lactams in the periplasm is therefore a crucial issue and depends on the number of porin entry channels or the efflux pump overexpression that can extrude molecules and therefore increase enzyme efficacy.
To study the impact of ACT-1 and ACT-28 expression in a porin-deficient strain, pTOPO-ACT-1 and pTOPO-ACT-28 were electroporated into E. coli HB4 lacking porins OmpF and OmpC (26). Expression of the blaACT-1 and blaACT-28 genes conferred high-level resistance to all β-lactams, including carbapenems, with MICs of 12 μg/ml and >32 μg/ml, respectively (Table 1). The higher MICs for imipenem conferred by ACT-28 compared to ACT-1 are in agreement with our biochemical results (steady-state kinetic and Carba NP test results).
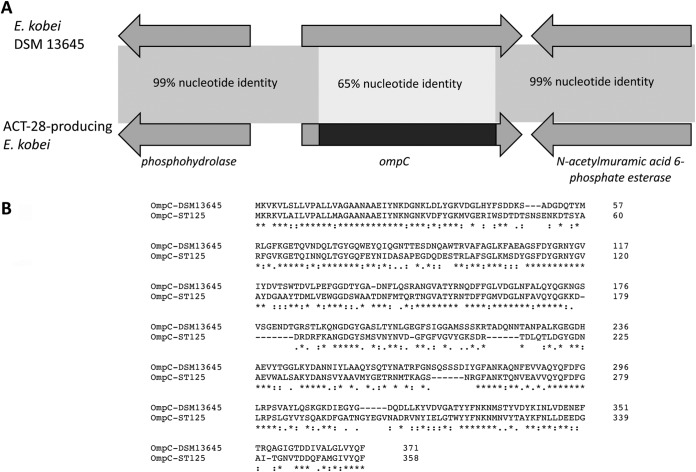
To evaluate the role of membrane permeability defect in the global resistance phenotype of ACT-28-producing E. kobei, in silico analysis of porins was performed. Using WGS data, outer membrane protein sequences of the ompF and ompC types were compared to that of the E. kobei DSM 13645 reference strain. These porins are homologs of K. pneumoniae ompK35 and ompK36 porins, respectively. In both species, alterations of these porins are known to be involved in decreased susceptibility to carbapenems and notably ertapenem (6). ompF shared 100% nucleotide identity to the corresponding gene in E. kobei DSM 13645. Surprisingly, analysis of ompC revealed an allelic replacement of 967 bp that occurred within the gene in all ACT-28-producing E. kobei ST125 isolates (Fig. 4A). Intriguingly, this allelic replacement was in phase with the identical 5′ end of ompC and led to a complete coding sequence. This ompC chimera, likely the result of homologous recombination, was 1,077 bp instead of 1,143 bp for ompC from DSM 13645. All ACT-28 producers possessed this peculiar modified OmpC, and BLAST analysis revealed that this genetic event was specific to ACT-28-producing E. kobei ST125. This in-phase allelic replacement, resulting in OmpC from ACT-28 producers sharing only 53% amino acid sequence identity with OmpC from DSM 13645, might modify the functionality of the porin (Fig. 4B).
FIG 4.
An allelique replacement of ompC occurred in E. kobei ST125. (A) Schematic representation of the ompC region found in all ACT-28-producing E. kobei isolates and E. kobei DSM 13645, used as reference. (B) Amino acid alignment of OmpC of E. kobei ST125 and E. kobei DSM 13456. The two proteins shared only 53% amino acid identity.
WGS data were also used to investigate the molecular mechanism that led to AmpC depression in ACT-28-producing E. kobei ST125 isolates. Most ECC clinical isolates with AmpC derepressed have mutations in AmpD, a cytosolic amidase involved in cell wall recycling, and less commonly in AmpR (27). Alignment of AmpD proteins of the 8 ACT-28 producers and E. kobei 131G4 (AmpC not derepressed [data not shown]) to E. kobei DSM 13645 as a reference revealed mutations that led to truncated proteins due to early stop codons in 3 isolates (99G2, 145H5, and 154H6) (Fig. S1). Only the 48 final amino acids of AmpD were present in isolate 145F2, whereas the gene was totally absent in 121J9. In 149G8, the initial Met codon was absent; hence, AmpD expression could be decreased. In 145E9 and 151B8, AmpD is strictly identical to the sequence of DSM 13645 (Fig. S1). Otherwise, alignment of ampR of all ACT-28 producers revealed the presence of 7 SNPs in comparison to ampR of 131G4, but they do not impact amino acid sequence (data not shown). Therefore, it is unlikely that AmpR might be involved in AmpC derepression. At last, no mutations in ampC and ampD promoters were observed. Overall, mutations in AmpD were present in the majority of isolates but we cannot exclude that mutations in partners of the AmpC derepression pathway other than AmpD and AmpR might be implicated in the phenotype of ACT-28 producers (27).
Conclusions.
We have described a novel lineage of E. kobei ST125 that can be falsely identified as a member of the carbapenemase-producing Enterobacteriaceae (CPE) by several diagnostic tests. ACT-28, a specific chromosome-encoded cephalosporinase, was the only β-lactamase detected in this lineage. Kinetic study revealed its high affinity for imipenem, which might result in carbapenem resistance when associated with outer membrane permeability defect. We showed that ACT-28 led to a weak but significant carbapenemase activity as revealed with imipenem hydrolysis-based diagnostic tests (Carba NP test, MALDI-TOF-based assay, and rCIM test). As a consequence, patients carrying these isolates may be mistakenly categorized as CPE carriers and isolated with dedicated staff or even cohorted with true CPE carriers. With cAmpC-producing bacteria, biochemical tests have to be interpreted with susceptibility testing on a cloxacillin-containing plate. Indeed, in a strain with a cephalosporinase overproduced and impaired permeability, diameters around the β-lactam disks increase, whereas with true carbapenemase producers, diameters around imipenem change only slightly, if at all (28).
MATERIALS AND METHODS
Bacterial strains.
Eight ACT-28-producing E. kobei isolates (99G2, 121J9, 145E9, 145F2, 149H5, 149H6, 149G8, and 151B8) were addressed to the French National Reference Center for Antibiotic Resistance (F-NRC) between 2016 and 2017 for carbapenemase detection. Nineteen other ECC clinical isolates addressed to the F-NRC over the same period were used as controls (99B3, 99D9, 99G9, 99G10, 100A9, 103D9, 105A4, 105E9, 105H3, 105H6, 105I5, 105I8, 113I7, 131G4, 136E4, 148H3, 152E1, 153C2, and 168A8). These isolates were randomly picked among the 1,039 ECC collection isolates with reduced susceptibility to carbapenems but lacking main carbapenemases (OXA-48-like, KPC, NDM, VIM, and IMP enzymes) and lacking carbapenemase activity (negative Carba NP test).
Identification of ECC was performed using MALDI-TOF MS using the Bruker MS system (Bruker Daltonics, Bremen, Germany), according to the manufacturer’s instructions. In addition, analysis of the hsp60 sequences was performed to distinguish species among the Enterobacter cloacae complex (ECC) (2). ACT-1-producing K. pneumoniae was used as source of the blaACT-1 gene for PCR amplification prior to cloning experiments (19). Electrocompetent E. coli TOP10 and E. coli HB4 (a strain with permeability defect due to OmpC and OmpF major porin deficiency) were used as recipients for electroporation experiments, and E. coli BL21(DE3) (Invitrogen, Eragny, France) was used for overexpressing ACT-28 (29).
Susceptibility testing and MIC determinations.
Susceptibility testing was performed by the disk diffusion method on Mueller-Hinton agar plates (Bio-Rad, Marnes la Coquette, France) with or without cloxacillin (bioMérieux, La Balmes les Grottes, France) incubated for 18 h at 37°C and interpreted according to the EUCAST breakpoints, updated in 2018 (http://www.eucast.org). MICs for ticarcillin, cefoxitin, ceftazidime, cefotaxime, cefepime, imipenem, meropenem, and ertapenem were determined by Etest (bioMérieux). For E. coli HB4(pTOPO-ACT-1) and E. coli HB4(pTOPO-ACT-28), imipenem MICs were also determined using commercially available dry-form broth microdilution technique (Sensititre; Thermo Fisher Scientific, Courtaboeuf, France).
Plasmid extraction.
Natural plasmid extraction was attempted using the Kieser extraction method and subsequently analyzed by electrophoresis on a 0.7% agarose gel as previously described (30).
Biochemical, immunological, and molecular carbapenemase confirmatory tests.
Bacterial colonies of ECC isolates were recovered from Trypticase soy agar to perform th4e in-house Carba NP test as previously described (10) and the β-CARBA test (Bio-Rad) and RAPIDEC CARBA NP (bioMérieux) according to the manufacturer’s recommendations. The rapid CIM (rCIM) test was performed as previously described (12). Imipenem hydrolysis monitored by MALDI-TOF MS was done using the MBT STAR-Carba IVD kit (Bruker Daltonics, Bremen, Germany) according to the manufacturer’s recommendations (11). Briefly, 50 μl of MBT STAR-BL incubation buffer was added to a ready-to-use tube containing MBT STAR-BL antibiotic reagent. One to five individual bacterial colonies were resuspended in the tube by vortexing. Samples were incubated at 37°C for 30 min under agitation (900 rpm). The bacteria were pelleted by centrifugation for 2 min at 16,000 × g. One microliter of the supernatant was loaded on the MALDI target, dried under a stream of air (1 min), and overlaid with the MBT STAR-BL matrix solution. The spots were dried gently under a stream of air (less than 1 min). MALDI-TOF MS analysis was performed on a Microflex LT mass spectrometer (Bruker Daltonics). Final spectra were automatically interpreted using the MBT STAR-BL IVD software.
Carbapenemase production was investigated by lateral flow immunoassays using the RESIST-4 O.K.N.V. kit (CORIS BioConcept, Gembloux, Belgium) for the detection of KPC, NDM, VIM, and OXA-48-like enzymes and the NG-CARBA 5 test (NG Biotech, Guipry, France) for the detection of KPC, NDM, VIM, OXA-48-like, and IMP enzymes (13–15). Finally, genes for KPC, NDM, VIM, OXA-48-like, and IMP enzymes were sought using the Xpert Carba-R kit (Cepheid, Sunnyvale, CA) (16).
Sequencing, whole-genome sequencing, and bioinformatic analysis.
Recombinant plasmids pTOPO-ACT-28, pTOPO-ACT-1, pET41b-ACT1, and pET41b-ACT28 were purified using a QIAprep spin miniprep kit (Qiagen, Courtaboeuf, France) and were sequenced using an ABI3130 automated sequencer (Applied Biosystems Thermo Fisher, Courtaboeuf, France). The nucleotide and the deduced protein sequences were analyzed using software from the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov) and the BLDB (www.bldb.eu) (18).
The DNA libraries were prepared using the NexteraXT v3 kit (Illumina, San Diego, CA), according to the manufacturer’s instructions, and then run on the HiSeq system (Illumina) for generating paired-end 150-bp reads. Illumina read de novo assembly was performed using CLC genomic workbench 10.0 according to the manufacturer’s recommendations (Qiagen). The genome was annotated using the RAST tool (http://rast.nmpdr.org/rast.cgi) (29).
Resfinder online software (https://cge.cbs.dtu.dk/services/ResFinder/) was used for the detection of resistance determinants other than β-lactamase (31). Sequence type determination was performed using the Enterobacter cloacae MSLT scheme available online at https://pubmlst.org/ecloacae/. Average nucleotide identity (ANI) was calculated with the online tool http://enve-omics.ce.gatech.edu/ani/ (22). Phylogenetic trees based on hsp60 or ampC sequences were drawn with MEGA7 software using the maximum likelihood method based on the Tamura-Nei model.
Multiple-sequence alignment of ACT variants, AmpD, and OmpC was done online using the Clustal Omega program (https://www.ebi.ac.uk/Tools/msa/clustalo/). Sequences of AmpD from wild-type E. kobei 131G4 and E. kobei DSM 13645 (GenBank accession number CP017181.1) were used as references for molecular analysis of AmpC derepression. Sequences of OmpC and OmpF from E. kobei DSM 13645 were used as references for porin analysis.
The draft genomes of eight ACT-28-producing ECC isolates available online were included in bioinformatic analyses: E. kobei ICBEaBL-III-03(2) (NIHL01000275.1), E. kobei GN02825 (LEDC01000001.1), E. cloacae 1323 (JVTR01000062.1), E. cloacae e1425 (FJYX01000004.1), Enterobacter sp. strain MGH 37 (JCLI01000015.1), Enterobacter sp. MGH 25 (AYJF01000016.1), Enterobacter kobei CRE54 (PXKD01000001.1), and Enterobacter kobei CRE71 (PXKA01000001.1) (all accession numbers from GenBank). Identification E. kobei was confirmed after hsp60 and phylogenomic analyses.
CSI Phylogeny 1.4 was used for phylogenomic analysis based on SNP calling among the core genomes of the 16 ACT-28-producing E. kobei isolates (24). The 18 reference genomes of Chavda’s classification (A to R) and 28 non-ACT-28-producing E. kobei genomes available in NCBI database were added to the analysis (https://cge.cbs.dtu.dk/services/CSIPhylogeny/) (3, 24). All accession numbers of genomes used in the study are listed in Table S3.
Enzymatic activities.
AmpC expression in ACT-28-producing E. kobei and in wild-type ECC isolate CIP79.33, used as a reference, was studied using specific activity. This was determined using the supernatant of a whole-cell crude extract obtained from an overnight culture with an Ultrospec 2000 UV spectrophotometer (Amersham Pharmacia Biotech), as previously described (32). Cephalothin was used as a substrate at a concentration of 100 μM.
Cloning experiments.
The blaACT-28 and blaACT-1 genes were amplified using ACT-28F (5′-ATGAAGACAAAATCCCTTTGC-3′) and ACT-28R (5′-CAGGCAACCA-CTAAAAACGC-3′) primers and using ACT-1F (5′-ATGATGATGACTAAATCCCTTTGC-3′) and ACT-1R (5′-CTACAGCGCGCTCAAAATACG-3′) primers, respectively, and were subsequently cloned into pTOPO (Zero Blunt TOPO PCR cloning kit; Invitrogen, Courtaboeuf, France), resulting in plasmids pTOPO-ACT-28 and pTOPO-ACT-1, respectively. These plasmids were electroporated into E. coli TOP10 and E. coli HB4.
For β-lactamase production and purification, the blaACT-28 and blaACT-1 genes corresponding to the mature β-lactamase were PCR amplified using the primers ACT28-cloningpET41-For (5′-CTTTAAGAAGGAGATATACATATGGCGCCGATGTCAGAGAAACAGC-3′) and ACT28-cloningpET41-Rev (5′-GTGGTGGTGGTGGTGCTCGAGCTGCAGAGCGCTCAGAATACG) and primers ACT1-cloningpET41-For (5′-CTTTAAGAAGGAGATATACATATGGCTACCCCGATGTCAGAAAAACAGC-3′) and ACT1-cloningpET41-Rev (5′-GGTGGTGGTGGTGGTGCTCGAGCAGCGCGCTCAAAATACGGTATGC-3′), respectively, and cloned into the expression vector pET-41b(+) (Novagen, VWR International, Fontenay-sous-Bois, France) using the NEBuilder HiFiDNA assembly cloning kit (New England BioLabs Inc., United Kingdom), following the manufacturer’s instructions. Recombinant plasmids pET41b-ACT1 and pET41b-ACT28 were transformed into electrocompetent E. coli BL21(DE3) (32).
β-Lactamase purification and steady-state kinetics.
An overnight culture of E. coli strain BL21(DE3) harboring pET41b-ACT-28 or pET41b-ACT-1 recombinant plasmid was used to inoculate 2 liters of brain heart infusion (BHI) broth supplemented with kanamycin at 50 mg/liter. Bacteria were cultured at 37°C until reaching an optical density at 600 nm (OD600) of 0.6. Then expression of the β-lactamase genes was carried out overnight at 25°C with 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) as an inducer. Cultures were centrifuged at 6,000 × g for 15 min, and then the pellets were resuspended with 10 ml of buffer A (20 mM phosphate-buffered saline [PBS], 175 mM K2SO4, 40 mM imidazole [pH 7.40]) (32). Bacterial cells were disrupted by sonication; the bacterial pellet was removed by two consecutive centrifugation steps at 10,000 × g for 1 h at 4°C. The supernatant was further centrifuged at 48,000 × g for 1 h at 4°C. β-Lactamases were purified using a one-step pseudoaffinity chromatography nitrilotriacetic acid (NTA)-nickel column (GE Healthcare, Freiburg, Germany). Protein purity was estimated by SDS-PAGE, and pure fractions were pooled and dialyzed against 20 mM HEPES–50 mM K2SO4 (pH 7) and concentrated by using Vivaspin columns (GE Healthcare). Protein concentration was determined using the Bradford protein assay (Bio-Rad) (32).
Purified β-lactamases were used to determine the kinetic parameters (Km and kcat) of benzylpenicillin, cephalothin, cefotaxime, ceftazidime, and imipenem in 100 mM sodium phosphate (pH 7.4). Experiments were performed three times. The rates of hydrolysis were determined with an Ultrospec 2100 spectrophotometer and were analyzed using SWIFT II software (GE Healthcare, Velizy-Villacoublay, France). Km and kcat values were determined by analyzing the β-lactam hydrolysis under initial rate conditions using the Eadie-Hofstee linearization of the Michaelis-Menten equation as previously described (33).
Accession number(s).
All of the genomes determined in this study are available at NCBI under BioProject no. PRJNA472443. All genomes used in this study are listed in Table S3. New ACT and CMH alleles described in this study, listed as follows, have been deposited in GenBank (accession numbers in parentheses): ACT-55 (MH469274), ACT-56 (MH469275), ACT-57 (MH469278), ACT-58 (MH469279), ACT-59 (MH469280), ACT-60 (MH469281), ACT-61 (MH469283), ACT-62 (MH469270), ACT-63 (MH469271), ACT-64 (MH469272), CMH-4 (MH469276), and CMH-5 (MH469277).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Assistance Publique-Hôpitaux de Paris, by a grant from the Université Paris-Sud (EA 7361), and by the LabEx LERMIT, supported by a grant from the French National Research Agency (ANR-10-LABX-33). This work was also funded in part by a grant from Joint Programming Initiative on Antimicrobial Resistance (ANR-14-JAMR-0002).
L.D. is a coinventor of the Carba NP test, the patent for which has been licensed to bioMérieux (La Balmes les Grottes, France).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02388-18.
REFERENCES
- 1.Sanders WE, Sanders CC. 1997. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin Microbiol Rev 10:220–241. doi: 10.1128/CMR.10.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann H, Roggenkamp A. 2003. Population genetics of the nomenspecies Enterobacter cloacae. Appl Environ Microbiol 69:5306–5318. doi: 10.1128/AEM.69.9.5306-5318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chavda KD, Chen L, Fouts DE, Sutton G, Brinkac L, Jenkins SG, Bonomo RA, Adams MD, Kreiswirth BN. 2016. Comprehensive genome analysis of carbapenemase-producing Enterobacter spp.: new insights into phylogeny, population structure, and resistance mechanisms. mBio 7:e02093-16. doi: 10.1128/mBio.02093-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby GA. 2010. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacoby GA. 2009. AmpC β-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doumith M, Ellington MJ, Livermore DM, Woodford N. 2009. Molecular mechanisms disrupting porin expression in ertapenem-resistant Klebsiella and Enterobacter spp. clinical isolates from the UK. J Antimicrob Chemother 63:659–667. doi: 10.1093/jac/dkp029. [DOI] [PubMed] [Google Scholar]
- 7.Philippon A, Arlet G, Jacoby GA. 2002. Plasmid-determined AmpC-type β-lactamases. Antimicrob Agents Chemother 46:1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mammeri H, Guillon H, Eb F, Nordmann P. 2010. Phenotypic and biochemical comparison of the carbapenem-hydrolyzing activities of five plasmid-borne AmpC β-lactamases. Antimicrob Agents Chemother 54:4556–4560. doi: 10.1128/AAC.01762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JY, Jung HI, An YJ, Lee JH, Kim SJ, Jeong SH, Lee KJ, Suh P-G, Lee H-S, Lee SH, Cha S-S. 2006. Structural basis for the extended substrate spectrum of CMY-10, a plasmid-encoded class C β-lactamase. Mol Microbiol 60:907–916. doi: 10.1111/j.1365-2958.2006.05146.x. [DOI] [PubMed] [Google Scholar]
- 10.Dortet L, Agathine A, Naas T, Cuzon G, Poirel L, Nordmann P. 2015. Evaluation of the RAPIDEC CARBA NP, the Rapid CARB Screen and the Carba NP test for biochemical detection of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 70:3014–3022. doi: 10.1093/jac/dkv213. [DOI] [PubMed] [Google Scholar]
- 11.Dortet L, Tandé D, de Briel D, Bernabeu S, Lasserre C, Gregorowicz G, Jousset AB, Naas T. 2018. MALDI-TOF for the rapid detection of carbapenemase-producing Enterobacteriaceae: comparison of the commercialized MBT STAR-Carba IVD kit with two in-house MALDI-TOF techniques and the RAPIDEC CARBA NP. J Antimicrob Chemother 73:2352–2359. doi: 10.1093/jac/dky209. [DOI] [PubMed] [Google Scholar]
- 12.Muntean M-M, Muntean A-A, Gauthier L, Creton E, Cotellon G, Popa MI, Bonnin RA, Naas T. 2018. Evaluation of the rapid carbapenem inactivation method (rCIM): a phenotypic screening test for carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 73:900–908. doi: 10.1093/jac/dkx519. [DOI] [PubMed] [Google Scholar]
- 13.Sağıroğlu P, Hasdemir U, Altınkanat Gelmez G, Aksu B, Karatuna O, Söyletir G. 2018. Performance of “RESIST-3 O.K.N. K-SeT” immunochromatographic assay for the detection of OXA-48 like, KPC, and NDM carbapenemases in Klebsiella pneumoniae in Turkey. Braz J Microbiol Publ Braz Soc Microbiol 49:885–890. doi: 10.1016/j.bjm.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glupczynski Y, Jousset A, Evrard S, Bonnin RA, Huang T-D, Dortet L, Bogaerts P, Naas T. 2017. Prospective evaluation of the OKN K-SeT assay, a new multiplex immunochromatographic test for the rapid detection of OXA-48-like, KPC and NDM carbapenemases. J Antimicrob Chemother 72:1955–1960. doi: 10.1093/jac/dkx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boutal H, Vogel A, Bernabeu S, Devilliers K, Creton E, Cotellon G, Plaisance M, Oueslati S, Dortet L, Jousset A, Simon S, Naas T, Volland H. 2018. A multiplex lateral flow immunoassay for the rapid identification of NDM-, KPC-, IMP- and VIM-type and OXA-48-like carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 73:909–915. doi: 10.1093/jac/dkx521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dortet L, Fusaro M, Naas T. 2016. Improvement of the Xpert Carba-R kit for the detection of carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:3832–3837. doi: 10.1128/AAC.00517-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito R, Mustapha MM, Tomich AD, Callaghan JD, McElheny CL, Mettus RT, Shanks RMQ, Sluis-Cremer N, Doi Y. 2017. Widespread fosfomycin resistance in Gram-negative bacteria attributable to the chromosomal fosA gene. mBio 8:e00749-17. doi: 10.1128/mBio.00749-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naas T, Oueslati S, Bonnin RA, Dabos ML, Zavala A, Dortet L, Retailleau P, Iorga BI. 2017. Beta-lactamase database (BLDB)—structure and function. J Enzyme Inhib Med Chem 32:917–919. doi: 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford PA, Urban C, Mariano N, Projan SJ, Rahal JJ, Bush K. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC beta-lactamase, and the foss [sic] of an outer membrane protein. Antimicrob Agents Chemother 41:563–569. doi: 10.1128/AAC.41.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paauw A, Caspers MPM, Schuren FHJ, Leverstein-van Hall MA, Delétoile A, Montijn RC, Verhoef J, Fluit AC. 2008. Genomic diversity within the Enterobacter cloacae complex. PLoS One 3:e3018. doi: 10.1371/journal.pone.0003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamal W, Albert MJ, Rotimi VO. 2014. Real-time comparative evaluation of bioMerieux VITEK MS versus Bruker Microflex MS, two matrix-assisted laser desorption-ionization time-of-flight mass spectrometry systems, for identification of clinically significant bacteria. BMC Microbiol 14:289. doi: 10.1186/s12866-014-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. 2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 23.Izdebski R, Baraniak A, Herda M, Fiett J, Bonten MJM, Carmeli Y, Goossens H, Hryniewicz W, Brun-Buisson C, Gniadkowski M, Grabowska A, Nikonorow E, Derde LPG, Dautzenberg MJ, Adler A, Kazma M, Navon-Venezia S, Malhotra-Kumar S, Lammens C, Dumpis U, Giamarellou H, Muzlovic I, Nardi G, Petrikkos GL, Stammet P, Salomon J, Lawrence C, Legrand P, Rossini A, Salvia A, Samso JV, Fierro J, Paul M, Lerman Y. 2015. MLST reveals potentially high-risk international clones of Enterobacter cloacae. J Antimicrob Chemother 70:48–56. doi: 10.1093/jac/dku359. [DOI] [PubMed] [Google Scholar]
- 24.Kaas RS, Leekitcharoenphon P, Aarestrup FM, Lund O. 2014. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS One 9:e104984. doi: 10.1371/journal.pone.0104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vu H, Nikaido H. 1985. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother 27:393–398. doi: 10.1128/AAC.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mammeri H, Nordmann P, Berkani A, Eb F. 2008. Contribution of extended-spectrum AmpC (ESAC) beta-lactamases to carbapenem resistance in Escherichia coli. FEMS Microbiol Lett 282:238–240. doi: 10.1111/j.1574-6968.2008.01126.x. [DOI] [PubMed] [Google Scholar]
- 27.Guérin F, Isnard C, Cattoir V, Giard JC. 2015. Complex regulation pathways of AmpC-mediated β-lactam resistance in Enterobacter cloacae complex. Antimicrob Agents Chemother 59:7753–7761. doi: 10.1128/AAC.01729-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dortet L, Cuzon G, Plésiat P, Naas T. 2016. Prospective evaluation of an algorithm for the phenotypic screening of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 71:135–140. doi: 10.1093/jac/dkv308. [DOI] [PubMed] [Google Scholar]
- 29.Dabos L, Bogaerts P, Bonnin RA, Zavala A, Sacré P, Iorga BI, Huang DT, Glupczynski Y, Naas T. 2018. Genetic and biochemical characterization of OXA-519, a novel OXA-48-like β-lactamase. Antimicrob Agents Chemother 62:e00469-18. doi: 10.1128/AAC.00469-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieser T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36. doi: 10.1016/0147-619X(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 31.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dortet L, Oueslati S, Jeannot K, Tandé D, Naas T, Nordmann P. 2015. Genetic and biochemical characterization of oxa-405, an oxa-48-type extended-spectrum β-lactamase without significant carbapenemase activity. Antimicrob Agents Chemother 59:3823–3828. doi: 10.1128/AAC.05058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornish-Bowden A. 1995. Fundamentals of enzyme kinetics. Portland Press, Seattle, WA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.