Zidebactam and WCK 5153 are novel bicyclo-acyl hydrazide (BCH) agents that have previously been shown to act as β-lactam enhancer (BLE) antibiotics in Pseudomonas aeruginosa and Acinetobacter baumannii. The objectives of this work were to identify the molecular targets of these BCHs in Klebsiella pneumoniae and to investigate their potential BLE activity for cefepime and aztreonam against metallo-β-lactamase (MBL)-producing strains in vitro and in vivo.
KEYWORDS: β-lactam enhancer, BLE, CRE, Klebsiella pneumoniae, WCK 5153, multidrug resistance, zidebactam
ABSTRACT
Zidebactam and WCK 5153 are novel bicyclo-acyl hydrazide (BCH) agents that have previously been shown to act as β-lactam enhancer (BLE) antibiotics in Pseudomonas aeruginosa and Acinetobacter baumannii. The objectives of this work were to identify the molecular targets of these BCHs in Klebsiella pneumoniae and to investigate their potential BLE activity for cefepime and aztreonam against metallo-β-lactamase (MBL)-producing strains in vitro and in vivo. Penicillin binding protein (PBP) binding profiles were determined by Bocillin FL assay, and 50% inhibitory concentrations (IC50s) were determined using ImageQuant TL software. MICs and kill kinetics for zidebactam, WCK 5153, and cefepime or aztreonam, alone and in combination, were determined against clinical K. pneumoniae isolates producing MBLs VIM-1 or NDM-1 (plus ESBLs and class C β-lactamases) to assess the in vitro enhancer effect of BCH compounds in conjunction with β-lactams. Additionally, murine systemic and thigh infection studies were conducted to evaluate BLE effects in vivo. Zidebactam and WCK 5153 showed specific, high PBP2 affinity in K. pneumoniae. The MICs of BLEs were >64 μg/ml for all MBL-producing strains. Time-kill studies showed that a combination of these BLEs with either cefepime or aztreonam provided 1 to >3 log10 kill against MBL-producing K. pneumoniae strains. Furthermore, the bactericidal synergy observed for these BLE–β-lactam combinations translated well into in vivo efficacy even in the absence of MBL inhibition by BLEs, a characteristic feature of the β-lactam enhancer mechanism of action. Zidebactam and WCK 5153 are potent PBP2 inhibitors and display in vitro and in vivo BLE effects against multidrug-resistant (MDR) K. pneumoniae clinical isolates producing MBLs.
INTRODUCTION
The worldwide, growing incidence of carbapenem-resistant Klebsiella pneumoniae has prompted the CDC to classify this pathogen under the “urgent threat” category. According to the 2018 report of the European Centre for Disease Prevention and Control (ECDC), several European Union countries, including Greece, Italy, Romania, and Cyprus, show carbapenem resistance in the range of 15.5% to 64.7% in K. pneumoniae isolates (1). High metallo-β-lactamase (MBL)-mediated carbapenem resistance rates in K. pneumoniae have also been reported from India (19%) and China (18% to 33%) (2–5).
From the clinical point of view, the development of resistance in K. pneumoniae can increase abruptly and become a significant challenge to therapy, leading to mortality rates above 50% (6, 7). The rise of carbapenem resistance in K. pneumoniae has been associated with the production of carbapenem-hydrolyzing β-lactamases, such as Klebsiella pneumoniae carbapenemase (KPC)-type, OXA-type, and class B β-lactamases. Furthermore, these mechanisms are encoded in mobile genetic elements that can be readily spread intra- and interspecies (8). Additionally, the loss of outer membrane porins (OMPs) further contributes to the varied resistance mechanisms harbored by this pathogen (9, 10). Clinically available β-lactamase inhibitors (BLIs), such as clavulanic acid, tazobactam, sulbactam, avibactam, and vaborbactam (formerly RPX-7009), have no inhibitory activity against MBL enzymes (11–13). Therefore, newer therapeutic approaches that can tackle diverse β-lactam-impacting resistance mechanisms, including MBLs expressed in K. pneumoniae, are warranted.
In previous works, our group has shown that two novel bicyclo-acyl hydrazide (BCH) β-lactam enhancers (BLEs), zidebactam and WCK 5153, selectively bind penicillin binding protein 2 (PBP2) of Acinetobacter baumannii and Pseudomonas aeruginosa (14, 15). In these organisms, both compounds, through their PBP2 binding-driven β-lactam enhancer action, have demonstrated the ability to overcome several carbapenem resistance mechanisms in combination with β-lactams (13, 15–17). In the present study, we show for the first time the PBP binding profiles of BCH compounds and comparators for another clinically significant pathogen, K. pneumoniae, and the synergistic bactericidal action of these BLEs in combination with β-lactams (cefepime and aztreonam). Additionally, we examined the in vivo translation of the β-lactam enhancer effect of these two PBP2 inhibitors in combination with cefepime or aztreonam.
RESULTS
MICs of cefepime or aztreonam in combination with β-lactam enhancers.
The broth microdilution MICs of tested stand-alone agents and combinations against MBL- expressing K. pneumoniae strains are shown in Table 1. The MICs of cefepime were ≥32 μg/ml. Aztreonam was active against the solely VIM-1-producing K. pneumoniae strain 4338 (MIC of 1 μg/ml) but remained inactive against the other K. pneumoniae strains studied. On a stand-alone basis, zidebactam and WCK 5153 showed no antibacterial activity (MICs of >256 μg/ml). The addition of 4 μg/ml of zidebactam or WCK 5153 reduced the MICs of cefepime by >4 times against all the strains. It is worth mentioning that the combination of either zidebactam or WCK 5153 with cefepime or aztreonam reduced their MICs to the susceptible or intermediate range of ≤8 μg/ml (18) except in strain 7043, possibly owing to the outer membrane protein loss (OmpK35/-36) and/or the hyperexpression of the AcrAB-TolC efflux pump. The enhancer effect was superior for aztreonam, as the MICs were reduced >32 times against aztreonam-resistant strains. Imipenem exhibited a MIC of 4 μg/ml against VIM-1-producing K. pneumoniae 4338 and a MIC of 128 μg/ml against the other strains. The tigecycline MIC was 0.5 μg/ml against K. pneumoniae strain 1186 and in the range of 2 to 8 μg/ml against other strains. The meropenem MIC was 0.5 μg/ml for K. pneumoniae 4338, 64 μg/ml for K. pneumoniae strain 7043, and ≥128 μg/ml for the remaining strains.
TABLE 1.
Antimicrobial susceptibilities of MBL-expressing K. pneumoniae isolates
| K. pneumoniae strain | β-Lactamase(s) produced | MIC (μg/ml)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZID | WCK 5153 | FEP |
ATM |
IPM | MEM | TGC | ||||||
| Alone | + ZID (4 μg/ml) | + WCK 5153 (4 μg/ml) | Alone | + ZID (4 μg/ml) | + WCK 5153 (4 μg/ml) | |||||||
| 4338 | VIM-1 | >256 | >256 | 32 | 0.5 | 0.5 | 1 | ≤0.5 | ≤0.5 | 4 | 0.5 | 8 |
| 7043 | NDM-1, CTX-M-15 | >256 | 256 | >256 | 32 | 32-64 | >256 | 8 | 4 | 128 | 64 | 4 |
| NCTC 13443 | NDM-1, CMY, SHV, TEM, CTX-M-15 | >256 | >256 | >256 | 4 | 4 | >256 | 2 | 2 | 128 | >128 | 4 |
| AI 1185 | NDM, CMY, TEM, SHV | >256 | >256 | 128 | ≤0.25 | ≤0.25 | >256 | ≤0.25 | ≤0.25 | 128 | 128 | 4 |
| AI 1186 | NDM, CMY, SHV, TEM | >256 | >256 | >128 | ≤0.25 | ≤0.25 | >256 | ≤0.25 | ≤0.25 | 128 | 128 | 0.5 |
| AI 1460 | NDM, CMY SHV, TEM | >256 | >256 | >128 | ≤0.25 | ≤0.25 | >256 | ≤0.25 | ≤0.25 | 128 | 128 | 2 |
Microbroth dilution MICs were performed following CLSI M07-A10 guidelines (28). Modal MICs are provided. FEP, cefepime; ATM, aztreonam; ZID, zidebactam; IPM, imipenem; MEM, meropenem; TGC, tigecycline.
PBP binding profile of β-lactam enhancers.
Both zidebactam and WCK 5153 characteristically demonstrated exclusive and comparable K. pneumoniae PBP2 binding at substantially low concentrations. The PBP2 binding 50% inhibitory concentrations (IC50s; mean ± standard deviation) of zidebactam and WCK 5153 were 0.08 ± 0.02 and 0.07 ± 0.03 μg/ml, respectively (Table 2; Fig. S1 in the supplemental material). The PBP2 inhibitory activity of BLEs was ≈2-fold higher than that of amdinocillin, a well-known PBP2 binding β-lactam.
TABLE 2.
IC50s of zidebactam, WCK 5153, and reference drugs cefepime and amdinocillin for PBPs of reference strain K. pneumoniae 52145
| K. pneumoniae 52145 PBPa | Mean IC50 ± SD (μg/ml)b |
|||
|---|---|---|---|---|
| Cefepime | Amdinocillin | Zidebactam | WCK 5153 | |
| 1a | 1.2 ± 0.47 | >2 | >2 | >2 |
| 1b | >2 | >2 | >2 | >2 |
| 2 | 0.74 ± 0.27 | 0.18 ± 0.07 | 0.08 ± 0.02 | 0.07 ± 0.03 |
| 3 | 0.19 ± 0.08 | >2 | >2 | >2 |
| 4 | >2 | >2 | >2 | >2 |
| 5/6 | >2 | >2 | >2 | >2 |
PBP, penicillin binding protein.
Mean values ± standard deviations from at least 3 independent experiments are shown.
Similar to previous observations for Escherichia coli, cefepime displayed a multiple-PBP binding profile in K. pneumoniae, with the highest affinity for PBP3 (IC50 of 0.19 ± 0.08 μg/ml), followed by PBP2 (IC50 of 0.74 ± 0.27 μg/ml) and, to a lesser extent, PBP1a (IC50 of 1.2 ± 0.47 μg/ml) (19). Furthermore, as previously shown, amdinocillin displayed relatively higher affinity toward PBP2 than did cefepime; however, it did not bind to other PBPs.
Time-kill studies of cefepime or aztreonam in combination with β-lactam enhancers.
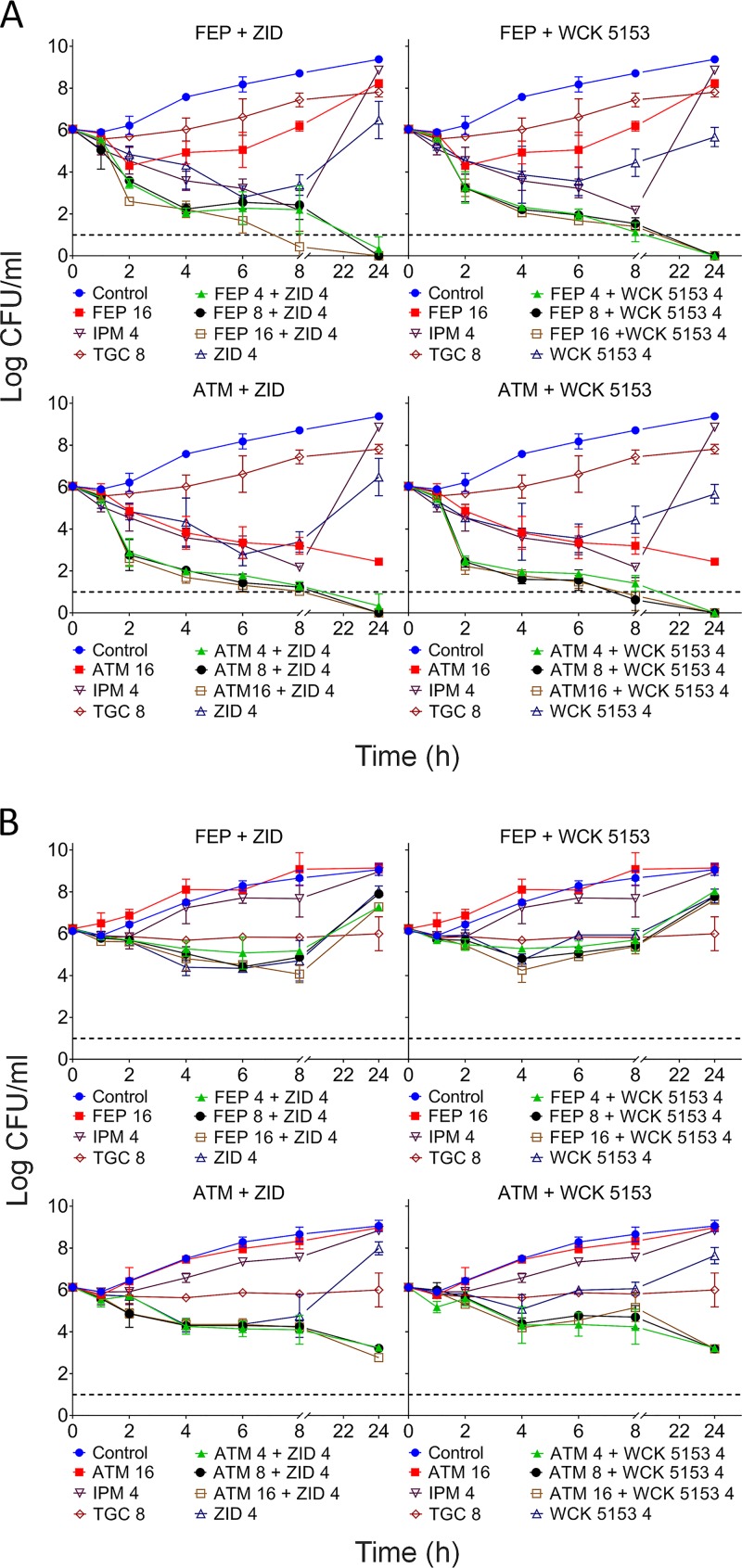
Figure 1 shows the time-kill curves for K. pneumoniae strains 4338 (Fig. 1A) and 7043 (Fig. 1B). As shown in Fig. 1A, cefepime concentrations as low as 4 μg/ml (1/8× MIC) in combination with 4 μg/ml of zidebactam or WCK 5153 (>1/64× MIC) showed an extensive bactericidal effect of about 4 log10 against VIM-1- expressing K. pneumoniae 4338 by 8 h and led to bacterial eradication below the detection limit by 24 h. Similarly, aztreonam at 4 μg/ml (1/4× MIC) in combination with 4 μg/ml of either zidebactam or WCK 5153 elicited approximately 4 log10 kill within 4 h and bacterial counts below the detection limit by 24 h.
FIG 1.
Time-kill kinetics of WCK 5153 and zidebactam in combination with β-lactams against K. pneumoniae MBL-producing strains. Killing curves are measured in terms of reduction of viable CFU/ml over time for MBL-producing K. pneumoniae strains 4338 (VIM-1) (A) and 7043 (NDM-1 and CTX-M-15) (B) for the combinations of cefepime (FEP) and aztreonam (ATM) with zidebactam (ZID) and WCK 5153. Stand-alone aztreonam, cefepime, imipenem (IPM), and tigecycline (TGC) were used as controls. Mean values from three experiments ± standard deviations are shown. Dashed lines represent the limit of detection.
For the NDM-1- and CTX-M-15-expressing K. pneumoniae strain 7043 (cefepime-plus-zidebactam MIC of 32 μg/ml), cefepime (4 to 16 μg/ml) with either zidebactam or WCK 5153 (4 μg/ml) showed >1.5 log10 kill by 8 h and regrowth thereafter. On the other hand, aztreonam (4 to 16 μg/ml) in combination with 4 μg/ml of zidebactam or WCK 5153 showed enhanced bactericidal activity with ≥3 log10 reduction in bacterial load after 24 h of incubation (Fig. 1B).
A comparable trend of enhanced bacterial killing activity of cefepime or aztreonam in the presence of zidebactam or WCK 5153 was observed for the other strains as well (Fig. S2). As expected, imipenem at 8 μg/ml was not bactericidal due to the presence of MBLs in all K. pneumoniae strains tested. No significant bactericidal activity was observed with tigecycline at 1× and 2× MICs.
In vivo efficacy of cefepime or aztreonam in combination with β-lactam enhancers.
The impact of BLEs on the in vivo pharmacodynamic activity of cefepime or aztreonam against cefepime- and aztreonam-resistant K. pneumoniae strains coexpressing NDM-1, class C β-lactamases, and extended-spectrum β-lactamases (ESBLs) was assessed in immunocompetent mouse peritonitis and neutropenic mouse thigh infection studies.
The peritonitis model study was performed with K. pneumoniae NCTC 13443. Cefepime at 100 mg/kg of body weight (3 doses) did not provide protection to infected mice (Table 3). The 50% effective dose (ED50) and ED90 of zidebactam or WCK 5153 in combination with 100 mg/kg of cefepime were 19.46 and 49.77 mg/kg, respectively. The efficacy of aztreonam in combination with BLEs was not evaluated in the peritonitis model, since the combination was studied in detail employing the thigh infection model.
TABLE 3.
Efficacy of cefepime in combination with β-lactam enhancers against K. pneumoniae NCTC 13443 in mouse peritonitis model
| Drug (3 doses at 3-h intervals) | MIC (μg/ml) | ED50 (mg/kg) | ED90 (mg/kg) |
|---|---|---|---|
| Cefepime | >32 | >200 | >200 |
| Cefepime + zidebactam | 4a | 100 + 19.46 | 100 + 49.77 |
| Cefepime + WCK 5153 | 4a | 100 + 19.46 | 100 + 49.77 |
| Meropenem + cilastatin | >32 | >50 | >50 |
| Ceftazidime | >32 | >200 | >200 |
| Ceftazidime + avibactam | >32b | >100 + 25 | >100 + 25 |
| Tigecycline | 2 | 3.43 | 7.35 |
MIC was determined with fixed dose of 4 μg/ml of zidebactam or WCK 5153.
MIC was determined with fixed dose of 4 μg/ml of avibactam.
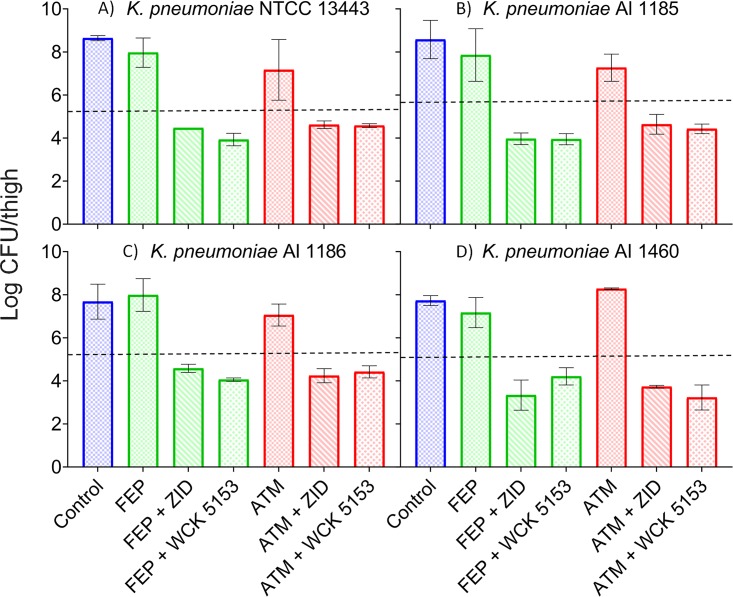
Neutropenic mouse thigh infection studies involving four K. pneumoniae strains coexpressing NDM, CMY, and ESBL β-lactamases were undertaken. The thigh bacterial loads at initiation of treatment (0 h) for all four strains ranged from 5.15 to 5.53 log10 CFU/thigh. The untreated group showed net bacterial growth of 2.41 to 3.36 log10 CFU/thigh by 24 h compared to the bacterial load at 0 h. Cefepime and aztreonam monotherapies (total daily doses of 1,200 and 900 mg/kg, respectively, fractionated into equal amounts every 2 h [q2h]) were not effective against any of the strains, showing bacterial loads in infected thighs ranging from 7.06 to 8.28 log10 CFU/thigh (net bacterial growth of >1 log10 CFU/thigh) at 24 h. Likewise, monotherapies of BLEs at a total daily dose of 900 mg/kg (q2h regimen) were ineffective, as evidenced by high thigh bacterial loads ranging from 6.61 to 8.0 log10 CFU (net bacterial growth of 1.08 to 2.25 log10 CFU/thigh) at 24 h. For all four strains, meropenem-cilastatin (total daily dose of 225 mg/kg administered as a q4h regimen) treatment led to high bacterial loads of 7.11 to 7.83 log10 CFU/thigh, indicating its ineffectiveness, possibly due to in vivo expression of NDM. Combinations of zidebactam or WCK 5153 (total daily dose of 900 mg/kg as a q2h regimen) with an otherwise inefficacious dosage regimen of cefepime or aztreonam (1,200 mg/kg and 900 mg/kg, respectively, as a q2h regimen) resulted in enhanced bactericidal activity. The combination of cefepime with zidebactam or WCK 5153 demonstrated significantly lower thigh bacterial loads, ranging from 3.34 to 4.47 log10 CFU at 24 h for all strains. Thus, the extent of killing at 24 h compared to the bacterial load at 0 h was 0.68 to 1.81 log10 CFU/thigh for cefepime combined with BLEs. Similarly, aztreonam and BLE combinations resulted in bacterial kill values of 0.66 to 1.92 log10 CFU (Fig. 2).
FIG 2.
Efficacy of cefepime and aztreonam in combination with BLEs in neutropenic mouse thigh infection model. Results are shown in terms of reduction of viable CFU/thigh at 24 h for MBL-producing K. pneumoniae strains NCTC 13443 (NDM-1, CMY, SHV, TEM, and CTX-M) (A), AI 1185 (NDM, CMY, SHV, and TEM) (B), AI 1186 (NDM, CMY, SHV and TEM) (C), and AI 1460 (NDM, CMY SHV, and TEM) (D) for the combinations of cefepime (FEP) and aztreonam (ATM) with zidebactam (ZID) and WCK 5153. Cefepime (FEP) and aztreonam (ATM) monotherapy groups received a 24-h dose of 1,200 mg/kg or 900 mg/kg, respectively (fractionated into a q2h regimen). Combination groups received the same dose regimen of cefepime and aztreonam in combination with zidebactam or WCK 5153 at a 24-h dose of 900 mg/kg (fractionated into a q2h regimen). The efficacy was assessed as the change in thigh bacterial load (mean value ± standard deviation) at 24 h compared to that at 0 h. Dashed lines represent log10 CFU/thigh at initiation of treatment (NCTC 13343, 5.28; AI 1185, 5.53; AI 1186, 5.27; AI 1460, 5.15).
DISCUSSION
It has been reported that the activities of piperacillin-tazobactam, amoxicillin-clavulanate, and ampicillin-sulbactam were challenged by the emergence of inhibitor-resistant β-lactamases (20). Furthermore, the continuous emergence of newer β-lactamases with an extended hydrolytic spectrum remains a persistent challenge for agents and therapies that rely on β-lactamase inhibition. The recent reports of on-therapy resistance development for ceftazidime-avibactam in KPC-expressing K. pneumoniae isolates (21) highlights the limitation of therapeutic approaches based on β-lactam–BLI combinations to tackle infections caused by multidrug-resistant (MDR) bacteria. In an attempt to overcome the limitations of BLI-based combinations, a novel approach based on BLE has been proposed. In vitro and in vivo studies have shown that this approach has a potential to circumvent the need for β-lactamase inhibition and still provide clinically significant improved activity against organisms harboring any of the four classes of β-lactamases (14, 15). Structure-activity relationship studies led to the identification of WCK 5107 (zidebactam) and WCK 5153 as potent β-lactam enhancers (22). The present study evaluated the in vitro and in vivo activities of cefepime and aztreonam in combination with BLEs against MBL-expressing K. pneumoniae.
Among the six carbapenem-resistant K. pneumoniae strains, one strain was susceptible to aztreonam due to solely expressing VIM-1, whereas all other strains coproduced ESBLs and/or class C β-lactamases along with MBLs. Cefepime MICs were higher for all of the strains.
Zidebactam and WCK 5153 do not possess β-lactamase-inhibitory activity against MBL enzymes (15). In the present study, neither BLE showed significant antibacterial activity against the K. pneumoniae strains tested. However, these BLEs were shown to induce spheroplast formation at sub-MICs (15). Such morphological change is evidence for BLE-mediated PBP2 dysfunction without concomitant growth inhibition. However, when coadministered with cefepime or aztreonam, the MICs were lowered significantly. Since BLEs were antibacterially inactive against the strains studied and also were noninhibitors of MBLs, the lowering of the MICs of PBP3 binding agents cefepime and aztreonam in combination with BLEs appears to be an outcome of inactivation of complementary PBPs, resulting in growth inhibition. In the past, PBP binding data of BLEs were published for E. coli, A. baumannii, and P. aeruginosa (14, 15), while in the present study, for the first time, we demonstrate high-affinity binding to K. pneumoniae PBP2. The time-kill studies showed that both zidebactam and WCK 5153 enhanced the bactericidal activity of cefepime and aztreonam. The effectiveness of BLEs at sub-MICs in combination with either cefepime or aztreonam against K. pneumoniae strains with diverse resistance mechanisms provides further evidence of synergy emanating from inactivation of multiple PBPs. In the past, it has been reported that sub-MICs of BLEs induce spheroplast formation irrespective of the resistance mechanism expressed (15, 23). Likewise, cefepime sub-MICs have been demonstrated to induce cell elongation as a result of PBP3 dysfunction (23). Previously, Satta et al. (24) described the relationship between the levels of saturation of multiple PBPs and their resultant bactericidal effects. Upon concurrent PBP2 and PBP3 inactivation by a combination of cefepime or aztreonam and BLEs, a rapid bactericidal action is triggered even against isolates producing metallo-β-lactamases that are not inhibited by BLE.
The enhancement of antibacterial effect by BLEs was also observed in animal efficacy studies for cefepime and aztreonam. In the peritonitis model, both zidebactam and WCK 5153 enabled an otherwise nonefficacious dose regimen of cefepime to protect mice infected with a lethal dose of the NDM- producing K. pneumoniae strain NCTC 13443.
The impact of BLEs on the pharmacodynamic activity of cefepime and aztreonam was investigated in a thigh infection study involving four cefepime-, aztreonam-, and meropenem-resistant, NDM-expressing K. pneumoniae strains. The clinically relevant meropenem-cilastatin dose regimen failed to show efficacy, indicating adequate expression of MBLs in vivo. Clinically relevant in vivo exposures of stand-alone cefepime or aztreonam were also ineffective, an indication of high in vivo MICs due to the expression of ESBLs, class C β-lactamases, and/or MBLs. In addition, on a stand-alone basis, BLEs failed to exert growth-inhibitory effects, possibly due to their solely PBP binding nature and lack of direct antibacterial activity. However, in vivo evidence of an enhancer effect was observed, as both cefepime and aztreonam in combination with inefficacious doses of BLEs invariably demonstrated bacterial kill. Recently, Avery et al. demonstrated pronounced killing of MDR A. baumannii with a cefepime-zidebactam combination (25). In their study, interestingly, the MICs of stand-alone cefepime and zidebactam for the A. baumannii strains were very high. The study ascribes the observed killing with the combination of zidebactam and cefepime to the concomitant spheroplast- and elongation-inducing effects, being the pharmacodynamic drivers of efficacy. Considering the fact that neither of the BLEs showed MBL inhibition, the thigh bactericidal effect of β-lactams in combination with BLE is strong evidence of the enhancer mechanism offering freedom from β-lactamase inhibition.
In summary, the present in vitro and in vivo studies reveal the β-lactam enhancer property of PBP2 binding zidebactam and WCK 5153 in driving the efficacy of β-lactams against MBL expressing K. pneumoniae.
MATERIALS AND METHODS
Bacterial isolates and susceptibility testing.
A total of six bacterial isolates were employed in this study. Two clinical isolates of MBL-producing K. pneumoniae (KP4338 [VIM-1] and KP7043 [NDM-1 and CTX-M-15]) were obtained from the Hospital Son Espases (Palma de Mallorca, Spain) strain collection; K. pneumoniae NCTC 13443 (NDM-1, CMY, SHV, TEM, and CTX-M-15) was obtained from Public Health England; and three clinical isolates of MBL-producing K. pneumoniae (AI 1185 [NDM-like, CMY, SHV, TEM], AI 1186 [NDM-like, CMY, SHV, TEM], and AI 1460 [NDM-like, CMY, SHV, and TEM]) were obtained from Indian tertiary care hospitals. The MICs of stand-alone cefepime, aztreonam, zidebactam, and WCK 5153 and combinations of cefepime or aztreonam with zidebactam or WCK 5153 were determined by the standard CLSI broth microdilution method (28). The MICs of other comparators, such as imipenem, meropenem, and tigecycline, were also determined. For combination MICs, zidebactam and WCK 5153 were added at a fixed concentration of 4 μg/ml.
PBP binding assay.
The PBP binding affinities of zidebactam, WCK 5153, and reference drugs cefepime and amdinocillin were determined by using membrane isolations from reference strain K. pneumoniae 52145 (26). Membrane preparations were obtained by following previously described protocols (15). PBP-containing membrane preparations were then incubated (30 min at 35°C) in the presence of increasing concentrations (0.0156 to 2 μg/ml) of zidebactam, WCK 5153, or reference drug cefepime or amdinocillin and labeled with Bocillin FL (25 μM). The reaction mixtures were denatured, and PBPs were separated by SDS-PAGE. Labeled PBPs were visualized and IC50s were determined (Typhoon FLA 9500 biomolecular imager and ImageQuant TL- GE; Healthcare Bio-Sciences AB, Uppsala, Sweden). All experiments were performed in triplicate.
Time-kill studies.
Time-kill studies for all six strains of K. pneumoniae were initiated by inoculating 100 μl of mid-log-phase (106 CFU/ml) culture in 96-well microplates with 100 μl of cation-adjusted Mueller-Hinton broth (caMHB) containing a final concentration of 4 μg/ml of each BLE (zidebactam and WCK 5153) alone and in combination with 4, 8, and 16 μg/ml of cefepime or aztreonam. The bactericidal actions of stand-alone cefepime or aztreonam (16 to 32 μg/ml), imipenem (4 μg/ml), and tigecycline (0.5 μg/ml) were assessed for comparison. The viable counts of cultures were determined by plating the serial dilutions at 2, 4, 6, 8, and 24 h. The lowest detection limit was 10 CFU/ml. All experiments were performed in duplicate.
Murine systemic infection.
The murine systemic infection study was performed using the NDM-1-expressing K. pneumoniae NCTC 13443 isolate. Male and female Swiss Albino mice (20 ± 2 g body weight) were obtained from Wockhardt’s animal-breeding facility. All animal experiments were approved by Wockhardt’s institutional animal ethics committee, constituted by the Committee for Purpose of Control and Supervision on Animal Experiments (CPCSEA), Government of India. Groups (n = 6 each) of male (n = 3) and female (n = 3) Swiss Albino mice were intraperitoneally infected with ≈106 CFU/mouse of bacterial inoculum in 5% hog gastric mucin that resulted in 100% mortality of untreated animals within 24 h. Subcutaneous treatment with drugs was initiated 30 min postinfection with three doses at an interval of 3 h. Survival patterns were monitored for 7 days. The ED50 and ED90 values were derived by probit analysis.
Neutropenic murine thigh infection study.
A total of four MBL (NDM-like)-producing strains (K. pneumoniae NCTC 13443, AI 1460, AI 1185, and AI 1186) were employed in the thigh infection studies. Male and female Swiss Albino mice (25 to 27 g) were rendered neutropenic (<100 neutrophils/mm3) by intraperitoneal injection of cyclophosphamide before infection. The infective inoculum was freshly prepared in normal saline by suspending an overnight culture taken from a Trypticase soy agar (TSA) plate. The inoculum was adjusted to contain a bacterial density of about 5 × 106 CFU/ml. Each animal’s right thigh was infected intramuscularly with 0.1 of freshly prepared inoculum. Treatment with test agents was initiated by 2 h postinfection (0 h). The control and treatment groups each consisted of 6 animals. All test agents were administered through subcutaneous injections with a volume of 0.25 ml. Stand-alone cefepime treatment groups received a total 24-h dose of 1,200 mg/kg, fractionated into a q2h (every 2 h) regimen. The same dose regimen of cefepime was combined with zidebactam or WCK 5153 in a total 24-h dose of 900 mg/kg fractionated into a q2h regimen. Similarly, stand-alone aztreonam groups received a total 24-h dose of 900 mg/kg fractionated into a q2h regimen, and the same dose regimen of aztreonam was combined with zidebactam or WCK 5153 at a total 24-h dose of 900 mg/kg fractionated into a q2h regimen. Mouse doses used in this study, corresponding to clinical exposures, were based on the pharmacokinetics studies reported earlier for cefepime and zidebactam (27) and unpublished data for aztreonam and WCK 5153.
In order to assess the optimal expression of NDM in terms of carbapenem ineffectiveness, a group of mice was treated with meropenem-cilastatin (1:1) at a total 24-h dose of 225 mg/kg fractionated into a q4h (every 4 h) regimen. Based on pharmacokinetics studies conducted at Wockhardt, the percentage of a 24-h time period that the unbound drug concentration exceeds the MIC (fT>MIC) of meropenem at this regimen for a MIC of 1 μg/ml (CLSI susceptibility breakpoint [21]) was 42%, indicating the adequacy of the doses to treat this otherwise-susceptible pathogen (data on file). For a meropenem MIC of 4 μg/ml (CLSI resistance breakpoint [21]), the regimen provided an fT>MIC of 26.2%. Separate groups of mice which received sterile normal saline every 2 h served as a growth control. At the time of initiation of treatment (0 h), a group of mice were humanely euthanized and thighs were aseptically removed. The thighs were homogenized in 5 ml normal saline, serially diluted, and plated on TSA plates. Thigh bacterial loads were determined for all treated groups at 24 h after initiation of treatment. For each group, the mean value ± standard deviation for the thigh bacterial load was calculated. The in vivo efficacy of treatment was assessed as the change in thigh bacterial load at 24 h compared to the load at 0 h.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Wockhardt Bio AG, Switzerland, and by the Ministerio de Economía y Competitividad of Spain, Instituto de Salud Carlos III, cofinanced by the European Regional Development Fund A Way to Achieve Europe ERDF through the Spanish Network for Research in Infectious Diseases (grants number RD12/0015 and RD16/0016).
B.M. and A.O. have received funds for research from Wockhardt Bio AG, Switzerland. M.P., S.B., P.J., S.P., H.P., K.U. and S.T. are employees of Wockhardt Research Centre. S.B. and M.P. are shareholders of Wockhardt Ltd. All other authors have nothing to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00128-19.
REFERENCES
- 1.European Centre for Disease Prevention and Control. 4 June 2018. Rapid risk assessment: carbapenem-resistant Enterobacteriaceae—first update. European Centre for Disease Prevention and Control, Stockholm, Sweden. [Google Scholar]
- 2.Gandra S, Joshi J, Trett A, Lamkang AS, Laxminarayan R. 2017. Scoping report on antimicrobial resistance in India. Center for Disease Dynamics, Economics & Policy, Washington, DC. [Google Scholar]
- 3.Li B, Xu XH, Zhao ZC, Wang MH, Cao YP. 2014. High prevalence of metallo-beta-lactamase among carbapenem-resistant Klebsiella pneumoniae in a teaching hospital in China. Can J Microbiol 60:691–695. doi: 10.1139/cjm-2014-0291. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Sun QL, Shen Y, Zhang Y, Yang JW, Shu LB, Zhou HW, Wang Y, Wang B, Zhang R, Wang S, Shen Z. 2018. Rapid increase in prevalence of carbapenem-resistant Enterobacteriaceae (CRE) and emergence of colistin resistance gene mcr-1 in CRE in a hospital in Henan, China. J Clin Microbiol 56:e01932-17. doi: 10.1128/JCM.01932-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin S, Fu Y, Zhang Q, Qi H, Wen JG, Xu H, Xu L, Zeng L, Tian H, Rong L, Li Y, Shan L, Xu H, Yu Y, Feng X, Liu HM. 2014. High incidence and endemic spread of NDM-1-positive Enterobacteriaceae in Henan Province, China. Antimicrob Agents Chemother 58:4275–4282. doi: 10.1128/AAC.02813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez GV, Master RN, Clark RB, Fyyaz M, Duvvuri P, Ekta G, Bordon J. 2013. Klebsiella pneumoniae antimicrobial drug resistance, United States, 1998-2010. Emerg Infect Dis 19:133–136. doi: 10.3201/eid1901.120310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 8.Cerqueira GC, Earl AM, Ernst CM, Grad YH, Dekker JP, Feldgarden M, Chapman SB, Reis-Cunha JL, Shea TP, Young S, Zeng Q, Delaney ML, Kim D, Peterson EM, O’Brien TF, Ferraro MJ, Hooper DC, Huang SS, Kirby JE, Onderdonk AB, Birren BW, Hung DT, Cosimi LA, Wortman JR, Murphy CI, Hanage WP. 2017. Multi-institute analysis of carbapenem resistance reveals remarkable diversity, unexplained mechanisms, and limited clonal outbreaks. Proc Natl Acad Sci U S A 114:1135–1140. doi: 10.1073/pnas.1616248114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wassef M, Abdelhaleim M, AbdulRahman E, Ghaith D. 2015. The role of OmpK35, OmpK36 porins, and production of beta-lactamases on imipenem susceptibility in Klebsiella pneumoniae clinical isolates, Cairo, Egypt. Microb Drug Resist 21:577–580. doi: 10.1089/mdr.2014.0226. [DOI] [PubMed] [Google Scholar]
- 10.Shi W, Li K, Ji Y, Jiang Q, Wang Y, Shi M, Mi Z. 2013. Carbapenem and cefoxitin resistance of Klebsiella pneumoniae strains associated with porin OmpK36 loss and DHA-1 beta-lactamase production. Braz J Microbiol 44:435–442. doi: 10.1590/S1517-83822013000200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lomovskaya O, Sun D, Rubio-Aparicio D, Nelson K, Tsivkovski R, Griffith DC, Dudley MN. 2017. Vaborbactam: spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob Agents Chemother 61:e01443-17. doi: 10.1128/AAC.01443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abboud MI, Damblon C, Brem J, Smargiasso N, Mercuri P, Gilbert B, Rydzik AM, Claridge TD, Schofield CJ, Frere JM. 2016. Interaction of avibactam with class B metallo-beta-lactamases. Antimicrob Agents Chemother 60:5655–5662. doi: 10.1128/AAC.00897-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N. 2017. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J Antimicrob Chemother 72:1373–1385. doi: 10.1093/jac/dkw593. [DOI] [PubMed] [Google Scholar]
- 14.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 2017. Potent beta-lactam enhancer activity of zidebactam and WCK 5153 against Acinetobacter baumannii, including carbapenemase-producing clinical isolates. Antimicrob Agents Chemother 61:e01238-17. doi: 10.1128/AAC.01238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 2017. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “beta-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-beta-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61:e02529-16. doi: 10.1128/AAC.02529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK. 2017. WCK 5222 (cefepime-zidebactam) antimicrobial activity tested against clinical isolates of gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 61:e00072-17. doi: 10.1128/AAC.00072-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bethel CR, Papp-Wallace KM, Barnes MD, Bonomo RA. 2016. Novel bridged diazabicyclooctanes (DBOs) are effective inhibitors of representative class A, C, and D β-lactamases expressed by multidrug resistant (MDR) pathogens, abstr 373. ASM Microbe, Boston, MA.
- 18.CLSI. 2017. Performance standards for antimicrobial susceptibility testing; 27th informational supplement. CLSI document M100-S27. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Sutaria DS, Moya B, Green KB, Kim TH, Tao X, Jiao Y, Louie A, Drusano GL, Bulitta JB. 2018. First penicillin-binding protein occupancy patterns of beta-lactams and beta-lactamase inhibitors in Klebsiella pneumoniae. Antimicrob Agents Chemother 62:e00282-18. doi: 10.1128/AAC.00282-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drawz SM, Bonomo RA. 2010. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev 23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humphries RM, Yang S, Hemarajata P, Ward KW, Hindler JA, Miller SA, Gregson A. 2015. First report of ceftazidime-avibactam resistance in a KPC-3-expressing Klebsiella pneumoniae isolate. Antimicrob Agents Chemother 59:6605–6607. doi: 10.1128/AAC.01165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshpande P, Bhavsar S, Joshi S, Pawar S, Kale R, Mishra A, Jadhav S, Pavase L, Gupta S, Yeole R, Rane V, Ahirrao V, Bhagwat S, Patel M. 2016. WCK 5107 (zidebactam, ZID): structure activity relationship (SAR) of novel bicyclo acyl hydrazide (BCH) pharmacophore active against gram-negatives including Pseudomonas (PA), abstr 479. ASM Microbe, Boston, MA.
- 23.Palwe SR, Biniwale SS, Khande HN, Joshi PR, Bhagwat SS, Patel MV. 2016. Cefepime (FEP) and WCK 5107 (zidebactam, ZID) mediated dual PBP Engagement at sub-MIC concentrations drive cidality against diverse β-lactamases expressing Gram-negatives, abstr 442. ASM Microbe, Boston, MA.
- 24.Satta G, Cornaglia G, Mazzariol A, Golini G, Valisena S, Fontana R. 1995. Target for bacteriostatic and bactericidal activities of beta-lactam antibiotics against Escherichia coli resides in different penicillin-binding proteins. Antimicrob Agents Chemother 39:812–818. doi: 10.1128/AAC.39.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avery LM, Abdelraouf K, Nicolau DP. 2018. Assessment of the in vivo efficacy of WCK 5222 (cefepime-zidebactam) against carbapenem-resistant Acinetobacter baumannii in the neutropenic murine lung infection model. Antimicrob Agents Chemother 62:e00948-18. doi: 10.1128/AAC.00948-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes G, Borrell N, de Astorza B, Gomez C, Sauleda J, Alberti S. 2002. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun 70:2583–2590. doi: 10.1128/IAI.70.5.2583-2590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi PR, Khande HN, Takalkar SS, Kulkarni AM, Chavan RP, Zope VS, Palwe SR, Biniwale SS, Bhagwat SS, Patel MV. 2016. WCK 5222 [cefepime (FEP)-WCK 5107 (zidebactam, ZID)]: in vitro and in vivo coverage of OXA-carbapenemases expressing-acinetobacter (OXA-AB), abstr 440 ASM Microbe, Boston, MA. [Google Scholar]
- 28.CLSI. 2017. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed. CLSI document M07-A10 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.