Dalbavancin is a lipoglycopeptide with potent activity against Gram-positive microorganisms, a long half-life, a favorable safety profile, and a high concentration in bone, which makes it an interesting alternative for treatment of osteoarticular infections. We performed a multicentric retrospective study of all patients with an osteoarticular infection (septic arthritis, spondylodiscitis, osteomyelitis, or orthopedic implant-related infection) treated with at least one dose of dalbavancin between 2016 and 2017 in 30 institutions in Spain.
KEYWORDS: bone and joint infections, dalbavancin, osteomyelitis, prosthetic joint infection
ABSTRACT
Dalbavancin is a lipoglycopeptide with potent activity against Gram-positive microorganisms, a long half-life, a favorable safety profile, and a high concentration in bone, which makes it an interesting alternative for treatment of osteoarticular infections. We performed a multicentric retrospective study of all patients with an osteoarticular infection (septic arthritis, spondylodiscitis, osteomyelitis, or orthopedic implant-related infection) treated with at least one dose of dalbavancin between 2016 and 2017 in 30 institutions in Spain. In order to evaluate the response, patients with or without an orthopedic implant were separated. A total of 64 patients were included. Staphylococcus epidermidis and Staphylococcus aureus were the most frequent microorganisms. The reasons for switching to dalbavancin were simplification (53.1%), adverse events (25%), or failure (21.9%). There were 7 adverse events, and no patient had to discontinue dalbavancin. In 45 cases, infection was related to an orthopedic implant. The implant material was retained in 23 cases, including that in 15 (65.2%) patients that were classified as cured and 8 (34.8%) that presented improvement. In 21 cases, the implants were removed, including those in 16 (76.2%) cases that were considered successes, 4 (19%) cases were considered improved, and 1 (4.8%) case that was considered a failure. Among the 19 cases without implants, 14 (73.7%) were considered cured, 3 (15.8%) were considered improved, and 2 (10.5%) were considered failures. The results show that dalbavancin is a well-tolerated antibiotic, even when >2 doses are administered, and is associated with a high cure rate. These are preliminary data with a short follow-up; therefore, it is necessary to gain more experience and, in the future, to establish the most appropriate dose and frequency.
INTRODUCTION
Adult bone and joint infections, including osteomyelitis, septic arthritis, and orthopedic implant-related infections, are common and require prolonged antibiotic treatment to eradicate planktonic, biofilm, intracellular, and intracanaliculus bacteria that are frequently involved in these infections (1–3). Although the current guidelines for native vertebral osteomyelitis and prosthetic joint infections (PJI) recommend at least 6 weeks of intravenous treatment, antimicrobial therapy with high oral bioavailability is an alternative to intravenous administration (4–6). Good outcomes have been reported with the oral combination of a fluoroquinolone and rifampin (7–10), particularly for implant-associated infections. However, other oral alternatives combined with rifampin have been associated with lower remission rates (8, 11).
In addition, a recent article measuring the postdischarge adherence to oral antibiotics by an electronic bottle cap that recorded each pill bottle opening demonstrated a significant difference between self-reported adherence and electronically measured adherence (57% versus 96%; P < 0.0001) (12). In this study, poor adherence was associated with a lower clinical response at 30 days. It is reasonable to expect an even lower adherence rate when the antibiotic duration is longer than 2 weeks. Therefore, other alternatives for bone and joint infections are necessary, most especially for those caused by fluoroquinolone-resistant staphylococci (13) and for patients at risk of low adherence to oral treatment (e.g., older people, homeless people, drug addicts).
The aim of the present study was to review the clinical characteristics, outcome, and adverse events observed in patients with bone and joint infections treated with at least one dose of dalbavancin, a long-acting lipoglycopeptide, since it was approved in Spain.
RESULTS
A total of 64 patients were included in the study. The mean age was 63 (standard deviation [SD], 15.7) years, and 39 (60.9%) were male. The most common comorbidities were diabetes mellitus in 14 cases (21.9%), chronic obstructive pulmonary diseases (COPD) in 7 cases (10.9%), cardiac disease in 7 cases (10.9%), chronic renal failure in 6 cases (9.4%), malignant tumor in 6 cases (9.4%), and rheumatoid arthritis in 5 (7.8%). The most common microorganisms were Staphylococcus epidermidis (n = 30, 46.9%) and Staphylococcus aureus (n = 14, 21.9%), which were methicillin resistant in 93.3% (28 out of 30) and 64.3% (9 out of 14) of the cases, respectively (Table 1). All enterococci were susceptible to vancomycin. Five (11.1%) patients had a polymicrobial infection, and in two of them a Gram-negative organism was also isolated. The reasons for switching to dalbavancin were simplification of the regimen (n = 34, 53.1%), adverse events related to the previous antibiotic (n = 16, 25%), or failure during treatment with the previous antibiotic (n = 14, 21.9%). The first dose was 1,000 mg in 50 cases, 1,500 mg in 12, and 500 mg and 750 mg in 1 case each. In 9 cases, a single dose was administered, in 54 cases, dalbavancin was administered once weekly (500 mg) for a median (interquartile range [IQR]) of 5 (3 to 7) doses, and in 1 case, 4 doses of 1,500 mg were administered biweekly. There were 7 adverse events: 3 gastrointestinal problems, 1 self-limited rash, 1 phlebitis, 1 self-reported asthenia, and 1 case with a documented increase of 0.5 mg/dl of serum creatinine. In none of the cases did treatment with dalbavancin have to be stopped due to the adverse events.
TABLE 1.
Etiology according to type of infection
| Microorganism(s) | No. (%) of patients with: |
|
|---|---|---|
| Implant-associated infection (n = 45) | Bone or joint infection (n = 19) | |
| Staphylococcusepidermidis | 26 (57.7) | 4 (21) |
| Staphylococcusaureus | 4 (8.9) | 10 (52.6) |
| Staphylococcuslugdunensis | 2 (4.4) | 0 |
| Staphylococcuscapitis | 1 (2.2) | 0 |
| Streptococcuspneumoniae | 1 (2.2) | 0 |
| Enterococcusfaecalis | 4 (8.9) | 1 (5.2) |
| Enterococcusfaecium | 3 (6.6) | 1 (5.2) |
| Corynebacterium striatum | 2 (4.4) | 1 (5.2) |
| Streptococcus spp.a | 0 | 3 (15.7) |
| Anaerobesb | 2 (4.4) | 1 (5.2) |
| Gram negativesc | 2 (4.4) | 0 |
| Polymicrobial | 5 (11.1) | 3 (15.7) |
| Negative culture | 3 (6.6) | 1 (5.2) |
Streptococcus spp. included one S. agalactiae, one S. pyogenes, and one S. sanguinis.
Anaerobes included one Clostridium celerecrescens and two Propionibacteriumacnes.
Gram negatives included one Escherichiacoli and one Pseudomonasaeruginosa (both were isolated with a Gram-positive microorganism).
The characteristics and outcomes of patients grouped as cases with implant-associated infections (n = 26 prosthetic joint infections; n = 19 other-implant infections) and bone or joint infections (n = 19) are depicted in Tables 2 and 3.
TABLE 2.
Characteristics and outcomes of patients with implant-associated infections (n = 45)
| Variablef | Value |
|---|---|
| Age (yrs), mean (SD) | 64 (15) |
| Male sex, no. (%) | 24 (53.3) |
| Comorbidity, no. (%) | |
| Diabetes mellitus | 7 (15.5) |
| Rheumatoid arthritis | 3 (6.6) |
| Chronic renal failure | 5 (11.1) |
| Cancer | 5 (11.1) |
| COPD | 4 (8.8) |
| Liver cirrhosis | 2 (4.4) |
| Cardiac disease | 3 (6.6) |
| Type of implant, no. (%) | |
| Joint prosthesis | 26 (57.8) |
| Hip | 13 |
| Knee | 10 |
| Shoulder | 3 |
| Other implant | 19 (42.2) |
| Spine | 11 |
| Long bone | 5 |
| Other | 3 |
| Median (IQR) no. of days from implantation to infection diagnosis | 115 (27–424) |
| Fever, no. (%) | 13 (28.8) |
| Local signs of infection at admission, no. (%) | 31 (68.8) |
| Wound drainage, no. (%) | 21 +(46.6) |
| Fistula, no. (%) | 11 (24.4) |
| Median (IQR) leukocyte count (cells/mm3) | 7,300 (5,750–9,925) |
| Median (IQR) SCr (mg/dl) before dalbavancin treatmenta | 1 (0.6–1) |
| Median (IQR) highest SCr (mg/dl) during dalbavancina | 1 (0.6–1) |
| Baseline CRP (mg/dl)b | 5 (2.7–11.7) |
| Last control CRP (mg/dl)c | 1 (0.3–1.3) |
| Median (IQR) no. of days of antibiotics prior to dalbavancin treatment | 41 (21–87) |
| Reason for starting dalbavancin, no. (%) | |
| Failure to prior antibiotic | 12 (26.6) |
| Simplification | 23 (51.1) |
| Toxicity to prior antibiotic | 10 (22.2) |
| Median (IQR) no. of dalbavancin doses | 5 (3–8) |
| Other concomitant antibiotic, no. (%) | |
| Rifampin | 8 (17.7) |
| Other | 7 (15.5) |
| Outcome, no. (%)d | |
| Implant retention | 23 (52.3) |
| Success | 15 |
| Improvement | 8 |
| Failure | 0 |
| Implant removal | 21 (47.7) |
| Success | 16 |
| Improvement | 4 |
| Failure | 1 |
| Death, no. (%)e | 1 (2.2) |
| Median (IQR) no. of days of follow-up | 157 (75.5–273.5) |
Measured in 36 patients.
Measured in 42 patients.
Measured in 41 patients.
Evaluation from 44 cases. One case was lost during follow-up.
Not related to the infection.
COPD, chronic obstructive pulmonary disease; SCr, serum creatinine; CRP, C-reactive protein. No. (%), number (percent) of patients.
TABLE 3.
Characteristics and outcomes of patients with bone or joint infections
| Variabled | Value |
|---|---|
| Age (yrs), mean (SD) | 61 (17.5) |
| Male sex, no. (%) | 15 (78.9) |
| Comorbidity, no. (%) | |
| Diabetes mellitus | 7 (36.8) |
| Rheumatoid arthritis | 2 (10.5) |
| Chronic renal failure | 1 (5.2) |
| Cancer | 1 (5.2) |
| COPD | 3 (15.7) |
| Liver cirrhosis | 0 |
| Cardiac disease | 4 (21) |
| Type of bone or joint infection (%) | |
| Osteomyelitis | 12 (63.1) |
| Septic arthritis or spondylodiscitis | 7 (36.9) |
| Fever, no. (%) | 3 (15.7) |
| Local signs of infection at admission, no. (%) | 11 (57.9) |
| Wound drainage, no. (%) | 5 (26.3) |
| Fistula, no. (%) | 3 (15.7) |
| Median (IQR) leukocyte count (cells/mm3) | 9,545 (8,235–14,085) |
| Median (IQR) SCr (mg/dl) before dalbavancin treatmenta | 1 (0.6–1) |
| Median (IQR) highest SCr (mg/dl) during dalbavancin treatmenta | 1 (0.7–1.2) |
| Baseline CRP (mg/dl) | 14 (4.4–13.7) |
| Last control CRP (mg/dl)b | 1 (0.2–1.3) |
| Median (IQR) no. of days of antibiotics prior to dalbavancin treatment | 32 (21–46) |
| Reason for starting dalbavancin, no. (%) | |
| Failure to prior antibiotic | 2 (10.5) |
| Simplification | 11 (57.9) |
| Toxicity to prior antibiotic | 6 (31.5) |
| Median (IQR) no. of dalbavancin doses | 2 (2–4) |
| Other concomitant antibiotic, no. (%) | |
| Rifampin | 0 |
| Other | 6 (31.5) |
| Outcome, no. (%) | |
| Success | 14 (73.6) |
| Improvement | 3 (15.7) |
| Failure | 2 (10.5) |
| Death, no. (%)c | 3 (15.7) |
| Median (IQR) no. of days in follow-up | 164 (93–262.5) |
Measured in 17 patients.
Measured in 18 patients.
Not related to the infection.
COPD, chronic obstructive pulmonary disease; SCr, serum creatinine; CRP, C-reactive protein. No. (%), number (percent) of patients.
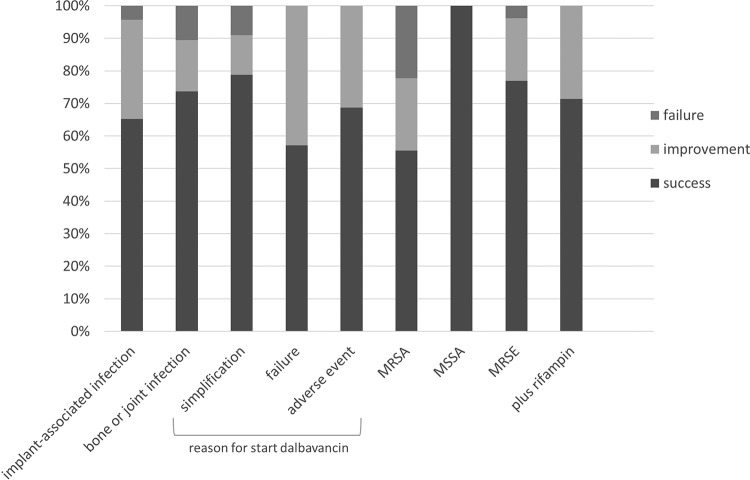
Among the 45 cases with an orthopedic implant infection, the outcomes were evaluated in 44 cases because one patient was lost during follow-up. In 52.3% (n = 23) of the cases, the implant was retained. Of these, 15 cases (65.2%) were classified as successes and 8 cases (34.8%) were classified as improvements. In 47.7% (n = 21), the implant was removed and 16 (76.2%) were classified as successes, 4 (19%) were classified as improvements, and 1 (4.8%) was classified as a failure after a median (IQR) follow-up of 157 (75.5 to 273.5) days after the last dalbavancin dose. Among the 19 cases with a bone or joint infection and after a median (IQR) follow-up of 164 (93 to 262.5) days after the last dalbavancin dose, 14 (73.7%) were classified as successes, 3 (15.8%) were classified as improvements, and 2 (10.5%) were classified as failures. In one failure, the infection persisted and the patient died 5 months after finishing dalbavancin for an unrelated event, and the other failure was a patient with sacral osteomyelitis due to methicillin-resistant S. aureus who had received a single dose of dalbavancin to simplify the antibiotic treatment and had relapsed after 1 month. The outcomes of patients according to the type of infection, the reason for starting dalbavancin, the type of microorganism, and the concomitant use of rifampin are summarized in Fig. 1.
FIG 1.
Outcome of patients according to the type of infection, the reason for starting dalbavancin, etiology, and concomitant use of rifampin.
DISCUSSION
Dalbavancin is a new semisynthetic lipoglycopeptide approved in the United States and Europe for acute bacterial skin and skin and soft tissue infections with potent activity against staphylococci, including methicillin-resistant, heterogeneous vancomycin-intermediate S. aureus (hVISA), and VISA strains and vancomycin-susceptible enterococci (MIC90 ≤ 0.12 mg/liter) (14, 15). The main advantage of this antibiotic is its long half-life (180 to 240 h), which allows a once weekly or biweekly intravenous administration, reducing the use of vascular catheters and saving hospital stays (16). Bone and joint infections, particularly when the implant is not removed, require at least 6 to 12 weeks of antibiotic treatment according to the majority of current guidelines. A recent unblinded and randomized trial (OVIVA) confirmed that oral antibiotic treatment is as effective as intravenous administration in bone, joint, or metalware-associated infections (17). The better results with oral antibiotics have been demonstrated with levofloxacin plus rifampin; however, other oral alternatives combined with rifampin have been associated with lower remission rates (8, 11), in part due to a worse safety profile but also to a reduction in the serum concentration of companion drugs like clindamycin, co-trimoxazole, linezolid, or fusidic acid (18–21), in contrast to what has been shown with fluoroquinolones (10). Therefore, more alternatives are needed for the treatment of bone and joint infections (22). The first conclusion of our study is that dalbavancin is well tolerated, with minor adverse events without any treatment interruption or evidence of nephrotoxicity. Indeed, dalbavancin is a derivative of teicoplanin, and it is significantly less toxic than vancomycin (23). In line with this, dalbavancin was infused over 30 min, and in only one case, a skin rash was reported after the first dose but did not recur after the subsequent ones. This makes dalbavancin an attractive drug for infusion at home by OPAT (outpatient parenteral antibiotic treatment) systems. About 50% of the cases received dalbavancin once the infection was controlled in order to complete antibiotic treatment, and the success rate was almost 80%. These results support a recent report showing a >90% success rate with dalbavancin as a sequential treatment for infective endocarditis caused by Gram-positive cocci (24). On the other hand, the success rate among patients that received dalbavancin after failure with a prior antibiotic was >50%, suggesting a role for dalbavancin as a salvage therapy in some cases. The efficacy of dalbavancin in these infections could be partly attributed to its efficacy against biofilms (25, 26) and its bone diffusion. Dunne et al. (27) determined the concentrations in synovial fluid and bone after 14 days of 1,000 mg of dalbavancin to be 14 mg/liter and 4 μg/g, respectively, both above the MIC90 of dalbavancin for staphylococci and enterococci. This result suggests that a biweekly regimen is possible and even more convenient. Finally, although the number of patients that received rifampin concomitantly with dalbavancin was low, the success rate was 70%, suggesting that this combination is not subjected to pharmacokinetic interactions and might be a valid alternative to the regimen of levofloxacin plus rifampin for orthopedic implant infections. These results are in agreement with a not-yet-published randomized, open-label trial comparing dalbavancin at 1,500 mg on days 1 and 8 versus the standard of care (SOC) for osteomyelitis (excluding implant infections). The researchers showed a clinical cure rate of 94% versus 88% after 1 year of follow-up, and the drug was well tolerated (U. Rappo, V. Shevchenko, O. Shevchenko, A. Jandourek, P. Gonzalez, S. Puttagunta, M. Dunne, A. Suen, V. MasCasullo, D. Melnick, R. Miceli, M. Kovacevic, and G. De Bock, presented at the 28th European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], 21 to 24 April 2018).
Our study has some limitations. The first limitation is the retrospective nature of the study as well as the heterogeneity of the patients. Second, the majority of treatments began after the infection was under control, which could lead to overestimation of the success rate, and the follow-up period, which in general should be 2 years for these infections, was short.
In conclusion, ≥2 doses of dalbavancin is a safe regimen for treating bone and joint infections, and our results show an acceptable success rate that should be confirmed in future prospective studies.
MATERIALS AND METHODS
Study design.
This was an observational and retrospective study in which participant centers were invited to include all patients with a bone or joint infection who had been treated with at least one dose of dalbavancin from March 2016 to November 2017.
The clinical medical records were retrospectively reviewed, and the following variables were collected: age, sex, comorbidities (diabetes mellitus, rheumatoid arthritis, chronic obstructive pulmonary disease, liver cirrhosis, cardiovascular disease, and neoplasia), type of infection (septic arthritis, osteomyelitis, or orthopedic implant-related infection), location of the infection, clinical manifestations (fever, inflammatory signs, ulcer, wound drainage, or fistula), radiology performed for the diagnosis (computer tomography, magnetic resonance, scintigraphy), laboratory tests (leukocyte count, hemoglobin, platelet count, baseline and highest serum creatinine level during dalbavancin treatment, C-reactive protein, erythrosedimentation rate), microbiology data (sample, type of microorganism, and susceptibility pattern), surgical treatment (debridement, implant removal), antibiotic treatment prior to dalbavancin administration, initial dalbavancin and maintenance dose, frequency and number of doses, and potentially related adverse events. Each physician decided the dose and frequency of administration. In cases with a polymicrobial infection, all microorganisms isolated received adequate antibiotic treatment.
Outcome variable definitions.
Treatment success was defined as the absence of clinical signs of infection at the latest medical visit, without the need for additional surgeries or antibiotic treatment for the same infection during or after finishing dalbavancin. The infection was considered improved when no evidence of infection was present at the latest medical visit but suppressive antibiotic therapy was initiated after finishing dalbavancin. Failure was defined as persistent or reappearing signs of infection, the need to perform additional surgeries to control the infection after starting dalbavancin, or occurrence of adverse events requiring the discontinuation of dalbavancin treatment or infection-related death.
Ethics.
The study was approved by our institutional review board, which waived the requirement of informed consent owing to the design of the study.
Statistical analysis.
Variables were gathered in an Excel file, and a descriptive analysis was performed using mean and standard deviation (SD) or median and interquartile range (IQR) as descriptors of the cohort. For the outcome description, patients were divided in two groups, those with an orthopedic implant infection and those with a bone or joint infection, including osteomyelitis, septic arthritis, and spondylodiscitis.
ACKNOWLEDGMENTS
This study was supported by the Bone and Joint Infection Study Group (SGR 253) of the Agència de Gestió d’Ajuts Universitaris I de Recerca (AGAUR) and by the Red Española de Investigación en Patología Infecciosa (REIPI). L. Morata is the recipient of a Rio-Hortega grant (CM 15/00129) from the Instituto de Salud Carlos III.
REFERENCES
- 1.Trouillet-Assant S, Lelièvre L, Martins-Simões P, Gonzaga L, Tasse J, Valour F, Rasigade J-P, Vandenesch F, Muniz Guedes RL, Ribeiro de Vasconcelos AT, Caillon J, Lustig S, Ferry T, Jacqueline C, Loss de Morais G, Laurent F. 2016. Adaptive processes of Staphylococcus aureus isolates during the progression from acute to chronic bone and joint infections in patients. Cell Microbiol 18:1405–1414. doi: 10.1111/cmi.12582. [DOI] [PubMed] [Google Scholar]
- 2.Stoodley P, Nistico L, Johnson S, Lasko L-A, Baratz M, Gahlot V, Ehrlich GD, Kathju S. 2008. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J Bone Joint Surg Am 90:1751–1758. doi: 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Mesy Bentley KL, MacDonald A, Schwarz EM, Oh I. 2018. Chronic osteomyelitis with Staphylococcus aureus deformation in submicron canaliculi of osteocytes: a case report. JBJS Case Connect 8:e8. doi: 10.2106/JBJS.CC.17.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM, Petermann GW, Osmon DR, Infectious Diseases Society of America. 2015. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 61:e26–e46. doi: 10.1093/cid/civ482. [DOI] [PubMed] [Google Scholar]
- 5.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR, Infectious Diseases Society of America. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803.. [DOI] [PubMed] [Google Scholar]
- 6.Ariza J, Cobo J, Baraia-Etxaburu J, Benito N, Bori G, Cabo J, Corona P, Esteban J, Horcajada JP, Lora-Tamayo J, Murillo O, Palomino J, Parra J, Pigrau C, Del Pozo JL, Riera M, Rodríguez D, Sánchez-Somolinos M, Soriano A, Del Toro MD, de la Torre B, Spanish Network for the Study of Infectious Diseases and the Sociedad Española de Enfermedades Infecciosas, Microbiología Clínica (SEIMC). 2017. Executive summary of management of prosthetic joint infections. Clinical practice guidelines by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Enferm Infecc Microbiol Clin 35:189–195. doi: 10.1016/j.eimc.2016.08.012.. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 8.Senneville E, Joulie D, Legout L, Valette M, Dezèque H, Beltrand E, Roselé B, d'Escrivan T, Loïez C, Caillaux M, Yazdanpanah Y, Maynou C, Migaud H. 2011. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis 53:334–340. doi: 10.1093/cid/cir402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sánchez-Somolinos M, Baraia-Etxaburu JM, Rico A, Palomino J, Rodríguez-Pardo D, Horcajada JP, Benito N, Bahamonde A, Granados A, del Toro MD, Cobo J, Riera M, Ramos A, Jover-Sáenz A, Ariza J, Euba G, Cabo X, Pedrero S, Goenaga MÁ, Elola M, Moreno E, García-Ramiro S, Martínez-Pastor JC, Tornero E, García-Lechuz JM, Marín M, Villanueva M, López I, Cisterna R, Santamaría JM, Gómez M-J, Puente A, Cano P, Pigrau C, Sordé R, Flores X, Sorlí L, González-Miguez P, Puig L, Franco M, Jordán M, Coll P, Amador-Mellado J, Fuster-Foz C, García-Paíno L, Nieto I, Muniain MÁ, Suárez A-I, Maseguer MA, Garagorri E, Pintado V, Marinescu C, Ramírez A, Múñez E, Álvarez T, García R, Barcenilla F, Prat L, Pérez F, REIPI Group for the Study of Prosthetic Infection. 2013. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 56:182–194. doi: 10.1093/cid/cis746. [DOI] [PubMed] [Google Scholar]
- 10.Viale P, Furlanut M, Scudeller L, Pavan F, Negri C, Crapis M, Zamparini E, Zuiani C, Cristini F, Pea F. 2009. Treatment of pyogenic (non-tuberculous) spondylodiscitis with tailored high-dose levofloxacin plus rifampicin. Int J Antimicrob Agents 33:379–382. doi: 10.1016/j.ijantimicag.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Tornero E, Morata L, Martínez-Pastor JC, Angulo S, Combalia A, Bori G, García-Ramiro S, Bosch J, Mensa J, Soriano A. 2016. Importance of selection and duration of antibiotic regimen in prosthetic joint infections treated with debridement and implant retention. J Antimicrob Chemother 71:1395–1401. doi: 10.1093/jac/dkv481. [DOI] [PubMed] [Google Scholar]
- 12.Eells SJ, Nguyen M, Jung J, Macias-Gil R, May L, Miller LG. 2016. Relationship between adherence to oral antibiotics and postdischarge clinical outcomes among patients hospitalized with Staphylococcus aureus skin infections. Antimicrob Agents Chemother 60:2941–2948. doi: 10.1128/AAC.02626-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benito N, Franco M, Ribera A, Soriano A, Rodriguez-Pardo D, Sorlí L, Fresco G, Fernández-Sampedro M, Dolores del Toro M, Guío L, Sánchez-Rivas E, Bahamonde A, Riera M, Esteban J, Baraia-Etxaburu JM, Martínez-Alvarez J, Jover-Sáenz A, Dueñas C, Ramos A, Sobrino B, Euba G, Morata L, Pigrau C, Coll P, Mur I, Ariza J, Barcenilla F, Pérez-Villar F, Prats-Gispert L, Cisterna R, Ibarra S, López Í, Santamaría JM, Cabo J, García D, Lora-Tamayo J, Murillo O, Pedrero S, Álvarez-Parrondo S, Muedra-Font R, Raya-Fernández C, Rodríguez-Alonso C, Moreno A, Blanco-Martínez-de-Morentin MA, Cabo-Magadan R, Combalia A, García S, Martínez-Pastor JC, Tornero E, Merino-Pérez J, Montejo JM, Alier A, Horcajada JP, Plasencia V, Puig L, Auñon Á, Blanco A, García-Cañete J, Sandoval E, Fakkas-Fernández M, Garcés-Zarzalejo C, Fariñas-Alvarez C, Fariñas MC, Martinez-Martinez L, Salas-Venero C, Cobo J, Ruiz-Carbajosa P, Jordán M, Crusi X, Marinescu C, Montaner F, Ramírez A, Corona PS, Lung M, Muniain-Ezcurra MÁ, Peñas-Espinar C, Suárez AI, Álvarez R, Cordero J-A, López-Pliego M, Palomino J, Puente A, REIPI (Spanish Network for Research in Infectious Disease) Group for the Study of Prosthetic Joint Infections. 2016. Time trends in the aetiology of prosthetic joint infections: a multicentre cohort study. Clin Microbiol Infect 22:732.e1–8. doi: 10.1016/j.cmi.2016.05.004.. [DOI] [PubMed] [Google Scholar]
- 14.Biedenbach DJ, Bell JM, Sader HS, Turnidge JD, Jones RN. 2009. Activities of dalbavancin against a worldwide collection of 81,673 gram-positive bacterial isolates. Antimicrob Agents Chemother 53:1260–1263. doi: 10.1128/AAC.01453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sader HS, Mendes RE, Duncan LR, Pfaller MA, Flamm RK. 2018. Antimicrobial activity of dalbavancin against Staphylococcus aureus with decreased susceptibility to glycopeptides, daptomycin, and/or linezolid from U.S. medical centers. Antimicrob Agents Chemother 62:e02397-17. doi: 10.1128/AAC.02397-17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson VR, Keating GM. 2008. Dalbavancin. Drugs 68:639–648. discussion 649–51. doi: 10.2165/00003495-200868050-00006. [DOI] [PubMed] [Google Scholar]
- 17.Li HK, Scarborough M, Zambellas R, Cooper C, Rombach I, Walker AS, Lipsky BA, Briggs A, Seaton A, Atkins B, Woodhouse A, Berendt A, Byren I, Angus B, Pandit H, Stubbs D, McNally M, Thwaites G, Bejon P. 2015. Oral versus intravenous antibiotic treatment for bone and joint infections (OVIVA): study protocol for a randomised controlled trial. Trials 16:583. doi: 10.1186/s13063-015-1098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeller V, Dzeing-Ella A, Kitzis M-D, Ziza J-M, Mamoudy P, Desplaces N. 2010. Continuous clindamycin infusion, an innovative approach to treating bone and joint infections. Antimicrob Agents Chemother 54:88–92. doi: 10.1128/AAC.01081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribera E, Pou L, Fernandez-Sola A, Campos F, Lopez RM, Ocaña I, Ruiz I, Pahissa A. 2001. Rifampin reduces concentrations of trimethoprim and sulfamethoxazole in serum in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45:3238–3241. doi: 10.1128/AAC.45.11.3238-3241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandelman K, Zhu T, Fahmi OA, Glue P, Lian K, Obach RS, Damle B. 2011. Unexpected effect of rifampin on the pharmacokinetics of linezolid: in silico and in vitro approaches to explain its mechanism. J Clin Pharmacol 51:229–236. doi: 10.1177/0091270010366445. [DOI] [PubMed] [Google Scholar]
- 21.Pushkin R, Iglesias-Ussel MD, Keedy K, MacLauchlin C, Mould DR, Berkowitz R, Kreuzer S, Darouiche R, Oldach D, Fernandes P. 2016. A randomized study evaluating oral fusidic acid (CEM-102) in combination with oral rifampin compared with standard-of-care antibiotics for treatment of prosthetic joint infections: a newly identified drug-drug interaction. Clin Infect Dis 63:1599–1604. doi: 10.1093/cid/ciw665. [DOI] [PubMed] [Google Scholar]
- 22.Bouza E, Valerio M, Soriano A, Morata L, Carus EG, Rodríguez-González C, Hidalgo-Tenorio MC, Plata A, Muñoz P, Vena A, Alvarez-Uria A, Fernández-Cruz A, Nieto AA, Artero A, Allende JMB, Morell EB, Candel-González FJ, Castelo L, Cobo J, del Carmen Gálvez Contreras M, Fernández RG, Horcajada JP, Guisado-Vasco P, Losa JE, Hervás R, Iftimie SM, Mejías MEJ, Jover F, Ferreiro JLL, Serrano ABL, Malmierca E, Masiá M, Sempere MRO, Nieto AR, Rodriguez-Pardo D, Alvarez SJR, San Juan R, Cepeda CS, Berrocal MAS, Sobrino B, Sorlí L, DALBUSE Study Group (Dalbavancina: Estudio de su uso clinico en España). 2018. Dalbavancin in the treatment of different gram-positive infections: a real-life experience. Int J Antimicrob Agents 51:571–577. doi: 10.1016/j.ijantimicag.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Svetitsky S, Leibovici L, Paul M. 2009. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother 53:4069–4079. doi: 10.1128/AAC.00341-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobudic S, Forstner C, Burgmann H, Lagler H, Ramharter M, Steininger C, Vossen MG, Winkler S, Thalhammer F. 2018. Dalbavancin as primary and sequential treatment for Gram-positive infective endocarditis: 2-year experience at the General Hospital of Vienna. Clin Infect Dis 67:795–798. doi: 10.1093/cid/ciy279. [DOI] [PubMed] [Google Scholar]
- 25.Neudorfer K, Schmidt-Malan SM, Patel R. 2018. Dalbavancin is active in vitro against biofilms formed by dalbavancin-susceptible enterococci. Diagn Microbiol Infect Dis 90:58–63. doi: 10.1016/j.diagmicrobio.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Fernández J, Greenwood-Quaintance KE, Patel R. 2016. In vitro activity of dalbavancin against biofilms of staphylococci isolated from prosthetic joint infections. Diagn Microbiol Infect Dis 85:449–451. doi: 10.1016/j.diagmicrobio.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Dunne MW, Puttagunta S, Sprenger CR, Rubino C, Van Wart S, Baldassarre J. 2015. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother 59:1849–1855. doi: 10.1128/AAC.04550-14. [DOI] [PMC free article] [PubMed] [Google Scholar]