Abstract
Objectives
Emerging studies have demonstrated that microRNAs (miRNAs) play crucial roles in carcinogenesis of many developing human tumours. However, the functions and mechanisms of miR‐297 in lung cancer have, up to now, been largely undefined.
Materials and methods
Here, miR‐297 expression was measured in lung adenocarcinoma tissues and cell lines, using qRT‐PCR. Lung adenocarcinoma cell line was treated with an miR‐297 mimic. MTT and colony analysis were performed to detect cell proliferation and colony formation. The direct target gene of miR‐297 was assessed by qRT‐PCR, Western blotting and luciferase assays.
Results
We demonstrated that miR‐297 expression was upregulated in lung adenocarcinomas compared to adjacent normal tissues. Expression of miR‐297 was also upregulated in tested lung adenocarcinoma cell lines. Ectopic expression of miR‐297 enhanced lung adenocarcinoma cell proliferation and colony formation. Furthermore, overexpression of miR‐297 promoted cell migration and invasion. In addition, we identified Glypican‐5 (GPC5) as a direct target gene of miR‐297 in lung adenocarcinoma cells. Expression of GPC5 was downregulated in both lung adenocarcinoma tissues and cell lines. Moreover, expression of GPC5 was inversely associated with expression of miR‐297 in lung adenocarcinoma tissues.
Conclusions
These results suggest that miR‐297 acted as an oncogenic miRNA, partly by targeting GPC5, adenocarcinoma of the lung.
1. Introduction
Lung cancer is one of the most common malignancies and the leading cause of cancer‐related death worldwide.1, 2, 3, 4 Lung cancer contains four histological types: adenocarcinoma, large cell carcinoma, squamous cell carcinoma, small cell and bronchoalveolar carcinoma.5, 6, 7, 8 Among them, lung adenocarcinoma is the most common type with high metastasis and invasive abilities.9, 10, 11 The 5‐year overall survival rate of lung cancer is about 14%, dependent on histology, cancer stage, general health status and other factors.12, 13, 14 However, no biomarker is available for early lung cancer diagnosis and prognosis. Therefore, it is important to find new biomarkers and targets forearly diagnosis of lung cancer.
MicroRNAs (miRNAs) are a new series of endogenous, conserved and small RNAs that inhibit protein translation through binding to the 3′‐UTR (3′‐untranslated region) of mRNAs (messenger RNAs).15, 16, 17, 18 miRNAs are involved in many processes such as cell proliferation, development, apoptosis, differentiation, inflammation, migration and stress response.11, 13, 19, 20, 21, 22, 23, 24 Increasing studies have demonstrated that miRNAs participate in the development of various tumours such as gastric cancer, hepatocellular carcinoma, gallbladder carcinoma, bladder cancer, cutaneous squamous cell carcinoma and colorectal cancer.25, 26, 27, 28, 29 Therefore, investigations of miRNAs in lung cancer is important to find new diagnostic biomarker for lung cancer diagnosis.
Recently, increasing studies have proved that miR‐297 plays an important role in the tumour development.30, 31, 32 For example, Xu et al.30 demonstrated that the expression of miR‐297 was downregulated in MDR (multidrug resistance) colorectal carcinoma cell line HCT116/L‐OHP compared with its parental cells. Overexpression of miR‐297 suppressed MRP‐2 (MDR‐associated protein 2) expression and sensitized cells to anti‐cancer drugs in MDR colorectal carcinoma cell. Kefas et al.31 showed that miR‐297 was a cytotoxic miRNA with minimal cytotoxicity to normal astrocytes in glioblastoma. However, the role of miR‐297 was still unknown in lung cancer. In this study, we demonstrated that miR‐297 expression was upregulated in lung adenocarcinoma tissues and cell lines. Ectopic expression of miR‐297 promoted the lung adenocarcinoma cell proliferation, migration and invasion. We also identified Glypican‐5 (GPC5) as a direct target gene of miR‐297 in lung cancer.
2. Materials and methods
2.1. Clinical tissues and cell culture and transfection
Thirty‐five lung adenocarcinoma tissues and their adjacent non‐tumour tissues were collected from patients who underwent surgery in our hospital. This experiment was approved by the Ethical Committee of Hebei province Cangzhou Hospital of integrated traditional and Western Medicine and was in accordance with the Helsinki Declaration. Tissue was promptly frozen in the liquid nitrogen. No patients underwent chemotherapy or radiotherapy before surgery. Written informed consent from patient was obtained. Lung adenocarcinoma cell lines (H1299, SPC‐A1, H23 and A549) and one bronchial epithelial cell line (16HBE) were collected from Cell Resource of CAMS (Chinese Academy of Medical Sciences) (Beijing, China) and cultured in the RPMI 1640 medium. miR‐297 mimic and scramble oligoribonucleotides and GPC5 vector was synthesized from RiboBio (Guangzhou, China). Cell transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer's protocol.
2.2. Real‐time quantitative PCR
Total RNA from samples or cells was extracted using TRIzol (Invitrogen) according to the manufacturer's protocol. The miR‐297 and the GPC5 expression were measured by real‐time quantitative PCR (RT‐qPCR) according to the previous protocol. The following primers were used in this study: GAPDH Forward: 5′‐AATGGGCAGCCGTTAGGAAA‐3′; Reverse: 5′‐TGAAGGGGTCATTGATGGCA‐3′, GPC5 Forward: 5′‐AGACCACCACAAGGAACAGTG‐3′; Reverse: 5′‐AGACTGGGCTTTGATTCCATT‐3′. miR‐297 expression was normalized to RNU6B. GPC5 expression was normalized to GAPDH.
2.3. Western blot
The protein from cell or tissues was extracted using BCA kit. Total proteins were separated using 12% SDS‐PAGE and transferred to the PVDF membranes (Millipore, Boston, MA, USA). The membrane was blocked with non‐fat milk and then incubated with primary antibody. The primary antibody was used as following: GPC5, GAPDH (Sigma, St. Louis, MO, USA). Detection of GAPDH was used as the loading control.
2.4. Scratch‐wound assays and invasion analysis
Cell migration was measured by the scratch‐wound analysis. The scratch was created by a sterile pipette tip. The wound was washed for three times to remove the debris cell. The cells were continuing cultured for another 24 or 48 hours. The wound width was measured by using microscope (Nikon, Tokyo, Japan).
2.5. Cell proliferation and colony formation
Cell proliferation was detected using MMT following the manufacturer's protocol. Cells were cultured into 96‐well plate after transfection for 24, 48 and 72 hours. The absorbance was determined at a 450 nm wavelength. For cell colony formation assay, cells were cultured in the 12‐well plate after transfection for 2 weeks. The colonies were detected with crystal violet and counted (Sigma‐Aldrich).
2.6. Luciferase assays
Cells were cultured in the 24‐well plates and were cotransfected with miR‐297 mimic or scramble and Renilla vector, wild‐type or mutant 3′ UTR of GPC5 luciferase reporter. The luciferase assay was performed as previously described. Luciferase activity was detected 24 hours after transfection.
2.7. Statistical analysis
Results are shown as the means ± SD (standard deviation). ANOVA test was used to measure the difference between more than two groups and Student's t‐test was used to detect the difference between two groups. Differences were shown significant when P<.05.
3. Results
3.1. miR‐297 expression was upregulated in lung adenocarcinoma tissues
As shown in Fig. 1a, miR‐297 expression was upregulated in lung adenocarcinoma tissues compared with the adjacent normal tissues. Among them, miR‐297 expression was higher in 26 lung adenocarcinoma tissues (74%, 26/35) than in normal tissues (Fig. 1b). In addition, miR‐297 expression in the metastatic lymph node was higher in lung adenocarcinoma tissues than in the adjacent normal tissues (Fig. 1c).
Figure 1.
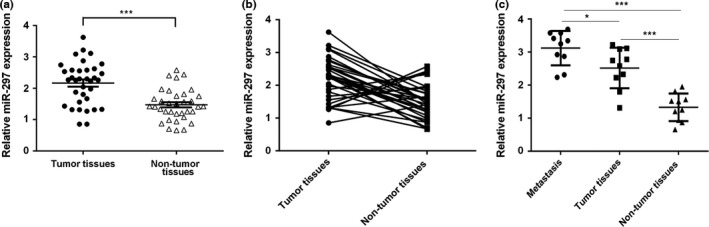
miR‐297 expression is upregulated in lung adenocarcinoma tissues. (a) The expression of miR‐297 was measured by qRT‐PCR. (b) Among them, miR‐297 expression was higher in 26 lung adenocarcinoma tissues (74%, 26/35) than in normal tissues. (c) miR‐297 expression in the metastatic lymph node was higher compared to lung adenocarcinoma tissues and adjacent normal tissues. *P<.05 and ***P<.001
3.2. miR‐297 enhanced lung adenocarcinoma cell proliferation and colony formation
miR‐297 expression was upregulated in the lung adenocarcinoma cell lines (H1299, SPC‐A1, H23 and A549) compared with one bronchial epithelial cell line (16HBE) (Fig. 2a). miR‐297 expression was obviously enhanced in the A549 cells after transfection with miR‐297 mimics compared with transfection with scramble (Fig. 2b). Ectopic expression of miR‐297 enhanced the cell proliferation of A549 cells (Fig. 2c). Meanwhile, overexpression of miR‐297 promoted the cyclin D1 expression in the A549 cells (Fig. 2d,e). Ectopic expression of miR‐297 promoted cell colony information in the A549 cells (Fig. 2f).
Figure 2.
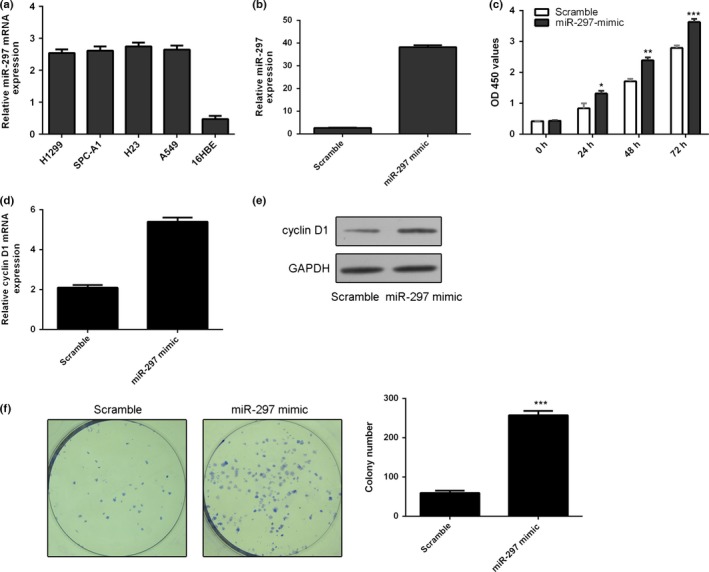
miR‐297 enhances lung adenocarcinoma cell proliferation and colony formation. (a) The expression of miR‐297 in the lung adenocarcinoma cell lines (H1299, SPC‐A1, H23 and A549) and one bronchial epithelial cell line (16HBE) was measured by qRT‐PCR. (b) The expression of miR‐297 in the A549 cells transfected with miR‐297 mimic was detected using qRT‐PCR. (c) Ectopic expression of miR‐297 enhanced the A549 cells proliferation. (d) Overexpression of miR‐297 promoted the cyclin D1 mRNA expression. (e) The protein expression of cyclin D1 was measured by Western blot. (f) Ectopic expression of miR‐297 promoted cell colony information in the A549 cells. *P<.05, **P<.01 and ***P<.001
3.3. miR‐297 promoted lung adenocarcinoma cell migration and invasion
Cell invasion and migration are two important processes of tumour metastasis. Therefore, we measured the invasive and migrative ability of lung adenocarcinoma cells transfected with miR‐297 mimic and found that the A549 cells transfected with miR‐297 mimic presented less migrative and invasion ability than those with control miRNA. As shown in the Fig. 3a, invasion assays showed that ectopic expression of miR‐297 promoted the A549 cells invasion. In addition, overexpression of miR‐297 increased the A549 cells migration (Fig. 3b).
Figure 3.
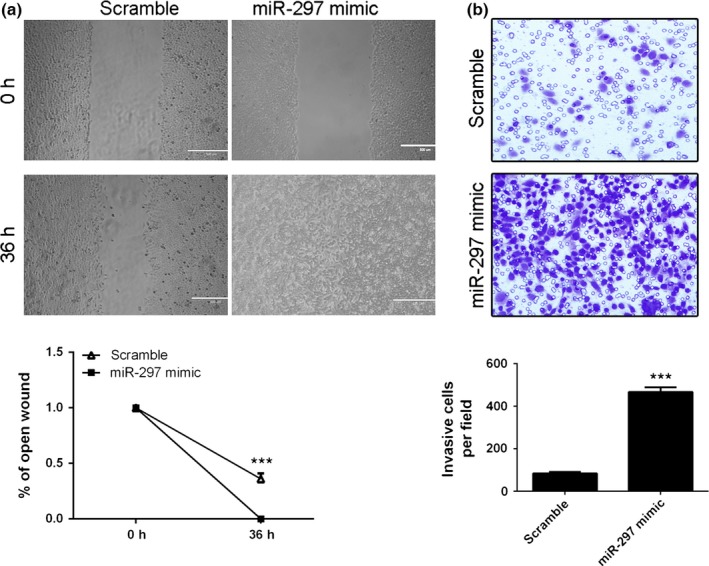
miR‐297 promotes lung adenocarcinoma cell migration and invasion. (a) Overexpression of miR‐297 promoted the A549 cells invasion. (b) miR‐297 overexpression increased the A549 cells migration. ***P<.001
3.4. GPC5 was a direct target of miR‐297
We found the potential targets of miR‐297 using TargetScan. The 3′UTR of GPC5 mRNA contained a potential site for the seed region of miR‐297 (Fig. 4a). As shown in Fig. 4b, ectopic expression of miR‐297 inhibited the luciferase activity of GPC5 3′UTR by about 60% in the A549 cells (Fig. 4b). Overexpression of miR‐297 suppressed the expression of GPC5 in the A549 cells (Fig. 4c,d). The expression of GPC5 was lower than in the lung adenocarcinoma cell lines (H1299, SPC‐A1, H23 and A549) compared with 16HBE (Fig. 4e,f).
Figure 4.
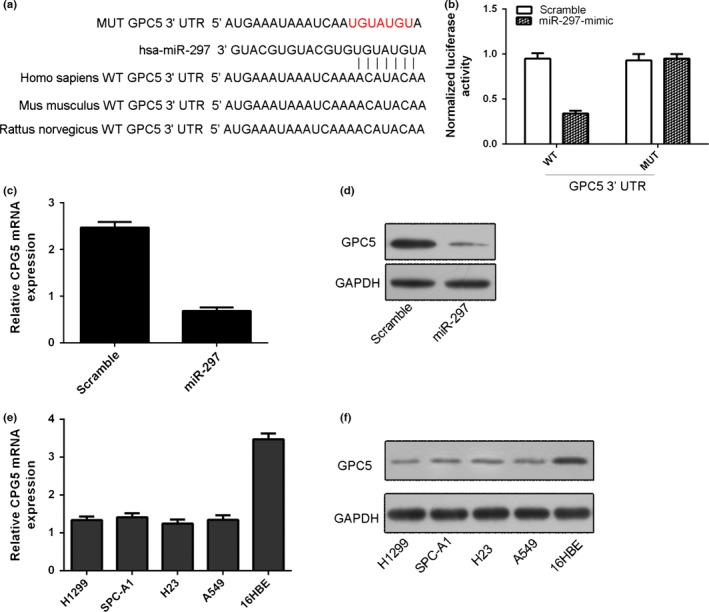
GPC5 is a direct target of miR‐297. (a) The 3′UTR of GPC5 mRNA contained a potential site for the seed region of miR‐297 using TargetScan. (b) Ectopic expression of miR‐297 inhibited the luciferase activity of GPC5 3′UTR by about 60% in the A549 cells by using Luciferase reporter analysis. (c) Overexpression of miR‐297 inhibited the GPC5 mRNA in the A549 cells. (d) Overexpression of miR‐297 inhibited the GPC5 protein expression in the A549 cells. (e) The mRNA expression of GPC5 in the lung adenocarcinoma cell lines (H1299, SPC‐A1, H23 and A549) and one bronchial epithelial cell line (16HBE) was measured by qRT‐PCR. (f) The protein expression of GPC5 in the lung adenocarcinoma cell lines (H1299, SPC‐A1, H23 and A549) and one bronchial epithelial cell line (16HBE) was measured by Western blot
3.5. The expression of GPC5 was inversely correlated with the expression of miR‐297 in the lung adenocarcinoma tissues
GPC5 expression was downregulated in the lung adenocarcinoma tissues compared with the adjacent normal tissues (Fig. 5a). Among them, GPC5 expression was lower in 26 lung adenocarcinoma tissues (77%, 27/35) than in the normal tissues (Fig. 5b). Furthermore, the expression level of GPC5 was inversely associated with that in the lung adenocarcinoma tissues (Fig. 5c).
Figure 5.
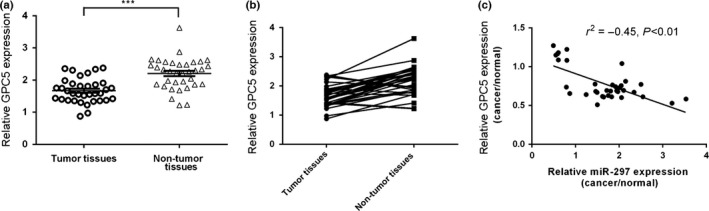
The expression of GPC5 was inversely correlated with the expression of miR‐297 in the lung adenocarcinoma tissues. (a) The expression of GPC5 was measured by qRT‐PCR in the lung adenocarcinoma tissues and adjacent normal tissues. (b) Among them, GPC5 expression was lower in 26 lung adenocarcinoma tissues (77%, 27/35) than normal tissues. (c) The expression of GPC5 was inversely associated with the expression of miR‐297 in the lung adenocarcinoma tissues. ***P<.001
3.6. Overexpression of GPC5 abrogated miR‐297‐induced cell proliferation and invasion
The protein expression of GPC5 was overexpressed after treatment with pcDNA‐GPC5 in the A549 cells (Fig. 6a). We next treated the miR‐297‐overexpression A549 cells with pcDNA‐GPC5 or control. Overexpression of GPC5 abrogated miR‐297‐induced cell growth (Fig. 6b). Moreover, ectopic expression of GPC5 inhibited the miR‐297‐induced cell invasion (Fig. 6c,d).
Figure 6.
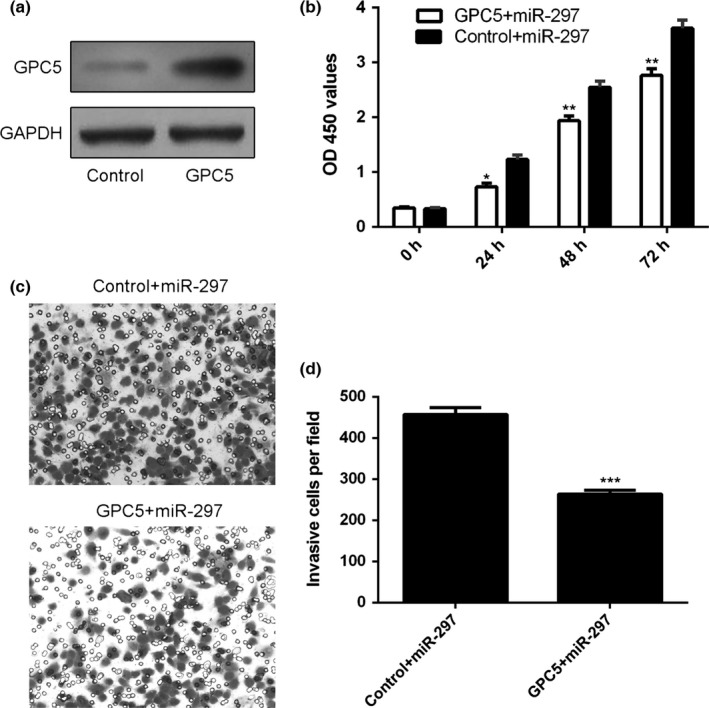
Overexpression of GPC5 abrogated miR‐297‐induced cell proliferation and invasion. (a) The protein expression of GPC5 in the A549 cells was detected by Western blot. (b) The A549 cells proliferation was measured by MMT analysis. (c) Ecotpic expression of GPC5 inhibited the miR‐297‐induced cell invasion. (d) Relative ratio of invasive cells per field is shown. *P<.05, **P<.01 and ***P<.001
4. Discussion
In this study, we demonstrated that miR‐297 expression was upregulated in lung adenocarcinoma tissues compared with the adjacent normal tissues. In addition, miR‐297 expression was also upregulated in lung adenocarcinoma cell lines. Ectopic expression of miR‐297 enhanced cell proliferation and colony formation in lung adenocarcinoma. Furthermore, overexpression of miR‐297 promoted lung adenocarcinoma cell migration and invasion. We identified GPC5 as a direct target gene of miR‐297 in the lung adenocarcinoma cell. The expression of GPC5 was downregulated in the lung adenocarcinoma tissues compared with the adjacent normal tissues. Moreover, the expression of GPC5 was inversely associated with the expression of miR‐297 in the lung adenocarcinoma tissues. These results suggested that miR‐297 acted as an oncogenic miRNA partly by targeting GPC5 expression in lung adenocarcinoma.
Recently, increasing evidences have showed that miR‐297 plays an important role in the development of tumour.30, 31, 32 For example, Xu et al.30 demonstrated that the expression of miR‐297 was downregulated in MDR (multidrug resistance) colorectal carcinoma cell line HCT116/L‐OHP compared with its parental cells. Overexpression of miR‐297 suppressed MRP‐2 (MDR‐associated protein 2) expression and sensitized cells to anti‐cancer drugs in MDR colorectal carcinoma cell. Kefas et al.31 showed that miR‐297 was a cytotoxic miRNA with minimal cytotoxicity to normal astrocytes in glioblastoma. Overexpression of miR‐297 suppressed the glioblastoma cell invasion and tumour formation by targeting DGK‐α. However, the role of miR‐297 was still unknown in lung cancer. In this study, we found that miR‐297 expression was upregulated in lung adenocarcinoma tissues compared with the adjacent normal tissues. Among them, miR‐297 expression was higher in 26 lung adenocarcinoma tissues (74%, 26/35) than in normal tissues. Moreover, the expression of mir‐297 was higher in the metastatic lymph node than that in lung adenocarcinoma tissues and adjacent normal tissues. In addition, ectopic expression of miR‐297 promoted the lung adenocarcinoma cell proliferation, migration and invasion.
Glypicans (GPCs) are a class of HSPGs (heparan sulphate proteoglycans) that bind to the plasma membrane by a GPI (glycosyl‐phosphatidylinositol) anchor.33, 34 Increasing evidences have demonstrated that GPCs, especially GPC5 and GPC3, act a crucial role in the development of various tumours.35, 36, 37 Recent study has demonstrated that GPC5 plays important roles in the progression of lung cancer.38, 39, 40 For example, Li et al.41 found that GPC5 expression was upregulated in non‐small cell lung cancer (NSCLC) cell lines and overexpression of GPC5 promoted the NSCLC cell migration and invasion. Moreover, GPC5 expression was upregulated in NSCLC tissues and the GPC5 expression level was correlated with poor differentiation, metastasis, vascular invasion and TNM stage. However, Zhao et al.40 demonstrated that GPC5 expression was downregulated in lung adenocarcinoma cell and inhibition of miR___620 suppressed the lung adenocarcinoma cell migration, proliferation and invasion by inhibiting the expression of GPC5. Furthermore, Zhang et al.39 showed that GPC5 expression was upregulated in SACC‐M (high lung‐metastatic cell line) compared with SACC‐2 and SACC‐83 (low lung‐metastatic cell lines) cells. In line with these results, we also found that GPC5 expression was downregulated in the lung adenocarcinoma tissues compared with the adjacent normal tissues. We identified GPC5 was a direct target gene of miR‐297 in lung adenocarcinoma cell. Moreover, the expression of GPC5 was inversely associated with the expression of miR‐297 in the lung adenocarcinoma tissues.
In conclusion, our results demonstrated that miR‐297 expression was upregulated in lung adenocarcinoma tissues and cell lines. Ectopic expression of miR‐297 promoted the lung adenocarcinoma cell proliferation, migration and invasion. We also identified that GPC5 was a direct target gene of miR‐297 in lung cancer. These data suggested that miR‐297 played an oncogenic role in the development and progression of lung adenocarcinoma.
References
- 1. Zhang J, Xu L, Yang Z, et al. MicroRNA‐10b indicates a poor prognosis of non‐small cell lung cancer and targets E‐cadherin. Clin Transl Oncol. 2015;17:209–214. doi: 10.1007/s12094-014-1213-7. [DOI] [PubMed] [Google Scholar]
- 2. Zhang L, Xu B, Qiang Y, et al. Overexpression of deubiquitinating enzyme USP28 promoted non‐small cell lung cancer growth. J Cell Mol Med. 2015;19:799–805. doi: 10.1111/jcmm.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao C, Xu Y, Zhang Y, et al. Downregulation of miR‐145 contributes to lung adenocarcinoma cell growth to form brain metastases. Oncol Rep. 2013;30:2027–2034. doi: 10.3892/or.2013.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu SH, Zhang CL, Dong FS, Zhang YM. miR‐99a suppresses the metastasis of human non‐small cell lung cancer cells by targeting AKT1 signaling pathway. J Cell Biochem. 2015;116:268–276. doi: 10.1002/jcb.24965. [DOI] [PubMed] [Google Scholar]
- 5. Yu T, Li J, Yan M, et al. MicroRNA‐193a‐3p and ‐5p suppress the metastasis of human non‐small‐cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene. 2014;34:413–423. doi: 10.1038/onc.2013.574. [DOI] [PubMed] [Google Scholar]
- 6. Yu L, Todd NW, Xing L, et al. Early detection of lung adenocarcinoma in sputum by a panel of microRNA markers. Int J Cancer. 2010;127:2870–2878. doi: 10.1002/ijc.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ye XW, Yu H, Jin YK, et al. miR‐138 inhibits proliferation by targeting 3‐phosphoinositide‐dependent protein kinase‐1 in non‐small cell lung cancer cells. Clin Resp J. 2015;9:27–33. doi: 10.1111/crj.12100. [DOI] [PubMed] [Google Scholar]
- 8. Yang Y, Liu L, Zhang Y, et al. MiR‐503 targets PI3K p85 and IKK‐beta and suppresses progression of non‐small cell lung cancer. Int J Cancer. 2014;135:1531–1542. doi: 10.1002/ijc.28799. [DOI] [PubMed] [Google Scholar]
- 9. Wang H, Zhu LJ, Yang YC, Wang ZX, Wang R. MiR‐224 promotes the chemoresistance of human lung adenocarcinoma cells to cisplatin via regulating G(1)/S transition and apoptosis by targeting p21(WAF1/CIP1). Br J Cancer. 2014;111:339–354. doi: 10.1038/bjc.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vucic EA, Thu KL, Pikor LA, et al. Smoking status impacts microRNA mediated prognosis and lung adenocarcinoma biology. BMC Cancer. 2014;14:778. doi: 10.1186/1471-2407-14-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song Q, Xu Y, Yang C, et al. miR‐483‐5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014;74:3031–3042. doi: 10.1158/0008-5472.CAN-13-2193. [DOI] [PubMed] [Google Scholar]
- 12. Skrzypski M, Czapiewski P, Goryca K, et al. Prognostic value of microRNA expression in operable non‐small cell lung cancer patients. Br J Cancer. 2014;110:991–1000. doi: 10.1038/bjc.2013.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothschild SI, Tschan MP, Federzoni EA, et al. MicroRNA‐29b is involved in the Src‐ID1 signaling pathway and is dysregulated in human lung adenocarcinoma. Oncogene. 2012;31:4221–4232. doi: 10.1038/onc.2011.578. [DOI] [PubMed] [Google Scholar]
- 14. Mataki H, Seki N, Chiyomaru T, et al. Tumor‐suppressive microRNA‐206 as a dual inhibitor of MET and EGFR oncogenic signaling in lung squamous cell carcinoma. Int J Oncol. 2015;46:1039–1050. doi: 10.3892/ijo.2014.2802. [DOI] [PubMed] [Google Scholar]
- 15. Yu X, Li Z, Shen J, et al. MicroRNA‐10b promotes nucleus pulposus cell proliferation through RhoC‐Akt pathway by targeting HOXD10 in intervetebral disc degeneration. PLoS ONE. 2013;8:e83080. doi: 10.1371/journal.pone.0083080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Yu X, Li Z. MicroRNA expression and its implications for diagnosis and therapy of tongue squamous cell carcinoma. J Cell Mol Med. 2015;20:10–16. doi: 10.1111/jcmm.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Z, Yu X, Shen J, Liu Y, Chan MT, Wu WK. MicroRNA dysregulation in rhabdomyosarcoma: a new player enters the game. Cell Prolif. 2015;48:511–516. doi: 10.1111/cpr.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen HY, Lin YM, Chung HC, et al. miR‐103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4. Cancer Res. 2012;72:3631–3641. doi: 10.1158/0008-5472.CAN-12-0667. [DOI] [PubMed] [Google Scholar]
- 19. Wan L, Zhang L, Fan K, Wang J. miR‐27b targets LIMK1 to inhibit growth and invasion of NSCLC cells. Mol Cell Biochem. 2014;390:85–91. doi: 10.1007/s11010-013-1959-1. [DOI] [PubMed] [Google Scholar]
- 20. Shan N, Shen L, Wang J, He D, Duan C. MiR‐153 inhibits migration and invasion of human non‐small‐cell lung cancer by targeting ADAM19. Biochem Biophys Res Commun. 2015;456:385–391. doi: 10.1016/j.bbrc.2014.11.093. [DOI] [PubMed] [Google Scholar]
- 21. Liu C, Yang H, Xu Z, et al. microRNA‐548l is involved in the migration and invasion of non‐small cell lung cancer by targeting the AKT1 signaling pathway. J Cancer Res Clin Oncol. 2015;141:431–441. doi: 10.1007/s00432-014-1836-7. [DOI] [PubMed] [Google Scholar]
- 22. Kong D, Piao YS, Yamashita S, et al. Inflammation‐induced repression of tumor suppressor miR‐7 in gastric tumor cells. Oncogene. 2012;31:3949–3960. doi: 10.1038/onc.2011.558. [DOI] [PubMed] [Google Scholar]
- 23. Li K, Du Y, Jiang BL, He JF. Increased microRNA‐155 and decreased microRNA‐146a may promote ocular inflammation and proliferation in Graves' ophthalmopathy. Med Sci Monit. 2014;20:639–643. doi: 10.12659/MSM.890686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li H, Yang BB. Stress response of glioblastoma cells mediated by miR‐17‐5p targeting PTEN and the passenger strand miR‐17‐3p targeting MDM2. Oncotarget. 2012;3:1653–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Z, Lei H, Luo M, et al. DNA methylation downregulated mir‐10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54. doi: 10.1007/s10120-014-0340-8. [DOI] [PubMed] [Google Scholar]
- 26. Li Z, Yu X, Wang Y, et al. By downregulating TIAM1 expression, microRNA‐329 suppresses gastric cancer invasion and growth. Oncotarget. 2015;6:17559–17569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han Y, Liu Y, Zhang H, et al. Hsa‐miR‐125b suppresses bladder cancer development by down‐regulating oncogene SIRT7 and oncogenic long noncoding RNA MALAT1. FEBS Lett. 2013;587:3875–3882. doi: 10.1016/j.febslet.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 28. Yu X, Li Z. The role of miRNAs in cutaneous squamous cell carcinoma. J Cell Mol Med. 2016;20:3–9. doi: 10.1111/jcmm.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang L, Pickard K, Jenei V, et al. miR‐153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Cancer Res. 2013;73:6435–6447. doi: 10.1158/0008-5472.CAN-12-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu K, Liang X, Shen K, et al. miR‐297 modulates multidrug resistance in human colorectal carcinoma by down‐regulating MRP‐2. Biochem J. 2012;446:291–300. doi: 10.1042/BJ20120386. [DOI] [PubMed] [Google Scholar]
- 31. Kefas B, Floyd DH, Comeau L, et al. A miR‐297/hypoxia/DGK‐alpha axis regulating glioblastoma survival. Neuro‐oncology. 2013;15:1652–1663. doi: 10.1093/neuonc/not118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fang Z, Xu C, Li Y, et al. A feed‐forward regulatory loop between androgen receptor and PlncRNA‐1 promotes prostate cancer progression. Cancer Lett. 2016;374:62–74. doi: 10.1016/j.canlet.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 33. Filmus J, Capurro M. The role of glypicans in Hedgehog signaling. Matrix Biol. 2014;35:248–252. doi: 10.1016/j.matbio.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 34. De Robertis M, Arigoni M, Loiacono L, et al. Novel insights into Notum and glypicans regulation in colorectal cancer. Oncotarget. 2015;6:41237–41257. doi: 10.18632/oncotarget.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Veugelers M, Vermeesch J, Reekmans G, Steinfeld R, Marynen P, David G. Characterization of glypican‐5 and chromosomal localization of human GPC5, a new member of the glypican gene family. Genomics. 1997;40:24–30. doi: 10.1006/geno.1996.4518. [DOI] [PubMed] [Google Scholar]
- 36. Li F, Shi W, Capurro M, Filmus J. Glypican‐5 stimulates rhabdomyosarcoma cell proliferation by activating Hedgehog signaling. J Cell Biol. 2011;192:691–704. doi: 10.1083/jcb.201008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luo C, Shibata K, Suzuki S, et al. GPC3 expression in mouse ovarian cancer induces GPC3specific T cell‐mediated immune response through M1 macrophages and suppresses tumor growth. Oncol Rep. 2014;32:913–921. doi: 10.3892/or.2014.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Y, Yang P. GPC5 gene and its related pathways in lung cancer. J Thorac Oncol. 2011;6:2–5. doi: 10.1097/JTO.0b013e3181fd6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Wang J, Dong F, Li H, Hou Y. The role of GPC5 in lung metastasis of salivary adenoid cystic carcinoma. Arch Oral Biol. 2014;59:1172–1182. doi: 10.1016/j.archoralbio.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 40. Zhao Z, Han C, Liu J, Wang C, Wang Y, Cheng L. GPC5, a tumor suppressor, is regulated by miR‐620 in lung adenocarcinoma. Mol Med Rep. 2014;9:2540–2546. doi: 10.3892/mmr.2014.2092. [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Miao L, Cai H, et al. The overexpression of glypican‐5 promotes cancer cell migration and is associated with shorter overall survival in non‐small cell lung cancer. Oncol Lett. 2013;6:1565–1572. doi: 10.3892/ol.2013.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]


