Abstract
Due to an increasing number of skin diseases as a result of exposure to ultraviolet (UV) radiation, it is necessary to evaluate the effectiveness of new skin care formulations with broad‐spectrum sunscreens.
Objectives: This study aims to assess the status of nerve fibres in healthy human skin, to quantify effects of UV radiation on nerve endings, and to evaluate neuroprotective effects of new skin care formulations against UV exposure damage.
Methods: Samples were obtained from 34 female patients enrolled for plastic surgery and were immediately treated (10 min) with three emulsions: Cream 1, Cream 2 (placebo) and a sunscreen with sun protection factor 15 (SPF15). Control samples and those treated with the cream emulsions were exposed to UVA and UVB for 60 min. Nerve fibres were identified by immunofluorescence using a monoclonal antibody (anti‐human CD56/NCAM). Cell damage was assessed by image analysis.
Results: Several cellular nervous structures were identified in the skin samples, including free nerve endings. UVA and UVB significantly decreased (40–60%) density of nerve endings in the control samples and those treated with placebo (Cream 2) or SPF15 (all P < 0.001). Cream 1 completely blocked effects of UV radiation on nerve endings (P > 0.05 vs. control).
Conclusions: Quantification of cell damage induced by UV radiation provides useful information for identification of new skin care compounds with neuroprotective properties.
Introduction
Solar ultraviolet (UV) radiation is one of the most powerful natural physical carcinogens, able to disrupt integrity of the skin. It is responsible for chronic inflammation, skin ageing and development of skin cancer. UV radiation penetrates the epidermis and induces a variety of biological effects in nervous system components of the skin (1). The dermis is linked to the central nervous system through both autonomic (sympathetic) and sensory peripheral innervation. Sensory innervation is derived from neurons located in the dorsal root ganglia; sympathetic innervation is provided by peripheral fibres of post‐ganglionic sympathetic neurons. The free nerve endings of these fibres project into the epidermis, where they come into close contact with keratinocytes, melanocytes and Langerhans cells (2). Further characterization of these skin sensory structures will lead to better understanding of physiological and pathological conditions related to UV exposure (3).
Changes in skin nerve endings have been related to neuropathies and other skin‐related diseases. Chronic UV exposure is a major environmental factor that promotes skin ageing (photo‐ageing and/or extrinsic ageing). This form of photo‐ageing overlaps chronological ageing and can be easily compared to body areas that remain protected from solar exposure (1). Although significant progress has been made in understanding the mechanisms involved in intrinsic and extrinsic ageing (4), effects of UV radiation on dermal nerve fibres remain largely unknown.
There is also a growing need for development of new techniques to quantify cell damage produced by UV exposure. Previous studies have investigated cutaneous changes produced by UV exposure, by qualitative methods. For instance, application of preparations which have a sun protection factor (SPF) is the most common qualitative method used by the cosmetics industry and measures required time to develop erythema. Recently, immunohistochemistry and immunofluorescence techniques have been developed for identification of intra‐epidermal nerve fibres (2, 3, 5). These methods are specific for study of peripheral nerve fibres, both in health and in pathological conditions (6). In this study, we have investigated free nerve endings in healthy human skin, quantified the effects of UV radiation on nerve endings, and evaluated neuroprotective effects of new skin care formulations against UV irradiation.
Materials and methods
Subjects
Thirty‐four female patients (all Caucasian), with a mean age 33 ± 12 years, were recruited from the Plastic Surgery Unit of the Hospital São Lucas (Porto Alegre, Brazil). All patients underwent cosmetic plastic surgery to reduce either breast (mammoplasty; n = 15) or abdomen (abdominoplasty; n = 19). Written informed consent was presented to and signed by all patients. The investigation was approved by the Ethics Committee of the Pontifical Catholic University of Rio Grande do Sul (PUCRS).
Exclusion criteria included participation in indoor tanning over the last 6 months, diabetes, any neoplasia or infection, or chronic inflammatory disease (e.g. arthritis, lupus, psoriasis, pityriasis, vitiligo).
Skin biopsies
Biopsies consisted of skin fragments of approximately 7 cm2. Tissues were divided into smaller pieces (1 cm2) to be used for all treatments, at room temperature. They were immersed in saline solution following the surgical interventions then were immediately taken to the laboratory for processing.
Skin care formulations
Skin care formulations used were Cream 1, Cream 2 (placebo) and a commercially available SPF 15 sunscreen (SPF15). Cream 1 and Cream 2 were supplied by O Boticário® (São José dos Pinhais, Brazil) and consisted of complex mixtures with several bioactive compounds that were only added to the test cream, including ethylhexyl methoxycinnamate, diethylamino hydroxybenzoyl hexyl benzoate, ethylhexyl triazone, Hypnea musciformis extracts, Gellidiela acerosa, Cucumis sativa, Citrus unshiu, Peg‐6 isostearate, hesperetin laurate, sodium ascorbyl phosphate, retinyl palmitate and tocopheryl acetate. Emulsions were gently applied manually to the skin samples (2 mg/cm2) in Petri dishes containing saline (that did not cover up the samples), 10 min prior to UV exposure.
UV irradiation
Samples were left untreated, treated with emulsions only (Cream 1, Cream 2 or SPF 15), exposed to UVA or UVB, or pretreated with emulsions (Cream 1, Cream 2 or SPF 15) and then exposed to UVA or UVB, ex vivo. UVA and UVB 45 cm lamps (Phillips, 15 W Sylvania Co., Germany) were used to irradiate the skin samples for 60 min, in sterile conditions, provided in a laminar flow cabinet. Untreated samples were left untouched (in saline) on the bench for the same period of time. Irradiances were duly measured before treatments (2.18 ± 0.10 W/m2– UVA; 2.48 ± 0.11 W/m2– UVB). These measurements were performed using a spectro‐radiometer, by the Radiation Physics Group of the Faculty of Physics of PUCRS.
Immunofluorescence
Skin samples were treated with 99% isopentane (Vetec Química Fina Ltd., Rio de Janeiro, Brazil) and were frozen in liquid nitrogen for 10 s. Four 15‐µm longitudinal sections of epidermis per treatment were cut using a cryostat (Cryotome SME, Shandon Scientific Ltd., Astmoor, WA7 1PR, UK). Sections were heated on a hot plate at 42 °C for 5 min, and fixed in acetone for 10 min. At this point, the sections could be either used or frozen at –20 °C. Frozen sections were warmed up at room temperature for 10 min in a moist chamber (Erviegas, São Paulo, Brazil), followed by rehydration in phosphate‐buffered saline (PBS) for 10 min. Fc blocking (solution of 10% sheep serum, 10% human serum in PBS) was performed for 20 min. Sections were washed (×4) in PBS and incubated overnight with primary antibody [anti‐CD56 (neural‐cell adhesion molecule or NCAM), FK Biotec, Porto Alegre, Brazil] diluted 1 : 200 in Fc block. NCAM is expressed on both skin unmyelinated fibres and non‐myelinating Schwann cells (7, 8). Samples were washed (×4) in PBS and were incubated in secondary antibody [goat anti‐mouse immunoglobulin G labelled with fluorescein isothiocyanate (FITC); Sigma, St. Louis, MO, USA] diluted 1 : 50 in Fc block, for 30 min. Counterstaining was performed with DAPI (1 : 2000 in PBS) for 2 min. Sections were mounted in Vectasheild (Vector Labs, Burlingame, CA, USA) for analysis using a fluorescence microscope (Zeiss Axioskop 40, Carl Zeiss do Brasil Ltd., São Paulo, Brazil).
Image analysis
Five fields (×100) per treatment were captured using the fluorescence microscope (Zeiss Axioskop 40) equipped with an Image Pro Plus Capture Kit digital camera (Media Cybernetics, Silver Spring, MD, USA). Cells were located using excitation in blue (488‐nm) light, and images were sequentially acquired using selective FITC (green) and DAPI (blue) filter blocks. Images were captured with Image Pro‐plus 4.5 software (Media Cybernetics). Acquisition time was fixed by the software and time from cell localization to initiation of image capture was less than 5 s, to minimize bleaching effects. No fluorescence bleaching was observed during the acquisitions. Density of nerve endings was evaluated by automatic pixel selection (colour range function, Adobe Photoshop CS3) on the acquired images. Results are expressed as total number of green pixels (stained neurofilaments). All images had spatial resolution of 4 pixel per µm2. Evaluation of damage was performed in a double‐blind fashion: the investigator responsible for analysing the images was not aware of treatments used on the various skin specimens.
Statistical analysis
Variables were tested for normality of distribution by the Kolmogorov–Smirnov test. Data were analysed by factorial analysis of variance, including three levels for the variable cream (absent, Cream 1 and Cream 2) and three levels for irradiation (absent, UVA and UVB). Differences between treatments were investigated by Tukey contrast analysis, with significance of α = 95%. Data are presented as mean ± standard error and analysed using SPSS 15.0 software (SPSS Inc., Chicago, IL, USA).
Results
In this study, it was possible to identify various NCAM‐positive cutaneous nerve fibres, generally responsible for sensory stimulation. Neural structures identified during fluorescence microscopy were of free and diffused nerve endings, and innervation of hair follicles and sebaceous glands was apparent (Fig. 1). The NCAM staining pattern corresponds to distribution of sensory fibres (free nerve endings and hair follicles) as well as autonomic sympathetic nervous system of the skin (for sebaceous glands and blood vessels). These findings are clearly in line with previous studies that identified skin nerve fibres with anti‐NCAM (3,7,8) or anti‐PGP 9.5 antibodies (3).
Figure 1.
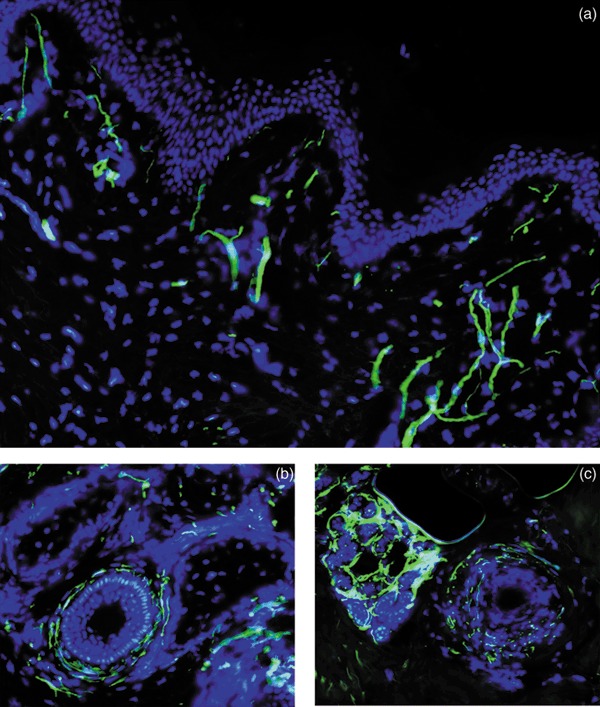
Nerve endings in human skin. Free nerve endings in human skin (a), hair follicle innervation (b) and sebaceous glands (c) were identified by fluorescence microscopy (green, FITC, anti‐NCAM). Nuclei stained with DAPI (blue). Magnification ×200.
We then quantified effects of UV radiation on NCAM‐positive densities and assessed neuroprotective effects of new skin care formulations. As shown in Fig. 3, UVA or UVB significantly reduced (40–60%) nerve ending densities in untreated skin samples (control) and those treated with Cream 2 (placebo) or with a SPF15, all P < 0.001 (Fig. 3b). No morphological alterations were observed in cutaneous nerve fibres exposed to UV radiation, as assessed by fluorescence microscopy. Samples treated with Cream 1 showed complete blockage of UV‐related damaging effects on nerve endings (P > 0.05, vs. samples not exposed to UV) (Fig. 2).
Figure 3.
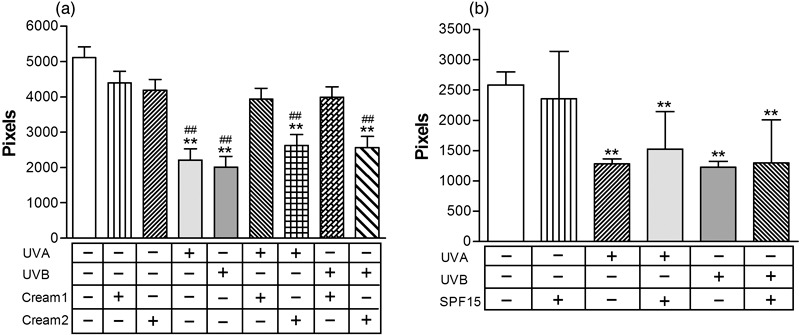
Quantitative analysis of nerve damage induced by ultraviolet (UV) radiation and assessment of neuroprotection. Skin samples left untreated, treated with emulsions only (Cream 1, Cream 2 or SPF 15), exposed to UVA or UVB, or pretreated with emulsions (Cream 1, Cream 2 or SPF 15) then exposed to UVA or UVB ex vivo. Statistically significant differences indicated: **P < 0.001 vs. untreated samples; ##P < 0.001 vs. samples treated with emulsions only.
Figure 2.
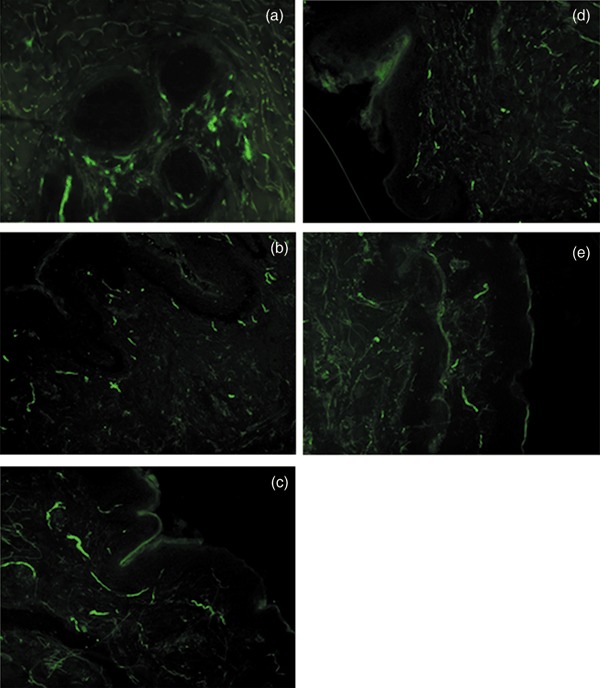
Effects of ultraviolet A (UVA) radiation on free nerve endings. Skin samples left untreated (a), exposed to UVA for 60 min (b), pretreated for 10 min with Cream 1 (c), Cream 2 (d) or SPF15 (e) then exposed to UVA for 60 min. Free nerve endings identified with anti‐NCAM (green, FITC) and assessed by fluorescence microscopy. Magnification ×200.
We performed additional statistical analyses in order to test whether the reported changes were tissue‐specific. We performed skin biopsies on breasts and abdomen of the participants, which could theoretically present a differential pattern of innervation or solar exposure. However, UV‐induced effects were similarly observed in breast (n = 15) and abdominal samples (n = 19) when analysed separately, all P < 0.001, vs. control (Fig. 4). The broad‐spectrum neuroprotective effects of Cream 1 were also observed on breast (UVA, P = 0.83; UVB, P = 0.84) or abdomen (UVA, P = 0.17; UVB, P = 0.10) samples when analysed separately.
Figure 4.
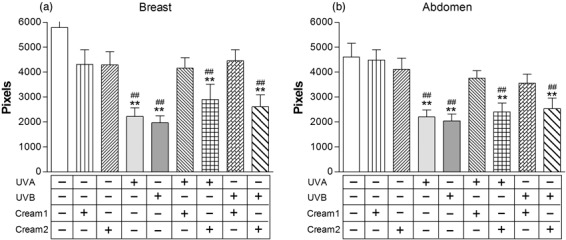
Effects of ultraviolet (UV) radiation on skin samples obtained from breast (n = 15) or abdomen (n = 19) tissues. Skin samples left untreated, treated with emulsions only (Cream 1, Cream 2), exposed to UVA or UVB, or pretreated with emulsions (Cream 1, Cream 2) then exposed to UVA or UVB ex vivo. Statistically significant differences indicated: **P < 0.001 vs. untreated samples; ##P < 0.001 vs. samples treated with emulsions only.
Discussion
In this study, we assessed damage caused by UV radiation to cutaneous nerve fibres. This method has the advantage of dispensing with the use of experimental animals, instead, employing plastic surgery ‘leftovers’ that would otherwise be discarded. This technique allowed identification of various cutaneous nerve fibres, corresponding to distribution of both sensory and sympathetic nervous system components of skin. This technique also enabled development of new neuroprotective preparations against UV‐related damage. UVA or UVB exposure significantly reduced density of NCAM‐positive skin nerve endings, and one new skin care formulation was identified as being neuroprotective.
Our results are in accordance with previous work which has reported that UV phototherapy in vivo is capable of reducing the number of both epidermal and calcitonin gene‐related peptide‐positive dermal nerve fibres (9). Reduction in skin nerve fibre density may contribute to beneficial effects of UV irradiation in attenuating itching (pruritus) in several skin diseases, including psoriasis and atopic dermatitis.
The underlying molecular mechanisms involved with UV‐related effects on skin nerve densities are not completely understood. Initial molecular events that follow UV exposure to skin include erythema, direct DNA modification by action of free radicals, and increased p53 production. Cells react to this damage by repairing DNA, increasing melanin production, and inducing heat shock proteins, among other protective mechanisms (10). Depending on wavelength, irradiance, and cell type involved, apoptosis is induced. In some cases, these alterations result in neoplastic development, however, such changes are related more to chronic or repeated UV exposure. Induction of photoproducts and increase in protein associated with cell damage can also result in apoptosis of skin neurons. The induction of photoproducts and the increase in proteins associated with cell damage can also result in apoptosis of skin neurons and reduced nociceptor function (10). Lower levels of immunostaining observed here could be a result of downregulation of NCAM molecules rather than disappearance of nerve endings, since nerve cell body degeneration may take days or weeks. Analysis of UV‐treated samples using transmission electron microscopy should also be performed to shed light on this issue. Further studies can be performed to analyse the molecular mechanisms of UV‐related cell damage, including formation of pyrimidine dimers and induction of heat shock proteins or neural growth factors. In particular, neurotrophins are a group of diverse proteins secreted by target neurons, which influence survival and maintenance of neurons. Neuronal growth factor promotes growth and differentiation of sensory neurons. Induction of cutaneous inflammation, as shown by erythema induced by UV exposure, correlates with rapid increase in neuronal growth factor production by the inflamed tissues (10). Other studies have associated peripheral nerve endings with exacerbation or maintenance of inflammatory diseases, due to secretion of neurotrophins (11).
Some shortcomings of this study can be discussed. Further studies are necessary to compare the observed ex vivo with in vivo effects of radiation. There is a growing need for development of new techniques to quantify cell damage produced by UV exposure in vivo. Some recent studies indicate that skin pigmentation and melanin distribution can be considered indicative of damage when compared to other methods (12). It has been shown that susceptibility to alter pigmentation is probably indicative of a tendency to develop deleterious damage due to chronic exposure to UV, photo‐ageing and skin cancer, while natural pigmentation could be related to protection (13). However, in vivo studies are more difficult to perform due to invasiveness of biopsy procedures needed, and because of natural solar exposure, which is considered as an intervening variable. Also, acute UV exposure effects need to be compared to chronic exposure effects. There is very limited information regarding effects of chronic UV exposure on skin nerve fibres. Only one study has evaluated effects of repeated sub‐inflammatory UVB irradiation exposure on skin nerve fibres of hairless mice (2). It was shown that chronic UVB irradiation significantly increased the number of sensory nerve fibres (immunoreactive to calcitonin gene‐related peptide), without altering total number of epidermal nerve fibres, and increased skin content of calcitonin gene‐related peptide. Finally, analysis of pixels performed here is more related to intensity of staining than to actual number of fibres. Although specific care was taken to process and analyse these images, further studies should correlate pixel intensity with number of fibres. Taking into account limitations aforementioned, the present study has two important clinical implications: (a) quantification of UV‐induced cell damage in skin nerve fibres and (b) identification of a new skin care compound with neuroprotective properties.
References
- 1. Nielsen KP, Zhao L, Stamnes JJ, Stamnes K, Moan J (2006) The importance of the depth distribution of melanin in skin for DNA protection and other photobiological processes. J. Photochem. Photobiol. B. 82, 194. [DOI] [PubMed] [Google Scholar]
- 2. Legat FJ, Jaiani LT, Wolf P, Wang M, Lang R, Abraham T, Solomon AR, Armstrong CA, Glass JD, Ansel JC (2004) The role of calcitonin gene‐related peptide in cutaneous immunosuppression induced by repeated subinflammatory ultraviolet irradiation exposure. Exp. Dermatol. 13, 242. [DOI] [PubMed] [Google Scholar]
- 3. Tschachler E, Reinisch CM, Mayer C, Paiha K, Lassmann H, Weninger W (2004) Sheet preparations expose the dermal nerve plexus of human skin and render the dermal nerve end organ accessible to extensive analysis. J. Invest. Dermatol. 122, 177. [DOI] [PubMed] [Google Scholar]
- 4. Yaar M, Eller MS, Gilchrest BA (2002) Fifty years of skin aging. J. Invest. Dermatol. Symp. Proc. 7, 51. [DOI] [PubMed] [Google Scholar]
- 5. Nedelec B, Hou Q, Sohbi I, Choiniere M, Beauregard G, Dykes RW (2005) Sensory perception and neuroanatomical structures in normal and grafted skin of burn survivors. Burns 31, 817. [DOI] [PubMed] [Google Scholar]
- 6. Jung K, Seifert M, Herrling T, Fuchs J (2008) UV‐generated free radicals (FR) in skin: Their prevention by sunscreens and their induction by self‐tanning agents. Spectrochim. Acta A Mol. Biomol. Spectrosc. 69, 1423. [DOI] [PubMed] [Google Scholar]
- 7. Niedecken H, Wehrmann W, Bauer R, Kreysel HW (1988) Anti‐Leu 19 monoclonal antibody detects an antigen on autonomic nerves in human skin. J. Cutan. Pathol. 15, 212. [DOI] [PubMed] [Google Scholar]
- 8. Le Forestier N, Lescs MC, Gherardi RK (1993) Anti‐NKH‐1 antibody specifically stains unmyelinated fibres and non‐myelinating Schwann cell columns in humans. Neuropathol. Appl. Neurobiol. 19, 500. [DOI] [PubMed] [Google Scholar]
- 9. Wallengren J, Sundler F (2004) Phototherapy reduces the number of epidermal and CGRP‐positive dermal nerve fibres. Acta Derm. Venereol. 84, 111. [DOI] [PubMed] [Google Scholar]
- 10. Chen WP, Chang YC, Hsieh ST (1999) Trophic interactions between sensory nerves and their targets. J. Biomed. Sci. 6, 79. [DOI] [PubMed] [Google Scholar]
- 11. Legat FJ, Wolf P (2006) Photodamage to the cutaneous sensory nerves: role in photoaging and carcinogenesis of the skin? Photochem. Photobiol. Sci. 5, 170. [DOI] [PubMed] [Google Scholar]
- 12. Latreille J, Gardinier S, Ambroisine L, Mauger E, Tenenhaus M, Guéhenneux S, Morizot F, Tschachler E, Guinot C (2007) Influence of skin colour on the detection of cutaneous erythema and tanning phenomena using reflectance spectrophotometry. Skin Res. Technol. 13, 236. [DOI] [PubMed] [Google Scholar]
- 13. Del Bino S, Sok J, Bessac E, Bernerd F (2006) Relationship between skin response to ultraviolet exposure and skin color type. Pigment Cell Res. 19, 606. [DOI] [PubMed] [Google Scholar]


