Abstract
Objectives: This study is to explore the role of Notch signalling during the regeneration of rat tracheal epithelium after injury induced by 5‐fluorouracil (5‐FU).
Materials and methods: We developed an ex vivo model of rat tracheal epithelial regeneration using 5‐FU to induce injury. Expression levels of members of the Notch signalling pathway, ABCG2, CK19, and proliferating cell nuclear antigen (PCNA) were examined by reverse transcription–polymerase chain reaction, Western blot, and immunofluorescence. One group of tracheas were cultured in the medium with a γ‐secretase inhibitor or Jag‐1 peptide after 5‐FU treatment and another group were pre‐treated with the γ‐secretase inhibitor or Jag‐1 peptide before 5‐FU treatment. The expression changes of ABCG2, CK19, and PCNA were examined by Western blot or immunofluorescence and the morphologic changes were observed by haematoxylin and eosin stain during the recovery process.
Results: Expression levels of Notch3, Jagged1, and Hey1 were increased in rat tracheal epithelial cells after treatment with 5‐FU. During injury recovery, disruption of Notch signalling by treatment with the γ‐secretase inhibitor reduced expression of ABCG2 and PCNA, but promoted expression of CK19, while persistent activation of Notch signalling promoted expression of ABCG2 and PCNA, but reduced expression of CK19. Under both conditions, recovery from injury was reduced. However, blocking Notch signalling prior to 5‐FU treatment led to the complete blockage of recovery, while activating Notch signalling before 5‐FU treatment promoted recovery.
Conclusions: During tracheal epithelial regeneration, Notch signalling maintains an undifferentiated state and promotes proliferation among a population of tracheal epithelial cells.
Introduction
Trachea is the essential organ of the respiration system and it is lined by a pseudostratified epithelium composed of basal, ciliated, and secretory cells. Despite the efficient defence system of the airway epithelium, it is frequently injured because of its permanent contact with the external milieu, which contains toxic agents, viruses, microorganisms, and pollutants (1). Immediately after injury, the airway epithelium initiates a repair process in order to restore the barrier integrity. The epithelial cells participate to the closure of the wound and to the functional regeneration of the epithelium. In order to more specifically determine the cellular and molecular mechanisms involved in the airway epithelial repair and regeneration, we constructed an ex vivo model for tracheal regeneration, using damage induced by treatment with 5‐fluorouracil (5‐FU).
5‐Fluorouracil, a member of the antimetabolite family of drugs, resembles other nutrients used by proliferating cells. Proliferating cells take up 5‐FU, which then interferes with their growth and ultimately causes death. In contrast, cells in the resting stage of the cell cycle are survived (2, 3, 4). Treatment of the tracheal epithelium with 5‐FU has been shown to lead to a series of morphological changes (5). Shortly after treatment, the normally proliferating tracheal epithelium desquamates, and only a few cells in the G0 phase of the cell cycle remain in the basal membrane. Thereafter, the tracheal epithelia became cuboidal and returned to its original appearance about 48 h after removal of 5‐FU. However, the precise molecular mechanisms regulating this process remain unclear.
Notch comprises a family of highly conserved receptors (6), whose activation is induced by specific ligands, Delta and Jagged, through cell–cell interactions (7). Once activated, the Notch intracellular domain (NICD) is cleaved by γ‐secretase, which leads to translocation of the NICD into the nucleus (8, 9). Subsequently, NICD becomes associated with the transcription factor RBP‐J which together form a transactivation complex (10, 11) that initiates transcription of primary target genes such as the hairy/enhancer of split (Hes) and Hes‐related repressor protein (Hey, also called HERP/Hesr/HRT/CHF/gridlock) (12, 13, 14). Hes and Hey act as transcriptional repressors and suppress expression of downstream target genes so as to regulate cell proliferation and differentiation. DAPT is a highly specific inhibitor of γ‐secretase, and can inhibit activation of Notch signal by preventing γ‐secretase‐mediated Notch cleavage (15). Jag‐1 is a synthetic peptide corresponding to the domain‐specific language domain of Jagged1 ligand and has been shown to act as a Notch agonist (16, 17, 18).
Components of the Notch signalling pathway are widely expressed in mammals and include the receptors Notch1, Notch2, Notch3 and Notch4, ligands Jagged1, Jagged2, Dll1, Dll3 and Dll4, and primary target genes such as Hes1, Hes3, Hes5, Hey1 and Hey2 (19). Notch signalling is involved in various aspects of cellular regulation, including stem cell maintenance and tissue regeneration, although it plays diverse, tissue‐specific roles in this process. In haematopoietic tissue (20, 21, 22, 23), nervous tissue (24), intestine (25), and muscle (26), Notch signalling promotes self‐renewal and maintains the undifferentiated state of stem cells, while in other tissues, such as skin, Notch signalling promotes differentiation of epidermal stem cells (27). The role of Notch signalling and expression of signalling pathway members in the regeneration of tracheal epithelium remains unclear, as is its role in regulating stem cell behaviour. Here, we show that Notch signalling plays a role in the regeneration of tracheal epithelium by maintaining an undifferentiated state and promoting proliferation among a population of tracheal epithelial cells and provide a framework for future studies into the role of this important pathway in the trachea biology and regeneration medicine.
Materials and methods
Model for tracheal epithelial regeneration and DAPT and Jag‐1 peptide treatment
Male and female Wistar rats (~200 g) were used according to the guidelines of the Animal Care Committee at the China Medical University. Tissue culture plastic and reagents were purchased from Invitrogen (Carlsbad, CA, USA). The DAPT (Sigma‐Aldrich, St. Louis, MO, USA) and Jag‐1 (AnaSpec, San Jose, CA, USA) were both dissolved in dimethyl sulfoxide (DMSO). Tracheas were excised sterilely and cultured in 1 : 1 mix of Dulbecco's modified Eagle's medium and Ham's F‐12 medium (DMEM/F12) containing 120 mg/mL 5‐FU and 10% foetal bovine serum (FBS) for 12 h at 37 °C. Following removal of 5‐FU, tracheas were cultured in DMEM/F12 containing 10% FBS with or without 5 µm DAPT (Sigma‐Aldrich) or 40 µm Jag‐1 peptide (AnaSpec). The tracheas cultured in DMEM/F12 containing DMSO alone were used as vehicle controls for the DAPT‐ or Jag‐1‐treated group. Tracheas were removed at 0, 3, 6, 9, 12, 24, and 48 h after removing 5‐FU and analysed by one of several methods. Another group of tracheas were firstly cultured in DMEM/F12 containing 10% FBS with or without 5 µm DAPT or 40 µm Jag‐1 peptide for 12 h and cultured for another 12 h in DMEM/F12 containing 10% FBS with 5‐FU, then the tracheas were cultured in DMEM/F12 containing 10% FBS for 48 h. The tracheas cultured in DMEM/F12 containing DMSO alone before 5‐FU treatment were used as vehicle controls for the DAPT or Jag‐1 pre‐treated group. Tracheas were removed immediately after DAPT or Jag‐1 treatment and at 0, 3, 6, 9, 12, 24, and 48 h after removing 5‐FU, respectively. For reverse transcription–polymerase chain reaction (RT‐PCR) and Western blot analyses, tracheal epithelia were digested and stored at –80 °C until use. For immunofluorescent analysis, tracheas were fixed in 4% paraformaldehyde, and prepared as paraffin‐embedded tissue sections for haematoxylin and eosin stain and immunofluorescent staining.
Purification of tracheal epithelial cells
Epithelial cells were isolated from treated and untreated tracheas. Tracheas were digested by filling lumens of ligated trachea with 1 mg/mL proteinase XIV solution (Sigma‐Aldrich) in DMEM/F12 without serum at 4 °C overnight. Enzymatic digestion was terminated by adding 10 mL of DMEM/F12 containing 10% FBS. Cell aggregates were collected for further purification by a flow cytometer FACSort (Becton Dickinson, New Jersey, CA, USA). Firstly, these cells were mixed with antivimentin (a mesenchymal cell marker) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by a secondary fluorescein isothiocyanate‐conjugated antibody (Santa Cruz Biotechnology), then the cells were washed and sorted by flow cytometer. The negative fractions were collected and stored at –70 °C for further analysis by RT‐PCR and Western blot.
Indirect immunofluorescence
Indirect immunofluorescence staining was performed to the serial tissue sections from tracheas during the recovery from injury with Notch3, Jagged1, ABCG2, CK19, and proliferating cell nuclear antigen (PCNA) antibodies (Santa Cruz Biotechnology), respectively. The method was basically as described previously (28) and the thickness of the sections is 3 µm. Briefly, rabbit anti‐Notch3, rabbit anti‐CK19, goat anti‐Jagged1, goat anti‐ABCG2, and goat anti‐PCNA (dilution 1 : 100) were used as primary antibodies. Fluorescein isothiocyanate‐conjugated goat anti‐rabbit immunoglobulin G and rhodamine isothiocyanate‐conjugated rabbit anti‐goat immunoglobulin G (dilution 1 : 100; HuaMei, Beijing, China) were used as secondary antibodies. Both antibodies were diluted with 1% bovine serum albumin–phosphate‐buffered saline (BSA‐PBS). After sections were treated with the secondary antibody, they were incubated with 0.5 µg/mL 4′,6‐diamidino‐2‐phenylindole (Sigma‐Aldrich) for nuclear counterstaining. Specimens were examined with an epi‐illumination fluorescence microscope BX50 (Olympus, Tokyo, Japan).
For serum controls, 1% BSA‐PBS was used instead of the primary antibody as a negative control.
Western blot analysis and densitometric analysis
Total cell homogenates were prepared by lysing cells in NP‐40 lysis buffer [1% NP‐40, 10% glycerol, 20 mm Tris‐HCl (pH 8.0), 137 mm NaCl and 4% complete protease inhibitor cocktail mix (Roche, Mannheim, Germany)]. Eighty micrograms of total protein was subjected to SDS‐PAGE, followed by blotting to polyvinylidene difluoride (Immobilon, Millipore Corp., Billerica, MA, USA). Membranes were blocked with 5% non‐fat dried milk in PBS for 1 h with shaking, and were then washed in PBS with 0.1% Tween‐20, three times, for 10 min each. Membranes were incubated with primary antibody (all antibodies from Santa Cruz Biotechnology) in 1% BSA in PBS overnight at 4 °C, with shaking. Membranes were washed as described above, and were then secondary antibodies (Table 1) for 2 h at room temperature. Membranes were washed again as described above and then incubated with 3,3′‐diaminobenzidine at room temperature. When bands reached the desired intensity (2–5 min), membranes were washed briefly in water, followed by PBS. Finally, membranes were dried and photographed and scanned. After scanning, the densitometric analysis was performed using ImageJ 1.33 software (National Institutes of Health, Bethesda, MD, USA).
Table 1.
Antibodies used in Western blot analyses
| Primary antibody | Secondary antibody (horseradish peroxidase‐conjugated) | |||||
|---|---|---|---|---|---|---|
| Name | Source | Dilution | Product no. | Name | Dilution | Product no. |
| β‐Actin | Rabbit | 1 : 200 | sc‐7210 | goat anti‐rabbit IgG | 1 : 2000 | sc‐2004 |
| Notch3 | 1 : 100 | sc‐5593 | ||||
| Hey1 | 1 : 100 | sc‐28764 | ||||
| Jagged1 | Goat | 1 : 100 | sc‐6011 | rabbit anti‐goat IgG | 1 : 2000 | sc‐2768 |
| ABCG2 | 1 : 200 | sc‐25156 | ||||
| CK19 | 1 : 200 | sc‐33119 | ||||
| PCNA | Mouse | 1 : 200 | sc‐25280 | goat anti‐mouse IgG | 1 : 2000 | sc‐2005 |
RNA extraction and RT‐PCR analysis
Total RNA was extracted from cells harvested with TRIzol reagent (Invitrogen). The RT‐PCR was performed with the TaKaRa RNA PCR Kit (AMV) version 3.0 (Takara Bio, Otsu, Japan), according to the manufacturer's protocol. PCR primers were designed span exons, to exclude possibility of genomic contamination (Table 2), and β‐actin was used as an endogenous control. PCR conditions were as follows: 94 °C for 2 min, 94 °C for 30 s, variable temperature (Table 2) for 40 s, and 72 °C for 1 min, for 35 cycles. Reverse transcription reactions lacking reverse transcriptase served as negative controls. PCR products were visualized by ethidium bromide staining on 2% agarose gels on a gel scanner (UVP Life Sciences, Cambridge, UK).
Table 2.
RT‐PCR primer sequences and product sizes
| Gene | Primer sequence (5′→3′) | Accession number | Size (bp) | Annealing temperature (°C) |
|---|---|---|---|---|
| Notch1 | AGTGCCTGCCCTTTGAGTCTTC CCCCGTTCTTGCAGTTGTTTC | X57405 | 348 | 59 |
| Notch2 | GCAGCCGCCGTCAATAATGTG GCGTGGGTGAGGCCTGTAAGAT | NM_024358 | 595 | 59.1 |
| Notch3 | GACCGTGTGGCCTCTTTCTATTGTG GCAGCTGAAGCCATTGACTCTATCCT | NM_020087 | 489 | 57.1 |
| Notch4 | GAGGCTATCTCAGGGACAAGG GCCGGTTGGACAACGACA | NM_001002827 | 430 | 58 |
| Jagged1 | TAGCAACACTGGCCCTGACAAATAC CGCATAGCCAGGTGGACAGATACAG | NM_019147 | 505 | 56.2 |
| Jagged2 | GGAAACAGTTGTTATGGGTG CTGGGGTCTTTGGTGAAT | XM_343119 | 429 | 55.9 |
| Dll1 | AGAGTCAAGGGCGTCCAG TGTTGCGAGGTCGTCAGG | NM_032063 | 433 | 57.4 |
| Dll3 | CGTGAGTCCCTAACCGTATGTC GGATCTTCACCGCCAACAC | NM_053666 | 359 | 57.8 |
| Dll4 | GCTCCGGCATCTTCCAGT GCAGTCTTGTGAGGGTGTTA | XM_001080829 | 426 | 54.5 |
| Hes1 | CAAAGTAGCCCTAAGACATAA CAGTGTTTTCAGTTGGCTC | NM_024360 | 336 | 49.8 |
| Hes3 | CACCAGATACGGAAGCGAAAG GAGGCAGGGGCTGAGAAC | NM_022687 | 279 | 58.2 |
| Hes5 | CACCAGCCCAACTCCAAACT AGAGGCCGCAGGCAGATT | NM_024383 | 340 | 60.1 |
| Hey1 | AAGCTGAGATCTTGCAGATG GGCGGCGACAGTTTGGAG | AY059383 | 490 | 56.2 |
| Hey2 | TGACATCCTCCATGTCCC ACTGATAACGGTGGGCTG | AY059382 | 427 | 55.8 |
| β‐actin | CCAAGGCCAACCGCGAGAAGATGAC AGGGTACATGGTGGTGCCGCCAGAC | NM_031144 | 587 | 58 |
Statistical analysis
Data from at least three independent experiments were used for statistical analysis by SPSS 11.5 (SPSS Inc., Chicago, IL, USA). All values were expressed as mean ± standard deviation. Statistical analyses were preformed by one‐way anova, where P < 0.05 was considered significant.
Results
Morphological changes in rat tracheal epithelium after ex vivo injury induced by 5‐FU
Tissue sections of normal rat trachea and rat tracheas at 0, 3, 6, 12, 24, and 48 h after removal of 5‐FU were analysed by haematoxylin and eosin staining to investigate the morphological changes in rat tracheal epithelium (Fig. 1). Immediately following removal of 5‐FU (0 h), only a few cells with less cytoplasm were observed distributed at intervals on the basement membrane. Three hours later, the cytoplasm extended along the basal membrane and cells appeared flattened. At 6 h, the number of flat cells had increased, while cells appeared to have become cuboidal by 12 h. The number of cells increased sharply at the 24‐h time point. At about 48 h, cilia had appeared on the surface of the tracheal epithelium, which appeared pseudostratified and almost completely normal.
Figure 1.
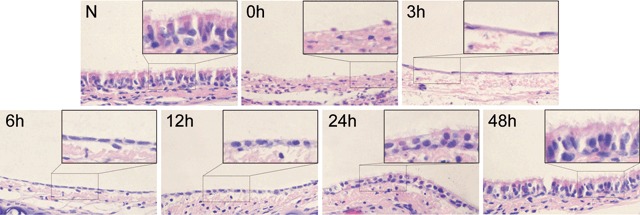
Morphological changes in rat tracheal epithelium after injury induced by 5‐FU observed by HE stain. (N) Normal rat tracheal epithelium is pseudostratified epithelium. (0 h) Only a few cells with less cytoplasm than normal tracheal epithelial cells remained in the basal membrane immediately after 5‐FU treatment. (3 h) The cytoplasm of these cells extended along the basal membrane and cells became flat. (6 h) The number of flat cells increased and the cytoplasm became short. (12 h) Cells appeared cuboidal. (24 h) The number of cells increased sharply. (48 h) Cilia appeared on the luminal side of trachea and the tracheal epithelium became pseudostratified and appeared almost completely recovered (original magnification ×400).
Expression levels of ABCG2, CK19, and PCNA proteins in rat tracheal epithelium during recovery from injury induced by 5‐FU
To evaluate the proliferation and differentiation of tracheal epithelial cells during recovery from tracheal epithelial injury, we examined the expression of three proteins: ABCG2, a commonly used marker for stem cells (29, 30); CK19, a marker for differentiated epithelial cells (31); and PCNA, a marker for cell proliferation (32, 33) by Western blot (Fig. 2a). Densitometric analysis was performed to quantify expression levels of ABCG2, CK19, and PCNA (Fig. 2b). We found that in untreated rat tracheal epithelia, almost no ABCG2 and PCNA were detected, while levels of CK19 were very high relative to levels in 5‐FU‐treated tracheal epithelia. However, immediately following removal of 5‐FU, levels of ABCG2 and PCNA increased. Thus, expression of ABCG2 and PCNA increased together and reached peak levels at the same time, 6 h after removal of 5‐FU, and both decreased gradually, returning to normal levels about 48 h after removal of 5‐FU. In contrast, expression of CK19 was lowest at 0 h and increased slowly until 6 h; thereafter, the expression of CK19 increased sharply and nearly returned to normal level about 48 h after removal of 5‐FU.
Figure 2.
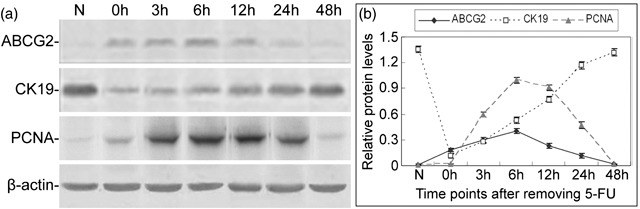
Expression levels of ABCG2, CK19, and PCNA proteins in rat tracheal epithelium during recovery from injury induced by 5‐FU. (a) Western blot analysis of ABCG2, CK19, and PCNA proteins in normal trachea and tracheas after treatment with 5‐FU. (b) Expression levels of ABCG2, CK19, and PCNA relative to β‐actin. Data are presented as means ± SD of three independent experiments.
These observations indicate that in the untreated rat tracheal epithelium, differentiated epithelial cells are predominant and stem cells are very rare, and cell proliferation is quite slow. After 5‐FU‐induced injury, the number of stem cells increased and the number of differentiated epithelial cells decreased greatly. Meanwhile, cells underwent proliferation.
Expression of Notch signalling pathway components in the rat tracheal epithelium
The Notch signalling pathway consists of many components, but their expression in the tracheal epithelium has not been characterized. We examined mRNA expression of four receptors, Notch1, Notch2, Notch3 and Notch4, five ligands, Jagged1, Jagged2, Dll1, Dll3 and Dll4, and five primary target genes, Hes1, Hes3, Hes5, Hey1 and Hey2 (Fig. 3). It showed that levels of Notch1, Notch3, Jagged1, Dll4, Hes1, Hey1, and Hey2 were very low in the untreated rat tracheal epithelium. Following treatment with 5‐FU, expression levels of Notch1, Dll4, Hes1, and Hey2 did not change obviously, while Notch3, Jagged1, and Hey1 levels changed markedly. Expression of Notch2, Notch4, Jagged2, Dll1, Dll3, Hes3, and Hes5 was not detected in the rat tracheal epithelium during this process (Fig. 3).
Figure 3.
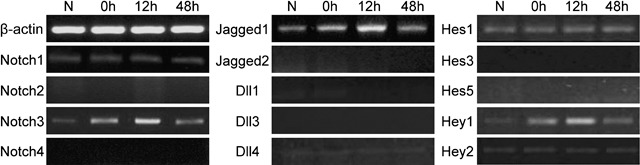
Expression of Notch signalling pathway members in the rat tracheal epithelium. The mRNA levels of Notch receptors (Notch1, Notch2, Notch3 and Notch4), ligands (Jagged1, Jagged2, Dll1, Dll3 and Dll4) and primary target genes (Hes1, Hes3, Hes5, Hey1 and Hey2) in the rat tracheal epithelium before and after 5‐FU treatment were examined by RT‐PCR.
These results suggest that Notch1, Notch3, Jagged1, Dll4, Hes1, Hey1, and Hey2 are probably involved in the maintenance of normal rat tracheal epithelium. Moreover, Notch3, Jagged1, and Hey1 may play a role in the proliferation and differentiation of rat tracheal stem cells (Table 3).
Table 3.
The expression of Notch signalling pathway components in rat tracheal epithelium
| Receptors | Ligands | Primary target genes | |
|---|---|---|---|
| Untreated tracheal epithelium | Notch1, Notch3 | Jagged1, Dll4 | Hes1, Hey1, Hey2 |
| Recovery of tracheal epithelium injury | Notch3 | Jagged1 | Hey1 |
Expression levels of N3ICD, Jagged1, and Hey1 in the rat tracheal epithelium during injury recovery
To confirm further characterize changes in Notch signalling during the regeneration of tracheal epithelium, we examined protein levels of the intracellular domain of Notch3, N3ICD, Jagged1, and Hey1 by Western blot analysis (Fig. 4a) in rat tracheal epithelial cells after injury induced by 5‐FU. Densitometric analysis showed that changes in expression of N3ICD, Jagged1, and Hey1 were similar. Expression levels of N3ICD, Jagged1, and Hey1 were very low in the normal rat tracheal epithelium. After treatment with 5‐FU, expression levels increased and reached peak levels at 6, 6, and 12 h, respectively, and decreased gradually thereafter to return to normal levels by about 48 h (Fig. 4b).
Figure 4.
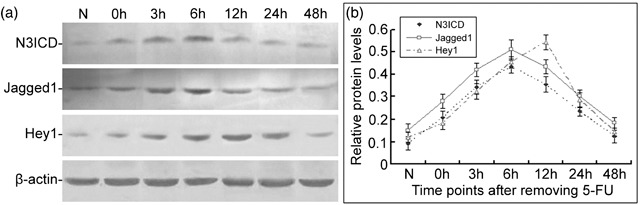
Changes in expression of N3ICD, Jagged1, and Hey1 in the rat tracheal epithelium during the injury recovery process. (a) Protein levels of N3ICD, Jagged1 and Hey1 in normal rat tracheal epithelium and tracheal epithelium after 5‐FU treatment were examined by Western blot. (b) Relative levels of N3ICD, Jagged1, and Hey1protein. Data are presented as means ± SD of three independent experiments.
Interestingly, during the recovery process following injury induced by 5‐FU, changes in expression of Notch pathway members were similar to changes in ABCG2 and PCNA (2, 4), suggesting that Notch signalling pathway plays a correlative role in maintaining an undifferentiated state and promoting proliferation among a population of tracheal epithelial cells.
Spatiotemporal localization of Jagged1, Notch3, ABCG2, CK19, and PCNA in the tracheal epithelium during regeneration induced by 5‐FU
To examine the localization of Jagged1, Notch3, ABCG2, CK19, and PCNA, we performed immunofluorescence on serial sections from the tracheal epithelium treated with and without 5‐FU. The numbers of ABCG2‐positive cells, CK19‐positive cells, and PCNA‐positive cells were counted to provide insight into the differentiation and proliferation state of the tracheal epithelium during regeneration.
Immediately following removal of 5‐FU, very few Jagged1‐positive cells were detected. Subsequently, the number of Jagged1‐positive cells increased gradually and reached peak levels at about 6 h, at which point expression began to decrease. Jagged1 was mainly localized to the cell membrane in cells on the basal surface of the tracheal epithelium (Fig. 5a). Nuclear localization of Notch3 is indicative of active Notch signalling (10, 11). After treatment with 5‐FU, most of the cells remaining in the basement membrane exhibiting nuclear Notch3 increased. By about 6 h of treatment, the proportion of cells with nuclear Notch3 reached peak levels, and decreased thereafter. By 48 h, the rate returned to baseline levels (Fig. 5b). Interestingly, most Jagged1‐positive cells were interspersed with nuclear Notch3‐positive cells, and these were distributed within similar regions within the tracheal epithelium.
Figure 5.
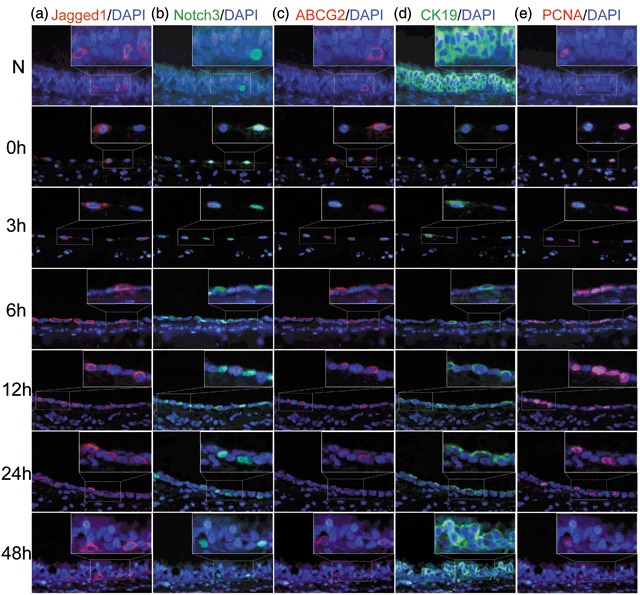
Immunofluorescence staining of Jagged1, Notch3, ABCG2, CK19, and PCNA during recovery of injury induced by 5‐FU. (a) In the untreated rat tracheal epithelium, only a few cells were Jagged1‐positive. The number of Jagged1‐positive cells increased gradually, reaching its peak at about 6 h, and decreasing thereafter. (b) Few cells with nuclear localized Notch3 were detected in the untreated rat tracheal epithelium. After 5‐FU treatment, the proportion of cells of Notch3 nuclear‐positive increased significantly, and proportion peaked by about 6 h. By 48 h, the proportion had returned to normal levels. Most of cells with nuclear Notch3 neighboured Jagged1‐positive cells. (c) ABCG2 was mainly localized to the cell membrane and exhibited correlated with nuclear Notch3 localization. Most ABCG2‐positive cells also exhibited nuclear Notch3. (d) CK19‐positive cells were predominant in normal rat tracheal epithelium. After 5‐FU treatment, the number of CK19‐positive cells decreased sharply, then increased slowly, and returned almost to baseline levels by 48 h. (e) PCNA was localized to the cell nuclear. In normal rat tracheal epithelium, few cells were PCNA‐positive. After 5‐FU treatment, the number of PCNA‐positive cells increased and peaked by 6 h, then decreased gradually and returned to its normal levels by about 48 h (original magnification ×400).
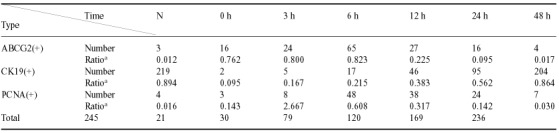
The expression of ABCG2 was consistent with the results from Western blot analysis and ABCG2 was mainly localized to the cell membrane. In the normal rat trachea, almost no ABCG2‐positive cells were detected. Immediately after 5‐FU treatment, the number of ABCG2‐positive cells increased sharply and peaked at about 6 h, then decreased gradually to baseline levels at about 48 h (Table 4). Interestingly, most of the ABCG2‐positive cells also exhibited nuclear Notch3 expression (Fig. 5b,c), confirming that Notch signalling is involved in the maintenance of tracheal stem cells. CK19 was localized to the cell membrane and CK19‐positive cells were mainly distributed on the luminal surface of the trachea. In untreated rat tracheal epithelia, most cells were CK19‐positive. Immediately following treatment with 5‐FU, almost no CK19‐positive cells were detectable, and then the number of CK19‐positive cells increased gradually, returning to almost baseline levels by 48 h after removal of 5‐FU (Fig. 5d and Table 4). PCNA was localized to the cell nuclear. In normal rat tracheal epithelium and rat tracheal epithelium immediately after 5‐FU treatment, very few cells were PCNA‐positive. The number of PCNA‐positive cells increased gradually and peaked by about 6 h, then decreased and returned its normal level by about 48 h after removing 5‐FU (Fig. 5e and Table 4).
Table 4.
Cell number of different cell types in tracheal epithelium per 5 fields (×400) during regeneration
Disruption of Notch signalling leads to reduced proliferation and increased differentiation of tracheal stem cells, while persistent activation of Notch signalling leads to increased proliferation and reduced differentiation of tracheal stem cells
To demonstrate the role of Notch signalling pathway in the regeneration of tracheal epithelium, we treated the rat tracheas with a pharmacological inhibitor of γ‐secretase, DAPT, or Jag‐1 peptide so as to block or activate Notch signalling, and then explored the effects on the regeneration of tracheal epithelium.
After 5‐FU treatment, rat tracheas were cultured in the medium with 5 µm DAPT or 40 µm Jag‐1 peptide, or with DMSO alone for 48 h. To determine their efficacies on Notch signalling, we examined N3ICD and Hey1 protein levels with and without DAPT or Jag‐1 treatment by Western blot analysis. We found that in the DAPT‐treated group, N3ICD and Hey1 protein levels were a little lower than the untreated group 3 h after treatment. Six hours later, differences with the control group became statistically significant, and 12 h later, N3ICD was undetectable, then 24 h later both N3ICD and Hey1 were undetectable. In the Jag‐1‐treated group, 6 h after culturing with Jag‐1, the expression of N3ICD and Hey1 became significantly higher than the control group and this was sustained since then (Fig. 6a,b,c). There was no significant difference between the DMSO vehicle control group and the group treated by 5‐FU alone (data not shown). These observations suggest that 5 µm DAPT could reduce Notch signalling within 6 h of treatment, and block Notch signalling within 12–24 h of treatment, while 40 µm Jag‐1 peptide could activate Notch signalling significantly within 6 h of treatment.
Figure 6.
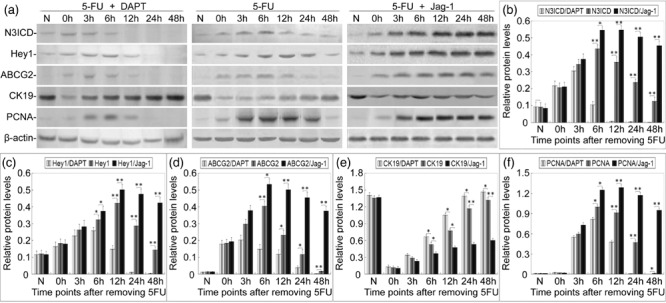
Expression of N3ICD, Hey1, ABCG2, CK19, and PCNA in rat tracheal epithelia with and without DAPT or Jag‐1 treatment. (a) Expression of N3ICD, Hey1, ABCG2, CK19, and PCNA proteins in rat tracheal epithelia with and without DAPT or Jag‐1 treatment were examined by Western blot. (b–e) Relative expression levels of N3ICD, Hey1, ABCG2, CK19, and PCNA proteins in rat tracheal epithelia with and without DAPT or Jag‐1 treatment were analysed. Data are presented as means ± SD of three independent experiments. The level of significance is indicated: *P < 0.05, **P < 0.01.
To examine the effects of blocking or activating Notch signalling on the proliferation and differentiation of tracheal epithelial cells, we investigated the expression of ABCG2, CK19, and PCNA in tracheal epithelia with and without DAPT or Jag‐1 by Western blot. In the DAPT‐treated group, expression of ABCG2 increased more slowly, and peaked at a lower level compared with the control group at 3 h. From 3 h and on, expression of ABCG2 ceased to increase, and decreased sharply from 6 h (Fig. 6a,d). The expression of CK19 increased rapidly and maintained a high level from 12 h, and then peaked at a higher level compared with non‐DAPT‐treated samples (Fig. 6a,e). PCNA expression was first detectable at 3 h, and increased slightly, then decreased from 6 h to 24 h after removing 5‐FU, PCNA expression was not detected. The expression of PCNA decreased compared with non‐DAPT‐treated samples (Fig. 6a,f). No significant difference between the DMSO control group and the group treated by 5‐FU alone was observed (data not shown). These results suggest that disruption of Notch signalling leads to a reduction in the undifferentiated state of tracheal epithelium and cell proliferation, with a concomitant increase in differentiation.
In the Jag‐1‐treated group, expression levels of ABCG2 and PCNA were significantly higher than in the group treated by 5‐FU alone from 6 h after Jag‐1 treatment, and high levels were sustained thereafter. While expression of CK19 was lower compared with the group treated by 5‐FU alone, low levels were sustained from 6 h and on. These observations indicate that active Notch signalling can promote the proliferation, but inhibit differentiation of tracheal stem cells.
Both disruption and persistent activation of notch signalling lead to reduced recovery of tracheal epithelium from 5‐FU‐induced injury
To examine the role of Notch signalling in regeneration of tracheal epithelium, tissue sections from 5‐FU‐treated rat tracheal epithelia treated with DAPT or Jag‐1 were stained with haematoxylin and eosin to detect morphological changes, compared with 5‐FU treatment alone. Cell numbers within the tracheal epithelium during the recovery process were counted among these groups, to compare the proliferation rates.
In the DAPT‐treated group, morphological differences with the group treated by 5‐FU alone appeared beginning around 6 h after treated with DAPT. DAPT‐treated tracheal epithelial cells became cuboidal around 6 h earlier than the group treated by 5‐FU alone. By 12 h, cilia had already appeared on the surface of the tracheal epithelium, earlier than in the group treated by 5‐FU alone (Fig. 7a). The changes in DMSO vehicle controls were the same as the group treated with 5‐FU alone (data not shown). These observations suggest that cellular differentiation occurs much earlier in the DAPT‐treated group. From 24 h to 48 h, there was no obvious increase in the number of epithelial cells in the DAPT‐treated group. In contrast, the cell number increased rapidly in the group treated by 5‐FU alone, which suggested that cell proliferation was apparently slower than in the control group (Table 5).
Figure 7.
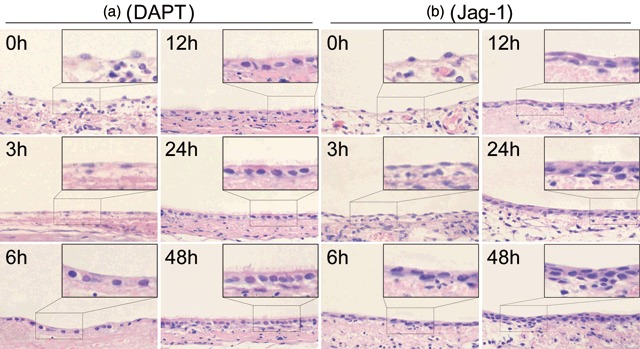
Morphological changes in rat tracheal epithelium treated with DAPT or Jag‐1 and 5‐FU observed by HE stain. (a) In the DAPT‐treated group, morphological differences with the control group appeared beginning around 6 h after removal of 5‐FU. (6 h) The number of tracheal epithelia increased gradually and cells appeared cuboidal. (12 h) No change was observed in the shape of the tracheal epithelia, while cilia appeared on the surface of the tracheal epithelium earlier than the group treated by 5‐FU alone. (24 h) The number of epithelial cells increased slightly and more cilia appeared on the surface of the tracheal epithelium. (48 h) There was no marked increase in the number of epithelial cells and cilia were evenly distributed on the surface of the tracheal epithelium. (b) In the Jag‐1‐treated group, the cells appeared to proliferate more rapidly than the control group. (3 h) There appeared more flat cells on the basement membrane than the control group. (6–24 h) The cell number increased more rapidly than the control group. (48 h) The epithelium appeared to be multilayers, but no cillia was observed on the surface of tracheal epithelium (original magnification ×400).
Table 5.
Cell number in tracheal epithelium per 5 fields (×400) with DAPT or Jag‐1 treatment during regeneration

In the Jag‐1‐treated group, the number of epithelial cells increased faster than the control group since 6 h after culturing with Jag‐1 which indicated a higher proliferation activity in the Jag‐1‐treated group (Table 5); however, no cilia was observed even after 48 h which suggested that the cell differentiation was obviously slower than the control group (Fig. 7b).
As a result, in both the DAPT‐treated group and the Jag‐1‐treated group, the tracheal epithelium did not revert to its original appearance by 48 h as in the control group (1, 7).
No recovery of the tracheal epithelium following pre‐treatment with DAPT, while Jag‐1 pre‐treatment promoted regeneration of the tracheal epithelium
Although almost no ABCG2 was detected in our experiments, very few stem cells are present in the untreated rat tracheal epithelium and it was just through the proliferation and differentiation of these cells that the tracheal epithelium recovered from injury induced by 5‐FU. The previous study also indicated a causal role of Notch signal in the maintenance of tracheal stem cells during tracheal epithelium regeneration. To concretely demonstrate the role of Notch signalling in determining tracheal stem cell fate, we pre‐treated rat tracheas with DAPT or Jag‐1 peptide prior to 5‐FU treatment. Tracheas cultured in medium with DMSO alone were used as vehicle controls. We compared changes in ABCG2 protein expression levels with respect to the group treated by 5‐FU alone by immunofluorescence. Haematoxylin and eosin stain was performed on tissue sections from tracheas treated with and without DAPT or Jag‐1 to observe morphological changes during regeneration of the tracheal epithelium.
Haematoxylin and eosin stain showed that immediately after DAPT pre‐treatment, the cell number in tracheal epithelium decreased after 5‐FU treatment, almost no cells remained within the basement membrane and no recovery trend was observed (Fig. 8a and Table 6). In the Jag‐1 pre‐treatment group, the number of ciliated cells seemed to decrease after culture with Jag‐1 peptide, and more cells remained in the basement membrane after 5‐FU treatment compared with the control group. A similar recovery process was observed as for the group treated by 5‐FU alone, although the tracheal epithelium had almost completely restored its original appearance at 24 h earlier than the control group (Fig. 8b,c).
Figure 8.
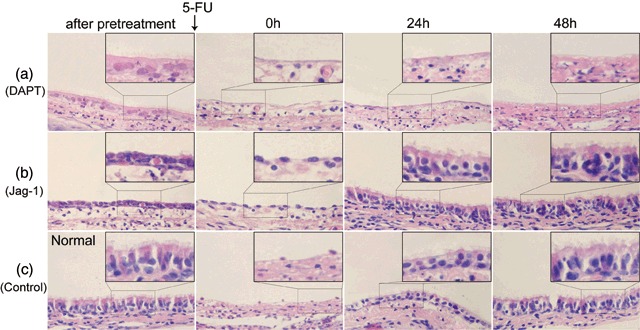
Morphological changes in rat tracheal epithelium pre‐treated with and without DAPT or Jag‐1 observed by haematoxylin and eosin stain. (a) After pre‐treated by DAPT, the cell number in tracheal epithelium decreased obviously. After 5‐FU treatment, almost no cell remained in the basement membrane. No recovery trend was observed. (b) Immediately after Jag‐1 pre‐treatment, cilia cells appeared to decrease significantly. After 5‐FU treatment, more cells remained in the basement membrane compared with the group without Jag‐1 pre‐treatment. Twenty‐four hours later, the tracheal epithelium almost revert to its origin appearance. (c) Morphological changes in rat tracheal epithelium without pre‐treatment during recovery (original magnification ×400).
Table 6.
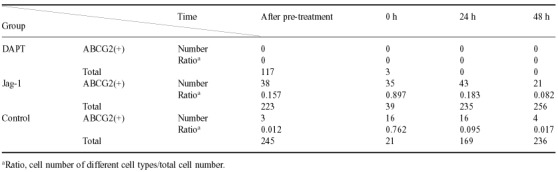
Cell number in tracheal epithelium per 5 fields (×400) with DAPT or Jag‐1 pre‐treatment during regeneration
Immunofluorescence with anti‐ABCG2 showed that the expression of ABCG2 was not detectable after DAPT treatment in the group pre‐treated with DAPT (Fig. 9a). In the group pre‐treated with Jag‐1 peptide, the number of ABCG2‐positive cells increased immediately after treatment with Jag‐1 peptide and a high expression level of ABCG2 was sustained compared with the control group (Fig. 9b,c and Table 6).
Figure 9.
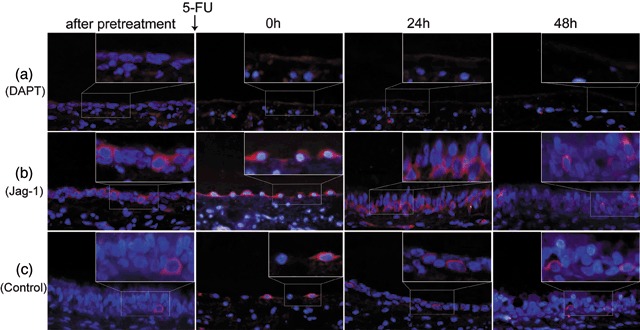
The expression changes of ABCG2 in rat tracheal epithelium pre‐treated with and without DAPT or Jag‐1 examined by immunofluorescence. (a) After pre‐treated by DAPT, no ABCG2‐positive cell was detected in tracheal epithelium. After 5‐FU treatment, nearly no cell remained in the basement membrane. (b) Immediately after Jag‐1 pre‐treated, more ABCG2‐positive cells were detected than normal rat tracheal epithelium. After 5‐FU treatment, most of the cells remaining in the basement membrane were ABCG2‐positive. Twenty‐four hours later, tracheal epithelium seemed to returned to its normal appearance and more ABCG2‐positive cells were detected than the control group. At about 48 h, the number of ABCG2‐positive cells decreased than before, but were still more than that of the control group. (c) The expression changes of ABCG2 in rat tracheal epithelium treated by 5‐FU alone (original magnification ×400).
The changes in the DMSO vehicle control group were the same as the group treated by 5‐FU alone (data not shown).
These observations indicate that blocking Notch signalling prior to 5‐FU injury leads to exhaustion of tracheal stem cells and disruption of the recovery process. Moreover, activating Notch signalling before injury mobilized tracheal stem cells in normal rat tracheal epithelium and promoted the recovery process.
Discussion
Immediately after treatment with 5‐FU, the normally proliferating tracheal epithelium desquamates, and only a few cells in resting phase of the cell cycle remain in the basal membrane. It has been shown that the cells in resting phase mainly include two types (34): terminally differentiated cells that will never divide and ultimately senesce, and stem cells that participate in tissue homeostasis by returning to the cell cycle following stimulation. It was previously shown that it is just through the proliferation and differentiation of tracheal stem cells that the tracheal epithelium returns to its original pseudostratified, ciliated, columnar epithelial morphology (5). Although tracheal stem cell has not been isolated succussfully till now, the regeneration process of tracheal epithelium provides insight into the proliferation and differentiation of tracheal stem cells.
The expression of ABCG2, CK19, and PCNA provides insight into the proliferative and differentiative statuses of the rat tracheal epithelium during recovery from 5‐FU‐induced injury. Based on the expression of these markers, the number of stem cell is likely to be low in the untreated rat tracheal epithelium. In the early stages of recovery from 5‐FU‐induced injury (0–6 h), the number of stem cells increased rapidly, while the number of differentiated cells increased slowly. In the late stages of recovery (12–48 h), the number of stem cell decreased while the number of differentiated cells increased quickly, especially after 24 h. PCNA expression remained relatively high from 3 to 24 h and peaked at 6 h, indicating that in both early and late stages of recovery, active proliferation occurred among tracheal epithelial cells. This observation, combined with the changes in the number of stem cells and differentiated cells during the recovery, suggests that proliferation of tracheal stem cells to undifferentiated cells was preponderant during the early stage, while proliferation towards differentiated cell types was preponderant in the late stage of recovery.
In the normal rat tracheal epithelium, expression of N3ICD and Hey1 was very low, suggesting that Notch activity is low, and this may lead to slow self‐renewal of the normal tracheal epithelium. During the injury recovery, changes in Notch activity were similar to changes in expression levels of ABCG2 and PCNA; moreover, immunofluorescence to the serial sections showed that most ABCG2‐positive cells were also Notch3‐nuclear positive which strongly suggesting that Notch signalling pathway plays a correlative role in the maintenance of an undifferentiated state and the promotion of proliferation among a population of tracheal epithelial cells. Consistent with this proposal, reduction of Notch signalling led to reduced proliferation and increased differentiation of tracheal stem cells; in contrast, persistent activation of Notch signalling led to increased proliferation and reduced differentiation of tracheal epithelial cells. As a consequence, under both conditions, the tracheal epithelium was unable to restore its normal structure at the same rate as the group with intact Notch signalling. These observations suggest that intact Notch signalling pathway is indispensable for the regeneration of tracheal epithelium and play a causal role in the maintenance and proliferation of tracheal stem cells.
Additional studies confirmed these conclusions. Pre‐treating normal tracheas with DAPT before 5‐FU treatment led to the exhaustion of tracheal stem cells, leaving a shortage behind to participate in regeneration. Similarly, pre‐treating normal tracheas with Jag‐1 prior to 5‐FU treatment mobilized tracheal stem cells, and thereby promoting the recovery process. These findings strongly support the hypothesis that Notch signalling regulates regeneration of the tracheal epithelium through regulation of tracheal stem cell fate.
Notch signalling is activated by Jagged ligands expressed on neighbouring cells. The expression of Jagged1 was up‐regulated and reached peak expression 6 h after removal of 5‐FU, and decreased gradually thereafter, returning to normal levels by 48 h. Interestingly, changes in expression of Jagged1 mirrored changes in Notch signalling. However, it is not clear why Jagged1 levels changed. Notch signalling has been shown to interact with other signalling pathways such as TGF‐β and Wnt (35, 36, 37, 38). Recently, Jagged1 was shown to be a target of Wnt signalling (39), suggesting a possible mechanism for increased Jagged1 expression during early injury recovery in our model. Immunofluorescence showed that most Jagged1‐positive cells were interspersed with nuclear Notch3‐positive cells which is consist with the idea of lateral inhibition (40, 41). Interestingly, we also found that changes in Notch signalling mirrored changes in ABCG2 expression and most ABCG2‐positive cells were also Notch3 nuclear‐positive cells during injury recovery; meanwhile, most Jagged1‐positive cells were adjacent to Notch3 nuclear‐positive cells and distributed to the basal side of the epithelium. These observations are consistent with the results of others that cells with active Notch signalling are stem cells, while those expressing Jagged ligands were progenitors (42). Although future studies are warranted, we predict that injury induces upstream signalling causing up‐regulation of Jagged1, which in turn leads to Hey1‐mediated Notch signalling and changes in the proliferation and differentiation of tracheal stem cells. This process ultimately contributes to repair and injury recovery in the tracheal epithelium.
Taken together, our data suggest a model in which Notch signalling maintains the slow self‐renewal and homeostasis of the normal tracheal epithelium. After injury induced by 5‐FU, however, the injury recovery process is achieved through the proliferation and differentiation of tracheal stem cells. This process appears to be regulated by a complex signalling network, including the Notch signalling pathway, which is critical for maintaining an undifferentiated state and promoting the undifferentiated proliferation among a population of tracheal epithelial cells.
Acknowledgement
This work was supported by National Natural Science Foundation of China (grant 30170407 to X.‐S. Jia).
References
- 1. Man SFP, Hulbert WC (1988) Airway repair and adaptation to inhalation injury In: Loke J, ed. Pathophysiology and Treatment of Inhalation Injuries. Lung Biology in Health and Disease. New York: Dekker, pp. 1–47. [Google Scholar]
- 2. Donowitz GR, Quesenberry P (1986) 5‐Fluorouracil effect on cultured murine stem cell progeny and peripheral leukocytes. Exp. Hematol. 14, 207–214. [PubMed] [Google Scholar]
- 3. Stewart FM, Temeles D, Lowry P, Thraves T, Grosh WW, Quesenberry PJ (1993) Post‐5‐fluorouracil human marrow: stem cell characteristics and renewal properties after autologous marrow transplantation. Blood 81, 2283–2289. [PubMed] [Google Scholar]
- 4. Paiushina OV, Damaratskaia EI, Bueverova EI, Nikonova TM, Butorina NN, Molchanova EA et al (2006) Analysis of sensitivity of stromal stem cells (CFU‐f) from rat bone marrow and fetal liver to 5‐fluorouracil. Izv. Akad. Nauk. Ser. Biol. 6, 660–666. [PubMed] [Google Scholar]
- 5. Ding Q, Jia XS (2004) Observation of rat tracheal stem cells during the injure and regeneration induced by 5‐FU. Acta Anat. Sinica 3, 106–108. [Google Scholar]
- 6. Wharton KA, Johansen KM, Xu T, Artavanis‐Tsakonas S (1985) Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF‐like repeats. Cell 43, 567–581. [DOI] [PubMed] [Google Scholar]
- 7. Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch MA et al (1990) Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF‐homologous genes in Drosophila. Cell 61, 523–534. [DOI] [PubMed] [Google Scholar]
- 8. Schroeter EH, Kisslinger JA, Kopan R (1998) Notch‐1 signalling requires ligand‐induced proteolytic release of intracellular domain. Nature 393, 382–386. [DOI] [PubMed] [Google Scholar]
- 9. Brou C, Logeat F, Gupta N, Bessia C, Lebail O, Doedens JR et al (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin‐metalloprotease TACE. Mol. Cell 5, 207–216. [DOI] [PubMed] [Google Scholar]
- 10. Kitagawa M, Oyama T, Kawashima T, Yedvobnick B, Kumar A, Matsuno K et al (2001) A human protein with sequence similarity to Drosophila mastermind coordinates the nuclear form of notch and a CSL protein to build a transcriptional activator complex on target promoters. Mol. Cell. Biol. 21, 4337–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lai EC (2002) Keeping a good pathway down: transcriptional repression of Notch pathway target genes by CSL proteins. EMBO Report 3, 840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Struhl G, Adachi A (1998) Nuclear access and action of Notch in vivo . Cell 93, 649–660. [DOI] [PubMed] [Google Scholar]
- 13. Iso T, Kedes L, Hamamori Y (2003) HES and HERP families: multiple effectors of the Notch signaling pathway. J. Cell. Physiol. 194, 237–255. [DOI] [PubMed] [Google Scholar]
- 14. Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. (2005) Roles of bHLH genes in neural stem cell differentiation. Exp. Cell Res. 306, 343–348. [DOI] [PubMed] [Google Scholar]
- 15. Kan T, Tominari Y, Rikimaru K, Morohashi Y, Natsugari H, Tomita T et al (2004) Parallel synthesis of DAPT derivatives and their gamma‐secretase‐inhibitory activity. Bioorg. Med. Chem. Lett. 14, 1983–1985. [DOI] [PubMed] [Google Scholar]
- 16. Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P et al (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780. [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK et al (2004) Involvement of Notch signaling in hippocampal synaptic plasticity. Proc. Natl. Acad. Sci. USA 101, 9458–9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weijzen S, Velders MP, Elmishad AG, Bacon PE, Panella JR, Nickoloff BJ et al (2002) The Notch ligand Jagged‐1 is able to induce maturation of monocyte‐derived human dendritic cells. J. Immunol. 169, 4273–4278. [DOI] [PubMed] [Google Scholar]
- 19. Sparrow DB, Clements M, Withington SL, Scott AN, Novotny J, Sillence D et al (2002) Diverse requirements for Notch signalling in mammals. Int. J. Dev. Biol. 46, 365–374. [PubMed] [Google Scholar]
- 20. Dzierzak E (2003) Ontogenic emergence of definitive hematopoietic stem cells. Curr. Opin. Hematol. 10, 229–234. [DOI] [PubMed] [Google Scholar]
- 21. Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami‐Yamaguchi E et al (2003) Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18, 699–711. [DOI] [PubMed] [Google Scholar]
- 22. Hadland BK, Huppert SS, Kanungo J, Xue Y, Jiang R, Gridley T et al (2004) A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood 104, 3097–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duncan AW, Rattis FM, Dimascio LN, Congdon KL, Pazianos G, Zhao C et al (2005) Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 6, 314–322. [DOI] [PubMed] [Google Scholar]
- 24. Androutsellis‐Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW et al (2006) Notch signalling regulates stem cell numbers in vitro and in vivo . Nature 442, 823–826. [DOI] [PubMed] [Google Scholar]
- 25. Stanger BZ, Datar R, Murtaugh LC, Melton DA (2005) Direct regulation of intestinal fate by Notch. Proc. Natl. Acad. Sci. USA 102, 12443–12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shawber C, Nofziger D, Hsieh JJ, Lindsell C, Bögler O, Hayward D, Weinmaster G (1996) Notch signaling inhibits muscle cell differentiation through a CBF1‐independent pathway. Development 122, 3765–3773. [DOI] [PubMed] [Google Scholar]
- 27. Lowell S, Jones P, Le Roux I, Dunne J, Watt FM (2000) Stimulation of human epidermal differentiation by delta‐notch signalling at the boundaries of stem‐cell clusters. Curr. Biol. 10, 491–500. [DOI] [PubMed] [Google Scholar]
- 28. Wang LL, Jia LL, Jia XS (2006) Expression of ABCG2 transporter during tracheal regeneration in rats. Acta Anat. Sinica 37, 163–167. [Google Scholar]
- 29. Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ et al (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side‐population phenotype. Nat. Med. 7, 1028–1034. [DOI] [PubMed] [Google Scholar]
- 30. Scharenberg CW, Harkey MA, Torok‐Storb B (2001) The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 99, 507–512. [DOI] [PubMed] [Google Scholar]
- 31. Herrmann H, Harris JR (1998) Intermediate Filaments. New York: Plenum Press, p. 205. [Google Scholar]
- 32. Takasaki Y, Deng JS, Tan EM (1981) A nuclear antigen associated with cell proliferation and blast transformation. J. Exp. Med. 154, 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bravo R, Frank R, Blundell PA, Macdonald‐Bravo H (1987) Cyclin/PCNA is the auxiliary protein of DNA polymerase‐delta. Nature 326, 515–517. [DOI] [PubMed] [Google Scholar]
- 34. Lodish H, Baltimore D, Berk A, Zipursky L, Matsudaira P, Darnell J (2004) Molecular Cell Biology, 5th edn. New York: Freeman Co, pp. 855–856. [Google Scholar]
- 35. Ross DA, Kadesch T (2001) The notch intracellular domain can function as a coactivator for LEF‐1. Mol. Cell. Biol. 21, 7537–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio‐Donahue CA et al (2003) Notch mediates TGF alpha‐induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 3, 565–576. [DOI] [PubMed] [Google Scholar]
- 37. Zavadil J, Cermak L, Soto‐Nieves N, Bottinger EP (2004) Integration of TGF‐beta/Smad and Jagged1/Notch signalling in epithelial‐to‐mesenchymal transition. EMBO J. 23, 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I et al (2005) Activation of Notch1 signaling is required for beta‐catenin‐mediated human primary melanoma progression. J. Clin. Invest. 115, 3166–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Katoh M, Katoh M (2006a) Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int. J. Mol. Med. 17, 681–685. [PubMed] [Google Scholar]
- 40. Cabrera CV (1990) Lateral inhibition and cell fate during neurogenesis in Drosophila: the interactions between scute, Notch and Delta. Development 110, 733–742. [PubMed] [Google Scholar]
- 41. Schweisguth F (1995) Suppressor of Hairless is required for signal reception during lateral inhibition in the Drosophila pupal notum. Development 121, 1875–1884. [DOI] [PubMed] [Google Scholar]
- 42. Katoh M, Katoh M (2006b) NUMB is a break of WNT‐Notch signaling cycle. Int. J. Mol. Med. 18, 517–521. [PubMed] [Google Scholar]


