Abstract
Objectives: The aim of this study was to evaluate whether hypoxia and/or erythropoietin would be able to modulate proliferation/differentiation processes of rat and human myoblasts.
Materials and methods: Rat L6 and primary human myoblasts were grown in 21% or 1% O2 in the presence or absence of recombinant human erythropoietin (RhEpo). Presence of erythropoietin receptors (EpoR) was assayed using RT‐PCR and Western blotting techniques. Cell proliferation was evaluated by determining the doubling time and kinetics of cultures by counting cells. Cell differentiation was analysed by determining myogenic fusion index using antibodies against the myosin heavy chain. Expression of myogenin and myosin heavy chain (MHC) proteins were evaluated using the Western blotting technique.
Results: After 96 h culture in growth medium for 2.5 and 9 h, doubling time of L6 and human primary myoblasts respectively, had increased in 1% O2 conditions (P < 0.01). Kinetics of culture showed alteration in proliferation at 72 h in L6 myoblast cultures and at 4 days in human primary myoblasts. The myogenic fusion index had reduced by 30% in L6 myoblasts and by 20% in human myoblasts (P < 0.01). Expression of myogenin and MHC had reduced by around 50%. Despite presence of EpoR mRNA and protein, RhEpo did not counteract the effects of hypoxia either in L6 cells or in human myoblasts.
Conclusions: The data show that exposure to hypoxic conditions (1% O2) of rat and human myoblasts altered their proliferation and differentiation processes. They also show that Epo is not an efficient growth factor to counteract this deleterious effect.
Introduction
Mature skeletal muscles develop cellular and molecular adaptations to low oxygen conditions. A classical adverse response to prolonged hypobaric hypoxia is loss of muscle mass, at least partly accounted for by changes in protein metabolism (1). Adult skeletal muscle has the remarkable capacity to regenerate after injury, and the effects of hypoxia on newly formed myofibres may also be questioned. It has been shown previously that ischaemia negatively affected the size of newly developed muscle fibres (2). Such a specific effect of hypoxia could be a result of changes in protein metabolism and/or altered activation, proliferation and fusion of myogenic precursor cells. It has been reported that myoblast differentiation is altered by decreased O2 availability in the C2C12 cell line (3) and the influence of hypoxia on myogenic cell properties needs to be examined. In media with 3% and 5% O2, proliferation of myogenic precursor cells increased compared to cultures grown in medium with 21% O2 (3, 4). However, Di Carlo et al. indicated that hypoxia (1% O2) inhibits both growth and differentiation of C2C12 myoblasts by inducing degradation of MyoD in the proteasome. At levels below 2% O2, expression of MyoD and myogenin is affected, resulting in delay of myogenic cell fusion and myosin heavy chain (MHC) expression (5).
Erythropoietin (Epo) is known to be involved in regulation of red blood cell mass, depending on oxygen availability. This glycoprotein hormone is mostly produced by fibroblast‐like type 1 renal interstitial cells in the adult (6), but also in several other tissues (reviewed (7)). Use of null‐mutant animals for Epo or its receptor EpoR, as well as studies dealing with expression of EpoR have demonstrated a role for Epo as a growth factor in vascular tissue or as protector against ischaemia in the nervous system (8, 9).
In humans, the effects of Epo on skeletal muscles have been suggested to be ergogenic, as in some athletes, Epo improves performance much more than expected from the increment in haematocrit (10). EpoR has been recently identified in C2C12 muscle cells (11), but so far, the role of Epo–EpoR interactions in muscle cells has been poorly investigated. In both C2C12 cells and primary cultures of mouse myoblasts, Epo activates proliferation of myogenic precursor cells and inhibits myogenic cell differentiation. During proliferation, the earliest markers of myogenic commitment (MyoD/Myf5) and a marker of early cell differentiation (myogenin) are up‐regulated (11); these data indicate that Epo plays a growth factor role that enhances myogenic precursor cell accumulation, suggesting that Epo might be involved in muscle development and repair (11). More recently, Rotter et al. (12) have shown that injection of recombinant human Epo (RhEpo) improves the regeneration process of rat soleus muscle. Conversely, Lundby et al. (13) have demonstrated that injection of RhEpo into human muscles has no effect on muscle structure and capillarity.Taken together, these results suggest a role for both hypoxia and Epo on development and repair of skeletal muscle, but nothing is known concerning their combined effects.
From these data, we can propose the hypothesis that if severe hypoxia inhibits mammalian myoblast proliferation and differentiation processes, Epo could counteract this deleterious effect. To evaluate this hypothesis, effects of hypoxia (1% O2) on proliferation and differentiation of rat L6 and human myoblasts were determined in presence or absence of various concentrations of RhEpo. Results show that hypoxic exposure considerably alters both myoblast proliferation and differentiation and that Epo is not efficient at counteracting this deleterious effect.
Materials and methods
Reagents, cells
For our experiments, we used both NeoRecormon (Roche, Meylan, France) and Eprex (Janssen‐cilag, Issy les Moulineaux, France) RhEpo. Both these RhEpo resources were tested by injecting 600 IU/kg (12 IU) into mice which were able to increase haematocrit. In culture experiments, a concentration range of 0.5–10 IU/mL was tested and as a result, 2 mL of medium was used.
Myoblasts from the L6 line were supplied by ATCC (US). Primary human satellite cells were isolated from muscle biopsies and were enriched using an antibody directed against NCAM/CD56 as described previously (14). As proliferative and differentiation capacities were shown to be stable between population doubling (PD) 1 and PD 15, all experiments were carried out between PD 7 and PD 12. Myogenicity was regularly checked by immunostaining using anti‐desmin antibody and was stable at 95%.
Cell culture
Human myoblasts were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 4 mm l‐glutamine, 1 mm sodium pyruvate, 10% of foetal bovine serum (FBS), 1% ultroser (Pall, Cergy, France), were seeded on collagen‐coated dishes (growth medium, GM). They were allowed to differentiate in DMEM medium supplemented with 4 mm l‐glutamine, 1 mm sodium pyruvat, 1% of ultroser and 2% FBS (differentiation medium, DM).
Rat myoblasts from the L6 line were grown in DMEM supplemented with 4 mm l‐glutamine, 1 mm sodium pyruvate and 10% of FBS (GM). DM was obtained by supplementing DMEM medium with 4 mm l‐glutamine, 1 mm sodium pyruvate and 2% FBS.
Hypoxic conditions were obtained using a chamber in which air was flushed out by 95% N2/5% CO2 until 1% O2 is attained. The percentage of oxygen was controlled using an O2 analyser (QS System, Rendsburg, Germany). Culture media were incubated for at least 6 h in hypoxic conditions before use.
Cell proliferation. Dose–response to Epo (0.5–10 U/mL) was performed as follows; myoblasts were seeded at a concentration of 30 × 103 cells per well in 12‐well plates. RhEpo doses were added to the medium at the time of seeding and at every 48 h of culture. At 96 h culture, cells were counted using a cell counter (ZX Coulter Beckman, Villepinte, France) and ‘Malassez cell’. Doubling time was calculated as t = 96/((ln y/x)/ln2) where y is number of cells after 96 h culture and x the number of seeded cells. Kinetics were obtained by seeding 30 × 103 cells/well in 12‐well plates. RhEpo doses were added to the medium at the time of seeding and at every 48 h of culture. Every 24 h, double counting was performed – first using a Coulter counter (ZX Coulter Beckman) then using the Malassez cell method after trypan blue staining. Presented data are results of three different cultures.
Cell differentiation. For differentiation experiments, cells were allowed to grow until they reached 80% confluence in GM at 21% O2. They were then incubated for 96 h in DM one part in 21% O2 and the other part in 1% O2. To ascertain that the DM was equilibrated at 1% O2, it was pre‐incubated for at least 6 h in 1% O2 before incubation with cells. As Ogilvie et al. (11) have demonstrated that RhEpo is able to induce accumulation of myogenic precursor cells (myogenin positive) in GM, RhEpo doses were added at the time of seeding and at 48 h of culture in GM. Myoblasts were then transferred to DM medium at 80% confluence and were incubated in 21% O2 or 1% O2 in the first experiment. In the second experiment, RhEpo was added at the time of seeding and at the time of incubation in DM (data not shown). We did not observe any differences in results between these two protocols. Results presented were obtained with incubation of RhEpo at the time of seeding and at 48 h culture in GM. Ninety‐six hours after induction of differentiation, cells were rinsed with cold PBS, pH 7.4, and fixed in cold methanol. They were then immunostained using anti‐fast MHC (MY‐32; Sigma, Saint Quentin Fallavier, France) and counterstained in haematoxylin to visualize myotubes and nuclei in the culture. Anti‐fast MHC antibody recognized both fast MHC and developmental myosin heavy chains and then was able to stain all the myotubes. Stained sections were studied by light microscopy connected to a digital camera (Coolpix 990, Champigny/marne Nikon); Explora Nova image analysing software (Explora Nova Morpho, La Rochelle, France) was used to analyse the images. Myogenic fusion index was determined by dividing number of nuclei in multinucleate myotubes by total number of nuclei, in a given microscopic field. Ten fields per culture were counted in three independent cultures.
Western blotting
For whole cell extracts, cells were cultured for 48 h in GM for EpoR and for 96 h in DM, as described above for myogenin and MHC. They were washed three times in cold PBS and then treated with lysis buffer (50 mm TRIS pH 8; 150 mm NaCl, 1% Nonidet‐P40, 0.25% sodium desoxycholate and protease inhibitors). Protein samples were separated on 10% SDS/PAGE then transferred to a polyvinylidene difluoride membrane. Membranes were probed with 1:1000 dilution anti‐mouse EpoR rabbit polyclonal antibody (M‐20; Santa‐Cruz Biotechnology Inc., Santa‐Cruz, CA, USA), 1:200 dilution anti‐mouse myogenin rabbit polyclonal antibody (M‐225; Santa‐Cruz Biotechnology Inc.), 1:2000 dilution mouse anti‐fast MHC monoclonal antibody (MY‐32; Sigma), 1:2000 dilution mouse anti‐β‐actin monoclonal antibody (AC‐15; Sigma) followed by horseradish peroxidase‐coupled secondary antibodies, developed by ECL (Santa‐Cruz Biotechnology Inc.). The films were scanned using scan Epson stylus 3650 (Epson, Nanterre, France) and were analysed using ImageJ software (NIH, Bethesda, USA). The Western blotting results presented below are representative of those obtained on three different cultures.
RNA extraction and reverse transcription‐polymerase chain reaction
RNA was extracted from myoblasts cultured for 48 h in GM, and was purified using an RNeasy Mini kit (Qiagen, Courtaboeuf, France). Reverse transcription of RNA was performed using Superscript III (Invitrogen, Illkirch, France) according to the manufacturers’ instructions. RNA was incubated with 5 μm random hexamers for 50 min at 50 °C. PCR amplifications were performed with 75 ng cDNA incubated with 1.5 mm MgCl2, 0.2 mm dNTP, 2.5 U of Platinum® Taq DNA polymerase (Invitrogen) and 0.2 μm of each primer. Twenty‐four cycles were carried out at hybridization temperatures of 55 °C and 56 °C for rat and human EpoR respectively. Primers used were as follows: 5′‐CTCTTACCAGCTCGAAGGTGA‐3′ and 5′‐TCCAGGACCTCCACCCTTTGT‐3′ for Rat EpoR; and 5′‐ACCGTGTCATCCACATCAAT‐3′ and 5′‐GCCTTCAAACTCGCTCTCTG‐3′ for human EpoR.
PCR products were run through 2% agarose gel and were visualized using ethidium bromide. DNA was then bi‐directionally sequenced using an automated sequencer and dye‐terminator chemistry (ABI Prism DNA 310 machine, Applied Biosystems, Courtabeuf, France).
Statistical analysis
Statistical significances of data are presented as mean ±SEM. Effect of O2 and RhEpo on myoblast proliferation was evaluated using the two‐parameter test of analysis of variance (2D‐anova). The Newman–Keuls test was used for post hoc testing. All statistical analyses were carried out using Stastistica software (StatSoft Inc, Tulsa, OK, USA); all statistical testing considered 0.05 to indicate statistical significance.
Results
Rat and human myoblasts express the erythropoietin receptor
Both rat and human myoblasts expressed the erythropoietin receptor; it is known to be present in human skeletal muscle (13), but it had not yet been described in rat skeletal muscle cells. As we wanted to evaluate effects of Epo on both proliferation and differentiation of muscle cells when cultured in normoxic and hypoxic conditions, we first investigated whether the myoblasts used in this in vitro study, expressed it.
Reverse transcription‐polymerase chain reaction (RT‐PCR) was performed with total RNA extracted from both rat and human myoblast cultures. Analysis of the RT‐PCR products revealed a 363 bp fragment in L6 myoblasts and a 485 bp fragment in human primary myoblasts (Fig. 1a). Sequencing of these fragments identified the codes of rat and human EpoR cDNA respectively (data not shown).
Figure 1.
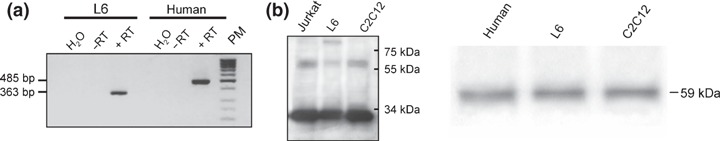
Identification of mRNA encoding EpoR and EpoR protein in rat L6 myoblasts and human myoblasts. L6 and human myoblasts were cultured for 48 h in growth medium and mRNA was extracted for PCR analyses. (a) Amplification products were visualized using agarose gel electropohoresis. Size of the amplification product of EpoR mRNA was 363 bp for rat myoblasts and 485 bp for human myoblasts, H2O PCR control water, RT control without reverse transcriptase, PM molecular weight. (b) Western blotting of Jurkat cells and C2C12 cells used as positive controls and L6 rat muscle, stained with anti‐mouse EpoR rabbit polyclonal antibody. Western blotting revealed the 59 KDa band in human myoblasts and L6 myoblasts (C2C12 cells used as control).
Western blotting analyses were carried out from protein extracts of 48 h myoblast cultures using M20 anti‐EpoR antibodies. As it is known that Jurkat and C2C12 cells express EpoR (Santa‐Cruz Biotechnology Inc. (11)), these cell types were used as positive controls. There were two major bands at 33 and 59 kDa in Jurkat cells, C2C12 and also in L6 myoblasts (Fig. 1b) and the 59 kDa band was also found in human myoblasts (Fig. 1b). We also detected a third band of around 100 kDa in L6 myoblasts (Fig. 1a). As the 59 kDa band has been described to correspond to the EpoR (15), the results indicate that it is expressed in 48 h cultures of rat and human myoblasts.
Effects of hypoxia and erythropoietin on human and rat myoblast growth
To evaluate the effects of hypoxia on proliferation of L6 and human myoblasts, cells were grown in an atmosphere of 21% (control) or 1% O2 (hypoxia) for 96 h and the kinetics of growth and doubling time of cells were assessed. It has been shown previously that stimulation of mouse C2C12 myoblasts with Epo enhances proliferation (11). We therefore tested whether Epo would able to stimulate or to restore rat and human myoblast proliferation under normoxia and hypoxia respectively.
For this purpose, we injected several doses of RhEpo ranging between 1 and 10 IU/mL, in culture medium, at both 21% and 1% O2. Fig. 2a1, provides results of the kinetics of cultures obtained with doses of 0, 2 and 5 IU RhEpo/mL. In 1% O2 cultures, L6 myoblast proliferation was reduced by a factor of 1.3 (P < 0.01) and 1.5 (P < 0.01) after 72 h and 96 h of culture respectively, regardless of dose of Epo injected in the media. After 96 h culture, doubling time of L6 myoblasts had increased by around 2.5 h (P < 0.01) at 1% O2 in the presence or absence of RhEpo in the media (Fig. 2a2). All kinetic parameters of the human primary myoblast culture decreased by a factor of 1.5 after 4 days of culture in hypoxia (P < 0.01) (Fig. 2b1). At this point their doubling time had lengthened by 9 h (P < 0.01) without any effect due to different doses of RhEpo tested (Fig. 2b2). These data indicate that hypoxia reduced rat and human myoblast growth and promoted doubling time of cells. They also show that RhEpo did not promote proliferation of these cells when they were growing in either normoxia or hypoxia.
Figure 2.
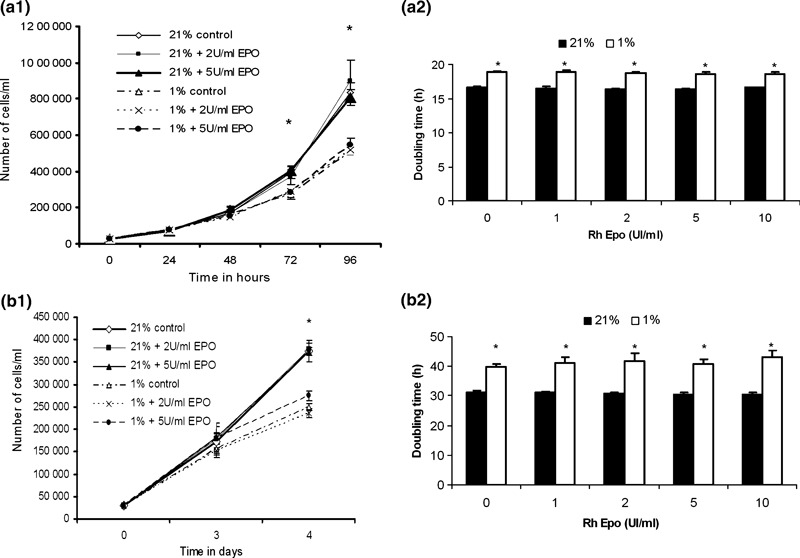
Effects of hypoxia and RhEpo on growth of cultured human and rat L6 myoblasts. (a1) Population growth kinetics of L6 myoblasts cultured in both 21% and 1% O2 conditions in growth medium containing 0, 2 and 5 IU/mL of RhEpo. RhEpo was added to the medium at T0 and T48h. (a2) Determination of doubling time of L6 myoblasts grown for 96 h in both 21% and 1% conditions in growth media containing RhEpo injected at doses ranging from 0 to 10 IU/mL at T0 and T48h. (b1) Population growth kinetics of human primary myoblasts cultured in both 21% and 1% O2 conditions in growth media containing 0, 2 and 5 IU/mL of RhEpo. RhEpo was added to medium at T0 and T48h. (b2) Determination of doubling time of L6 myoblasts grown for 96 h in both 21% and 1% conditions in growth medium containing RhEpo, injected at doses ranging from 0 to 10 IU/mL at T0 and T48. Values are mean ± SE of three separate experiments in triplicate. *P ≤ 0.01, 21% versus 1% O2.
Effects of hypoxia and erythropoietin on human and rat myoblast differentiation
The above results show that in hypoxia myoblast growth was altered, regardless of Epo availability. In the second part, effects of hypoxia and Epo on differentiation of rat and human myoblasts were examined when they differentiated in normoxic and hypoxic conditions, by determining the myogenic fusion index. Human and L6 myoblasts were allowed to grow in GM at 21% O2 and then were transferred to DM with 21% or 1% O2 and a range of RhEpo concentrations from 0 to 10 IU/mL. In 21% O2 cultures, the myogenic fusion indices were 40% and 60% for rat and human myoblasts respectively (Fig. 3a,b). In 1% O2, we observed that the myogenic fusion index was 30% lower and 20% lower (P < 0.01) in rat and human myoblasts respectively, independently of RhEpo doses (Fig. 3a,b). These data indicate that hypoxia reduced both rat and human myoblast fusion suggesting that it affects myoblast differentiation. They also indicate that Epo is not able to restore this effect.
Figure 3.
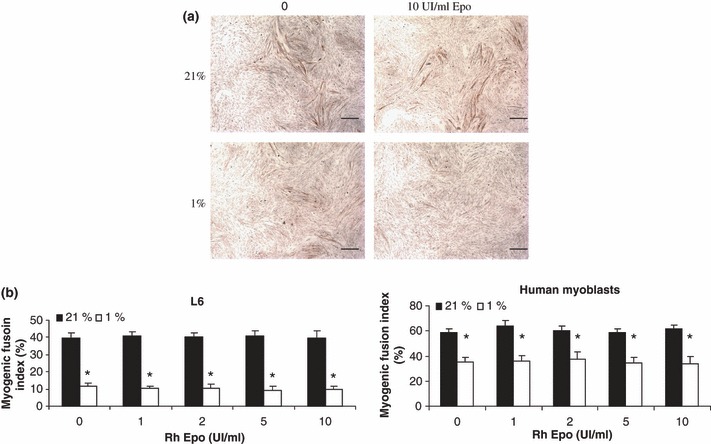
Effects of hypoxia and RhEpo on differentiation of human and rat L6 myoblasts. (a) Immunostaining of L6 myoblasts grown to 80% confluence in growth medium at 21% O2 containing 0 or 10 IU/mL RhEpo, and incubated for 96 h in differentiation medium for part one in 21% O2 and part two in 1% O2 in antibody raised against fast myosin heavy chain; counterstaining was carried out using haematoxylin, bar scale = 2mm. (b) Myogenic fusion index was determined by dividing number of nuclei in multinucleate myotubes by total number of nuclei in a given microscopic field. Ten fields per culture were counted in three independent cultures using MorphoPro software (Explora Nova). Data are presented as mean ± SEM. *P ≤ 0.01, 21% versus 1% O2.
Hypoxia altered expression of myogenin and fast myosin heavy chain
To confirm that hypoxia alters the differentiation of myoblasts and that Epo does not restore this effect, the expression of myogenin as an early marker of differentiation and of fast myosin heavy chain as a marker of late differentiation was analysed for rat myoblasts. Western blotting analyses of myogenin and fast myosin heavy chain showed that rat myoblasts expressed both the proteins in 21% and 1% O2 (4, 5). Quantitative analysis with β‐actin as standard revealed reduced expression of each protein by around 50% (P < 0.001) when L6 myoblasts were allowed to differentiate in medium containing 1% O2 (4, 5). When RhEpo was added at doses ranging from 1 to 10 IU/mL in the media, no qualitative nor quantitative modifications of myogenin and fast myosin heavy chain expression were detected for L6 myoblasts in medium containing 1% O2 (4, 5). These data show that hypoxia reduced rat myoblast differentiation and that Epo was not able to restore this process.
Figure 4.
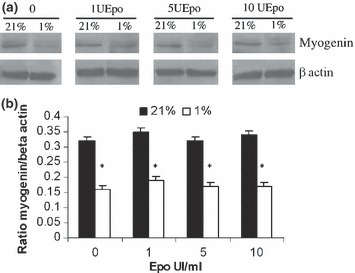
Effects of hypoxia and RhEpo on expression of myogenin and fast myosin heavy chain. L6 myoblasts were cultured for 72 h in growth medium containing 0–10 IU/mL RhEpo at 21% O2 and for 96 h in differentiation medium at 21% or 1% O2. (a) Western blotting using anti‐mouse myogenin rabbit polyclonal antibody and beta‐actin. (b) Quantification of myogenin expression was carried out as ratio of myogenin/β‐actin. Western blots are representative of those obtained from three different cultures. Data are presented as mean ± SEM. *P ≤ 0.01, 21% versus 1% O2.
Figure 5.
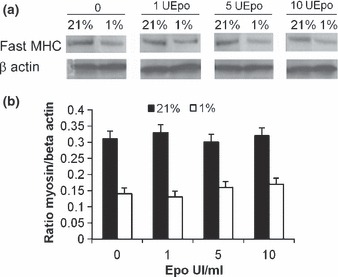
Expression of fast myosin heavy chain altered by hypoxia: RhEpo did not prevent this. L6 myoblasts were cultured for 72 h in growth medium containing 0–10 IU/mL RhEpo at 21% O2 and for 96 h in differentiation medium at 21% or 1% O2. (a) Western blotting shows expression of fast MHC and β‐actin in L6 myoblasts non‐treated (0) or treated, with a range from 1 to 10 IU/mL of RhEpo. (b) Quantification of myosin heavy chain expression was carried out as ratio of myogenin/β‐actin. Western blots are representative of those obtained from three different cultures. Data are presented as mean ± SEM. *P ≤ 0.01, 21% versus 1% O2.
Discussion
It has been established previously that hypoxia (1% O2) inhibits both C2C12 myoblast proliferation and differentiation (5). Conversely, it has been suggested that Epo would be able to improve accumulation of myogenic precursor cells. Therefore, the aim of this study was to evaluate the effects of hypoxia (1%) and Epo on both proliferation and differentiation of muscle cells. Our results show (i) that hypoxia increased the doubling time and reduced myogenic fusion indices in both human primary and L6 myoblast cultures, (ii) that human and L6 rat myoblasts expressed both EpoR mRNA and protein and (iii) that Epo did not prevent the effects of hypoxia.
Previous studies have established that with 3% and 6% O2 present, corresponding to physiological O2 concentrations, proliferation of mouse myogenic precursor cells increases compared to that with 21% O2 (3, 4). Under 2% O2, which corresponds to physiological hypoxia (3), differentiation of mouse skeletal muscle cells changes, and in 1% O2, both proliferation and differentiation of mouse myoblast are inhibited (5). Our results show that in 1% O2, proliferation of human and rat muscle cells was reduced, but not inhibited. As Di Carlo et al. (5) found impaired culture of C2C12 cells under the same conditions, 1% O2 seems critical for adaptation of skeletal muscle cells. However, after 96 h of culture, differentiation of both human and L6 muscle cells was dramatically impaired in 1% O2. Our results demonstrate that 1% O2 altered myogenin expression, which resulted in altered myotube formation and decrease in size of newly‐formed myotubes. Thus, our results are in agreement with experiments performed by Yun et al. and Gustafsson et al. (3, 16). In the present study, we found that human myoblasts, similar to rat and mouse myoblasts, are sensitive to oxygen conditions.
Also here, we have identified presence of both EpoR mRNA and EpoR protein in rat and human skeletal muscle cells. These results add to the list of cells that express EpoR, that is, endothelial cells (8, 17), cardiomyocytes (18, 19), neurons and astrocytes in rat and mouse (20, 21) and mouse skeletal muscle cells (11). Several antibodies have been described to be directed against EpoR (15). Among them, the monoclonal antibody M20 (anti‐mouse EpoR, SC697) is suitable for detection of EpoR using the Western blotting technique (15). With this antibody we discovered two major bands at 33 and 59 kDa in Jurkat cells (used as a control) as well as in rat myoblasts, and we detected the 59 kDa band in human myoblasts also. It is worth noting that a third band at 100 kDa was detected in L6 myoblasts. The 30 kDa polypeptide might correspond to a soluble form of the receptor (33 kDa) that could be involved in regulation of Epo/EpoR binding to the cell surface (22) and the band at 59 kDa has been described to be the EpoR (15). Based on the molecular mass, the 100 kDa polypeptide could correspond to an artefactual dimerization of EpoR during protein extraction procedures. This possibility is unlikely as Elliot et al. (15) have shown that in some tumour cell lines, the 100 kDa protein was neither EpoR nor derived from EpoR.
Our results did not demonstrate any effect of Epo on L6 rat myoblast proliferation and differentiation when cultured in either 21% or 1% O2. We conclude that in our experimental conditions, Epo does not counteract the blunting effect of hypoxia either on proliferation or on differentiation of human or rat muscle cells. These results are not in agreement with those of Ogilvie et al. (11) who demonstrated a role of 1 IU/mL Epo on accumulation of myogenic precursor cells in the C2C12 line. However, our results are in agreement with those of Lundby et al. (13) who identified EpoR in human skeletal muscle, but did not find any effect of acute or chronic RhEpo treatment on skeletal muscle structure and capillarity. Moreover, Chanseaume et al. (23) have demonstrated that RhEpo treatment did not improve engraftment of skeletal myoblasts in infarcted myocardium. In fact, these authors have shown that grafted skeletal myoblasts were sensitive to ischaemia irrespective of their treatment with RhEpo. In the same way, preliminary results of our group do not demonstrate any protection by RhEpo (at doses ranged between 0.5 and 10 IU/mL) against H2O2‐induced apoptosis in human myoblast cultures.
Finally, EpoR could be involved in other roles. Lately, Rotter et al. (12) have demonstrated that a high dose of RhEpo (50 IU/mL) was able to slightly reduce apoptosis of L8 myoblasts, induced by serum deprivation in the medium. Injections of RhEpo in rats induced transition from fast glycolytic phenotype to slow oxidative phenotype (24). These results suggest that the Epo receptor might rather be involved in protection of myoblasts from substrate deprivation or otherwise in regulation of skeletal muscle cell metabolism.
In conclusion, we show that hypoxia (1% O2) impairs both proliferation and differentiation processes of rat L6 and primary human myoblasts by altering expression of myogenin, the myosin heavy chain and myoblast fusion. This suggests a role of oxygen availability in both accumulation and fusion of human myoblasts, which could explain in part the smaller size of re‐formed fibres in ischaemic muscles. However, our study did not support the idea that Epo is an efficient growth factor for myoblast proliferation and differentiation process.
Acknowledgements
We would like to thank Prof. J Mercier and Dr G. Carnac for the kind gift of human myoblasts and Prof. A. X. Bigard for valuable comments on the manuscript. This study was supported by “l’Association Française de lutte contre les Myopaties” (AFM).
References
- 1. Preedy VR, Smith DM, Sugden PH (1985) The effects of 6 hours of hypoxia on protein synthesis in rat tissues in vivo and in vitro. Biochem. J. 228, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scholz D, Thomas S, Sass S, Podzuweit T (2003) Angiogenesis and myogenesis as two facets of inflammatory post‐ischemic tissue regeneration. Mol. Cell. Biochem. 246, 57–67. [PubMed] [Google Scholar]
- 3. Yun Z, Lin Q, Giaccia AJ (2005) Adaptive myogenesis under hypoxia. Mol. Cell. Biol. 25, 3040–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chakravarthy MV, Spangenburg EE, Booth FW (2001) Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscles. Cell. Mol. Life Sci. 58, 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Carlo A, De Mori R, Martelli F, Pompilio G, Capogrossi MC, Germani A (2004) Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. J. Biol. Chem. 279, 16332–16338. [DOI] [PubMed] [Google Scholar]
- 6. Maxwell PH, Osmond MK, Pugh CW, Heryet A, Nicholls LG, Tan CC et al. (1993) Identification of the renal erythropoietin‐producing cells using transgenic mice. Kidney Int. 44, 1149–1162. [DOI] [PubMed] [Google Scholar]
- 7. Maiese K, Li F, Chong ZZ (2005) New avenues of exploration for erythropoietin. JAMA 293, 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M (1990) Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc. Natl. Acad. Sci. USA 87, 5978–5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M et al. (1998) In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl. Acad. Sci. USA 95, 4635–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scoppetta C, Grassi F (2004) Erythropoietin: a new tool for muscle disorders? Med. Hypotheses 63, 73–75. [DOI] [PubMed] [Google Scholar]
- 11. Ogilvie M, Yu X, Nicolas‐Metral V, Pulido SM, Liu C, Ruegg UT et al. (2000) Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J. Biol. Chem. 275, 39754–39761. [DOI] [PubMed] [Google Scholar]
- 12. Rotter R, Menshykova M, Winkler T, Matziolis G, Stratos I, Schoen M et al. (2008) Erythropoietin improves functional and histological recovery of traumatized skeletal muscle tissue. J. Orthop. Res. 26, 1618–1626. [DOI] [PubMed] [Google Scholar]
- 13. Lundby C, Hellsten Y, Jensen MB, Munch AS, Pilegaard H (2008) Erythropoietin receptor in human skeletal muscle and the effects of acute and long‐term injections with recombinant human erythropoietin on the skeletal muscle. J. Appl. Physiol. 104, 1154–1160. [DOI] [PubMed] [Google Scholar]
- 14. Kitzmann M, Bonnieu A, Duret C, Vernus B, Barro M, Laoudj‐Chenivesse D et al. (2006) Inhibition of Notch signaling induces myotube hypertrophy by recruiting a subpopulation of reserve cells. J. Cell. Physiol. 208, 538–548. [DOI] [PubMed] [Google Scholar]
- 15. Elliott S, Busse L, Bass MB, Lu H, Sarosi I, Sinclair AM et al. (2006) Anti‐Epo receptor antibodies do not predict Epo receptor expression. Blood 107, 1892–1895. [DOI] [PubMed] [Google Scholar]
- 16. Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J et al. (2005) Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 9, 617–628. [DOI] [PubMed] [Google Scholar]
- 17. Kertesz N, Wu J, Chen TH, Sucov HM, Wu H (2004) The role of erythropoietin in regulating angiogenesis. Dev. Biol. 276, 101–110. [DOI] [PubMed] [Google Scholar]
- 18. Wagner KF, Katschinski DM, Hasegawa J, Schumacher D, Meller B, Gembruch U et al. (2001) Chronic inborn erythrocytosis leads to cardiac dysfunction and premature death in mice overexpressing erythropoietin. Blood 97, 536–542. [DOI] [PubMed] [Google Scholar]
- 19. Smith KJ, Bleyer AJ, Little WC, Sane DC (2003) The cardiovascular effects of erythropoietin. Cardiovasc. Res. 59, 538–548. [DOI] [PubMed] [Google Scholar]
- 20. Masuda S, Nagao M, Takahata K, Konishi Y, Gallyas F Jr, Tabira T et al. (1993) Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J. Biol. Chem. 268, 11208–11216. [PubMed] [Google Scholar]
- 21. Lappin TR, Maxwell AP, Johnston PG (2002) EPO’s alter ego: erythropoietin has multiple actions. Stem Cells 20, 485–492. [DOI] [PubMed] [Google Scholar]
- 22. Todokoro K, Kuramochi S, Nagasawa T, Abe T, Ikawa Y (1991) Isolation of a cDNA encoding a potential soluble receptor for human erythropoietin. Gene 106, 283–284. [DOI] [PubMed] [Google Scholar]
- 23. Chanseaume S, Azarnoush K, Maurel A, Bellamy V, Peyrard S, Bruneval P et al. (2007) Can erythropoietin improve skeletal myoblast engraftment in infarcted myocardium? Interact. Cardiovasc. Thorac. Surg. 6, 293–297. [DOI] [PubMed] [Google Scholar]
- 24. Cayla JL, Maire P, Duvallet A, Wahrmann JP (2008) Erythropoietin induces a shift of muscle phenotype from fast glycolytic to slow oxidative. Int. J. Sports Med. 29, 460–465. [DOI] [PubMed] [Google Scholar]


