Plazomicin is an aminoglycoside with activity against multidrug-resistant Enterobacteriaceae. Plazomicin is dosed on a milligram-per-kilogram-of-body-weight basis and administered by a 30-min intravenous infusion every 24 h, with dose adjustments being made for renal impairment and a body weight (BW) of ≥125% of ideal BW.
KEYWORDS: aminoglycosides, plazomicin, population pharmacokinetics
ABSTRACT
Plazomicin is an aminoglycoside with activity against multidrug-resistant Enterobacteriaceae. Plazomicin is dosed on a milligram-per-kilogram-of-body-weight basis and administered by a 30-min intravenous infusion every 24 h, with dose adjustments being made for renal impairment and a body weight (BW) of ≥125% of ideal BW. A population pharmacokinetic analysis was performed to identify patient factors that account for variability in pharmacokinetics and to determine if dose adjustments are warranted based on covariates. The analysis included 143 healthy adults and 421 adults with complicated urinary tract infection (cUTI), acute pyelonephritis, bloodstream infection, or hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia (HABP/VABP) from seven studies (phases 1 to 3). A three-compartment structural pharmacokinetic model with a zero-order rate constant for the intravenous infusion and linear first-order elimination kinetics best described the plasma concentration-time profiles. The base structural model included creatinine clearance (CLCR) as a time-varying covariate for clearance. The covariates included age, BW, height, body surface area, body mass index, sex, race, and disease-related factors. The ranges of the α-, β-, and γ-phase half-lives for the analysis population were 0.328 to 1.58, 2.77 to 5.38, and 25.8 to 36.5 h, respectively. Total and renal clearances in a typical cUTI or HABP/VABP patient were 4.57 and 4.08 liters/h, respectively. Starting dose adjustments for CLCR are sufficient for minimizing the variation in plasma exposure across patient populations; adjustments based on other covariates are not warranted. The results support initial dosing on a milligram-per-kilogram basis with adjustments for CLCR and BW. Subsequent adjustments based on therapeutic drug management are recommended in certain subsets of patients, including the critically ill and renally impaired.
INTRODUCTION
Gram-negative bacteria, including common members of the Enterobacteriaceae family, have become increasingly resistant to multiple antibiotics over the past decade (1–4). An infection with multidrug-resistant (MDR) pathogens can translate to increased mortality, health care costs, and hospital length of stay (3, 5–8). The limited treatment options currently available for infections due to MDR Enterobacteriaceae (9) pose an urgent threat to patients in health care settings; the development of new antibacterial agents is therefore a critical public health priority (1).
Aminoglycosides are an important class of antimicrobials for the treatment of serious bacterial infections due to their proven activity against Gram-negative and Gram-positive pathogens (10–15). Plazomicin is an aminoglycoside that was engineered to overcome aminoglycoside-modifying enzymes, the most common aminoglycoside resistance mechanism in Enterobacteriaceae (16). In vitro, plazomicin displays rapid bactericidal activity against MDR Enterobacteriaceae (17), including isolates that produce extended-spectrum β-lactamases, carbapenemases (18), and/or aminoglycoside-modifying enzymes (19, 20). Plazomicin is approved for the treatment of complicated urinary tract infections (cUTI), including acute pyelonephritis (AP), in patients who have limited or no alternative treatment options (21).
The clinical pharmacokinetic (PK) characteristics of plazomicin are generally consistent with those of other aminoglycoside antibiotics (22). Plazomicin displays linear and dose-proportional PK (23, 24), does not undergo metabolism, and is eliminated from the body primarily via urinary excretion of the parent drug (25). An in vitro, equilibrium dialysis experiment that evaluated plazomicin at 5, 50, and 100 μg/ml in human plasma showed that plasma protein binding is concentration independent and low (≈20%; data on file). In a human mass-balance study, 97.5% of the intravenously administered dose of plazomicin was recovered as the parent drug in the urine (25). Given the consistency in PK characteristics with other aminoglycoside antibiotics, plazomicin dosing in patient studies has generally followed typical aminoglycoside dosing recommendations (26, 27), which consider creatinine clearance (CLCR) to be a measure of renal function and use adjusted body weight (ABW) when body weight (BW) exceeds the ideal body weight (IBW). Thus, throughout the development program, plazomicin was administered on a milligram-per-kilogram-of-body-weight basis (with ABW in the two phase 3 studies), and dose adjustments were performed in patients based on CLCR. The recommended starting dose regimen in the drug product label (21) is plazomicin at 15 mg/kg administered every 24 h (q24h) as a 30-min intravenous infusion in patients with normal renal function or mild renal impairment (CLCR > 60 ml/min).
Given that therapeutic drug management (TDM) is recommended to optimize exposures and clinical outcomes in patients treated with aminoglycosides (28, 29), additional dose adjustments based on TDM were implemented in one of the phase 3 studies (30). In this study, patients with serious infection (bloodstream infection [BSI], hospital-acquired bacterial pneumonia [HABP], ventilator-associated bacterial pneumonia [VABP], cUTI, or AP) due to carbapenem-resistant Enterobacteriaceae (CRE) received starting doses based on CLCR and ABW or IBW. Plazomicin doses after the initial dose were determined based on TDM, in which dose adjustments were implemented to maintain the area under the plasma concentration-time curve (AUC) within a target range. AUC-based TDM was considered appropriate for this critically ill patient population; critical illness may be associated with a high PK variability of antibiotics, particularly those that are cleared by the kidneys (31–33).
Although not tested clinically, the plazomicin product label recommends trough concentration-based TDM in patients with cUTI who have a CLCR of >30 to 90 ml/min (i.e., mild to severe renal impairment) to maintain plasma trough concentrations below 3 μg/ml (21). Initial doses of plazomicin are based on CLCR and ABW or IBW in all patients with cUTI, and dose adjustments are necessary in renally impaired cUTI patients. Subsequent doses are informed by the trough concentrations of plazomicin. The rationale for this approach is that cUTI patients with renal impairment may be at increased risk of nephrotoxicity, and trough concentration-based TDM dose adjustments may mitigate this risk (21).
Herein, we describe an analysis that represents the culmination of several population PK modeling activities that were carried out to support the plazomicin clinical development program. The initial population PK model was developed using data from the phase 1 first-in-human study (23), and this model was then more fully elaborated upon with the availability of additional data from phase 1 and 2 studies in healthy subjects and cUTI patients (34–36). Development of the final population PK model, described herein, ensued upon completion of a phase 1 thorough QT study (24) and two phase 3 studies (37, 38). The analysis includes data from patients with cUTI, including AP, and patients with BSI or HABP/VABP due to CRE. The objective of this analysis was to identify patient factors that account for sources of variability in plazomicin PK and to determine if dose adjustments are warranted based on covariates.
RESULTS
PK analysis population.
Summary statistics of the baseline characteristics for the analysis population are presented in Table 1. The demographics were diverse and representative of the broader population of adult patients with cUTI/AP and serious infections caused by CRE.
TABLE 1.
Summary of subject characteristics of the PK analysis population
| Variablea | Values from the following studies: |
|||
|---|---|---|---|---|
| Phase 1 (n = 143) | Phase 2 (n = 92) | Phase 3 (n = 329) | Total (n = 564) | |
| Median (range) age (yr) | 29 (18–75) | 39.9 (18.3–77.4) | 64 (18–90) | 39 (18–90) |
| Median (range) BMI (kg/m2) | 25.9 (18.9–33.5) | 25.6 (16.9–38.8) | 26.9 (15.4–58.7) | 26.0 (15.4–58.7) |
| Median (range) BSA (m2) | 1.87 (1.49–2.44) | 1.70 (1.32–2.21) | 1.89 (1.29–2.58) | 1.86 (1.29–2.58) |
| Median (range) height (cm) | 172 (146–191) | 160 (142–183) | 168 (142–194) | 170 (142–194) |
| Median (range) CLCR (ml/min/1.73 m2) | 93.4 (7.37–159) | 81.3 (21.8–212) | 64.6 (8.7–226) | 90.2 (7.37–226) |
| Median (range) body weight (kg) | 74.6 (53.5–116) | 66.0 (42–100) | 76.0 (40.5–165) | 75.0 (40.5–165) |
| No. of subjects with the following characteristics/total no. of subjects (%): | ||||
| Male | 92/143 (64.3) | 15/92 (16.3) | 159/329 (48.3) | 266/564 (47.2) |
| Female | 51/143 (35.7) | 77/92 (83.7) | 170/329 (51.7) | 298/564 (52.8) |
| Race | ||||
| White | 101/143 (70.6) | 18/92 (19.6) | 324/329 (98.5) | 443/564 (78.5) |
| Black | 37/143 (25.9) | 13/92 (14.1) | 2/329 (0.608) | 52/564 (9.22) |
| Asian | 2/143 (1.4) | 21/92 (22.8) | 1/329 (0.304) | 24/564 (4.26) |
| American Indian/Alaskan Native | 1/143 (0.699) | 39/92 (42.4)b | 0 | 40/564 (7.09) |
| Other | 2/143 (1.4) | 1/92 (1.09) | 2/329 (0.608) | 5/564 (0.887) |
| Infection type | ||||
| cUTI | 0 | 40/92 (43.5) | 173/329 (52.6) | 213/564 (37.8) |
| AP | 0 | 52/92 (56.5) | 112/329 (34.0) | 164/564 (29.1) |
| BSI | 0 | 0 | 29/329 (8.81) | 29/564 (5.14) |
| HABP/VABP | 0 | 0 | 15/329 (4.56) | 15/564 (2.66) |
| Healthy | 143/143 (100) | 0 | 0 | 143/564 (25.4) |
| No. of subjects in the following renal function group/total no. of subjects (%)c: | ||||
| Normal renal function | 97/143 (67.8) | 39/92 (42.4) | 101/329 (30.7) | 237/564 (42.0) |
| Mild renal impairment | 32/143 (22.4) | 34/92 (37.0) | 120/329 (36.5) | 186/564 (33.0) |
| Moderate renal impairment | 8/143 (5.59) | 19/92 (20.7) | 101/329 (30.7) | 128/564 (22.7) |
| Severe renal impairment | 6/143 (4.2) | 0 | 7/329 (2.13) | 13/564 (2.30) |
| No. of subjects with the following characteristics/total no. of subjects (%): | ||||
| Positive pressure ventilation | 0 | 0 | 24/329 (7.29) | 24/564 (4.26) |
| Vasopressor used | 0 | 0 | 24/329 (7.29) | 24/564 (4.26) |
| History of traumae | 0 | 0 | 9/329 (2.74) | 9/564 (1.60) |
| Diabetes | 0 | 16/92 (17.4) | 47/329 (14.3) | 63/564 (11.2) |
The covariate analysis included the following continuous descriptors: age, body weight, height, BSA, and BMI. Evaluated categorical descriptors included sex, race, infection type, positive pressure ventilation, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, history of trauma, vasopressor use, and history of diabetes.
The phase 2 study case report form recorded geographic ancestry and grouped the following into one category: Americas (Native North American/Native South American/Canadian First Peoples). South American sites were high enrollers and likely account for many of the patients in this category.
Normal, CLCR ≥ 90 ml/min/1.73 m2; mild impairment, CLCR = 60 to 89 ml/min/1.73 m2; moderate impairment, CLCR = 30 to 59 ml/min/1.73 m2; severe impairment, CLCR < 30 ml/min/1.73 m2. In one of the phase 3 studies (study 007), 2/16 patients with mild renal impairment, 3/12 with moderate impairment, and 4/6 with severe impairment received continuous renal replacement therapy at some time during treatment with plazomicin.
Vasopressor use was defined as administration of adrenaline, dobutamine, etilefrine, isoproterenol, noradrenaline, or norepinephrine at any time during the study. Vasopressors were used as part of the medical management of shock in the critically ill patient population in study 007. Vasopressor use was handled as a categorical covariate (yes/no) to indicate use at any time during the study.
History of trauma was broadly defined and was commonly unrelated to the clinical condition at the time of plazomicin administration.
PK data description and outlier analysis.
The final population PK analysis data set included 564 subjects and 4,990 plazomicin plasma concentrations (see Table S1 in the supplemental material), which represented 98.4 and 97.0% of the possible subjects and plasma concentrations available for analysis, respectively. Reasons for sample exclusions included a plazomicin plasma concentration below the lower limit of quantification (BLQ) (46 samples) or designation as an outlier (106 samples).
Population PK analysis.
(i) Development of the structural population PK model. A three-compartment model with zero-order input and first-order elimination best described the pooled plasma plazomicin concentration-time data from the seven studies. Interindividual variability (IIV) was described for total clearance (CL), volume of the central compartment (Vc), distributional clearance to peripheral compartment 1 (CLd1), volume of peripheral compartment 1 (Vp1), distributional clearance to peripheral compartment 2 (CLd2), and volume of peripheral compartment 2 (Vp2) using log-normal parameter distributions. Residual variability was described using a combined additive-plus-constant coefficient of variation (CCV) error model. CLCR was evaluated as a time-varying covariate for CL in the base structural model, and it was determined that a sigmoidal Hill-type function best described the relationship between CL and CLCR. Handling of clearance due to continuous renal replacement therapy (CRRT) is described in Materials and Methods. Given that this model provided an unbiased fit to the data, alternative structural models were not explored further.
All model parameters were estimated with acceptable precision according to the asymptotic errors from the population PK model (standard error of the estimate [SEE], <67%). Goodness-of-fit plots for the base structural population PK model (data not shown) demonstrated that the three-compartment model including the relationship between CL and CLCR provided an excellent fit to the plasma plazomicin concentration-time data across renal function groups. There were no noticeable biases in the model fit when examining these plots, and there was good agreement between both the population mean (coefficient of determination [r2] = 0.738) and the individual post hoc (r2 = 0.935) predicted concentrations with the observed concentrations.
(ii) Covariate analysis. A complete summary of the parameter-covariate relationship determined to be the most statistically significant in each step of forward selection is provided in Table S2. The full multivariable model included 17 parameter-covariate relationships, in addition to the relationship between CL and CLCR, which was included a priori as part of the structural PK model.
IIV models for the full multivariable model showed that the distributions of each of the individual error terms were normally distributed and symmetric around zero. Revisions were made to the model as part of the full multivariable model assessment. The first step tested a full variance-covariance matrix. Various reduced models estimating different combinations of covariance terms were then evaluated at this stage of the analysis. Parsimony was achieved with the model in which the covariance terms between IIV on clearance (ηCL) and IIV on volume of the central compartment (ηVc), ηCL and IIV on the distributional clearance to peripheral compartment 1 (ηCLd1), and ηVc and ηCLd1 were estimated, resulting in a 364-unit decrease in the minimum value of the objective function (MVOF) (P < 0.000001). Subsequently, the model with the above-mentioned covariance terms was modified by adding separate error terms for each study phase (phase 1, 2, or 3); this model resulted in a 588-unit drop in the objective function (P < 0.000001). The final step of model refinement was to test interoccasion variability (IOV) on various model parameters; the occasions were categorized as days 1 to 2, 3 to 6, 6 to 9, and >9. The only IOV term that was found to be statistically significant was the one relating to CL. This refined model was used as the comparator model for backward elimination.
The full multivariable population PK model was then subjected to a backward elimination procedure in which each covariate effect in the model except CLCR was removed in a univariate fashion and tested for statistical significance (α = 0.001). Five rounds of backward elimination were required, and four parameter-covariate relationships (between infection type and Vp2 and between age and each of Vc, CLd1, and CL) were removed (Table S2).
(iii) Final covariate model refinement. After completing the backward elimination, the model was refined to make it more parsimonious. First, proportional shifts in CL for HABP/VABP and cUTI appeared to have only a minor impact (low fractional change) and were dropped from the model, which resulted in no statistically significant changes (an increase of ≈2 units in the MVOF). Second, proportional shifts in PK parameters based on infection type that had similar estimates (i.e., cUTI and AP, HABP/VABP and BSI) were evaluated as a single group and tested for statistical differences. Finally, the sieving coefficient was reestimated, as it had been fixed to the value from the base structural model (0.926) during covariate analysis; the resultant fitted value was 0.734.
The model that resulted from the steps described above was then subjected to a nonparametric bootstrap procedure. The relationships between Vp1 and vasopressor use, CLd1 and HABP/VABP infection type, CLd1 and BSI type, and Vp1 and each of HABP/VABP infection and BSI types were dropped from the model due to unacceptably poor precision based on the percent SEE.
(iv) Final population PK model. The final population PK model for plazomicin included a fixed zero-order input for the intravenous infusion and first-order elimination. CRRT CL (CLCRRT) was estimated only during those periods when CRRT was operative, utilizing the patient-specific and ultrafiltrate flow rate (UFR) and dialysate flow rate (DFR) and the estimated sieving coefficient. IIV was estimated for CL, Vc, CLd1, Vp1, CLd2, and Vp2 using exponential error models. Random IOV on CL was retained in the model, as it resulted in a statistically significant improvement in the MVOF, despite the small magnitude of the effect (coefficient of variation [CV], 3.59%), which suggests that CL is relatively stable across occasions. An additive-plus-CCV error model best described residual variability with separate CCV error terms for the phase 1 studies, the phase 2 study, and the phase 3 studies.
The population PK parameter estimates and their associated precision (percent SEE) for the fit of the three-compartment model are provided in Table 2. Goodness-of-fit plots (Fig. S1) for the final model showed stronger agreement between the observed plasma concentrations and the population predicted concentrations from the final population PK model (r2 = 0.793) than from the base structural three-compartment population PK model (r2 = 0.738). However, the agreement between the observed plasma plazomicin concentrations and the individual post hoc predicted concentrations decreased marginally (r2 = 0.919 versus 0.935 in the base model).
TABLE 2.
Final population PK model parameter estimatesa
| Parameter | Final model |
Bootstrap statistics (n = 200) |
||
|---|---|---|---|---|
| Final estimate | % SEE | Mean | 90% CI | |
| CL (liters/h) | ||||
| Nonrenal CL (liters/h) | 0.491 | 24.9 | 0.458 | 0.210 to 0.577 |
| CLR maximum (liters/h) | 4.80 | 7.39 | 4.86 | 4.44 to 5.48 |
| Baseline CLCR50 (ml/min/1.73 m2) | 45.3 | 5.27 | 45.3 | 41.9 to 49.8 |
| Hill coefficient | 2.49 | 13.9 | 2.50 | 2.01 to 2.93 |
| CL-weight power | 0.529 | 14.0 | 0.533 | 0.397 to 0.651 |
| Proportional increase for AP patients | 0.130 | 22.9 | 0.128 | 0.0776 to 0.179 |
| Proportional increase for BSI patients | –0.189 | 41.0 | –0.187 | –0.300 to 0.0648 |
| Vc (liters) | ||||
| Coefficient | 9.10 | 4.07 | 9.07 | 8.54 to 9.64 |
| Vc-BSA power | 1.23 | 17.5 | 1.25 | 0.869 to 1.59 |
| Proportional increase for cUTI and AP patients | 1.05 | 10.6 | 1.05 | 0.867 to 1.23 |
| Proportional increase for BSI and HABP/VABP patients | 1.55 | 17.0 | 1.56 | 1.14 to 1.99 |
| CLd1 (liters/h) | ||||
| Coefficient | 8.05 | 7.97 | 8.06 | 7.09 to 9.15 |
| Proportional increase for cUTI and AP patients | –0.831 | 4.85 | –0.823 | –0.880 to 0.748 |
| Vp1 (liters) | ||||
| Coefficient | 8.71 | 3.97 | 8.71 | 8.19 to 9.16 |
| Vp1-BSA power | 1.17 | 22.2 | 1.16 | 0.670 to 1.72 |
| Vp1-age slope | 0.00954 | 11.1 | 0.00949 | 0.00796 to 0.0111 |
| Proportional increase for cUTI and AP patients | –0.437 | 14.6 | –0.426 | –0.530 to 0.309 |
| CLd2 (liters/h) | ||||
| Coefficient | 0.199 | 3.64 | 0.199 | 0.186 to 0.215 |
| CLd2-height power | 3.38 | 17.5 | 3.36 | 2.15 to 4.43 |
| Proportional increase for cUTI and AP patients | –0.299 | 46.0 | –0.310 | –0.533 to 0.0699 |
| Proportional increase for BSI and HABP/VABP patients | 2.86 | 31.7 | 3.04 | 1.62 to 5.00 |
| Vp2 (liters) | ||||
| Coefficient | 6.98 | 9.21 | 7.00 | 5.99 to 8.23 |
| Vp2-weight power | 1.62 | 20.8 | 1.53 | 0.881 to 2.13 |
| Proportional increase for vasopressor use | 3.90 | 36.0 | 4.15 | 2.05 to 6.99 |
| CLCRRT (liters/h) | ||||
| Sum of UFR and DFR (liters/h) | 1.14–1.8 | NA | NA | NA |
| Sieving coefficient | 0.734 | 94.7 | 0.728 | 0.405 to 0.999 |
| ω2 for CL | 0.103 | 9.52 | 0.103 | 0.0870 to 0.120 |
| ω2 for Vc | 0.211 | 14.8 | 0.210 | 0.156 to 0.270 |
| ω2 for CLd1 | 0.0661 | 47.8 | 0.0605 | 0.00196 to 0.120 |
| ω2 for Vp1 | 0.0678 | 20.9 | 0.0697 | 0.0491 to 0.0998 |
| ω2 for CLd2 | 0.0350 | 24.2 | 0.0307 | 0.0165 to 0.0469 |
| ω2 for Vp2 | 0.170 | 34.9 | 0.128 | 0.0433 to 0.221 |
| IOV on CL | 0.00129 | 61.1 | 0.00149 | 0.000413 to 0.00287 |
| Covariance between CL and Vc | 0.0931 | 15.6 | 0.0938 | 0.0701 to 0.122 |
| Covariance between CL and Vp1 | 0.0734 | 15.1 | 0.0752 | 0.0589 to 0.0952 |
| Covariance between Vc and Vp1 | 0.0649 | 19.6 | 0.0670 | 0.0491 to 0.0906 |
| Residual variability, σ2 | ||||
| Additive component | 0.0000414 | 51.5 | 0.000101 | 0.0000511 to 0.000179 |
| CCV component for phase 1 studies | 0.0297 | 8.99 | 0.0299 | 0.0256 to 0.0345 |
| CCV component for phase 2 studies | 0.168 | 14.9 | 0.166 | 0.133 to 0.207 |
| CCV component for phase 3 studies | 0.0846 | 8.78 | 0.0849 | 0.0727 to 0.0967 |
NA, not applicable; σ2, residual variability (sigma squared); ω2, interindividual variability (omega squared); CI, confidence interval.
The relationship between renal clearance (CLR) and CLCR was described using a sigmoidal Hill-type function, and the relationship between total CL of plazomicin and CLCR included an intercept to represent nonrenal clearance. The other parameters describing the relationship between CL and CLCR were CLR maximum, CLCR50, and a Hill coefficient. CLR maximum is the maximum renal clearance (i.e., at a very high value of CLCR), CLCR50 is the CLCR value at which CLR is half-maximal, and the Hill coefficient defines the shape of the sigmoidal relationship. Based upon these relationships, the population mean total CL and CLR would be 4.57 liters/h (76.2 ml/min) and 4.08 liters/h (68.0 ml/min), respectively, in a typical cUTI or HABP/VABP patient with a BW of 75 kg, a body surface area (BSA) of 1.73 m2, and a CLCR of 90 ml/min.
As summarized in Table 2, several statistically significant relationships were identified between covariates and IIV for PK parameters: BW and infection type on CL; BSA and infection type on Vc; infection type on CLd1; BSA, age, and infection type on Vp1; height and infection type on CLd2; and BW and vasopressor use on Vp2.
Model evaluation.
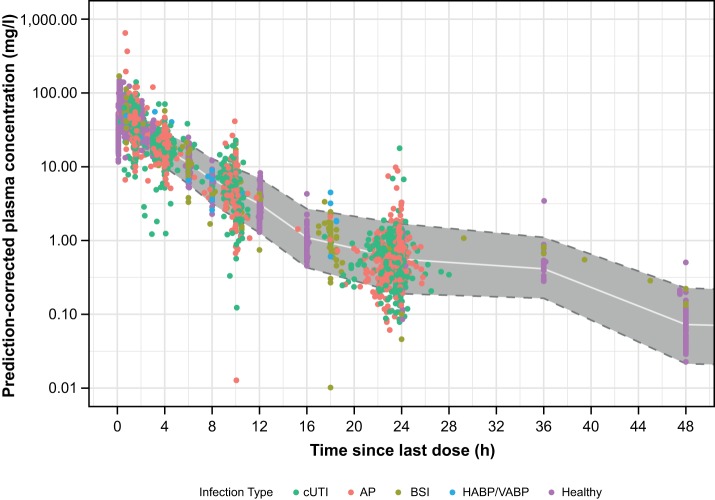
The prediction-corrected visual predictive check (PC-VPC) plots generally showed reasonable agreement between the observed concentrations and the individual simulated concentrations across time intervals and showed no bias with respect to renal function, infection type, or first versus multiple doses across healthy subjects and patients (Fig. 1), which suggests no substantial issues with respect to the fixed or random-effects parameters in the model and which supports the future use of this model for simulations. Furthermore, the bootstrap analysis showed that all of the population PK model parameters were estimated with reasonable precision (Table 2).
FIG 1.
Prediction-corrected visual predictive check for the final population PK model for plazomicin in healthy subjects and patients over the first 48 h after a dose, stratified by infection type. Dashed lines with gray zone, 5th and 95th percentiles of simulated concentrations; white line, median or 50th percentile of simulated concentrations; dots, observed plasma concentrations.
Plazomicin exposures and secondary PK parameter estimates.
Summary statistics for the key exposures and secondary PK parameters are provided in Table 3. The plazomicin half-lives (t1/2) associated with the three-compartment structural model indicate an initial distribution phase (α-phase half-life, 0.328 to 1.58 h), followed by a secondary distribution phase (β-phase half-life, 2.77 to 5.38 h) and a terminal elimination phase (γ-phase half-life, 25.8 to 36.5 h).
TABLE 3.
Summary of key plazomicin PK parameters in healthy subjects and patients, derived from the population PK model
| Parameter | Geometric mean value (% CV)d |
|||
|---|---|---|---|---|
| Study 006 | Study 002 | Study 009 | Study 007 | |
| AUC0–24 (mg·h/liter)a | 248 (16.0) | 233 (43.4) | 234 (38.5) | 235 (42.0) |
| CL (liters/h) | 4.50 (14.3) | 4.43 (41.3) | 3.91 (42.5) | 2.91 (60.5) |
| Cmax (mg/liter)b | 84.6 (21.0) | 54.5 (41.4) | 46.6 (43.0) | 37.1 (39.3) |
| Cmin (mg/liter)c | 0.372 (45.9) | 0.494 (104) | 0.880 (95.4) | 2.10 (99.4) |
| t1/2,α (h) | 0.328 (27.2) | 1.30 (26.7) | 1.58 (31.2) | 0.623 (45.7) |
| t1/2,β (h) | 2.77 (17.3) | 3.95 (26.2) | 5.17 (31.6) | 5.38 (46.9) |
| t1/2,γ (h) | 25.8 (37.6) | 36.5 (32.2) | 36.1 (31.3) | 28.6 (86.9) |
| Vss (liters) | 25.0 (23.9) | 27.9 (30.4) | 31.5 (32.9) | 56.6 (49.7) |
For phase 2/3 studies, AUC0–24 is the average daily AUC calculated via numerical integration of the concentration-time profile from the time of the first dose until 48 h divided by 2. For study 006, AUC0–24 is the AUC for first the 24 h after the single 15-mg/kg dose.
For phase 2/3 studies, Cmax is the highest concentration observed in the first 48 h of therapy (typically, the end of infusion after the second dose for patients receiving q24h dosing and the end of infusion after the first dose for those receiving q48h dosing). For study 006, Cmax is the highest concentration in the first 24 h after the single 15-mg/kg dose (always at the end of infusion).
For phase 2/3 studies, Cmin is the lowest concentration observed in the first 48 h of therapy (typically, the trough concentration after the first dose). For study 006, Cmin is the lowest concentration in the first 24 h after the single 15-mg/kg dose (always at 24 h).
In study 006, plazomicin was administered at 15 mg/kg to 54 healthy subjects. In study 002, plazomicin was administered at 15 mg/kg to 71 cUTI/AP patients. In study 009, the dose was based on the baseline renal function, as described in Table 4, footnote a, and was administered to 281 cUTI/AP patients. In study 007, the dose was based on the baseline renal function, as described in Table 4, footnote a, and was administered to 48 CRE patients. Two subjects in study 007 received q12h dosing starting at ≈24 h after the first dose of plazomicin, subsequent to therapeutic drug management.
Table 3 reports PK results for 54 healthy subjects and 71 patients with cUTI/AP who received plazomicin at 15 mg/kg by a 30-min infusion. The mean peak plasma concentration (Cmax) values were 84.6 mg/liter (CV, 21.0%) and 54.5 mg/liter (CV, 41.4%), respectively, indicating that the 15-mg/kg dose produced a higher Cmax in healthy subjects than in patients with cUTI/AP. This is likely attributed to a smaller Vc in healthy subjects than in patients with cUTI/AP. Overall, the mean volume of distribution at steady state (Vss) of plazomicin in healthy subjects and patients with cUTI/AP ranged from 25.0 to 31.5 liters, which is typical for an aminoglycoside and approximately twice the extracellular fluid volume (39). A larger Vss of 56.6 liters was observed in BSI and HABP/VABP patients with CRE infections, most likely due to the severity of illness in this patient group (40). The estimates of the mean plazomicin area under the plasma concentration-time curve from time zero to 24 h (AUC0–24) for a plazomicin dose of 15 mg/kg or a reduced dose based on the baseline renal function were generally consistent across the patient studies with moderate variability (38.5 to 43.4%). Mean plazomicin minimum plasma concentration (Cmin) values ranged from 0.494 to 0.880 mg/liter for patients with cUTI/AP and were higher (2.10 mg/liter) in patients with CRE infections.
To aid in identifying subgroups of patients who are likely to experience important differences in plazomicin exposures, the post hoc plazomicin PK estimates (AUC0–24, Cmax, Cmin, and CL) were investigated relative to patient covariates of interest (CLCR, BW, body mass index [BMI], age, sex, race, infection type, and vasopressor use). The assessments of clinical relevance focused on AUC and Cmin in patients in the phase 2 and phase 3 studies. AUC is of interest for plazomicin because the AUC/MIC ratio is the PK/pharmacodynamic (PD) index that best correlated with efficacy in an animal infection model (41). Cmin is of interest based on the precedent of clinical use with other aminoglycoside antibiotics, which commonly involve monitoring of Cmin to improve the safety profile. While the exposure results reported below are confined to AUC, the same conclusions were reached for exposure based on Cmin.
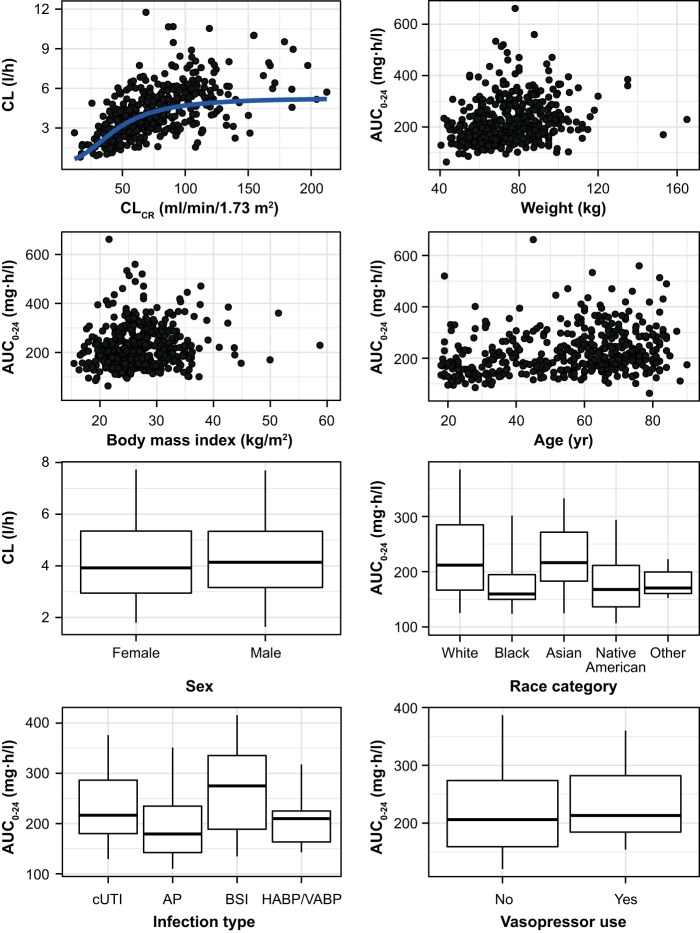
Figure 2 presents the relationships between post hoc parameter estimates for AUC0–24 and CL and the covariates. Total CL increased in a sigmoidal fashion with increasing CLCR. Given that the initial dose was selected based on the baseline CLCR in the phase 3 studies, AUC0–24 was similar across renal function groups. The scatter plots show no important trends between AUC0–24 and each of BW, BMI, or age. Box plots show broadly overlapping values of CL in males and females, as well as broadly overlapping values of AUC0–24 in patients who received vasopressors versus those who did not. Box plots of AUC0–24 versus race suggest that black patients may have slightly lower plasma exposures than patients of other races; however, race was not a significant covariate in the final population PK model, and apparent differences among racial categories are likely due to small sample sizes and potential imbalances in other factors (e.g., renal function, infection type). Box plots of AUC0–24 versus infection type suggest slightly higher plasma exposures in patients with BSI, which reflects an 18.9% decrease in CL relative to that in patients with cUTI or HABP/VABP (Table 2).
FIG 2.
Relationships between post hoc plazomicin parameter estimates (AUC or CL) following initial dosing and covariate category (CLCR, BW, BMI, age, sex, race, infection type, or vasopressor use) for patients in the phase 2 and 3 studies. The horizontal lines in box-whiskers plots are the median; the boxes show the 25th to 75th percentiles, and whiskers extend to the 5th and 95th percentiles.
DISCUSSION
The objective of this analysis was to identify patient factors that account for sources of variability in plazomicin PK and to determine if dose adjustments are warranted based on covariates. The population PK model for plazomicin that best described the data was a three-compartment model with a zero-order rate constant for the intravenous infusion and with first-order elimination kinetics. The relationship between CLR and CLCR, the most clinically significant covariate, was described by a sigmoidal Hill-type function. As glomerular filtration is the predominant mechanism of aminoglycoside elimination, the relationship between plazomicin CL and the term used to capture renal clearance might be expected to be linear. However, the observed data showed a sigmoidal relationship, where increases in plazomicin CL were less pronounced at the highest CLCR values (Fig. 2). It is important to consider that CLCR is used in this empirical population PK model as a surrogate of glomerular filtration rate (GFR), and CLCR may be less predictive of GFR at the upper range of CLCR estimates due to other factors which influence endogenous creatinine production. Despite this limitation, the sigmoidal relationship supports the objective of this analysis (i.e., identifying important patient covariates for plazomicin CL). It should also be noted that data were sparse for CLCR of >150 ml/min; therefore, caution should be exercised when using the model to predict exposures in subjects with CLCR above this level.
Plazomicin dosing was based on adjustments for the baseline CLCR in the phase 3 clinical studies (studies 007 and 009; Table 3), and these dosage adjustments are reflected in the product label (21). As the clearance of plazomicin is primarily through glomerular filtration, it may be reasoned that plazomicin dosing should be based on GFR. The gold standard for estimating GFR is by using inulin, which is highly precise but impractical for routine use in guiding drug dosing (42). The Cockcroft-Gault (CG) formula estimates CLCR as a surrogate for GFR, and most guidelines for aminoglycoside dosing recommend use of the CG formula. This formula requires the patient weight, which may be adjusted for IBW, lean BW, or BSA to account for differences in body size and obesity among patients (43). Alternative equations for estimated GFR (eGFR) are available, such as the Modification of Diet in Renal Disease (MDRD) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formulas (44, 45). These eGFR formulas were developed from well-conducted population studies and are primarily used to detect end-stage chronic kidney disease. While they represent an alternative to the CG formula to guide aminoglycoside dosing, they have not been as widely adopted as the CG formula. Moreover, studies suggest that the CG, MDRD, and CKD-EPI formulas are not interchangeable and may result in different renal function estimates, leading to differences in antimicrobial dosing. Given these caveats, the population PK analysis of plazomicin focused on CG, which was used in the phase 3 clinical studies (Table 4) and was thus integral to the overall assessment of benefit-risk in the intended patient populations.
TABLE 4.
Overview of the clinical studies included in the analysisc
| Study phase | Study no. (reference) | Plazomicin PK population | Study design | Plazomicin dosinga | PK sampling regimen type and times |
|---|---|---|---|---|---|
| Phase 1 | 001 (23) | Healthy subjects (n = 28) | Randomized, double blind, placebo controlled, parallel group | 1–15-mg/kg single dose; 4–15 mg/kg q24h | Intensive (predose and at 5, 10, 15, 20, 30, and 45 min and 1, 2, 3, 4, 6, 8, 12, 16, 24, and 48 h after the start of the infusion) |
| 003 (34) | Healthy subjects (n = 30) | Randomized, double blind, placebo controlled | 10.7- or 15-mg/kg single dose; 15 mg/kg q24h | Intensive (predose and at 10, 15, 30, and 45 min, and 1, 1.5, 2, 3, 6, and 10 h or 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 16, and 24 h after the start of the infusion) | |
| 004 (36) | Healthy subjects with normal renal function or with various degrees of renal impairment (n = 24) | Open label | 7.5-mg/kg single dose | Intensive (predose and at 36 and 45 min and 1, 1.5, 3, 6, 10, 16, 24, 36, 48, 72, and 96 h after the start of the infusion) | |
| 006 (24) | Healthy subjects (n = 61) | Randomized, double blind, placebo and positive controlled, crossover | 15- or 20-mg/kg single dose | Intensive (predose and at 36 and 45 min and 1, 2, 3, 6, 12, and 24 h after the start of the infusion) | |
| Phase 2 | 002 (35) | Patients with cUTI or AP (n = 92) | Double blind, comparator controlled | 10 or 15 mg/kg q24h | Sparse (at 35–55 min and 1.5–3 h after the start of the infusion on day 1 and just before the start of the infusion on days 2–5) |
| Phase 3 | 007 (38) | Patients with BSI, HABP, VABP, or cUTI due to CRE (n = 48) | Randomized, open label, compared with colistin | Initial dose of 8–15 mg/kg q12h, q24h, or q48h based on baseline renal function,b followed by doses based on TDM | At 1.5, 4, and 8, 10, or 18 h after the start of infusion on days 1 and 4 or at 0.75 and time points up to 12 (q12h), 24 (q24h), or 48 (q48h) h after the start of infusion on day 1 and at the end of therapy or early discontinuation |
| 009 (37) | Patients with cUTI, including AP (n = 281) | Randomized, double blind, compared with meropenem | 15 mg/kg q24h with dosing adjustments to 8–15 mg/kg q24h per renal functionb | Predose, 90 min, 4 h, and 10 h after the start of infusion relative to the first dose of study drug on the day of sampling |
In all studies except for studies 007 and 009, milligram-per-kilogram dosing was based strictly on total BW. In studies 007 and 009, milligram-per-kilogram dosing was based on BW except where BW was ≥125% of the ideal body weight (IBW). IBW was calculated based on sex and height, as described by Devine (53). Where BW was ≥125% of IBW, patients in studies 007 and 009 were dosed based on ABW, which was calculated as follows: ABW = IBW · 0.4 (BW – IBW) (27).
Renal function was determined by use of the calculated CLCR, as estimated by the equation of Cockcroft and Gault (46), with the use of ABW where BW was ≥125% of IBW, as described in footnote a. In studies 007 and 009, the dose schedules and the CLCR categories were as follows: 15 mg/kg q24h for >60 ml/min, 12 mg/kg q24h for >50 to 60 ml/min, 10 mg/kg q24h for >40 to 50 ml/min, 10 mg/kg q24h for >40 to 50 ml/min, and 8 mg/kg q24h for >30 to 40 ml/min. Study 007 included the following: 12 mg/kg q48h for >25 to 30 ml/min; 10 mg/kg q48h for >20 to 25 ml/min; 8 mg/kg q48h for >15 to 20 ml/min; 8 mg/kg q48h for ≤15 ml/min; 10 mg/kg q12h for CRRT, slow; and 11 mg/kg q24h for CRRT, fast. CRRT, slow, assumed residual plazomicin renal clearance of 0 ml/min and clearance attributed to a CRRT of 2.4 liters/h using slow dialysate and ultrafiltrate flow rates (5 to 15 ml/min) and a blood flow rate of 150 ml/min. CRRT, fast, assumed residual plazomicin renal clearance of 0 ml/min and clearance attributed to CRRT of 4.8 liters/h using fast dialysate and ultrafiltrate flow rates (30 to 40 ml/min) and a blood flow rate of 150 ml/min.
q12h, once every 12 h; q24h, once every 24 h; q48h, once every 48 h.
The population PK analysis used traditional covariate model-building techniques to identify patient descriptors that are associated with the IIV in plazomicin PK. After pooling of the data across phase 1, 2, and 3 studies, the resultant data set contained a large, diverse population, which facilitated the identification of several statistically significant relationships (Table 2). Infection type appeared to reduce IIV in a variety of PK parameters, especially CLd1, which may reflect changes in hemodynamic status and other critically ill patient characteristics with the inclusion of patient data from the phase 3 study in patients with serious infections due to CRE. The relatively small drop in IIV for CL is a consequence of the fact that CLCR, which was part of the base structural model, is by far the largest predictor of the IIV in plazomicin CL.
Post hoc assessments, which showed a compelling relationship between CL and CLCR, provided support for dose adjustments based on CLCR. In contrast, none of the other statistically significant covariates had a clinically meaningful impact on plazomicin exposure. Thus, dose adjustments in adults do not appear to be warranted on the basis of age, infection type, vasopressor use, or body size (further to milligram-per-kilogram dosing using either total BW or ABW).
Post hoc assessments further enabled comparison of AUC0–24 values in the two phase 3 studies (studies 007 and 009; Table 3). The geometric mean AUC0–24 was 235 mg·h/liter (CV, 42.0%) in patients with infections due to CRE in study 007 and 234 mg·h/liter (CV, 38.5%) in patients with cUTI/AP in study 009, indicating that the dosing paradigm produced only moderate variability in the exposure parameter that is considered most relevant to efficacy. This suggests that it is appropriate for plazomicin to be administered on a milligram-per-kilogram basis with adjustments for CLCR and BW of ≥125% of IBW.
In conclusion, a three-compartment structural PK model with a zero-order rate constant for the intravenous infusion and linear first-order elimination kinetics provided good fits of the plasma concentration-time data. A robust description of the plasma PK of plazomicin in the population of healthy subjects and patients was achieved, such that the derived measures of plazomicin exposure are expected to be both accurate and precise. The population PK analysis successfully identified patient factors that are sources of variability in PK parameters, while further showing that most of these covariate relationships do not have an important impact on plasma exposure to plazomicin. Post hoc estimates of AUC0–24 in the phase 3 studies suggest that it is appropriate for plazomicin to be administered on a milligram-per-kilogram basis with dose adjustments for CLCR and BW of ≥125% of IBW, while adjustments based on other covariates are not warranted. Although it was beyond the scope of the population PK analysis, it merits mention that initial dosing on a milligram-per-kilogram basis with adjustments for CLCR and BW of ≥125% of IBW is recommended in all patients, and subsequent adjustments based on TDM are recommended in certain patient subsets, including the critically ill and those with cUTI/AP and coexisting renal impairment.
MATERIALS AND METHODS
Study designs.
The clinical studies involved in this analysis included four phase 1 studies (studies 001 [ClinicalTrials.gov registration no. NCT00822978] [23], 003 [ClinicalTrials.gov registration no. NCT01034774] [34], 004 [ClinicalTrials.gov registration no. NCT01462136] [36], and 006 [ClinicalTrials.gov registration no. NCT01514929] [24]), one phase 2 study (study 002 [ClinicalTrials.gov registration no. NCT01096849] [35]), and two phase 3 studies (studies 007 [ClinicalTrials.gov registration no. NCT01970371] [38] and 009 [ClinicalTrials.gov registration no. NCT02486627] [37]).
Table 4 summarizes the clinical studies and their dosing and PK sampling schemes. The phase 1 studies were conducted in healthy subjects in whom plazomicin was administered strictly on a milligram-per-kilogram basis (no adjustments for CLCR or IBW). Studies 001 and 003 explored the safety, tolerability, and PK of plazomicin over single- and multiple-dose regimens. Study 004 explored the effect of renal impairment on the PK and tolerability of single-dose plazomicin. Study 006 assessed the potential of single-dose plazomicin to cause QT prolongation.
Three multiple-dose studies were performed in patients with cUTI, including AP (studies 002 and 009) or serious infection (BSI, HABP, VABP, cUTI, or AP), due to CRE (study 007). In all three patient studies, plazomicin blood samples were collected using sparse PK sampling schemes (Table 4). Studies 002 and 009 were randomized phase 2 and phase 3 cUTI trials, respectively, where plazomicin was compared with levofloxacin or meropenem, respectively. Study 007 was a randomized phase 3 trial of plazomicin versus colistin in critically ill patients with infections due to CRE. In study 002, plazomicin was administered at 10 or 15 mg/kg based strictly on BW, and in studies 007 and 009, plazomicin was administered at up to 15 mg/kg with adjustment for renal function and ABW where BW was ≥125% of IBW (see Table 4 footnotes). In study 007, plazomicin doses after the initial dose were determined based on therapeutic drug management, in which dose adjustments were implemented to maintain AUC within a target range.
Subject characteristics.
Subject demographic and disease characteristics recorded before administration of study drug were used to characterize the analysis population and informed assessments of IIV in key PK parameters.
Renal function was approximated using CLCR, as calculated by the CG equation (46) as follows: CLCR (in milliliters per minute) for males = (140 – age [in years] · BW [in kilograms])/(72 · SCr [in milligrams per deciliter]) and CLCR for females (in milliliters per minute) = male CLCR · 0.85, where the serum creatinine concentration (SCr) was capped to a lower bound of 0.50 mg/dl. The calculated CLCR values for each individual were normalized to a BSA of 1.73 m2, determined using the equation from Du Bois and Du Bois (47); BSA (in millimeters squared) = BW (in kilograms)0.425 · height (in centimeters)0.725 · 0.007184, to help control for body size differences when estimating renal function for the purposes of constructing a population PK covariate model.
If SCr data were available on different days during repeated dosing of plazomicin within a given patient, CLCR was calculated and updated for each day where SCr was measured and used as a time-changing covariate in the analysis data set. Before updating the CLCR calculation, linear interpolation was used to calculate SCr between actual measured SCr values.
For patients on CRRT, the timing of CRRT, UFR, and DFR was assigned based upon the source data.
Drug concentration assay.
In each study, blood samples for PK analysis were collected in Vacutainer tubes containing K2EDTA. Plasma was separated from whole-blood components by centrifugation and immediately frozen at –20°C or colder until analysis (Alturas Analytics, Inc. Moscow, ID, USA). Plasma plazomicin concentrations were determined using a validated liquid chromatography-tandem mass spectrometry method with a lower limit of quantification of 0.01 mg/liter. The same assay methodology was used for every study included in the population PK analysis.
Handling of outliers and samples assayed as having BLQ plazomicin plasma concentrations.
PK samples without both date and time information or with BLQ plazomicin concentrations were excluded from the population PK analysis.
An outlier was defined as an aberrant observation that substantially deviated from the rest of the observations within an individual. PK outlier concentrations were excluded from this analysis according to U.S. Food and Drug Administration guidance (48). The outlier detection was based primarily upon visual inspection of individual and pooled plasma concentration-time data for plazomicin. Searching for additional outliers during the analysis was based upon graphical exploration of individual and population conditional weighted residuals during structural PK model development.
Population PK analysis.
The population PK analysis was conducted using NONMEM software (v7.2; ICON Development Solutions, Ellicott City, MD, USA), implementing the first-order conditional estimation method with interaction. During various stages of model development, population PK models were minimally assessed using the following criteria: (i) evaluation of individual and population mean PK parameter estimates for plazomicin and their precision, as measured by the percent standard error of the population mean estimate; (ii) graphical examination of standard diagnostic and population analysis goodness-of-fit plots with possible stratification by various factors, such as patient population or plazomicin dose group; (iii) graphical examination of the agreement between the observed and individual post hoc predicted plazomicin concentration-time data; (iv) reductions in both residual variability and IIV; and (v) comparison of MVOF for nested models or Akaike’s information criterion for nonnested models (49).
(i) Development of the structural population PK model. For developing the structural population PK model, a previously developed three-compartment structural PK model using data from the phase 1 and 2 studies (studies 001, 003, 004, and 002), in which CL, Vc, Vp1 and Vp2, and CLd1 and CLd2 were estimated as model parameters, was refined in this analysis after including additional data from studies 006, 007, and 009 pooled with the previous phase 1 and 2 study data. As a general rule, other model structures (e.g., a two-compartment model) were attempted only if it was deemed necessary based upon the fit of the structural model to the pooled data set. Development of the base structural population PK model for the present analysis included CLCR as a time-varying covariate a priori for all subjects in the full data set who had more than one central laboratory SCr measurement. The functional form for the relationship between CLCR and CL was selected based upon the fit of different functional forms (i.e., linear, power, sigmoidal). Clearance due to CRRT was set to the sum of the actual patient-specific DFR and UFR, and multiplied by an estimate of the sieving coefficient (which represents the membrane permeation of the drug) on the study days when CRRT was used. For this submodel, DFR and UFR were fixed based on the source data and the sieving coefficient was a fitted parameter. The total CL for patients while on CRRT was equal to the sum of residual CL and the CRRT CL.
IIV was modeled for each PK parameter, where appropriate, using an exponential error model that assumed that these parameters are log-normally distributed and that the variance is constant. Residual variability was initially modeled by a combined additive-plus-CCV error model. Other models for residual variability were explored as necessary.
(ii) Covariate analysis. After constructing the structural population PK model, a covariate analysis was initiated. Patient factors were evaluated as continuous or categorical descriptors, as summarized in Table 1.
A formal univariate analysis of each covariate that demonstrated an observable trend with a structural PK model parameter and that had a biologically plausible relationship was performed in NONMEM during each step of forward selection. This was carried out to assess statistical significance based upon the resulting decrease in MVOF from the base structural model using a likelihood ratio test. The most statistically significant parameter-covariate relationship (α = 0.01) was added to the model during each step of forward selection; stepwise forward selection was concluded when none of the remaining covariates tested resulted in a statistically significant decrease in MVOF relative to that for the updated base structural model from the previous step of forward selection.
After completing forward selection, the refinement of the full multivariable model was conducted. The IIV models were first reevaluated through pairwise comparisons of the interindividual error terms (η) for each parameter, and potential adjustments to the IIV models or the variance-covariance matrix structure were made. Focus was then shifted toward correcting any potential biases or seeking ways to simplify the residual variability model, and if necessary, the additive-plus-CCV residual error model was simplified to a CCV error model at this stage of the analysis.
Univariate stepwise backward elimination was performed after all adjustments were made to the IIV and residual variability models by removing each parameter-covariate relationship and assessing whether the resulting increase in MVOF remained statistically significant (α = 0.001). The final population PK model for plazomicin was generated after it was determined that all parameter-covariate relationships in the model remained statistically significant.
(iii) Final model evaluation. The final population PK model analysis was qualified by performing a PC-VPC, which examined the agreement between the 5th, 50th, and 95th percentiles of the observed and the individual simulated plazomicin concentrations across time intervals. The PC-VPC normalizes both the observed and the simulated plasma concentration-time data by the median population mean predictions during each time interval to adjust for the differences due to independent variables in the final population PK model and to avoid having to stratify by single- versus multiple-dose data, dose group, or other significant covariate effects included in the model (50). This was also necessary due to the fact that patients could have doses altered secondary to TDM in study 007.
To assess the robustness of the final population PK model for plazomicin, a nonparametric bootstrap evaluation was performed (51, 52). Histograms of the bootstrap population mean PK parameter and variance estimates were also generated to assess the general shape of the distribution for each term in the model. The purpose of this was to further assess the precision of the final population PK parameter estimates in response to perturbations in the data and to assist with assessing potential areas of weaknesses and identifying limitations of the model.
Calculation of secondary PK parameters and exposure estimates.
The individual post hoc PK parameter estimates (CL, Vc, CLd1, CLd2, Vp1, and Vp2) were obtained from the final model and directly reported. These parameters were used to calculate secondary PK parameters, such as Vss (which is equal to Vc + Vp1 + Vp2), as well as the α-, β-, and γ-phase half-lives (t1/2,α, t1/2,β, and t1/2,γ, respectively). For most of the comparisons in this analysis, the individual post hoc PK parameters were also used to simulate predicted plasma plazomicin concentration-time data to generate plasma exposure estimates, such as Cmax, Cmin, and AUC0–24.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alturas Analytics (Moscow, ID, USA) for bioanalytical support and Allison Komirenko for her review and insights.
Editorial support was provided by Fran Brown and Shaw Yang of Certara and Kate Bradford and Jean Turner of Parexel.
This project was funded in whole or in part with federal funds from the Biomedical Advanced Research and Development Authority (BARDA), Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary, U.S. Department of Health and Human Services, under contract no. HHSO100201000046C. Support for the population PK analysis and funding were provided by Achaogen, Inc.
J.D.S., A.K., and J.A.G. are current employees or former employees of and stockholders in Achaogen, Inc. M.T., S.A.V.W., S.M.B., P.G.A., and C.M.R. are current or former employees of the Institute for Clinical Pharmacodynamics who received research funding from Achaogen, Inc.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02329-18.
REFERENCES
- 1.World Health Organization. 2014. Antimicrobial resistance: global report on surveillance 2014. http://www.who.int/antimicrobial-resistance/publications/surveillancereport/en/. Accessed August 2018.
- 2.Center for Disease Dynamics Economics and Policy (CDDEP). 2015. The state of the world's antibiotics 2015. https://cddep.org/wp-content/uploads/2017/06/swa_edits_9.16.pdf. Accessed August 2018.
- 3.U.S. Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/. Accessed August 2018.
- 4.European Centre for Disease Prevention and Control. 2017. Antimicrobial resistance surveillance in Europe 2016. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). https://ecdc.europa.eu/sites/portal/files/documents/AMR-surveillance-Europe-2016.pdf. Accessed August 2018.
- 5.MacVane SH, Tuttle LO, Nicolau DP. 2014. Impact of extended-spectrum beta-lactamase-producing organisms on clinical and economic outcomes in patients with urinary tract infection. J Hosp Med 9:232–238. doi: 10.1002/jhm.2157. [DOI] [PubMed] [Google Scholar]
- 6.Nelson RE, Slayton RB, Stevens VW, Jones MM, Khader K, Rubin MA, Jernigan JA, Samore MH. 2017. Attributable mortality of healthcare-associated infections due to multidrug-resistant gram-negative bacteria and methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 38:848–856. doi: 10.1017/ice.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaden JT, Li Y, Ruffin F, Maskarinec SA, Hill-Rorie JM, Wanda LC, Reed SD, Fowler VG Jr. 2017. Increased costs associated with bloodstream infections caused by multidrug-resistant gram-negative bacteria are due primarily to patients with hospital-acquired infections. Antimicrob Agents Chemother 61:e01709-16. doi: 10.1128/AAC.01709-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewardson AJ, Allignol A, Beyersmann J, Graves N, Schumacher M, Meyer R, Tacconelli E, De Angelis G, Farina C, Pezzoli F, Bertrand X, Gbaguidi-Haore H, Edgeworth J, Tosas O, Martinez JA, Ayala-Blanco MP, Pan A, Zoncada A, Marwick CA, Nathwani D, Seifert H, Hos N, Hagel S, Pletz M, Harbarth S, Timber Study Group. 2016. The health and economic burden of bloodstream infections caused by antimicrobial-susceptible and non-susceptible Enterobacteriaceae and Staphylococcus aureus in European hospitals, 2010 and 2011: a multicentre retrospective cohort study. Euro Surveill 21(33):pii=30319 10.2807/1560-7917.ES.2016.21.33.30319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. 2015. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis 2:ofv050. doi: 10.1093/ofid/ofv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig WA. 2011. Optimizing aminoglycoside use. Crit Care Clin 27:107–121. doi: 10.1016/j.ccc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. 2007. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis 45:753–760. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche J-D, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, et al. 2017. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 13.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJC, Baron EJ, O’Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. 2010. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 50:133–164. (Erratum, 50:1695.) doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 14.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, Raad II, Rijnders BJA, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. (Errata, 50:457 and 50:1079, 2010.) doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association of Urology. 2018. Urological infections guidelines. http://uroweb.org/guideline/urological-infections/. Accessed August 2018.
- 16.Aggen JB, Armstrong ES, Goldblum AA, Dozzo P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias RD, Lopez S, Gomez M, Wlasichuk KB, Diokno R, Miller GH, Moser HE. 2010. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob Agents Chemother 54:4636–4642. doi: 10.1128/AAC.00572-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thwaites M, Hall D, Shinabarger D, Serio AW, Krause KM, Marra A, Pillar C. 2018. Evaluation of the bactericidal activity of plazomicin and comparators against multidrug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 62:e00236-18. doi: 10.1128/AAC.00236-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galani I, Souli M, Daikos GL, Chrysouli Z, Poulakou G, Psichogiou M, Panagea T, Argyropoulou A, Stefanou I, Plakias G, Giamarellou H, Petrikkos G. 2012. Activity of plazomicin (ACHN-490) against MDR clinical isolates of Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. from Athens, Greece. J Chemother 24:191–194. doi: 10.1179/1973947812Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castanheira M, Davis AP, Mendes RE, Serio AW, Krause KM, Flamm RK. 2018. In vitro activity of plazomicin against Gram-negative and Gram-positive isolates collected from United States hospitals and comparative activity of aminoglycosides against carbapenem-resistant Enterobacteriaceae and isolates carrying carbapenemase genes. Antimicrob Agents Chemother 62:e00313-18. doi: 10.1128/AAC.00313-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox G, Ejim L, Stogios PJ, Koteva K, Bordeleau E, Evdokimova E, Sieron AO, Savchenko A, Serio AW, Krause KM, Wright GD. 2018. Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis 4:980–987. doi: 10.1021/acsinfecdis.8b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achaogen, Inc. 2018. Zemdri prescribing information. Achaogen, Inc, South San Francisco, CA. [Google Scholar]
- 22.Zhanel GG, Lawson CD, Zelenitsky S, Findlay B, Schweizer F, Adam H, Walkty A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2012. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev Anti Infect Ther 10:459–473. doi: 10.1586/eri.12.25. [DOI] [PubMed] [Google Scholar]
- 23.Cass RT, Brooks CD, Havrilla NA, Tack KJ, Borin MT, Young D, Bruss JB. 2011. Pharmacokinetics and safety of single and multiple doses of ACHN-490 injection administered intravenously in healthy subjects. Antimicrob Agents Chemother 55:5874–5880. doi: 10.1128/AAC.00624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gall J, Choi T, Riddle V, Van Wart S, Gibbons JA, Seroogy J. 16 January 2019. A phase 1 study of intravenous plazomicin in healthy adults to assess potential effects on the QT/QTc interval, safety, and pharmacokinetics. Clin Pharmacol Drug Dev. doi: 10.1002/cpdd.653. [DOI] [PubMed] [Google Scholar]
- 25.Choi T, Seroogy J, Sanghvi M, Dhuria SV. 2018. Mass balance, metabolism, and excretion of [14C]-plazomicin in healthy human subjects, poster 1400. Abstr IDWeek, San Francisco, CA. [Google Scholar]
- 26.University of California San Francisco Infectious Diseases Management Program. 2013. Aminoglycoside dosing and monitoring recommendations. http://idmp.ucsf.edu/aminoglycoside-dosing-and-monitoring-recommendations. Accessed August 2018.
- 27.Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. 1995. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 39:650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuner EA, Gallagher JC. 2017. Pharmacodynamic and pharmacokinetic considerations in the treatment of critically ill patients infected with carbapenem-resistant Enterobacteriaceae. Virulence 8:440–452. doi: 10.1080/21505594.2016.1221021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Lent-Evers NA, Mathot RA, Geus WP, van Hout BA, Vinks AA. 1999. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit 21:63–73. [DOI] [PubMed] [Google Scholar]
- 30.Trang M, Rubino CM, Hammel JP, Seroogy JD, Kim A, Forrest A, Bhavnani SM. 2018. Assessment of AUC-based therapeutic drug management algorithms for plazomicin therapy in patients with bloodstream infection, poster 84. Abstr ESCMID/ASM Conf Drug Dev Meet Challenge Antimicrob Resist, Lisbon, Portugal. [Google Scholar]
- 31.Bilbao-Meseguer I, Rodríguez-Gascón A, Barrasa H, Isla A, Solinís MÁ. 2018. Augmented renal clearance in critically ill patients: a systematic review. Clin Pharmacokinet 57:1107–1121. doi: 10.1007/s40262-018-0636-7. [DOI] [PubMed] [Google Scholar]
- 32.Sunder S, Jayaraman R, Mahapatra HS, Sathi S, Ramanan V, Kanchi P, Gupta A, Daksh SK, Ram P. 2014. Estimation of renal function in the intensive care unit: the covert concepts brought to light. J Intensive Care 2:31. doi: 10.1186/2052-0492-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong G, Sime FB, Lipman J, Roberts JA. 2014. How do we use therapeutic drug monitoring to improve outcomes from severe infections in critically ill patients? BMC Infect Dis 14:288. doi: 10.1186/1471-2334-14-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cass R, Kostrub CF, Gotfried M, Rodvold K, Tack KJ, Bruss J. 2013. A double-blind, randomized, placebo-controlled study to assess the safety, tolerability, plasma pharmacokinetics and lung penetration of intravenous plazomicin in healthy subjects, poster 1637. Abstr Eur Congr Clin Microbiol Infect Dis, Berlin, Germany. [Google Scholar]
- 35.Connolly LE, Riddle V, Cebrik D, Armstrong ES, Miller LG. 2018. A multicenter, randomized, double-blind, phase 2 study of the efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis. Antimicrob Agents Chemother 62:e01989-17. doi: 10.1128/AAC.01989-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komirenko AS, Riddle V, Gibbons JA, Van Wart S, Seroogy JD. 2018. A phase 1 study to assess the pharmacokinetics of intravenous plazomicin in adult subjects with varying degrees of renal function. Antimicrob Agents Chemother 62:e01128-18. doi: 10.1128/AAC.01128-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagenlehner FME, Cloutier DJ, Komirenko AS, Cebrik DS, Krause KM, Keepers TR, Connolly LE, Miller LG, Friedland I, Dwyer JP. 2019. Once daily plazomicin for complicated urinary tract infections. N Engl J Med 380:729–740. doi: 10.1056/NEJMoa1801467. [DOI] [PubMed] [Google Scholar]
- 38.McKinnell JA, Dwyer JP, Talbot GH, Connolly LE, Friedland I, Smith A, Jubb AM, Serio AW, Krause KM, Daikos DL. 2019. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med 380:791–793. doi: 10.1056/NEJMc1807634. [DOI] [PubMed] [Google Scholar]
- 39.Dager WE. 1994. Aminoglycoside pharmacokinetics: volume of distribution in specific adult patient subgroups. Ann Pharmacother 28:944–951. doi: 10.1177/106002809402800719. [DOI] [PubMed] [Google Scholar]
- 40.Pea F, Viale P, Furlanut M. 2005. Antimicrobial therapy in critically ill patients: a review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin Pharmacokinet 44:1009–1034. doi: 10.2165/00003088-200544100-00002. [DOI] [PubMed] [Google Scholar]
- 41.Louie A, Fikes S, Liu W, VanScoy B, Cirz R, Drusano G. 2012. Pharmacodynamics of plazomicin in a neutropenic murine pneumonia model against Klebsiella pneumoniae (KPN), poster 41. Abstr 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA American Society for Microbiology, Washington, DC. [Google Scholar]
- 42.Soveri I, Berg UB, Bjork J, Elinder CG, Grubb A, Mejare I, Sterner G, Back SE, SBU GFR Review Group. 2014. Measuring GFR: a systematic review. Am J Kidney Dis 64:411–424. doi: 10.1053/j.ajkd.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Shoker A, Hossain MA, Koru-Sengul T, Raju DL, Cockcroft D. 2006. Performance of creatinine clearance equations on the original Cockcroft-Gault population. Clin Nephrol 66:89–97. [DOI] [PubMed] [Google Scholar]
- 44.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 45.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 47.Du Bois D, Du Bois EF. 1989. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5:303–311. [PubMed] [Google Scholar]
- 48.Food and Drug Administration. 1999. Guidance for industry on population pharmacokinetics; availability. Food and Drug Administration, HHS. Notice Fed Regist 64:6663–6664. [PubMed] [Google Scholar]
- 49.Akaike H. 1998. A new look at the statistical model identification In Parzen E, Tanabe K, Kitagawa G (ed), Selected papers of Hirotugu Akaike. Springer, New York, NY. [Google Scholar]
- 50.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams PJ, Kim YH, 2007. Resampling techniques and their application to pharmacometrics In: Ette EI, Williams PJ (ed). Pharmacometrics: the science of quantitative pharmacology. John Wiley & Sons, Inc, New York, NY. [Google Scholar]
- 52.Ette EI. 1997. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 37:486–495. doi: 10.1002/j.1552-4604.1997.tb04326.x. [DOI] [PubMed] [Google Scholar]
- 53.Devine BJ. 1974. Gentamicin therapy. Drug Intell Clin Pharm 8:650–655. doi: 10.1177/106002807400801104. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.