Abstract
Objectives
Sclerotium rolfsii lectin (SRL), isolated from soil born phytopathogenic fungus Sclerotium rolfsii, exhibits exquisite binding specificity to the oncofoetal Thomsen‐Friedenreich (Galβ1,3GalNAcα‐O‐Ser/Thr, T or TF) antigen and associated glycans. In the present study, we report anti‐proliferative activity of SRL and investigate underlying mechanisms of SRL‐induced apoptosis, in the human ovarian cancer cell line PA‐1.
Materials and methods
SRL‐induced anti‐proliferative effects were determined using MTT assay and induction of apoptosis was determined by flow cytometry, confocal microscopy and western blot analysis.
Results
SRL inhibited population growth of PA‐1 cells in a dose‐ and time‐dependent manner with maximum inhibition (71.3 ± 1.9%) occurring at concentration of 50 μg/ml after 72 h incubation. Observed effects of SRL could be blocked by competing glycoproteins, asialomucin, mucin and fetuin. Treatment with SRL resulted in increase in hypodiploid cell population as determine by cell cycle analysis. Increase in numbers of annexin V‐PI positive cells, and cleavage of PARP confirmed apoptosis‐inducing activity of SRL. Involvement of caspases in SRL‐mediated apoptosis was determined by cleavage of caspases‐3, ‐8 and ‐9 in a time‐dependent manner, thereby suggesting possible involvement of both intrinsic and extrinsic caspase‐dependent pathways.
Conclusion
The present study demonstrates anti‐proliferative and apoptosis‐inducing activity of SRL that can be exploited for potential application in ovarian cancer research.
Introduction
Alteration in protein glycosylation is an important phenomenon in development and progression of malignancy. These altered glycans are known to be involved in many cell functions such as tumour proliferation, invasion and metastasis 1, which can be detected by using lectins. Lectins compose a class of proteins that are ubiquitous in nature, and have specific binding affinities to carbohydrates and glycoconjugates 2. They have diverse effects and wide application, and hence have attracted the attention of many researchers in the field of glycobiology. Fungal lectins are known to induce many biological effects such as mitogenic, anti‐proliferative, anti‐tumour, anti‐fungal and immunomodulatory activities 3, which are usually initiated by recognizing specific cell‐surface receptors. The property of apoptosis induction has been revealed in lectins of some of the higher fungi and plants 4, 5, 6, 7, but there are few reports on lectins from filamentous fungi. Sclerotium rolfsii is a soil‐born filamentous plant‐pathogenic fungus which secretes a developmentally regulated lectin designated as SRL 8. SRL recognizes oncofoetal antigen Galβ1‐3GalNAcα‐Ser/Thr (TF antigen) and its derivatives, specifically 9. TF antigen is a mucin‐type glycans and is known to be expressed by many types of cancer cell including those of ovarian cancer 10. In our previous studies, we have observed that SRL‐induced population growth‐inhibitory effects in human colon 11 and breast (Unpublished data; S.R. Inamdar, M.A. Savanur, S.M. Eligar, C. Chen, P. Mahajan, A. Borges, P. Shastry, B.M. Swamy, J.M. Rhodes, L.G. Yu.) cancer cells, by inducing apoptosis in vitro, and anti‐tumour activity in HT29 xenografts in vivo .
Ovarian cancer is one of the leading causes of gynaecological cancer death in many western countries 12 and many tumour markers with altered glycans, of epithelial ovarian cancer, have been identified. Increased expression of TF antigen has been observed in most, including in ovarian cancer 10. TF antigen is of mucin‐type core‐1 structure and is involved in malignant transformation, and SRL glycan array analysis reveals recognition of these cancer‐related oligosaccharides with high affinity. This has prompted us to investigate interactions of SRL with ovarian cancer cells. In the present study we report that SRL bound to the cell surface and induced apoptosis in our human ovarian cancer cell line PA‐1.
Materials and methods
Bovine serum albumin (BSA), bovine submaxillary mucin, fetuin, asialofetuin, phenylmethyl sulphonyl fluoride (PMSF), nonidet P‐40 (NP‐40), ethylenediaminetetra‐acetic acid (EDTA), 2‐mercaptoethanol, Ribonuclease A, Triton X‐100, trypan blue, glycine, DAPI, formaldehyde and propidum iodide were obtained from Sigma Chemicals Co., St. Louis, MO, USA. Acrylamide, bis‐acrylamide, Tris, sodium dodecyl sulphate (SDS) and MTT [3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide] were obtained from GE Life Sciences (Pittsburgh, PA, USA). Poly vinylene diflurodine (PVDF) membrane was from Millipore, Bedford, MA, USA. Protease inhibitor cocktail was from Roche (Mannheim, Germany). Mounting medium, annexin‐V detection kit, and tissue culture grade plastic‐ware were procured from BD Biosciences, San Jose, CA, USA. Antibodies to caspase‐8, and active caspase‐3 were from Epitomics, Burlingame, CA, USA and caspase‐9 and PARP were from Pierce (Rockford, IL, USA). β‐actin was from MP Biomedicals, Solon, OH, USA and species‐specific HRP‐labelled secondary antibodies were from Bio‐Rad (Hercules, CA, USA). All other chemical used were of analytical grade.
Purification of SRL from sclerotial bodies was carried out as described earlier 13. Conjugation of SRL with FITC was conducted as described by Goldman et al. 14; briefly, SRL (10 mg/ml) was incubated with FITC at 25 μg/mg concentration of protein in carbonate buffer (0.05 m, pH 9.5) with gentle stirring, overnight at 4 °C. Unbound excess FITC was removed by extensive dialysis against PBS and the labelled SRL was stored at 4 °C until further use.
Asialo bovine mucin (ABM) was prepared by acid hydrolysis of bovine submaxillary mucin, as described by Spiro 15. In brief, bovine mucin was dissolved in 0.05 m H2SO4 containing 0.15 m NaCl and the mixture was incubated at 80 °C for 1 h. Incubated samples were then centrifuged at 4500 g for 30 min at room temperature. Supernatant obtained was dialysed against distilled water extensively, and was lyophilized.
Cell culture
The human ovarian cancer cell line PA‐1 was procured from American Type Culture Collection (ATCC, Rockville, MD, USA) and maintained in MEM (Gibco‐BRL, NY, USA) supplemented with 10% heat inactivated foetal calf serum (FCS), 1 mm glutamine, 1 mm sodium pyruvate, 100 mg/ml streptomycin and 100 units/ml penicillin, at 37 °C in 5% CO2 and 95% humidified air.
Flow cytometry
PA‐1 cells were incubated with 3% BSA for blocking and then stained with FITC‐labelled SRL for 1 h at 4 °C. Carbohydrate‐mediated binding was analysed by pre‐incubating the FITC‐SRL with 100 μg/ml of competing glycoconjugates, asialomucin, fetuin and mucin for 1 h at 37 °C before cell staining. Data were acquired for 10 000 events using a BD FACS Calibur cytometer (Becton Dickinson, San Jose, CA, USA), and were analysed using Cell Quest Pro software. Unstained cells processed similarly were used as negative control.
Confocal microscopy
PA‐1 cells, cultured on coverslips for 24 h, were blocked with 3% BSA for 1 h at 4 °C. Cells were washed in phosphate‐buffered saline (25 mm, pH 7.2 – PBS) then incubated in FITC‐SRL for 1 h at 4 °C; they were then washed three times PBS. Cells were then fixed in 2% para‐formaldehyde for 10 min and stained with DAPI before mounting, using mounting medium. Stained cells were visualized by confocal laser scanning microscopy (Zeiss LSM 510, Göttingen, Germany) equipped with 488 and 560 nm Argon lasers.
Growth inhibitory studies
PA‐1 cells were seeded in 96‐well plates at 5 × 104 cells/ml density, and grown in complete medium for 24 h before lectin treatment, then cells in MEM with 0.5% FCS were treated with SRL at different concentrations (6.25–50 μg/ml) for different time points (12–72 h), maintained in humidified atmosphere (37 °C, 5% CO2). After each time point, 10 μl of MTT (5 mg/ml) was added to each well followed by lysis in 100 μl of 10% SDS in 0.01 N HCl. To observe effects of competing glycoprotein, SRL (25 μg/ml) was pre‐incubated in 100 μg/ml of ASM, for 1 h before being treated and processed as mentioned above. Finally, absorbance was measured at 570 nm, with reference wavelength of 640 nm, using ELISA plate readers. Percentage viable cell number was calculated, with respect to controls considered as 100%.
Cell cycle analysis
Cells were treated with or without SRL (25 μg/ml) for 12, 24 and 36 h and were harvested after gentle trypsinization. They were then washed in PBS, and fixed in 70% chilled ethanol for 30 min at 4 °C. Following this, they were washed once in PBS and treated with 50 μl Ribonuclease A (5 mg/ml in PBS, DNase‐free) for 10 min, RT, and stained with 450 μl propidium iodide (50 μg/ml in PBS) for 2 h in the dark. DNA content was analysed on FL‐2A channel of our flow cytometer (FACS Calibur; BD Biosciences) equipped with 488 nm argon laser at linear scale. Data were analysed using Cell Quest Pro software (BD Biosciences) for distribution of cells in different phases of the cell cycle.
Annexin‐V PI staining
FITC‐annexin V staining was used to determine SRL‐induced phosphatidylserine externalization of our PA‐1 cells. Those treated with SRL (25 μg/ml) for 12, 24 and 36 h were collected after gentle trypsinization and were resuspended in binding buffer at concentration of 1 × 106 cells/ml. Cell suspensions (100 μl) were incubated in FITC‐annexin V (5 μl) and propidium iodide (5 μl) for 15 min, at room temperature, in the dark. Binding buffer (1X – 400 μl) was added to each tube and analysed by flow cytometry. Percentages of cells positive for annexin V, PI alone and both annexin V and PI, were calculated by dot blot analysis using Cell Quest Pro software (BD).
Western blotting
Cells were treated with SRL (25 μg/ml) for different time intervals up to 48 h. At specific times they were lysed using RIPA lysis buffer (120 mm NaCl, 1.0% Triton X‐100, 20 mm Tris–HCl, pH 7.5, 100% glycerol, 2 mm EDTA, protease inhibitor cocktail; Roche), and total protein was electrophoresed on SDS–polyacrylamide gels, then blotted on to PVDF membranes (Millipore). After being blocked with 5% BSA, blots were probed with primary antibodies to caspases‐8, ‐9, active caspase‐3, and PARP, for 1 h at room temperature, followed by incubation of species‐specific secondary antibodies for 1 h. Bands were visualized using chemiluminescence with Super Signal West Femto Maximum Sensitivity substrate (Pierce). β‐actin was used as loading control.
Statistical analysis
Each experiment was performed at least three times, each time in triplicate. Results were analysed using one‐way ANOVA followed by ‘Newman–Keuls’ multiple comparisons with Stat Direct software, and data were considered significant when P < 0.05.
Results
Cell‐surface binding of SRL
SRL binding was determined by staining cells with FITC‐SRL, followed by flow cytometric analysis. A total of 90.49% cells were positive for SRL with MFI of 176.87 compared to unstained cells that had MFI of 3.12. Carbohydrate‐mediated binding of SRL was determined by using competing glycoconjugates or haptens, which resulted in significant reduction in binding of lectin to the cells. MFI decreased to 16.65, 23.66 and 28.54 for cells stained with FITC‐SRL pre‐incubated with asialomucin, mucin and fetuin respectively (Fig. 1a), which suggested that asialomucin would be the most effective inhibitor, followed by mucin and fetuin respectively.
Figure 1.
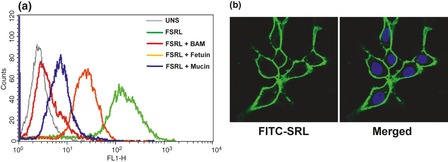
Binding of FITC ‐ SRL to PA ‐1 cells. (a) Human ovarian cancer PA‐1 cells were incubated with FITC‐SRL (2 μg/ml) in the presence or absence of competing glycoconjugates (100 μg/ml), and binding was analysed by flow cytometry. Overlays are representative data, with X‐axis and Y‐axis representing fluorescence intensity and cell counting, respectively. (b) PA‐1 cells stained with FITC‐SRL and DAPI and visualized using confocal microscopy. Images were captured at magnification 63×.
Surface binding was also visualized using confocal microscopy after staining with FITC‐SRL. Uniform and intense fluorescence on cell surfaces indicated high expression levels of SRL‐recognizing receptors (Fig. 1b).
Growth inhibitory effects of SRL
SRL induced cell population growth inhibitory effects in a dose‐ and time‐dependent manner. Cells were treated with serial concentrations of SRL (12.5, 25 and 50 μg/ml) for 24, 48 and 72 h. At the earliest time point (24 h), 25 and 50 μg/ml doses caused significant growth inhibition of 26 ± 8.7% and 34.5 ± 11.4% respectively. Treatment of cells for 48 h resulted in 32.4 ± 2.8%, 60.5 ± 10.8% and 68.6 ± 2.2% inhibition at 12.5, 25 and 50 μg/ml respectively, which further increased to 46.6 ± 2.6%, 66.3 ± 12.2% and 71.3 ± 1.9% inhibition at 72 h. Lower concentration of SRL (6.25 μg/ml) did not induce significant cell population growth inhibition, even after 72 h treatment (15%) (Fig. 2a). SRL (25 μg/ml) was pre‐incubated with the asialomucin (100 μg/ml) before treating cells for 48 h. Pre‐incubation of SRL with ASM resulted in complete inhibition of the cell population growth inhibitory effect compared to SRL only‐treated cells (Fig. 2b). These preliminary results suggest that SRL induced cell death in these PA‐1 cells in a dose‐ and time‐dependent manner and that interaction with cell‐surface glycoproteins was essential for exerting its effect.
Figure 2.
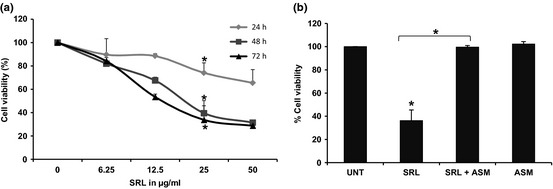
Effect of SRL on population growth of PA ‐1 cells. (a) SRL was incubated with human ovarian cancer PA‐1 cells at different concentrations (6.25–50 μg/ml) for different times (24–72 h). Cell viability after each time point was measured by MTT assay as described in the Materials and methods section. (b) SRL (25 μg/ml) was incubated in presence or absence of ASM (100 μg/ml) and cell viability was analysed by MTT assay after 48 h. Data are representative of three independent experiments performed in triplicate. *P < 0.05
Effect of SRL on the cell cycle
Cells treated with SRL (25 μg/ml) at different time points were stained with propidium iodide (PI) and were subjected to cell cycle analysis to determine percentages of cells undergoing apoptosis and distribution of cells in the different phases of cell cycle, by flow cytometry. Treatment with SRL for 12 and 24 h resulted in minimal increase in the hypodiploid population (6%) compared to untreated control cells. There was marginal increase in the G0/G1 population at 12 and 24 h time points, but at 36 h exposure 29.82% of the cells were in hypodiploid phase compared to 2.72% of untreated controls (Fig. 3). SRL also reduced G0/G1 and G2/M phases cell population to 46.02% and 12.05% compared to 62.75% and 20.11% of untreated cells, respectively.
Figure 3.
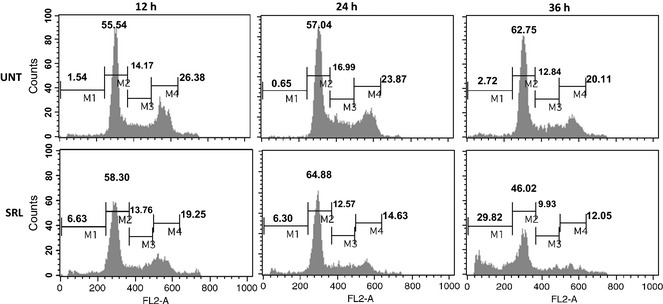
Effect of SRL on different phases of the cell cycle. Human ovarian cancer PA‐1 cells incubated with or without SRL (25 μg/ml) for 12, 24 and 36 h. Cells were stained with PI and data were acquired on FL2‐A channel of flow cytometer equipped with 488 nm laser. X‐axis represents DNA content of cells and the Y‐axis represents cell number. M1, M2, M3 and M4 represent cell population in hypodiploid/apoptotic, G0/G1, S and G2/M phases respectively.
Induction of apoptosis by SRL
The apoptotic potential of SRL was quantified using annexin‐V and PI staining, to distinguish viable, early and late stage apoptotic cells. Treatment with SRL resulted in a significant increase in annexin‐V positive cells to 35.51% and 38.35% at 12 and 24 h respectively, indicating induction of early apoptosis. Dot plot analysis revealed that 85% of the cells were in early/late apoptosis after 36‐h treatment (Fig. 4a,b).
Figure 4.
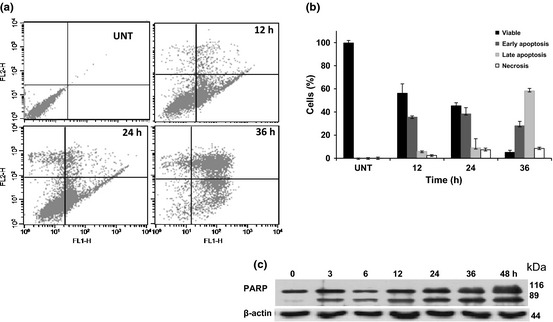
Induction of apoptosis by SRL . Human ovarian cancer PA‐1 cells treated with SRL (25 μg/ml) for 12, 24 and 36 h followed by FITC‐annexin‐V and PI staining. X‐axis depicts annexin‐V‐positive cells and Y‐axis depicts PI‐positive cells (a). Percentages of cells in early/late apoptosis and necrosis were compared to normal cells at different time points (b). Histoplots are representative of two similar experiments. Cleavage of PARP in SRL induced PA‐1 cells studied by western blot analysis at 25 μg/ml for different time points (c). Actin was used as loading control.
Detection of 89 or 24 kDa cleaved fragments of PARP is a hall mark of apoptosis. Effects of SRL on PARP cleavage were studied by western blot analysis and an 89 kDa cleaved fragment was observed in those treated with 25 μg/ml SRL for different time intervals (Fig. 4c). These results suggest that SRL‐mediated cell death by induction of apoptosis.
Involvement of caspases in SRL‐induced apoptosis
To delineate mechanism(s) of SRL‐induced apoptosis, effects of SRL on activation of initiator caspases‐8 and ‐9 and effector caspase‐3, were measured. Time‐dependent increase in active caspases‐8 and ‐9, with subsequent reduction in procaspase activity, was observed (Fig. 5). There was also a significant increase in expression of active caspase‐3 in a time‐dependent manner. Taken together, these results imply that both extrinsic and intrinsic apoptotic pathways were involved in SRL‐induced apoptosis.
Figure 5.
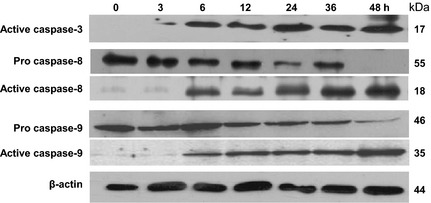
Activation of caspases by SRL . Human ovarian cancer PA‐1 cells exposed to SRL (25 μg/ml) for different time points, and whole cell lysate, transblotted to PVDF membrane and probed with anti‐active caspase‐3, caspases‐8 and ‐9 and PARP antibodies. β‐actin was used as loading control. Data are representative of two similar experiments.
Discussion
In the present study, we demonstrated that SRL exhibits population growth inhibitory effects on human ovarian cancer cells, in a dose‐ and time‐dependent manner. Our study also revealed that SRL treatment lead to activation of initiator caspases‐8 and ‐9 and effector caspase‐3, leading to induction of apoptosis. Activation of both caspase‐8 and ‐9 suggested possible involvement of extrinsic as well as intrinsic pathways for induction of apoptosis.
Expression of the TF antigen is known to increase in cells of many human malignancies, which is an important event in cancer progression and metastasis. Mucins are known to express TF antigen in many physiological conditions, including cell transformation. TF antigen expressed on mucins is a natural ligand to bind to galectins, which are known to be involved in cancer metastasis 10, 16, 17. Lectins from plants and fungi that are specific to TF antigen are known to induce proliferative/anti‐proliferative effects in vitro upon binding to many types of human cancer cell 18, 20. The surface binding and population growth inhibitory effect of SRL on PA‐1 cells can be effectively blocked by TF‐expressing ASM, suggesting possible involvement of TF‐expressing glycoconjugates for binding and inhibition of cell proliferation. Induction of apoptosis by SRL was demonstrated by increase in the hypodiploid cell population, annexin‐V binding, and cleavage of PARP. Many lectins are known to induce cell cycle arrest at either G0/G1, S, or G2/M phases, or in combination, to induce apoptosis. Agrocybe aegerita lectin (AAL) induces increase in hypodiploid population of cells (31.4%) after 48 h at 100 μg/ml and decrease in the G2/M population, without affecting cells in G0/G1, in HeLa cells 4; however, Astragalus mongholicus lectin induces cell cycle arrest in S phase in HeLa cells 5. Lectin from Legumi secchi has been shown to arrest the cell cycle in G2/M after 24‐h treatment of MCF7 cells, at 600 nm 7. In the present study, treatment of PA‐1 cells with SRL for 36 h resulted in increase in the hypodiploid cell population and subsequent reduction in G0/G1, S and G2/M phases, suggesting that SRL treatment does not preferentially induce apoptosis of cells at particular phases of the cycle. Annexin‐V‐FITC and PI double staining clearly showed increase in the early apoptotic cell population (annexin‐V positive, only) to 35.41% and 38.35% at 12 and 24 h respectively. At 36 h incubation with SRL, 86% of cells were in early/late phase of apoptosis (annexin‐V and PI positive). There are reports that demonstrate differential activities of lectins with respect to induction of early/late apoptosis, or necrosis, in different cell lines. For example, AAL‐treated HeLa cells underwent apoptosis and 6.65% of cells were annexin‐V positive at 24 h treatment 21. Haemagglutinin from Legumi secchi (LS)‐induced apoptosis in MCF‐7 cells and 88% of total population was in early/late apoptotic/necrotic phase after 24 h incubation 7. Mistletoe extracts MT‐A and MT‐P at 250 μg/ml induced elevation of apoptotic activity to 40% and 58.3% respectively 22. In comparison with these lectins, SRL induced apoptosis in 85% of treated cells, thereby indicating its high potency in inducing apoptosis.
Programmed cell death is an important phenomenon that involve participation of different caspases, which can engage in extrinsic and intrinsic pathways 23. Activation of initiator caspases may be triggered either by the extrinsic/receptor‐mediated pathway or the intrinsic/mitochondria‐mediated pathway 24. Commencement of the receptor‐mediated pathway is signalled by stimuli outside the cell, which with the aid of cell‐surface receptors, leads to activation of initiator caspase‐8 25. The intrinsic apoptotic pathway is triggered by signals within the cell that target mitochondria, resulting in release of apoptotic factors and activation of initiator caspase‐9 26. Here, SRL treatment resulted in activation of capsases‐8 and ‐9, followed by cleavage of executioner caspase‐3, suggesting possible involvement of both intrinsic and extrinsic caspase‐dependent pathways. SRL also induces a similar apoptotic effect in human colon 11 and breast (Unpublished data, S.R. Inamdar, M.A. Savanur, S.M. Eligar, C. Chen, P. Mahajan, A. Borges, P. Shastry, B.M. Swamy, J.M. Rhodes, L.G. Yu.) cancer cells after 72 h exposure.
In conclusion, TF antigen‐binding lectin SRL inhibits cell proliferation and induces apoptosis, in PA‐1 ovarian cancer cells. The observed effect could effectively be blocked by TF‐expressing ASM. We have also shown that SRL‐induced apoptosis involved activation of caspases‐8 and ‐9 and executioner caspase‐3, suggesting involvement of both extrinsic and intrinsic apoptotic pathways. The present study indicates the possible potential application of SRL in ovarian cancer research; however further detailed investigation would be warranted.
Acknowledgements
This work has been supported by funding from the Department of Biotechnology, India (BT/PR11017/MED/30/122/2008). SME is SRF under UGC Meritorious fellowship.
References
- 1. Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA (2001) Glycosylation and the immune system. Science 291, 2370–2376. [DOI] [PubMed] [Google Scholar]
- 2. Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth J (1999) Essentials of Glycobiology. New York: Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- 3. Konska G (2006) Lectins of Higher Fungi (Macromycetes) – their occurrence, physiological role, and biological activity. Int. J. Med. Mushr. 8, 19–30. [Google Scholar]
- 4. Zhao C, Sun H, Tong X, Qi Y (2003) An antitumour lectin from the edible mushroom Agrocybe aegerita . Biochem. J. 374, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yan Q, Li Y, Jiang Z, Sun Y, Zhu L, Ding Z (2009) Antiproliferation and apoptosis of human tumor cell lines by a lectin (AMML) of Astragalus mongholicus . Phytomedicine 16, 586–593. [DOI] [PubMed] [Google Scholar]
- 6. Yang Y, Xu HL, Zhang ZT, Liu JJ, Li WW, Ming H, et al (2011) Characterization, molecular cloning, and in silico analysis of a novel mannose‐binding lectin from Polygonatum odoratum (Mill.) with anti‐HSV‐II and apoptosis‐inducing activities. Phytomedicine 18, 748–755. [DOI] [PubMed] [Google Scholar]
- 7. Lam SK, Ng TB (2011) Apoptosis of human breast cancer cells induced by hemagglutinin from Phaseolus vulgaris cv Legumi secchi. Food Chem. 126, 595–602. [Google Scholar]
- 8. Swamy BM, Bhat AG, Hegde GV, Naik RS, Kulkarni S, Inamdar SR (2004) Immunolocalization and functional role of Sclerotium rolfsii lectin in development of fungus by interaction with its endogenous receptor. Glycobiology 14, 951–957. [DOI] [PubMed] [Google Scholar]
- 9. Chachadi VB, Inamdar SR, Yu LG, Rhodes JM, Swamy BM (2011) Exquisite binding specificity of Sclerotium rolfsii lectin toward TF‐related O‐linked mucin‐type glycans. Glycoconj. J. 28, 49–56. [DOI] [PubMed] [Google Scholar]
- 10. Yu LG (2007) The oncofetal Thomsen‐Friedenreich carbohydrate antigen in cancer progression. Glycoconj. J. 24, 411–420. [DOI] [PubMed] [Google Scholar]
- 11. Inamdar SR, Savanur MA, Eligar SM, Chachadi VB, Nagre NN, Chen C, Barclays M, Ingle A, Mahajan P, Borges A, Shastry P, Kalraiya RD, Swamy BM, Rhodes JM, Yu LG (2012) The TF‐antigen binding lectin from Sclerotium rolfsii inhibits growth of human colon cancer cells by inducing apoptosis in vitro and suppresses tumour growth in vivo . Glycobiology (In press ‐ doi: 10.1093/glycob/cws090. [DOI] [PubMed] [Google Scholar]
- 12. Parker SL, Tong T, Bolden S, Wingo PA (1996) Cancer statistics, 1996. CA Cancer J. Clin. 46, 5–27. [DOI] [PubMed] [Google Scholar]
- 13. Swamy BM, Hegde GV, Naik RS, Inamdar SR (2001) T‐antigen binding lectin from the phytopathogenic fungus Sclerotium rolfsii . Lect. Biol. Biochem. Clin. Biochem. 15, 45–55. Available at http://plab.ku.dk/tcbh/Lectins15/Swamy/paper.htm. [Google Scholar]
- 14. Goldman M (1968). Fluorescent Antibody Methods, pp. 101–161. New York: Academic Press. [Google Scholar]
- 15. Spiro RG (1960) Studies on fetuin, a glycoprotein of fetal serum I. Isolation, chemical composition, and physiochemical properties. J. Biol. Chem. 235, 2860–2869. [PubMed] [Google Scholar]
- 16. Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, et al (2007) Galectin‐3 interaction with Thomsen‐Friedenreich disaccharide on cancer‐associated MUC1 causes increased cancer cell endothelial adhesion. J. Biol. Chem. 282, 773–781. [DOI] [PubMed] [Google Scholar]
- 17. Zhao Q, Barclay M, Hilkens J, Guo X, Barrow H, Rhodes JM, et al (2010) Interaction between circulating galectin‐3 and cancer‐associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 9, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryder SD, Smith JA, Rhodes JM (1992) Peanut lectin: a mitogen for normal human colonic epithelium and human HT29 colorectal cancer cells. J. Natl. Cancer Inst. 84, 1410–1416. [DOI] [PubMed] [Google Scholar]
- 19. Yu L, Fernig DG, Smith JA, Milton JD, Rhodes JM (1993) Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 53, 4627–4632. [PubMed] [Google Scholar]
- 20. Yu LG, Milton JD, Fernig DG, Rhodes JM (2001) Opposite effects on human colon cancer cell proliferation of two dietary Thomsen‐Friedenreich antigen‐binding lectins. J. Cell. Physiol. 186, 282–287. [DOI] [PubMed] [Google Scholar]
- 21. Liang Y, Feng L, Tong X, Wang K, Li de F, Lin JC, et al (2009) Importance of nuclear localization for the apoptosis‐induced activity of a fungal galectin AAL (Agrocybe aegerita lectin). Biochem. Biophys. Res. Commun. 386, 437–442. [DOI] [PubMed] [Google Scholar]
- 22. Seifert G, Jesse P, Laengler A, Reindl T, Luth M, Lobitz S, et al (2008) Molecular mechanisms of mistletoe plant extract‐induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro. Cancer Lett. 264, 218–228. [DOI] [PubMed] [Google Scholar]
- 23. Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35, 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev. 17, 2481–2495. [DOI] [PubMed] [Google Scholar]
- 25. Ashkenazi A, Dixit VM (1998) Death receptors: signaling and modulation. Science 281, 1305–1308. [DOI] [PubMed] [Google Scholar]
- 26. Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922–2933. [PubMed] [Google Scholar]


