Abstract
Abstract. During development of Drosophila, cell proliferation and size are known to be regulated by insulin. Here we use Drosophila Kc cells to examine the molecular basis for the control of cell growth by insulin. Growing cells in the presence of insulin increased cell number above control levels at 16, 24, 48 and 72 h. We have demonstrated a novel anti‐apoptotic effect of insulin (∼50%) in these cells, measured by caspase 3‐like activity, which contributed to the increase in cell number. The anti‐apoptotic effect was observed both in control cells and those in which apoptosis was induced by ultraviolet irradiation. An approximately 2‐fold stimulation of bromodeoxyuridine incorporation demonstrated that insulin also increased Kc cell proliferation by stimulating new DNA synthesis. The ability of insulin to increase cell number, stimulate bromodeoxyuridine incorporation and reduce caspase 3‐like activity was prevented by PD98059, which inhibits activation of the Drosophila extracellular signal regulated kinase (DERK) pathway, and was unaffected by wortmannin, an inhibitor of Drosophila phosphatidylinositol 3‐kinase (DPI3K). Insulin also increased cell size approximately 2‐fold and this was prevented by wortmannin and rapamycin, an inhibitor of Drosphilia target of rapamycin (DTOR). In summary, we show that DERK plays an important role in mediating the effect of insulin to reduce apoptosis and increase DNA synthesis whereas the DPI3K/DTOR/Dp70S6 kinase pathway mediates effects of insulin on cell size in Drosophila Kc cells.
INTRODUCTION
Cell growth, division and survival are temporally co‐ordinated to regulate development in Drosophila melanogaster (Bangs & White, 2000; Johnston & Gallant 2002). Recent work has begun to characterize the mechanisms that control growth and organ size. The insulin‐signalling pathway has emerged as the principal regulator of cell growth and development in Drosophila (Brogiolo et al. 2001; Leevers 2001; Oldham et al. 2002; Oldham & Hafen 2003). For example, expression of an insulin‐like peptide leads to an increase in body mass (Brogiolo et al. 2001) and studies in the developing eye show that over‐expression of the insulin receptor increases growth (Brogiolo et al. 2001). Furthermore, when insulin signalling is inhibited, imaginal disc development and overall fly size are reduced (Edgar 1999). The association of mutations in the insulin receptor or defects in insulin signalling with leprechaunism in humans and with small flies in Drosophila suggests that an evolutionarily conserved function of insulin signalling is the control of cell growth and total body size (Brogiolo et al. 2001).
The insulin‐signalling system is very well conserved from Drosophila to humans (Garofalo 2002). This has led to Drosophila being exploited as an excellent system in which to investigate insulin signalling. There is one insulin receptor (DInR) (Fernandez et al. 1995) and seven insulin‐like peptides in Drosophila (Brogiolo et al. 2001). Downstream signalling pathways involve the homologue of the mammalian insulin receptor substrates (Oldham et al. 2000; Poltilove et al. 2000), phosphatidylinositol 3‐kinase (DPI3K) (Leevers et al. 1996), phosphatase and tensin homologues on chromosome 10 (DPTEN) (Goberdhan et al. 1999), protein kinase B/Akt (DAkt) (Verdu et al. 1999), p70 S6 kinase (DS6K) (Watson et al. 1996), extracellular signal‐regulated kinase (DERK) (Lim et al. 1999) and p38 mitogen‐activated kinase (Dp38) (Han et al. 1998) amongst others. Many studies have demonstrated a role for components of these insulin‐signalling pathways in the control of cell division or cell size.
It has also been shown that insulin can rescue many types of cell from apoptotic death (Bertrand et al. 1998; Lee‐Kwon et al. 1998; Yenush et al. 1998). Programmed cell death by apoptosis is a common means of destroying unwanted and superfluous cells (Vaux & Korsmeyer 1999). Apoptosis is mediated by a group of cysteine proteases, termed caspases (Thornberry & Lazebnik 1998). These enzymes, which cleave their substrates after an aspartate residue, are normally present as inactive precursors in cells. Upon receiving an apoptotic signal, the pro‐forms of caspases (pro‐caspases) undergo proteolytic processing to generate active enzymes (Kumar & Colussi 1999; Nicholson 1999; Kumar & Doumanis 2000; Richardson & Kumar 2002). There is a total of seven caspases in Drosophila; DCP‐1, DREDD, DRICE, DRONC, DECAY, DAMM and STRICA (Cakouros et al. 2002; Richardson & Kumar 2002).
The use of whole flies to study insulin signalling and growth control by insulin/insulin‐like growth factor I (IGF‐I) has been and will continue to be extremely useful. In addition, the availability of Drosophila‐derived cell lines which exhibit characteristics of the parent organism are ideal for this purpose. In this study, our aim was to examine the mitogenic and anti‐apoptotic effects of insulin on Drosophila Kc cells and to elucidate the underlying signalling mechanisms responsible for these effects. The results of the present investigation demonstrate that insulin increases Kc cell proliferation and suppresses apoptosis via DERK‐dependent and DPI3K‐independent signalling pathways.
MATERIALS AND METHODS
Determination of cell number
Kc cells (a kind gift from Dr Lucy Cherbas, University of Indiana, Indianapolis) were propagated at 26 °C in Schneider's Drosophila media (Invitrogen, Burlington, Ontario, Canada) supplemented with 10% foetal bovine serum, 100 units/ml penicillin, and 100 mg/ml streptomycin. Cells (0.5 × 106 in 2 ml; at a concentration of 0.25 × 106/ml) were grown in a six‐well culture dish for 16, 24, 48 and 72 h in the presence or absence of 1 µm insulin. Where indicated, cells were also pre‐treated for 30 min with PD98059 [Bioshop, Burlington, ON (5 mm)] or wortmannin (Calbiochem, La Jolla, CA; 100 nm). At each time point an aliquot of 50 µl was taken from each well and counted using a haemocytometer. Trypan blue dye exclusion was used to confirm viability of cells.
Bromodeoxyuridine (BrdUrd) incorporation assay
The effect of insulin on DNA synthesis was assessed by BrdUrd incorporation using a colorimetric enzyme‐linked immunosorbent‐based assay kit from Oncogene Research Products (La Jolla, CA). In brief, cells grown in 96‐well plates were stimulated with insulin 1 µm for 12 h; then BrdUrd label (1 : 2000 dilution) was added with subsequent incubation for an additional 12 h. Where indicated, cells were also treated with PD98059 (5 mm) or wortmannin (100 nm) for 30 min prior to the addition of insulin. Thereafter, DNA was denatured and cells were incubated with anti‐BrdUrd antibody followed by quantification of antibody binding by measuring absorbance at dual wavelengths of 450–540 nm.
Determination of apoptosis in Kc cells
Apoptosis was measured by determining caspase 3‐like activity using a kit from Medical & Biological Laboratories Co. Ltd. (Nagoya, Japan). This assay is based on spectrophotometric detection of the chromophore p‐nitroanilide (pNA) after cleavage from the labelled substrate DEVD‐pNA. Where indicated, 2 × 106 cells were then treated with UV light in the UV‐B range (302 nm) for 60 min to induce apoptosis and were subsequently grown for 24 h in the presence or absence of insulin 1 µm. Cells were also pre‐treated with PD98059 (5 mm) or wortmannin (100 nm) for 30 min prior to the addition of insulin where indicated. Caspase 3‐like activity was then determined according to the manufacturer's instructions.
Microscopy and image analysis to determine cell size
Cells (1 × 106) were grown in six‐well plates on sterile coverslips coated with poly d‐lysine. They were then incubated (where indicated with inhibitors: wortmannin: 100 nm, rapamycin: 20 ng/ml) for 1 h, followed by insulin (1 µm) for 24 h. Cells were identified using an Olympus FV300 laser scanning microscope (at 60× magnification). Images of cells were digitally aquired and the surface area of individual cells was measured using fluoview software Version 3.0 (Carsen Group, Markham, ON) as described previously (Lnenicka & Keshishian 2000; Cashion et al. 2003).
Statistical analysis
All data are expressed as the mean ± SEM. Statistical analysis was performed by one‐way analysis of variance (anova) with Tukey–Kramer multiple comparison test. The accepted level of significance was set at P < 0.05.
RESULTS
Insulin increases cell number via a DERK‐dependent signalling pathway
Figure 1 shows that at all time‐points measured over a 72‐h period (16, 24, 48 and 72 h) insulin significantly increased cell number, as assessed by cell counting using a haemocytometer. We also demonstrated that the specific inhibitor of DERK activation, PD98059, prevented the insulin‐induced increase in cell number at 16, 24, 48 and 72 h in a statistically significant manner (Fig. 1a). Importantly, PD98059 had no effect on the growth of cells cultured in the absence of insulin (Fig. 1a). We also used wortmannin to test the involvement of the DPI3K pathway in insulin‐induced increases in cell number. We found that wortmannin had no effect on cell number under basal or insulin‐treated conditions from 0 to 72 h (Fig. 1b).
Figure 1.
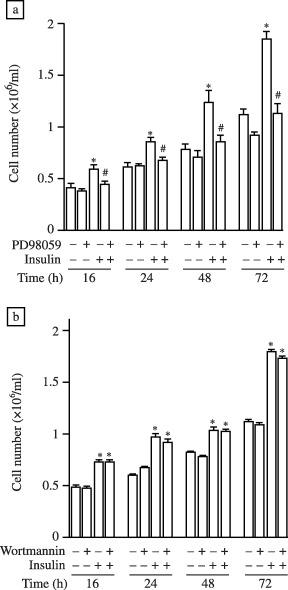
Insulin increases cell number via a DERK‐dependent signalling pathway. Cells (at a starting concentration of 0.25 × 106/ml) were grown in a six‐well culture dish for 16, 24, 48 and 72 h in the presence or absence of 1 µm insulin. Where indicated, cells were also pre‐treated for 30 min with (a) PD98059 (5 mm) or (b) wortmannin (100 nm). At each time‐point an aliquot of cells was removed and cell number was determined. Data are expressed as the mean ± SEM of three individual experiments. Statistical analysis was performed by one‐way analysis of variance and P < 0.05 indicated with respect to control (*) and insulin (#) at each time‐point.
Insulin increases BrdUrd incorporation in Kc cells in a DERK‐dependent manner
Having shown that insulin increased cell number (Fig. 1) we next determined whether an increase in cell proliferation was responsible for this effect. To examine DNA synthesis we utilized the BrdUrd incorporation assay. Figure 2 shows that insulin increased BrdUrd incorporation in Kc cells (2.06 ± 0.03 fold). The ability of insulin to increase BrdUrd incorporation was reduced in cells which were pre‐incubated with PD98059 (Fig. 2). Basal levels of BrdUrd incorporation were unaffected by pre‐treatment with PD98059 (Fig. 2). We also tested the effect of wortmannin and found that it had no effect on basal or insulin‐stimulated rates of DNA synthesis (Fig. 2).
Figure 2.
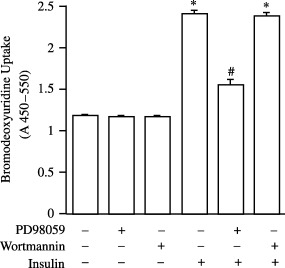
Insulin increases BrdUrd incorporation in Kc cells in a DERK‐dependent manner. Cells were stimulated with insulin 1 µm for 12 h, BrdUrd was added for a further 12 h then incorporation of BrdUrd was determined. Where indicated, cells were also treated with PD98059 (5 mm) or wortmannin (100 nm) for 30 min prior to the addition of insulin. Data are expressed as the mean ± SEM of four individual experiments. Statistical analysis was performed by one‐way analysis of variance and P < 0.05 indicated with respect to control (*) and insulin (#).
Insulin exerts anti‐apoptotic effects in Kc cells in a DERK‐dependent manner
The ability to protect cells from apoptosis may also contribute to the ability of insulin to increase cell numbers (Fig. 1). Therefore, we examined apoptosis using an assay kit to measure caspase 3‐like activity. Insulin was shown to decrease the levels of caspase 3‐like activity in Kc cells under both control and UV‐treated conditions by 50 and 49%, respectively (Fig. 3). The ability of insulin to reduce basal levels or to protect cells from UV‐induced apoptosis was prevented by prior incubation of cells with PD98059 but not wortmannin (Fig. 3). Control or UV‐induced levels of caspase 3‐like activity were unaffected by PD98059 or wortmannin (Fig. 3).
Figure 3.
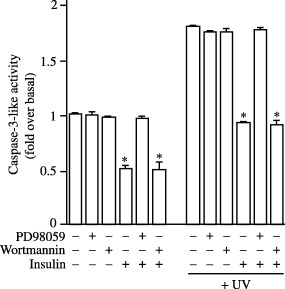
Insulin exerts anti‐apoptotic effects in Kc cells in a DERK‐dependent manner. Cells were treated with UV light (302 nm) for 60 min and subsequently grown for 24 h in the presence or absence of insulin 1 µm. Cells were also pre‐treated with PD98059 (5 mm) or wortmannin (100 nm) for 30 min prior to the addition of insulin where indicated. Caspase 3‐like activity was then determined. Data are expressed as the mean ± SEM of four individual experiments. Statistical analysis was performed by one‐way analysis of variance and P < 0.05 is indicated with respect to control (*).
Insulin increases cell size via DPI3K‐ and DTOR/Dp70S6K‐dependent signalling pathways
Cell size was determined by digitally acquiring images of cells grown under the indicated conditions and measuring surface area by image analysis using fluoview software. Treatment of cells with insulin for 24 h caused a statistically significant increase in cell size of 1.93‐fold (Fig. 4; control, 75.2 mm2 and insulin, 145.3 mm2). This effect was totally abolished when cells were pre‐treated with wortmannin (78.6 mm2) or rapamycin (75.8 mm2) whereas control cell size was not significantly altered by the presence of these inhibitors (Fig. 4).
Figure 4.
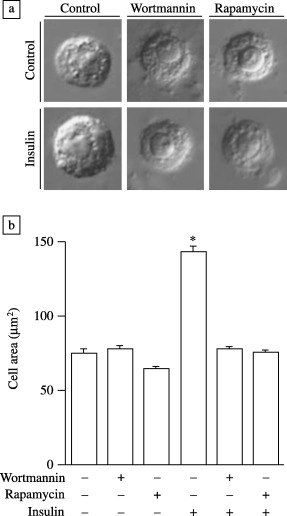
Insulin increases cell size via DPI3K‐ and Dp70S6K‐dependent signalling pathways. Cells (1 × 106) grown in six‐well plates were incubated where indicated with wortmannin (100 nm) or rapamycin (20 ng/ml) for 1 h followed by insulin 1 µm for 24 h. Representative images of cells are shown (a) and the surface area of 10 randomly chosen individual cells was measured, then the mean ± SEM was calculated (b). Statistical analysis was performed by one‐way analysis of variance and P < 0.05 is indicated with respect to control (*).
DISCUSSION
Accurate control of cell number and cell size plays a vital role in determining the development of multicellular organisms. Drosophila has often been used as a model system to study the molecular mechanisms controlling cell growth because the signalling pathways responsible are well conserved from Drosophila to man (Garofalo 2002). It is clear from studies to date that the insulin‐signalling pathway plays a fundamental role in the control of Drosophila cell growth. Over‐expression of one of the seven insulin‐like peptides expressed in Drosophila or the insulin receptor leads to increased growth in Drosophila (Brogiolo et al. 2001). Conversely, attenuation of insulin receptor function or insulin‐signalling pathways causes severe growth retardation (Edgar 1999). Thus, studies in whole flies have conclusively shown an important role for insulin in controlling cell growth. It is now necessary to develop a more detailed molecular understanding of the mechanisms whereby insulin controls cell growth and division.
Here we show that growing Drosophila Kc cells in the presence of insulin caused an increase in cell number. Furthermore, we have shown that insulin achieved this effect via a DERK‐dependent signalling pathway, in agreement with the fact that the role of ERK in regulating proliferation has been extensively characterized in various species (Fisher et al. 2001; Kavurma & Khachigian 2003; Masaki et al. 2003). Our results also support a recent report identifying a role of DERK in insulin‐stimulated cell growth in Drosophila Schneider's S2 cells (Kwon et al. 2002). It was also demonstrated recently that over‐expression of DAkt caused an increase in cell size but no alteration in the rate of cell proliferation (Verdu et al. 1999). One conclusion from this study was that Drosophila cell proliferation was independent of Akt. The inability of the PI3K inhibitor wortmannin to prevent insulin‐stimulated increases in cell number in our study supports the notion that the DPI3K/DAkt pathway does not play a role in insulin‐stimulated Kc cell proliferation.
The increase in cell number detected in cells grown in the presence of insulin could conceivably be the result of an increase in new DNA synthesis and thus cell proliferation, or of an ability of insulin to decrease rates of cell death. Therefore, we first examined the effect of insulin on new DNA synthesis by measuring BrdUrd incorporation in Kc cells. BrdUrd is a thymidine analogue which is incorporated into DNA, and this can be quantified using antibodies to BrdUrd (Dolbeare 1996). We found that insulin increased BrdUrd incorporation, via a DERK‐dependent signalling pathway, suggesting that DNA synthesis and cell division was increased by the hormone. Wortmannin did not prevent the insulin‐stimulated increase in cell number. To date, surprisingly little is known regarding control of DNA synthesis by insulin in Drosophila. A previous study using 32D cells transfected with a chimeric insulin receptor (human insulin binding domain and Drosophila intracellular domain) showed that although DPI3K could be activated by the chimaeric receptor, co‐transfection of insulin receptor substrate 1 (IRS‐1) to allow activation of ERK was necessary for insulin‐stimulated DNA synthesis (Yenush et al. 1996). Our studies use Drosophila Kc cells expressing only endogenous Drosophila insulin receptor and produce a similar conclusion; namely DERK, but not DPI3K, activation is necessary for insulin‐stimulated mitogenesis.
Accurate co‐ordination of cell growth and apoptosis is necessary for development of complex multicellular organisms (Vaux & Korsmeyer 1999; Tapon, Moberg & Hariharan 2001). Thus, it is conceivable that insulin increased cell number by regulating the rate of Kc cell apoptosis. Indeed, it has been reported previously that insulin can rescue several types of cells from apoptotic death (Bertrand et al. 1998, Lee‐Kwon et al. 1998; Yenush et al. 1998). However, here we tested, for the first time, the ability of insulin to control apoptosis in Kc cells and found that the basic rate of apoptosis or the increased rate induced by exposure to UV light were both reduced by insulin. Several studies have previously implicated components of the insulin‐signalling pathway in controlling apoptosis in Drosophila (Bohni et al. 1999; Huang et al. 1999; Verdu et al. 1999). Here we demonstrate using Drosophila Kc cells that insulin exerts a protective effect against apoptosis via a DERK‐signalling pathway. Our results also suggest that PI3K activation is not necessary for this effect of insulin.
In mice, deletion of the p70S6K gene generated an animal that was reduced in size (Shima et al. 1998). Many recent studies have established the important role of DTOR (Oldham & Hafen 2003) and Dp70S6K (Thomas 2002) in control of Drosophila growth and development. Importantly, in Drosophila mutation of p70S6K affects cell size but not cell number (Radimerski et al. 2002). Activation of TOR and p70S6K signalling is conventionally viewed as being PI3K‐dependent. However, a recent study suggested that DTOR‐mediated p70S6K activation is DPI3K‐independent (Radimerski et al. 2002). Indeed, the phosphorylation site required for murine TOR activation by Akt is absent in the Drosophila homologue of TOR (Oldham et al. 2000). Thus, it appears that DPI3K signalling diverges to regulate cell growth in Drosophila via both DAkt and Dp70S6K. Activation of p70S6K leads to translation of mRNAs which mostly encode ribosomal proteins and translation initiation factors. Thus, activation of this kinase is commonly associated with increased protein synthesis and cell growth. Increased cell growth in response to Akt activation is thought to result via phosphorylation and inactivation of the translation initiation factor eIF‐4E and subsequently increased cap‐dependent translation initiation (Toker 2000). Alternatively, Akt may act to stimulate progression of the cell cycle (Rossig et al. 2001). Here we demonstrate that inhibition of either DPI3K or DTOR prevents insulin‐induced increases in Kc cell size. This fits with the model proposed above. We also find that inhibition of either DPI3K or DTOR is sufficient to attenuate totally the increase in cell size caused by insulin. This suggests that a linear signalling pathway involving DPI3K and subsequently DTOR may mediate the ability of insulin to cause Drosophila Kc cell hypertrophy. It has been shown previously that DERK does not play a role in insulin‐stimulated increases in Drosophila cell size (Kwon et al. 2002).
In summary, we show that insulin exerts a mitogenic effect on Drosophila Kc cells. Importantly, the increased number of cells observed when cultures were grown in the presence of insulin ensues as a result of both increased DNA synthesis and reduced apoptosis. DERK plays an important role in mediating the effect of insulin to increase DNA synthesis and reduce apoptosis whereas the DPI3K/DTOR/Dp70S6 kinase pathway mediates the effect of insulin on cell size in Drosophila Kc cells.
ACKNOWLEDGEMENTS
We thank Dr Lucy Cherbas, Dr Tim Westwood and Scott Neal for providing, and advising on the use of, Drosophila Kc cells. Thanks also go to Dr Barry Loughton for his valuable input. This study was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) via a grant to A.J.H. and by the Canadian Diabetes Association (G.S. is supported by a Scholarship in honour of the late Mary A. Bodington and R.B.C. by a postdoctoral fellowship in honour of the late Norman J. Newell).
REFERENCES
- Bangs P, White K (2000) Regulation and execution of apoptosis during Drosophila development. Dev. Dyn. 218, 68. [DOI] [PubMed] [Google Scholar]
- Bertrand F, Atfi A, Cadoret A, L’Allemain G, Robin H, Lascols O, Capeau J, Cherqui G (1998) A role for nuclear factor kappaB in the antiapoptotic function of insulin. J. Biol. Chem. 273, 2931. [DOI] [PubMed] [Google Scholar]
- Bohni R, Riesgo‐Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E (1999) Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1‐4. Cell 97, 865. [DOI] [PubMed] [Google Scholar]
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E (2001) An evolutionarily conserved function of the Drosophila insulin receptor and insulin‐like peptides in growth control. Curr. Biol. 11, 213. [DOI] [PubMed] [Google Scholar]
- Cakouros D, Daish T, Martin D, Baehrecke EH, Kumar S (2002) Ecdysone‐induced expression of the caspase DRONC during hormone‐dependent programmed cell death in Drosophila is regulated by Broad‐Complex. J. Cell Biol. 157, 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashion AB, Smith MJ, Wise PM (2003) The morphometry of astrocytes in the rostral preoptic area exhibits a diurnal rhythm on proestrus: relationship to the luteinizing hormone surge and effects of age. Endocrinology 144, 274. [DOI] [PubMed] [Google Scholar]
- Dolbeare F (1996) Bromodeoxyuridine: a diagnostic tool in biology and medicine, Part III. Proliferation in normal, injured and diseased tissue, growth factors, differentiation, DNA replication sites and in situ hybridization. Histochem. J. 28, 531. [DOI] [PubMed] [Google Scholar]
- Edgar BA (1999) From small flies come big discoveries about size control. Nat. Cell Biol. 1, E191. [DOI] [PubMed] [Google Scholar]
- Fernandez R, Tabarini D, Azpiazu N, Frasch M, Schlessinger J (1995) The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO J. 14, 3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Liu B, Glennon PE, Southgate KM, Sale EM, Sale GJ, Lewis MJ, Groves PH (2001) Downregulation of the ERK 1 and 2 mitogen activated protein kinases using antisense oligonucleotides inhibits proliferation of porcine vascular smooth muscle cells. Atherosclerosis 156, 289. [DOI] [PubMed] [Google Scholar]
- Garofalo RS (2002) Genetic analysis of insulin signaling in Drosophila . Trends Endocrinol. Metab 13, 156. [DOI] [PubMed] [Google Scholar]
- Goberdhan DC, Paricio N, Goodman EC, Mlodzik M, Wilson C (1999) Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3‐kinase signaling pathway. Genes Dev. 13, 3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Choi KY, Brey PT, Lee WJ (1998) Molecular cloning and characterization of a Drosophila p38 mitogen‐activated protein kinase. J. Biol. Chem. 273, 369. [DOI] [PubMed] [Google Scholar]
- Huang H, Potter CJ, Tao W, Li DM, Brogiolo W, Hafen E, Sun H, Xu T (1999) PTEN affects cell size, cell proliferation and apoptosis during Drosophila eye development. Development 126, 5365. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Gallant P (2002) Control of growth and organ size in Drosophila . Bioessays 24, 54. [DOI] [PubMed] [Google Scholar]
- Kavurma MM, Khachigian LM (2003) ERK, JNK, and p38 MAP kinases differentially regulate proliferation and migration of phenotypically distinct smooth muscle cell subtypes. J. Cell Biochem. 89, 289. [DOI] [PubMed] [Google Scholar]
- Kumar S, Colussi PA (1999) Prodomains – adaptors – oligomerization: the pursuit of caspase activation in apoptosis. Trends Biochem. Sci. 24, 1. [DOI] [PubMed] [Google Scholar]
- Kumar S, Doumanis J (2000) The fly caspases. Cell Death Differ. 7, 1039. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Kim SH, Kim SE, Jang IH, Ahn Y, Lee WJ, Choi KY (2002) Drosophila extracellular signal‐regulated kinase involves the insulin‐mediated proliferation of Schneider cells. J. Biol. Chem. 277, 14853. [DOI] [PubMed] [Google Scholar]
- Lee‐Kwon W, Park D, Baskar PV, Kole S, Bernier M (1998) Antiapoptotic signaling by the insulin receptor in Chinese hamster ovary cells. Biochemistry 37, 15747. [DOI] [PubMed] [Google Scholar]
- Leevers SJ (2001) Growth control: invertebrate insulin surprises!. Curr. Biol. 11, R209. [DOI] [PubMed] [Google Scholar]
- Leevers SJ, Weinkove D, MacDougall LK, Hafen E, Waterfield MD (1996) The Drosophila phosphoinositide 3‐kinase Dp110 promotes cell growth. EMBO J. 15, 6584. [PMC free article] [PubMed] [Google Scholar]
- Lim YM, Nishizawa K, Nishi Y, Tsuda L, Inoue YH, Nishida Y (1999) Genetic analysis of rolled, which encodes a Drosophila mitogen‐activated protein kinase. Genetics 153, 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lnenicka GA, Keshishian H (2000) Identified motor terminals in Drosophila larvae show distinct differences in morphology and physiology. J. Neurobiol. 43, 186. [PubMed] [Google Scholar]
- Masaki T, Foti R, Hill PA, Ikezumi Y, Atkins RC, Nikolic‐Paterson DJ (2003) Activation of the ERK pathway precedes tubular proliferation in the obstructed rat kidney. Kidney Int. 63, 1256. [DOI] [PubMed] [Google Scholar]
- Nicholson DW (1999) Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ 6, 1028. [DOI] [PubMed] [Google Scholar]
- Oldham S, Hafen E (2003) Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 13, 79. [DOI] [PubMed] [Google Scholar]
- Oldham S, Montagne J, Radimerski T, Thomas G, Hafen E (2000) Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 14, 2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Stocker H, Laffargue M, Wittwer F, Wymann M, Hafen E (2002) The Drosophila insulin/IGF receptor controls growth and size by modulating PtdInsP (3) levels. Development 129, 4103. [DOI] [PubMed] [Google Scholar]
- Poltilove RM, Jacobs AR, Haft CR, Xu P, Taylor SI (2000) Characterization of Drosophila insulin receptor substrate. J. Biol. Chem. 275, 23346. [DOI] [PubMed] [Google Scholar]
- Radimerski T, Montagne J, Rintelen F, Stocker H, Van Der Kaay J, Downes CP, Hafen E, Thomas G (2002) dS6K‐regulated cell growth is dPKB/dPI (3) K‐independent, but requires dPDK1. Nat. Cell Biol. 4, 251. [DOI] [PubMed] [Google Scholar]
- Richardson H, Kumar S (2002) Death to flies: Drosophila as a model system to study programmed cell death. J. Immunol. Meth. 265, 21. [DOI] [PubMed] [Google Scholar]
- Rossig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S (2001) Akt‐dependent phosphorylation of p21 (Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol. Cell Biol. 21, 5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC (1998) Disruption of the p70 (s6k)/p85 (s6k): gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 17, 6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Moberg KH, Hariharan IK (2001) The coupling of cell growth to the cell cycle. Curr. Opin. Cell Biol. 13, 731. [DOI] [PubMed] [Google Scholar]
- Thomas G (2002) The S6 kinase signaling pathway in the control of development and growth. Biol. Res. 35, 305. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281, 1312. [DOI] [PubMed] [Google Scholar]
- Toker A (2000) Protein kinases as mediators of phosphoinositide 3‐kinase signaling. Mol. Pharmacol. 57, 652. [PubMed] [Google Scholar]
- Vaux DL, Korsmeyer SJ (1999) Cell death in development. Cell 96, 245. [DOI] [PubMed] [Google Scholar]
- Verdu J, Buratovich MA, Wilder EL, Birnbaum MJ (1999) Cell‐autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat. Cell Biol. 1, 500. [DOI] [PubMed] [Google Scholar]
- Watson KL, Chou MM, Blenis J, Gelbart WM, Erikson RL (1996) A Drosophila gene structurally and functionally homologous to the mammalian 70‐kDa s6 kinase gene. Proc. Natl Acad. Sci. USA 93, 13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenush L, Fernandez R, Myers MG Jr, Grammer TC, Sun XJ, Blenis J, Pierce JH, Schlessinger J, White MF (1996) The Drosophila insulin receptor activates multiple signaling pathways but requires insulin receptor substrate proteins for DNA synthesis. Mol. Cell Biol. 16, 2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenush L, Zanella C, Uchida T, Bernal D, White MF (1998) The pleckstrin homology and phosphotyrosine binding domains of insulin receptor substrate 1 mediate inhibition of apoptosis by insulin. Mol. Cell Biol. 18, 6784. [DOI] [PMC free article] [PubMed] [Google Scholar]


