Abstract
The aryl hydrocarbon receptor (AhR) is an important nuclear transcription factor that is best known for mediating toxic responses by adjusting numbers of metabolism‐related enzymes, including CYP1A1 and CYP1B1. Previous findings have revealed that, in addition to negatively regulating cell proliferation and survival, AhR may also positively regulate these pathways. Here, we review these findings and summarize distinct mechanisms by which AhR promotes cell proliferation and survival, including modulation of receptor expression, growth factor signalling and apoptosis, regulating the cell cycle and promoting cytokine expression. This review will aid better understanding the role of AhR in positive regulation of cell proliferation and survival.
1. Introduction
The aryl hydrocarbon receptor (AhR) is a low‐molecular‐weight cytosolic receptor belonging to the basic helix‐loop‐helix‐PER‐ARNT‐SIM (bHLH‐PAS) family1, 2 Upon treatment with an AhR ligand, the AhR binds to the AhR nuclear translocator (ARNT), and the ligand‐bound AHR/ARNT complex translocates from the cytoplasm into the nucleus to modulate the expression of target genes, such as CYP1A1.3 The AhR is best known for mediating the toxic response via adjusting several metabolism‐related enzymes, including cytochrome P450, glutathione S‐transferase‐α, NAD(P)H quinone reductase‐1 (NQO1) and UDP glucuronosyl transferase.4, 5, 6, 7
As a conservative nuclear transcription factor, the AhR is expressed in most human cell types and, in particular, is highly expressed in the lungs, thymus, kidney and liver.8 In recent years, AhR research has mainly focused on its influence on inflammation and cancer.2, 9, 10, 11, 12 However, studies have found that the AhR also plays a crucial role in regulating cell proliferation and survival. The formation and development of organs and tissues are important processes for life and are accompanied by extensive proliferation, differentiation and survival. In multicellular organisms, these processes occur in different areas and time periods. Thus, cell proliferation and survival may be differentially regulated in different tissues, cells and environments. Over the past several years, the signalling pathways and processes regulating cell proliferation and survival have been intensively studied. However, few studies on the role of AhR in the positive regulation of cell proliferation and survival have been proposed. In this review, we will mainly examine how AhR promotes cell proliferation and survival.
2. Regulation of AhR activation
The classic way to activate the AhR involves treatment with an AhR ligand, leading to the formation of a functional complex composed of the AhR and ARNT. Subsequently, the ligand‐bound AHR/ARNT complex translocates from the cytoplasm into the nucleus to modulate the expression of target genes, such as CYP1A1.3 However, recent studies suggest that AhR activity can also be regulated independent of a ligand. For example, in several tumour cell lines, high expression of the AhR enables the AhR to undergo dynamic nucleocytoplasmic shuttling, resulting in the activation of the AhR in the absence of ligand.2, 13 Oesch‐Bartlomowicz et al.14 found that the second messenger cAMP can activate AhR and allow for the endogenous function of the AhR. In lymphocytes, RORgt can also form a complex with the AhR to regulate the expression of IL‐22, following the activation of the AhR.15 Therefore, AhR activity can also be modulated independent of ligand.
3. AhR and cell proliferation and survival
The AhR is involved in many cellular processes, including apoptosis, the cell cycle, immunomodulation and also participates in barrier function.16, 17, 18, 19, 20 Many pollutants or chemical toxins, such as polychlorinated dibenzodioxins, polychlorinated dibenzofurans and coplanar polychlorinated biphenyls, can lead to cytotoxicity and thus influence the biological effects of a given cell. Therefore, it seems reasonable to suggest that treatment with AhR ligands may lead to negative control of cell proliferation and survival, and many publications that examine AhR ligands have shown an inhibition of cell proliferation and survival associated with various mechanisms. However, it is worth noting that increasing evidence suggests that AhR may also promote cell proliferation and survival.21, 22, 23, 24 We review these findings here, and summarize five distinct mechanisms by which the AhR promotes cell proliferation and survival. We expect that this review may help us to better understand the role of the AhR in cells.
4. Potential mechanisms
4.1. Modulation of receptor expression
The cytokine IL‐7 signals through the IL‐7/IL‐7R signalling pathway and is an important cytokine for innate lymphoid cell (ILC) development. IL‐7 is produced by intestinal epithelial cells and thymic stromal cells.25, 26 Previous studies have demonstrated that IL‐7 and IL‐7R are both vital for the differentiation and survival of RORγt+ ILC in IL‐7R‐ or IL‐7‐deficient mice.27, 28 Qiu et al.29 observed a reduction of both IL‐7 and IL‐7R in the large intestines of AhR−/− mice. This is consistent with the finding of enhanced apoptosis of RORγt+ ILCs in the absence of the AhR. In addition, the authors point out that the AhR defect may be, in part, due to the compromised expression of IL‐7 and IL‐7R. Consistent with their findings, our laboratory also found that FICZ (an endogenous ligand of AhR) may protect the intestinal barrier in an ischaemia‐reperfusion model by upregulating the expression of IL‐7R. This upregulation increased the number of IEL cells and promoted IEL survival (unpublished data). Previous studies have shown that environmental factors may also play an important role in the development of IL‐22‐producing ILC in the intestines. Moreover, further studies have shown that the expression of the AhR is elevated in human NKp46+ and LTi‐like ILCs.30 Deletion of the AhR can lead to a decrease in the number of NKp46+ cells and a partial decrease in the number of LTi‐like ILCs.22 Importantly, several reports have suggested that the AhR can directly bind to the promoter of the notch1 and notch2 genes, both of which are required for the maintenance of intestinal NKp46+ILCs.31, 32, 33 Lastly, Lee et al.22 demonstrated that the AhR, upon stimulation with TCDD, can activate the notch pathway by significantly inducing the expression of notch1 and notch2 to sustain the number of NKp46+ cells and, in part, LTi‐like ILCs. C‐kit is another crucial receptor for the maintenance of RORγt+ ILCs and intraepithelial γδ T cells via stem cell factor (SCF) signalling.34 In addition, further studies have suggested that the activation of the AhR may directly control c‐kit gene transcription.35, 36 Although these studies have not shown that AhR directly regulates c‐kit expression to influence cell number or cell survival, studies with a mutant c‐kit receptor have shown that it has a similar defect in the number of intraepithelial γδ T cells as the AhRko model.37 In intestinal epithelial cells, our previous studies have demonstrated that KGF/KGFR signalling protects the intestinal barrier from colitis and ischaemia‐reperfusion injury (I/R) by promoting epithelial cell proliferation.38 Importantly, we recently observed that AhR knockout mice are not sensitized to KGF‐induced intestinal epithelial cell proliferation (Fig. 1a–e). Based on these findings, we further detected a decrease in KGFR expression in AhR‐deficient mice and cells (Fig. 1f).
Figure 1.
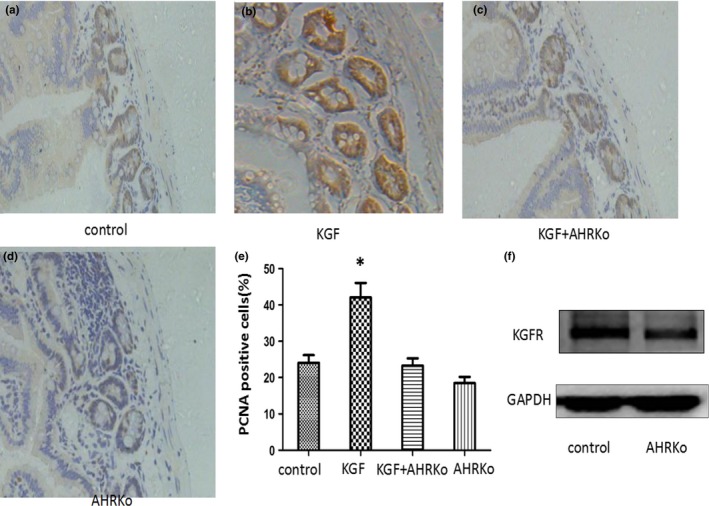
AhR knockout mice lack sensitivity to KGF in the small intestines. (a) PCNA expression was detected in the sham group. (b) PCNA expression was significantly increased in the KGF group compared with the sham group. (c) PCNA expression was significantly decreased in the KGF+AhRko group compared with the KGF group. (d) PCNA expression was detected in the AhRko group; original magnification: ×400. (e) PCNA expression is expressed as the mean and SD. (f) KGFR expression was examined by Western blotting
Although the regulation of lymphocyte proliferation and survival, particularly gut ILCs, has been intensively studied, until recently, the regulation of receptors associated with cell growth and survival have received much less attention. Thus, the AhR, as a conserved nuclear transcription factor, may play an essential role in promoting the growth of innate lymphoid cells and maintaining innate lymphoid cell (ILC) development by regulating the expression of receptors.
4.2. Participation of the AhR in growth factor signalling
A number of growth factors are both involved and produced in the process of tissues development and cell renewal. However, growth factor signalling pathways are very complex and involve many different molecules. There is increasing evidence to suggest that AhR may regulate several growth factor signalling pathways to induce cell proliferation and survival. Given its high expression as a cell growth factor in cancer tissue, IGF is likely involved in tumour development. Therefore, future research on the IGF signalling pathway may be useful for new cancer therapies. In an article examining human breast cancer, the author observed that AhR‐deficient MCF‐7 cells were less sensitized to IGF2 stimulation in vitro than their wild‐type counterparts.18 While examining the role of the AhR in IGF signalling‐mediated cell proliferation, Tomblin and Salisbury observed that treatment with IGF2 significantly upregulated and activated the AhR. Furthermore, the AhR may directly participate in the IGF2 signalling pathway as a downstream effector molecule. The role of the AhR in growth factor signalling has also been discussed previously.39 Vaziri et al. found that expression of the AhR was regulated by serum and mitogenic growth factors in murine 3T3 fibroblasts. Although the author did not examine if AhR deletion influenced cell proliferation stimulated by serum and mitogenic growth factors, the AhR may play a role during the cellular proliferative response. Importantly, the authors observed that treatment with an AhR ligand can disturb IGF‐ and FGF‐induced cell proliferation and tissue development. This result clearly suggests that endogenous AhR may participate in growth factor signalling, as an exogenous AhR ligand was able to interfere with this pathway. Interestingly, TCDD treatment might interfere with epidermal growth factor (EGF) signalling. However, in the absence of EGF, an AhR ligand may activate EGFR and extracellular signal‐regulated kinases (ERK)1/2, which regulate cell proliferation and cell survival in rat hepatocytes and immune cells.40, 41 Additionally, in the H508 and SNU‐C4 cell lines, TCDD can induce the phosphorylation of EGFR (Tyr845) through the regulation of c‐src kinase, which binds to phosphorylated EGF and further stimulates cell proliferation. This stimulation could be abolished by either CH223191 (an AhR antagonist) or AhR siRNA.42 Madhukar et al.43 observed that animals exposed to TCDD and EGF showed similar phenotypic changes, such as earlier eyelid opening and premature tooth eruption. We examined AhR and KGF expression in colon cancer tissue by real‐time PCR analysis and found that both genes were overexpressed. These results suggest that there may be a relationship between KGF and AhR. Our data also suggest that the AhR may directly participate in KGF signalling, as a downstream factor, to promote colon cancer cell growth in vitro.44
4.3. Anti‐apoptotic effects
Programmed cell death induced by apoptosis is very important in the course of tissue and cell development. AhR can influence apoptosis by controlling the expression of apoptosis genes.45 Many publications examining AhR ligands have shown an induction of apoptosis associated with various mechanisms involving AhR. However, there is increasing evidence that endogenous AhR, as well as several AhR ligands, can promote cell proliferation or maintain cell numbers by inhibiting apoptosis. In particular, the anti‐apoptotic effect of AhR is very important in cancer cell growth and development. Here, we will address lesser known reports that examine the impact of AhR on apoptosis.
In three different lymphoma cell lines, treatment with TCDD led to a loss of programmed cell death by increasing the expression of cyclooxygenase‐2 (COX‐2) and deregulating Bcl‐xl and Mcl‐1 expression.46 In addition, AhR‐mediated resistance to apoptosis was found in breast cancer cells.47 In tumours, the anti‐apoptotic effect of the AhR is well‐known, in part because exposure to environmental pollutants, such as pesticides and dioxins, can lead to the development of various tumours. Several AhR ligands, such as polyphenolic flavone chrysin and indoline, have anti‐cancer effects in a variety of cancer cell lines, including the induction of cancer cell programmed death.48, 49
However, in normal cells, AhR ligands still have anti‐apoptotic effects. Polychlorinated biphenyls (PCBs), environmental pollutants and exogenous ligands of the AhR, can alter cell proliferation and apoptosis. In vitro experiments have shown that treatment with PCB153 significantly decreases the expression of caspase‐3, caspase‐8 and caspase‐9, which can inhibit pituitary cell apoptosis and increase cell proliferation. Another ligand, PCB180, has the opposite effect.17 In the environment, there is a large amount of particulate matter (PM2.5) that may induce the apoptosis of normal human lung tissue or airway epithelial cells.21, 50 However, Ferecatu et al. found that low levels of Parisian PM2.5 is not cytotoxic for human bronchial epithelial cells, but instead has an anti‐apoptotic effect.51 Further studies have shown that several components of PM2.2 may contribute to the mitochondria‐mediated anti‐apoptotic effects of AhR on human bronchial epithelial cells. The different effects of PM on apoptosis may depend on the dosage.
Importantly, endogenous AhR also appears to have an anti‐apoptotic effect. Hecht et al.52 reported that the AhR‐dependent regulation of miR‐196a attenuated cigarette smoke‐induced apoptosis in lung fibroblasts. Furthermore, the deletion of AhR downregulated the cellular levels of miR‐196a, increased cigarette smoke‐induced apoptosis, and inhibited cell proliferation. Another study showed that the exposure of keratinocytes (KC) to ultraviolet (UV) radiation resulted in the initiation of apoptosis and inhibition of AhR signalling, as the UVB‐sensitized KC induced apoptosis.53 In that article, the author put forth a novel anti‐apoptotic pathway in KC: the AhR‐E2F1‐CHK1 axis.53
4.4. Regulation of the cell cycle
As mentioned above, previous studies have shown that ligand‐activated AhR can inhibit cell proliferation by arresting the cell cycle. However, several studies have also suggested that ligand‐activated AhR can accelerate cell proliferation by regulating the cell cycle. Here, we will give several examples to demonstrate that AhR may accelerate cell proliferation by regulating the cell cycle. In rat liver “stem‐like” cells, TNF‐alpha itself had no effect on cell proliferation. However, when a low concentration of either TCDD or PCB126 and TNF‐alpha were added together into rat liver WB‐F344 cells, there was an increased percentage of cells entering the S phase and an increased number of cells. The mRNA and protein levels of the cell cycle protein cyclin A also increased.54 Cyclin D1, another important cell cycle protein, can form a complex with CDK4/CDK6 to induce cell cycle progression from the G1 phase to S phase.55 In HAPI microglial cells, treatment with TCDD induced cell proliferation in a dose‐dependent and time‐dependent manner via the Akt/GSK‐3β/cyclin D1 signalling pathway.56 In our research, we found that KGF signalling can induce colon cancer cell proliferation, dependent on endogenous AhR, and treatment with AhR siRNA decreased the expression of cyclin D.44 Our data suggest that cyclin D1 expression may be regulated by the AhR. Furthermore, Tomblin and Salisbury18 proposed that the AhR may act as a DNA transcription factor and directly regulate the transcription of cyclin D1.
In addition to its role in vitro, studies using AhRko mice have provided evidence for the role of endogenous AhR in the regulation of the cell cycle. In the female reproductive system, Benedict et al.57 observed reduced fertility in AhR knockout mice and found that it was not due to fertility death. On that basis, Barnett et al.58 observed that the cell cycle progression was arrested in the G0 phase in AhRko mice compared with WT mice and further demonstrated that this cell cycle arrest was likely caused by a significant reduction in the levels of cyclin D2 and CDK4 in the context of fertility. In addition, there was a reduction in the cell number and an accumulation of 4N DNA content in mouse embryonic fibroblasts (MEFs) derived from AhR‐null mice compared with wild‐type MEFs.59 Considering the accumulation of 4N DNA content, the author examined the protein expression of AhR in the G(2)/M phase of the cell cycle and found a low level of Cdc2 and Plk expression; both of these proteins are important for the G(2)/M phase.
4.5. Promoting cell cytokine expression
The ability of the AhR to promote cell proliferation by regulating growth factors is important in cancer cells. For example, the growth regulator epiregulin (EREG) belongs to the EGF family and is directly regulated by the AhR.60, 61 In human lung adenocarcinomas, AhR activation and overexpression increased the expression of fibroblast growth factor‐9.62 Amphiregulin (AREG), EREG and platelet‐derived growth factor A (PDGFA) were found to be AhR ligands in head and neck squamous cell carcinoma (HNSCC) cell lines.61 Additionally, TCDD can induce AREG gene expression to promote the developing mouse ureter.63 Beyond the function of AhR in cancer cells, in mouse endothelial cells, AhR deficiency impaired angiogenesis induced by vascular endothelial growth factor, which exhibited a degree of AhR dependency with regard to its expression.64 In rheumatoid arthritis (RA) patients, fibroblast‐like synoviocytes (FLS) in the synovial tissue undergo hyperplasia, leading to joint destruction. However, inhibition of AhR activation can decrease RA‐FLS cell proliferation by attenuating growth factor release.65
In addition to growth factors, many cytokines can also maintain cell numbers either by promoting cell proliferation or by inhibiting apoptosis. Cytokines, including interleukin‐10 (IL‐10) and interleukin‐22 (IL‐22), play an important role in cell survival by regulating the pro‐survival genes Bcl‐2, Bcl‐XL and Mcl‐1, as well as the proliferative factors c‐Myc, cyclin D1 and Rb.66, 67, 68, 69 On the other hand, recent studies confirmed that the AhR is crucial for IL‐22/IL‐10 expression.70, 71, 72, 73 Interestingly, a recent study reported that Th22 cells were enriched in CRC tumour tissues, and the high expression of IL‐22 significantly promoted tumour growth in nude mice, as well as the proliferation of RKO cells.74 Furthermore, the authors found that the expression of the AhR was significantly higher in tumour tissue than in normal tissue. Therefore, it is possible that the enrichment of Th22 cells and high expression of IL‐22 are connected with the high expression of the AhR in CRC tumours, as there are AhR ligands generated by high tryptophan metabolism in tumour tissue.2 Il‐7, another cytokine mentioned in our article, is an important factor for the differentiation and survival of both RORγt+ILC and IEL. Qiu et al.29 observed a reduction in IL‐7 in the large intestine of AhR−/− mice, which is consistent with enhanced apoptosis of RORγt+ ILCs in the absence of the AhR. In human monocytes and murine dendritic cells, traffic‐related particulate matter (PM) can induce the expression of jag1 (a notch ligand) through the AhR and thereby induce monocyte and murine dendritic cell survival.75
5. Concluding remarks
In recent decades, various studies have reported that the AhR can both negatively and positively regulate cell proliferation and survival, the latter in either a ligand‐dependent or endogenous AhR‐dependent manner. The reason for these dual functions may be differences in the time frame, dosage of the ligand, category of ligand, cell types or whether the experiment was performed in vivo or in vitro. However, it is evident that endogenous AhR, which does not bind to a ligand, is very important for tissue development and for cell maintenance, by directly or indirectly regulating cell proliferation and apoptosis. In the article, we mainly summarized how the AhR promotes cell proliferation and survival through five distinct mechanisms: the modulation of receptor expression, its participation in growth factor signalling, anti‐apoptotic effects, regulation cell cycle and ability to promote cell cytokine expression. There may also be other mechanisms through which the AhR promotes cell proliferation and survival. For example, the AhR can increase cell numbers by regulating the expression of L‐type amino acid transporter 1 (LAT‐1), which promotes the absorption of amino acids.76 TCDD promotes palatal epithelial cell proliferation and survival via activating the MAPK pathway.77 Overall, there is a significant need to better understand the signalling pathway that involves the AhR because, as a conservative nuclear transcription factor, the AhR appears to play many roles in the cell.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (NSFC 81330013 to H.Y.). The authors declare no conflict of interest.
Contributor Information
Weidong Xiao, Email: weidong.xiao@126.com.
Hua Yang, Email: huayang@tmmu.edu.cn.
References
- 1. Noakes R. The aryl hydrocarbon receptor: a review of its role in the physiology and pathology of the integument and its relationship to the tryptophan metabolism. Int J Tryptophan Res. 2015;8:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14:801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poormasjedi‐Meibod MS, Elizei SS, Leung V, et al. Kynurenine modulates MMP‐1 and type‐I collagen expression via aryl hydrocarbon receptor activation in dermal fibroblasts. J Cell Physiol. 2016;999:1–12. [DOI] [PubMed] [Google Scholar]
- 4. Emi Y, Ikushiro S, Iyanagi T. Xenobiotic responsive element‐mediated transcriptional activation in the UDP‐glucuronosyltransferase family 1 gene complex. J Biol Chem. 1996;271:3952–3958. [DOI] [PubMed] [Google Scholar]
- 5. Favreau LV, Pickett CB. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991;266:4556–4561. [PubMed] [Google Scholar]
- 6. Fujisawa‐Sehara A, Sogawa K, Yamane M, Fujii‐Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug‐metabolizing cytochrome P‐450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987;15:4179–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rushmore TH, King RG, Paulson KE, Pickett CB. Regulation of glutathione S‐transferase Ya subunit gene expression: identification of a unique xenobiotic‐responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci U S A. 1990;87:3826–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel A, Zhang S, Moorthy B, Shivanna B. Omeprazole does not potentiate acute oxygen toxicity in fetal human pulmonary microvascular endothelial cells exposed to hyperoxia. Pharm Anal Acta. 2015;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monteleone I, MacDonald TT, Pallone F, Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: linking the environment to disease pathogenesis. Curr Opin Gastroenterol. 2012;28:310–313. [DOI] [PubMed] [Google Scholar]
- 10. Qiu J, Zhou L. Aryl hydrocarbon receptor promotes RORgammat(+) group 3 ILCs and controls intestinal immunity and inflammation. Semin Immunopathol. 2013;35:657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh NP, Singh UP, Singh B, et al. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL‐17 expression and amelioration of experimental colitis. PLoS ONE. 2011;6:e23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang Z, Jiang Y, Yang Y, et al. 3,3’‐Diindolylmethane alleviates oxazolone‐induced colitis through Th2/Th17 suppression and Treg induction. Mol Immunol. 2013;53:335–344. [DOI] [PubMed] [Google Scholar]
- 13. Ikuta T, Tachibana T, Watanabe J, et al. Nucleocytoplasmic shuttling of the aryl hydrocarbon receptor. J Biochem. 2000;127:503–509. [DOI] [PubMed] [Google Scholar]
- 14. Oesch‐Bartlomowicz B, Huelster A, Wiss O, et al. Aryl hydrocarbon receptor activation by cAMP vs. dioxin: divergent signaling pathways. Proc Natl Acad Sci U S A. 2005;102:9218–9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacPherson L, Ahmed S, Tamblyn L, et al. Aryl hydrocarbon receptor repressor and TiPARP (ARTD14) use similar, but also distinct mechanisms to repress aryl hydrocarbon receptor signaling. Int J Mol Sci. 2014;15:7939–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Florean C, Schnekenburger M, Lee JY, et al. Discovery and characterization of Isofistularin‐3, a marine brominated alkaloid, as a new DNA demethylating agent inducing cell cycle arrest and sensitization to TRAIL in cancer cells. Oncotarget. 2016;7:24027–24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raggi F, Russo D, Urbani C, et al. Divergent effects of dioxin‐ or non‐dioxin‐like polychlorinated biphenyls on the apoptosis of primary cell culture from the mouse pituitary gland. PLoS ONE. 2016;11:e0146729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tomblin JK, Salisbury TB. Insulin like growth factor 2 regulation of aryl hydrocarbon receptor in MCF‐7 breast cancer cells. Biochem Biophys Res Commun. 2014;443:1092–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang EJ, Stokes JV, Kummari E, Eells J, Kaplan BL. Immunomodulation by subchronic low dose 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin in experimental autoimmune encephalomyelitis in the absence of pertussis toxin. Toxicol Sci. 2016;151:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han B, Sheng B, Zhang Z, et al. Aryl hydrocarbon receptor activation in intestinal obstruction ameliorates intestinal barrier dysfunction via suppression of MLCK‐MLC phosphorylation pathway. Shock. 2016;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21. Dagher Z, Garcon G, Billet S, et al. Activation of different pathways of apoptosis by air pollution particulate matter (PM2.5) in human epithelial lung cells (L132) in culture. Toxicology. 2006;225:12–24. [DOI] [PubMed] [Google Scholar]
- 22. Lee JS, Cella M, McDonald KG, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tarnow P, Tralau T, Luch A. G protein‐coupled receptor 30 ligand G‐1 increases aryl hydrocarbon receptor signalling by inhibition of tubulin assembly and cell cycle arrest in human MCF‐7 cells. Arch Toxicol. 2015;90:1939–1948. [DOI] [PubMed] [Google Scholar]
- 24. Esakky P, Hansen DA, Drury AM, Moley KH. Cigarette smoke‐induced cell cycle arrest in spermatocytes [GC‐2spd(ts)] is mediated through crosstalk between Ahr‐Nrf2 pathway and MAPK signaling. J Mol Cell Biol. 2015;7:73–87. [DOI] [PubMed] [Google Scholar]
- 25. Satoh‐Takayama N, Lesjean‐Pottier S, Vieira P, et al. IL‐7 and IL‐15 independently program the differentiation of intestinal CD3‐NKp46+ cell subsets from Id2‐dependent precursors. J Exp Med. 2010;207:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cai YJ, Wang WS, Yang Y, et al. Up‐regulation of intestinal epithelial cell derived IL‐7 expression by keratinocyte growth factor through STAT1/IRF‐1, IRF‐2 pathway. PLoS ONE. 2013;8:e58647. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Vonarbourg C, Mortha A, Bui VL, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor‐expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meier D, Bornmann C, Chappaz S, et al. Ectopic lymphoid‐organ development occurs through interleukin 7‐mediated enhanced survival of lymphoid‐tissue‐inducer cells. Immunity. 2007;26:643–654. [DOI] [PubMed] [Google Scholar]
- 29. Qiu J, Heller JJ, Guo X, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL‐22 for mucosal immunity. Nature. 2009;457:722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dere E, Lo R, Celius T, Matthews J, Zacharewski TR. Integration of genome‐wide computation DRE search, AhR ChIP‐chip and gene expression analyses of TCDD‐elicited responses in the mouse liver. BMC Genom. 2011;12:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boverhof DR, Burgoon LD, Tashiro C, et al. Comparative toxicogenomic analysis of the hepatotoxic effects of TCDD in Sprague Dawley rats and C57BL/6 mice. Toxicol Sci. 2006;94:398–416. [DOI] [PubMed] [Google Scholar]
- 34. Chappaz S, Gartner C, Rodewald HR, Finke D. Kit ligand and Il7 differentially regulate Peyer's patch and lymph node development. J Immunol. 2010;185:3514–3519. [DOI] [PubMed] [Google Scholar]
- 35. Kiss EA, Vonarbourg C, Kopfmann S, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. [DOI] [PubMed] [Google Scholar]
- 36. Sun YV, Boverhof DR, Burgoon LD, Fielden MR, Zacharewski TR. Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic Acids Res. 2004;32:4512–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Puddington L, Olson S, Lefrancois L. Interactions between stem cell factor and c‐Kit are required for intestinal immune system homeostasis. Immunity. 1994;1:733–739. [DOI] [PubMed] [Google Scholar]
- 38. Cai Y, Wang W, Liang H, et al. Keratinocyte growth factor improves epithelial structure and function in a mouse model of intestinal ischemia/reperfusion. PLoS ONE. 2012;7:e44772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vaziri C, Schneider A, Sherr DH, Faller DV. Expression of the aryl hydrocarbon receptor is regulated by serum and mitogenic growth factors in murine 3T3 fibroblasts. J Biol Chem. 1996;271:25921–25927. [DOI] [PubMed] [Google Scholar]
- 40. Randi AS, Sanchez MS, Alvarez L, et al. Hexachlorobenzene triggers AhR translocation to the nucleus, c‐Src activation and EGFR transactivation in rat liver. Toxicol Lett. 2008;177:116–122. [DOI] [PubMed] [Google Scholar]
- 41. Kwon MJ, Jeong KS, Choi EJ, Lee BH. 2,3,7,8‐Tetrachlorodibenzo‐p‐dioxin (TCDD)‐induced activation of mitogen‐activated protein kinase signaling pathway in Jurkat T cells. Pharmacol Toxicol. 2003;93:186–190. [DOI] [PubMed] [Google Scholar]
- 42. Xie G, Peng Z, Raufman JP. Src‐mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1006–G1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Madhukar BV, Brewster DW, Matsumura F. Effects of in vivo‐administered 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin on receptor binding of epidermal growth factor in the hepatic plasma membrane of rat, guinea pig, mouse, and hamster. Proc Natl Acad Sci U S A. 1984;81:7407–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin J, Sheng B, Pu A, et al. Keratinocyte growth factor regulation of aryl hydrocarbon receptor activation in colorectal cancer cells. Dig Dis Sci. 2016;61:444–452. [DOI] [PubMed] [Google Scholar]
- 45. Park KT, Mitchell KA, Huang G, Elferink CJ. The aryl hydrocarbon receptor predisposes hepatocytes to Fas‐mediated apoptosis. Mol Pharmacol. 2005;67:612–622. [DOI] [PubMed] [Google Scholar]
- 46. Vogel CF, Li W, Sciullo E, et al. Pathogenesis of aryl hydrocarbon receptor‐mediated development of lymphoma is associated with increased cyclooxygenase‐2 expression. Am J Pathol. 2007;171:1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bekki K, Vogel H, Li W, et al. The aryl hydrocarbon receptor (AhR) mediates resistance to apoptosis induced in breast cancer cells. Pestic Biochem Physiol. 2015;120:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liou JP, Hsu KS, Kuo CC, Chang CY, Chang JY. A novel oral indoline‐sulfonamide agent, N‐[1‐(4‐methoxybenzenesulfonyl)‐2,3‐dihydro‐1H‐indol‐7‐yl]‐isonicotinamide (J30), exhibits potent activity against human cancer cells in vitro and in vivo through the disruption of microtubule. J Pharmacol Exp Ther. 2007;323:398–405. [DOI] [PubMed] [Google Scholar]
- 49. Ronnekleiv‐Kelly SM, Nukaya M, Diaz‐Diaz CJ, et al. Aryl hydrocarbon receptor‐dependent apoptotic cell death induced by the flavonoid chrysin in human colorectal cancer cells. Cancer Lett. 2016;370:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Agopyan N, Head J, Yu S, Simon SA. TRPV1 receptors mediate particulate matter‐induced apoptosis. Am J Physiol Lung Cell Mol Physiol. 2004;286:L563–L572. [DOI] [PubMed] [Google Scholar]
- 51. Ferecatu I, Borot MC, Bossard C, et al. Polycyclic aromatic hydrocarbon components contribute to the mitochondria‐antiapoptotic effect of fine particulate matter on human bronchial epithelial cells via the aryl hydrocarbon receptor. Part Fibre Toxicol. 2010;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hecht E, Zago M, Sarill M, et al. Aryl hydrocarbon receptor‐dependent regulation of miR‐196a expression controls lung fibroblast apoptosis but not proliferation. Toxicol Appl Pharmacol. 2014;280:511–525. [DOI] [PubMed] [Google Scholar]
- 53. Frauenstein K, Sydlik U, Tigges J, et al. Evidence for a novel anti‐apoptotic pathway in human keratinocytes involving the aryl hydrocarbon receptor, E2F1, and checkpoint kinase 1. Cell Death Differ. 2013;20:1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Umannova L, Zatloukalova J, Machala M, et al. Tumor necrosis factor‐alpha modulates effects of aryl hydrocarbon receptor ligands on cell proliferation and expression of cytochrome P450 enzymes in rat liver “stem‐like” cells. Toxicol Sci. 2007;99:79–89. [DOI] [PubMed] [Google Scholar]
- 55. Yang T, Zhang H, Qiu H, et al. EFEMP1 is repressed by estrogen and inhibits the epithelial‐mesenchymal transition via Wnt/beta‐catenin signaling in endometrial carcinoma. Oncotarget. 2016;7:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu G, Li Y, Yoshimoto K, et al. 2,3,7,8‐Tetrachlorodibenzo‐p‐dioxin stimulates proliferation of HAPI microglia by affecting the Akt/GSK‐3beta/cyclin D1 signaling pathway. Toxicol Lett. 2014;224:362–370. [DOI] [PubMed] [Google Scholar]
- 57. Benedict JC, Miller KP, Lin TM, et al. Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol Reprod. 2003;68:1511–1517. [DOI] [PubMed] [Google Scholar]
- 58. Barnett KR, Tomic D, Gupta RK, et al. The aryl hydrocarbon receptor affects mouse ovarian follicle growth via mechanisms involving estradiol regulation and responsiveness. Biol Reprod. 2007;76:1062–1070. [DOI] [PubMed] [Google Scholar]
- 59. Elizondo G, Fernandez‐Salguero P, Sheikh MS, et al. Altered cell cycle control at the G(2)/M phases in aryl hydrocarbon receptor‐null embryo fibroblast. Mol Pharmacol. 2000;57:1056–1063. [PubMed] [Google Scholar]
- 60. Patel RD, Kim DJ, Peters JM, Perdew GH. The aryl hydrocarbon receptor directly regulates expression of the potent mitogen epiregulin. Toxicol Sci. 2006;89:75–82. [DOI] [PubMed] [Google Scholar]
- 61. John K, Lahoti TS, Wagner K, Hughes JM, Perdew GH. The Ah receptor regulates growth factor expression in head and neck squamous cell carcinoma cell lines. Mol Carcinog. 2014;53:765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang CK, Chang H, Chen PH, et al. Aryl hydrocarbon receptor activation and overexpression upregulated fibroblast growth factor‐9 in human lung adenocarcinomas. Int J Cancer. 2009;125:807–815. [DOI] [PubMed] [Google Scholar]
- 63. Choi SS, Miller MA, Harper PA. In utero exposure to 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin induces amphiregulin gene expression in the developing mouse ureter. Toxicol Sci. 2006;94:163–174. [DOI] [PubMed] [Google Scholar]
- 64. Roman AC, Carvajal‐Gonzalez JM, Rico‐Leo EM, Fernandez‐Salguero PM. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF‐A depletion in the endothelium and transforming growth factor‐beta overexpression in the stroma. J Biol Chem. 2009;284:25135–25148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lahoti TS, Hughes JM, Kusnadi A, et al. Aryl hydrocarbon receptor antagonism attenuates growth factor expression, proliferation, and migration in fibroblast‐like synoviocytes from patients with rheumatoid arthritis. J Pharmacol Exp Ther. 2014;348:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kurowska E, Majkutewicz I. The interleukin‐10 in the central nervous system. Postepy Hig Med Dosw (Online). 2015;69:886–891. [DOI] [PubMed] [Google Scholar]
- 67. Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL‐22) plays a protective role in T cell‐mediated murine hepatitis: IL‐22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. [DOI] [PubMed] [Google Scholar]
- 68. Sugimoto K, Ogawa A, Mizoguchi E, et al. IL‐22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sonnenberg GF, Nair MG, Kirn TJ, et al. Pathological versus protective functions of IL‐22 in airway inflammation are regulated by IL‐17A. J Exp Med. 2010;207:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chinen I, Nakahama T, Kimura A, et al. The aryl hydrocarbon receptor/microRNA‐212/132 axis in T cells regulates IL‐10 production to maintain intestinal homeostasis. Int Immunol. 2015;27:405–415. [DOI] [PubMed] [Google Scholar]
- 71. Ple C, Fan Y, Ait YS, et al. Polycyclic aromatic hydrocarbons reciprocally regulate IL‐22 and IL‐17 cytokines in peripheral blood mononuclear cells from both healthy and asthmatic subjects. PLoS ONE. 2015;10:e0122372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wagage S, John B, Krock BL, et al. The aryl hydrocarbon receptor promotes IL‐10 production by NK cells. J Immunol. 2014;192:1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Abe H, Kimura A, Tsuruta S, et al. Aryl hydrocarbon receptor plays protective roles in ConA‐induced hepatic injury by both suppressing IFN‐gamma expression and inducing IL‐22. Int Immunol. 2014;26:129–137. [DOI] [PubMed] [Google Scholar]
- 74. Huang YH, Cao YF, Jiang ZY, Zhang S, Gao F. Th22 cell accumulation is associated with colorectal cancer development. World J Gastroenterol. 2015;21:4216–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xia M, Viera‐Hutchins L, Garcia‐Lloret M, et al. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor‐notch signaling cascade. J Allergy Clin Immunol. 2015;136:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tomblin JK, Arthur S, Primerano DA, et al. Aryl hydrocarbon receptor (AHR) regulation of L‐Type Amino Acid Transporter 1 (LAT‐1) expression in MCF‐7 and MDA‐MB‐231 breast cancer cells. Biochem Pharmacol. 2016;106:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gao Z, Bu Y, Liu X, et al. TCDD promoted EMT of hFPECs via AhR, which involved the activation of EGFR/ERK signaling. Toxicol Appl Pharmacol. 2016;298:48–55. [DOI] [PubMed] [Google Scholar]


