Abstract
Abstract. Cranial neural crest‐derived ectomesenchymal cells are multipotential progenitors that contribute to various tissue types during embryogenesis. Their potential to be expanded in culture as a monolayer and to be induced into different cell lineages in vitro has not been previously reported in detail. In this study, the ectomesenchymal cells in the first branchial arch were enzymatically isolated from the mandibular processes of BALB/c mice and were maintained in an intact state in a medium containing leukaemia inhibitory factor. Here, we first evaluated the proliferative activity of the cells after the third passage, using bromodeoxyuridine labelling and in situ hybridization of telomerase mRNA. Positive staining for expression of HNK‐1, S‐100 and vimentin confirmed that the population of stem cells originated from the ectomesenchyme, which did not express cytokeratin. Then we investigated the molecular and cellular characteristics of the ectomesenchymal cells during their differentiation towards neurogenic, endothelial, myogenic and odontogenic lineages. Expression of multiple lineage‐specific genes and proteins was detected by utilizing a range of molecular and biochemical approaches when the cells were transferred to inductive medium. Histological and immunohistochemical analysis of the induced cells at various intervals indicated obvious phenotypic alteration and presence of specific proteins for the differentiated lineages, for example nestin, factor VIII, α‐SMA and dentin sialophosphoprotein (DSPP), respectively. Correlatively, results of reverse transcription–PCR corroborated at mRNA level the expression of the characteristic molecules during differentiation. Therefore, it is suggested that the ectomesenchymal cells derived from the first branchial arch may represent a novel source of multipotential stem cells capable of undergoing expansion and variant differentiation in vitro.
INTRODUCTION
In the early embryo, cranial neural crest cells (CNCC) migrate from forebrain, midbrain and hindbrain regions to populate the fronto‐nasal, maxillary and mandibular processes. Except for the enamel of the tooth crown, cranial neural crest‐derived ectomesenchymal cells form the remaining structures of the tooth, including the dentine, pulp and periodontal tissues (Romer 1972; Tan & Morriss‐Kay 1986; Bronner‐Fraser 1993; Chai et al. 2000). Recent studies have well advanced our understanding of this important pluripotent cell population, and how genetic and epigenetic mechanisms mediate their subsequent lineage segregation, proliferation, differentiation and final contribution to a particular cell type (Chai et al. 2000; Tian et al. 2004; Yan et al. 2004; Jiang et al. 2005; Xie et al. 2005). During craniofacial development, the proliferative activity of these cells produces discrete swellings that demarcate each branchial arch. Following epithelial–mesenchymal interactions, post‐migratory ectomesenchymal cells differentiate into multiple phenotypes and contribute to the formation of various head and neck structures. Growth and transcription factors have been implicated during the specification and determination of neural crest cells (Yan et al. 2004; Jiang et al. 2005; Xie et al. 2005). It is clearly important to identify this novel source of mesenchymal stem cells, which may represent a promising pool of candidate cells for the engineered repair of tissues and organ systems.
Stem cells possess self‐renewal, long‐term cell viability and multilineage differentiation potential, all of which are taken as their significant characteristics. Bone mesenchymal stem cells have excellent capacity to proliferate in culture and to differentiate into a wide variety of cell types in response to an appropriate culture system (Bianco & Gehron Robey 2000; Gronthos et al. 2003). In this study, cranial neural crest‐derived ectomesenchymal cells were passaged in vitro to evaluate their proliferative potential using bromodeoxyuridine (BrdUrd) labelling. Meanwhile, expression of telomerase was measured by in situ hybridization, as active telomerase can prevent cellular senescence and extends the lifespan of cultured cells (Bodnar et al. 1998; Vaziri & Benchimol 1998). A key function of telomerase is to add telomere repeats to the ends of chromosomes during cell division. Telomerase activity remains high in stem cells and precursor cells (Tang et al. 2001; Xu et al. 2001), which are capable of infinite self‐replication and differentiation into a variety of cell types (Morrison et al. 1996; Weissman 2000).
According to previous reports, each brachial arch develops according to a unique program of spatial and temporal patterning. As the tooth germ develops from the bud to cap stage, ectomesenchymal cells are concentrated at the interface with the dental epithelium, suggesting critical function of these cells in epithelial–mesenchymal interactions during tooth germ development. At the cap stage, the dental epithelium forms an aggregated cell mass (the enamel knot), which serves as a signalling centre to guide cusp formation. As tooth development advances from the cap to the bell stage, the cranial neural crest‐derived dental mesenchyme and the inner dental epithelium begin to terminally differentiate into the pre‐odontoblast and pre‐ameloblast, respectively (Chai et al. 2000; Jernval & Thesleff 2000; Yan et al. 2004).
In view of the fact that the ectomesenchymal cells are regulated by growth factors and downstream transcription factors before they become committed to a number of different cell types (Noden 1983; Lumsden 1988; Le Douarin et al. 1993; Imai et al. 1996), the cultured undifferentiated cells were transferred to a specific medium with appropriate ingredients and were then induced to develop into a variety of lineages, including endothelial cells and neuroblasts. These findings on how the fate of the ectomesenchymal cells was determined were important for us to elucidate the mechanism of normal and abnormal orofacial development. Based on results of this study, we also could improve tissue‐engineering approaches to repair orofacial structures (Alsberg et al. 2001) and promote the molecular research on the signals that trigger periodontal (Ripamonti & Reddi 1997) or dental regeneration (Smith & Lesot 2001).
MATERIALS AND METHODS
Isolation and culture of ectomesenchymal cells
Eight‐week‐old BALB/c mice were used in line with the International Guiding Principles for Animal Research (1985). Noon of the day on which vaginal plugs were detected was considered as E0.5. The first branchial arches were dissected from embryos aged E9.5 (Sadler 2000), were minced and then incubated in the solution with 0.125% trypsin and 1 mm EDTA at 37 °C for 5 min. After neutralization of the trypsin, cells released from mandibular arch specimens were collected by centrifugation at 1000 g for 5 min. The cell pellet was resuspended and seeded in medium containing DMEM/F12 (1 : 1, Gibco), 10% foetal bovine serum (FBS, Gibco), 106 U/l leukaemia inhibition factor (LIF, Chemicon International, Temecula, CA) (Wright et al. 2003; Deng et al. 2004; , Tian et al. 2004; Jiang et al. 2005), 100 U/ml penicillin and 100 g/ml streptomycin. After incubation in plastic flasks for 20 min, most of the ectomesenchymal cells were attached and epithelial cells were still suspended in medium. The supernatant with these unattached cells was removed and was replaced with fresh medium. Cultures were maintained in a humidified atmosphere of 5% CO2 at 37 °C and cells were passaged three times prior to differentiation or measurement. When 80% confluent, monolayers were trypsinized (0.25% trypsin, 5 min) and the ectomesenchymal cells were further selected (because of their longer time detached from the flask compared to epithelial cells).
BrdUrd labelling for cell proliferation analysis
Cells at passage 3 were resuspended and were seeded into 6‐well plates with glass slides, at a density of 1 × 105/well. BrdUrd labelling was performed in 1.0 ml of medium containing 5 µm BrdUrd for 48 h. The slides with labelled cells were washed with PBS, fixed in 4% buffered paraformaldehyde for 20 min and were incubated in 1 m HCl for 30 min. Then cells were washed with PBS again, before being blocked in 1% bovine serum albumin and 1.5% normal goat serum at room temperature for 30 min. Slides were then incubated at 37 °C with monoclonal antibody against BrdUrd (Dako), and secondary antibody labelled with FITC to detect BrdUrd presence in nuclei of the cells.
In situ hybridization for telomerase mRNA
Slides covered with passage 3 cells were pre‐treated with 4% buffered paraformaldehyde and 0.1% (v/v) DEPC for 1 h. Hybridization was carried out according to the protocol of the kit (Santa Cruz, CA). Briefly, cells were digested with pepsin to expose the appropriate mRNA fragment and were fixed in 4% buffered paraformaldehyde again. First, slides were pre‐hybridized and then hybridized with the telomerase probe, labelled with digoxin overnight in a humidified chamber at 40 °C. Sequentially, they were incubated with secondary biotinylated antibody and horseradish peroxide conjugated streptavidin (Santa Cruz, CA). The peroxidase reaction was developed using 3,3′‐diaminobenzidine tetrahydrochloride (DAB) as chromogen.
TRAP analysis
To statistically assess telomerase activity in the cultured ectomesenchymal cells, we used a TRAP ELISA kit (Roche Molecular Biochemicals, Indianapolis, IN, USA); we performed the TRAP assay according to the manufacturer's instructions. Primary bone marrow stromal stem cells (BMSSCs) were taken as positive control. In our preliminary experiments, we found that for ectomesenchymal cells or BMSSCs, the amount of PCR‐amplified telomerase product detected with the TRAP ELISA kit increased linearly with the total cell number from 1 × 105 to 5 × 106. So 1 × 106 cells from each cell line were lysed and the extracted protein was used to compare the telomerase activity.
Identification of cultured cells
At passage 3, few epithelial cells remained; most of the culture was made up of mandibular arch ectomesenchymal cells. To verify that the majority were undifferentiated ectomesenchymal cells, cultures were stained with monoclonal antibody to HNK‐1 at 1 : 100 dilution, monoclonal antibody to vimentin at 1 : 30 dilution, monoclonal antibody to S‐100 at 1 : 100 dilution and monoclonal antibody to cytokeratin at 1 : 100 dilution. (All antibodies were obtained from Dako Cytomation (Carpinteria, CA).)
The proportion of positive staining cells was quantitatively analysed with a colour image analysis system (Media Cybernetics: Image‐Pro Plus 5.0). At least 10 000 cells in 100 different regions were counted per slide. These regions were equally distributed throughout the slides. The positive ratio, defined as the number of cells with positive staining divided by the total number of cells counted and expressed as a percentage, was calculated.
Multilineage differentiation of ectomesenchymal cells
The specific medium contained the following components listed in Table 1 to induce multilineage differentiation of ectomesenchymal cells. The medium was replaced every 3–4 days, and this process was repeated till differentiated cells were confluent and harvested for studies.
Table 1.
Lineage‐specific differentiation induced by medium supplementation
| Lineage | Medium | Serum (%) | Supplementation |
|---|---|---|---|
| Neurogenesis | DMEM | None | 25 m forskolin, 1% antibiotic/antimycotic |
| Endothelial cells | DMEM | 20% FBS | 10 ng/ml VEGF, 1 ng/ml bFGF and 2 ng/ml IGF‐I, 1% antibiotic/antimycotic |
| Myogenesis | DMEM | 10% FBS, 5% HS | 50 m hydrocortisone, 1% antibiotic/antimycotic |
| Odontogenesis | DMEM | 10% FBS | 12 ng/ml TGF‐β, 300 µg/ml BMP‐2, 200 µg/ml Dental Matrix non‐collagen proteins (DMNCPs) a , 1% antibiotic/antimycotic |
| Control | DMEM | 10% FBS | None |
The preparation of DMNCPs was according to Smith & Leaver 1979.
Histological and immunohistochemical analysis
Cellular morphological features of induced cells, in monolayers, were assessed with standard haematoxylin and eosin staining. Differentiated phenotypes of variant cells were viewed and compared by phase‐contrast microscopy. For immunohistochemical analysis, monolayers of cells were prepared on slides fixed in 4% buffered paraformaldehyde. Fixed cells on glass slides were incubated with 3% hydrogen peroxide in methanol for 30 min to inhibit endogenous peroxidase activity. After washing with PBS, they were blocked in 1% bovine serum albumin and 1.5% normal goat serum at room temperature for 30 min. Cells on slides were then incubated overnight at 4 °C with antibodies against nestin, factor VIII, α‐SMA (Dako Cytomation (Carpinteria, CA)) and DSPP (Fourth Military Medical University, Xi’an, China). Then, sections were incubated with secondary biotinylated antibodies, horseradish peroxide conjugated streptavidin, and were developed using DAB. After rinsing in distilled water, slides were dehydrated in ascending ethanol concentrations, were cleared in xylene and were covered with coverslips for microscopy.
RNA isolation and reverse transcription–PRC
Total RNA was extracted from all the specimens using the TRIzol reagent (Life Technologies, Rockville, MD) according to the manufacturer's protocol. About 1 g of total RNA was reversed transcribed by murine laeukemia virus reverse transcriptase (TaKaRa, Jap) and PCR amplification of target message RNA was performed with a TaKaRa PCR kit (TaKaRa, Jap). PCR oligonucleotide primers and annealing temperature are listed in Table 2. The products were electrophoresed on 1.5% agarose gels, stained with ethidium bromide and visualized with quantity one software (Bio‐Rad).
Table 2.
Specific primers for PCR amplication with expected fragments size and optimal annealing temperature
| Gene | Primers | Annealing temperature (°C) | Fragment (bp) | GenBank No. |
|---|---|---|---|---|
| β‐actin | 5′‐ACTCTTCCAGCCTTCCTTCC‐3′ | 55 | 313 | BC013835 |
| 5′‐ACTCGTCATACTCCTGCTTGC‐3′ | ||||
| Nestin | 5′CCCTCACCACTCTATTTTA‐3′ | 47 | 439 | NM_016701 |
| 5′‐ACTATCTAAACCTTTAGGAGAA‐3′ | ||||
| GFAP | 5′ACAGAGGAGTGGTATCGGTC‐3′ | 53 | 381 | NM_010277 |
| 5′‐GAAAACCGCATCACCATT‐3′ | ||||
| vWF | 5′GCCTGCGGACATTTTCTC‐3′ | 58 | 591 | NM_011708 |
| 5′‐GGTGGGAAATGAGGGTTG‐3′ | ||||
| α‐SMA | 5′‐CAGGGAGTAATGGTTGGA‐3′ | 54 | 502 | NM_007392 |
| 5′‐CTACTGCCGAGCGTGAGA‐3′ | ||||
| DSPP | 5′‐GAATAGCACCAACCATGAGG‐3′ | 63 | 313 | NM_010080 |
| 5′‐TCTAACGGAAGTGACGAAAG‐3′ | ||||
| DMP‐1 | 5′‐GTGGAGATGACACCTTTGGCGATGA‐3′ | 58 | 546 | NM_016779 |
| 5′‐TATGAGGTCGGAAGAATCTAAAGG‐3′ |
RESULTS
Proliferative potential of ectomesenchymal cells
Most of the primary cells harvested from the first branchial arch tissues (Fig. 1a) showed fibroblast‐like morphology with an elongated spindle‐shape (Fig. 1b). Cultured in 10% FBS‐containing medium, the undifferentiated ectomesenchymal cells had a striking proliferative potential with a high level of BrdUrd labelling (Fig. 1c). This suggested that the ectomesenchymal cells cultured as monolayers in vitro still maintained active renewal potential. In situ hybridization for telomerase mRNA provided the reason of why so few of the ectomesenchymal cells reached senescence or apoptosis after several passages (Fig. 1d). Expression of telomerase in isolated cells was at such a high level that it is only otherwise to be seen in cancer cells or in other types of stem cell (Smith & Leaver 1979; Drummond et al. 2005; Serakinci et al. 2004). Furthermore, telomerase activity in primary ectomesenchymal cells was maintained for a long period until passage 3 was reached (Fig. 1e).
Figure 1.
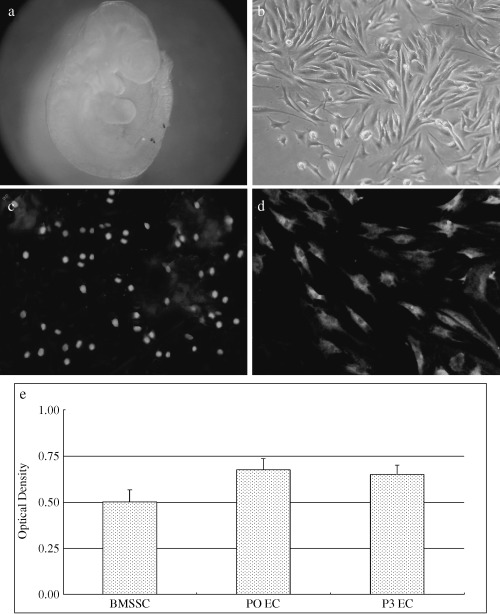
Proliferative potential of entomesenchymal cells. The first branchial arch primordia (a); most of the primary cells harvested from the first branchial arch tissues showed fibroblast‐like morphology with an elongated spindle‐shape (b). High levels of BrdUrd labelling indicate a striking proliferative potential of the undifferentiated ectomesenchymal cells (c). The ectomesenchymal cells express high levels of telomerase mRNA (d) and indicate correspondingly high levels of telomerase activity (e).
Characteristics of ectomesenchymal cells
Ectomesenchymal cells attached to the culture flask more rapidly than epithelial cells and remained as monolayers for longer time periods during trypsinization for subsequent passaging. This led to further purification of the ectomesenchymal cell population during this natural time period. By the third passage, cultured cells were positively stained with monoclonal antibody to HNK‐1 (Fig. 2a, more than 95% ectomesenchymal cells positive), vimentin (Fig. 2b, more than 90% ectomesenchymal cells positive) and S‐100 (Fig. 2c, ∼30% ectomesenchymal cells positive), all being markers of undifferentiated ectomesenchymal and neural crest cells, but most cells were not stained with the antibody to cytokeratin (Fig. 2d, most ectomesenchymal cells negative).
Figure 2.
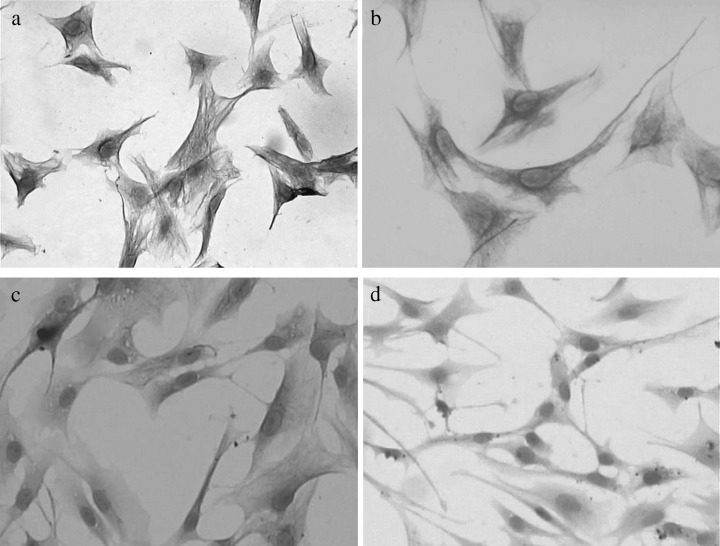
Characters of entomesenchymal cells. Ectomesenchymal cells positively stained with monoclonal antibodies to HNK‐1, vimentin, and S‐100, respectively (a,b,c), but almost nothing with the antibody to cytokeratin (d).
Neurogenesis
Cultured cells were induced to palisade‐like parallel arrangement after 2 days in the medium containing 25 m forskolin. Then they became bipolarand larger. Treated after 4 days, morphological changes in the cells were distinguished; they became long and asymmetrical with time (Fig. 3a). Results of the immunohistochemical assays showed that most induced cells expressed nestin (Fig. 3b, ∼60% ectomesenchymal cells positive). Meanwhile, reverse transcription (RT)‐PCR also confirmed neurogenesis in the cell cultures with the expression of nestin and GFAP at mRNAs (Fig. 5).
Figure 3.
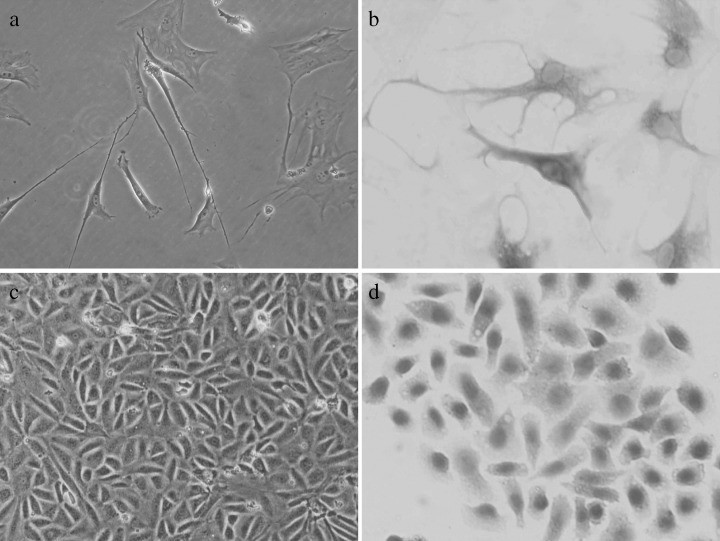
Neurogenic and endothelial potential of entomesenchymal cells. After 4 days of neurogenic culture, morphological changes of cells were distinct; they became long and asymmetric with increasing time (a). Immunohistochemical assay showed that a high proportion of the induced cells expressed nestin (b). After culture in endothelial medium for 14 days, ectomesenchymal cells became shortened and rounded like paving stones, during differentiation (c), and became stained with the antibody to factor VIII protein (d).
Figure 5.
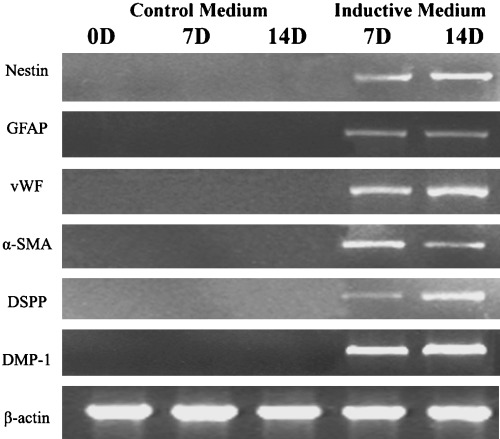
Expression of specific genes was analysed by RT‐PCR in inductive and control groups. Each group was assayed at different time points including 0, 7 and 14 days. Results in each group were reproducible.
Induction of the endothelial phenotype
Morphology of induced cells indicated that they developed the endothelial phenotype in conditioned medium with 20% FBS, 10 ng/ml VEGF, 1 ng/ml bFGF and 2 ng/ml IGF‐I after 14 days culture. Cells shortened and rounded like paving stones during the differentiational process (Fig. 3c) and were strongly positively stained with the antibody to factor VIII protein (Fig. 3d, ∼90% ectomesenchymal cells positive). Intensities of vWF bands in RT‐PCR analysis further verified the expression of endothelial cell marker genes (Fig. 5), whereas non‐inducted negative controls were stable with no sign of phenotypic alteration.
Myogenesis
After being in the myogenic medium for 3 days, the ectomesenchymal cells exhibited changes in their morphological features. They became larger and flatter like myofibroblasts. With increasing culture time, this trend became more obvious, finally with almost all cells taking on a flattened morphology at 7 days (Fig. 4a). The majority of the flattened cells expressed α‐SMA, a well‐characterized smooth muscle marker (Fig. 4b, ∼70% ectomesenchymal cells positive). Prolonging the culture period with induction medium, to 2 weeks, could increase the proportion of α‐SMA positive cells. In contrast, the cells remaining without myogenic supplements did not stain with the antibody to α‐SMA. RT‐PCR analysis of mRNA using α‐SMA specific primers showed that the induced ectomesenchymal cells had a high level expression, but no expression was observed in the control group (Fig. 5).
Figure 4.
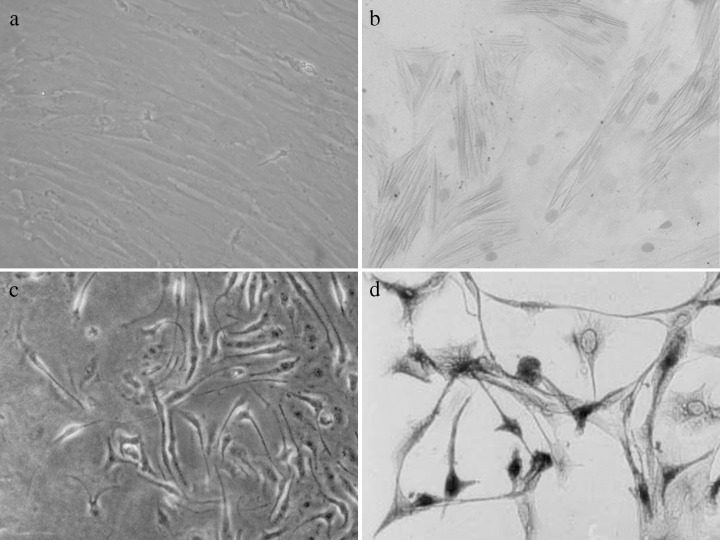
Myogenic and odontogenic potential of entomesenchymal cells. After being placed in myogenic medium for 7 days, ectomesenchymal cells became larger and flatter resembling myofibroblasts (a). The majority of the flattened cells expressed α‐SMA, a well‐characterized smooth muscle cell marker (b). After 14 days of odontogenetic inductive culture, ectomesenchymal cells became polygonal in shape and were characterized by long cytoplasmic processes (c). Immunocytochemical staining demonstrated that DSPP was expressed (d).
Odontogenesis
After 2 weeks of induction, ectomesenchymal cells had significantly switched to a bipolar morphology when compared to intact controls (Fig. 4c). Accompanied by the changes in phenotype, DSPP expression at mRNA and protein levels both increased dramatically (Fig. 4d, ∼65% ectomesenchymal cells was positive). Measurement of mRNA encoding DMP‐1 and DSPP indicated that there was high expression in odontogenic cells (Fig. 5). These genes were not observed expressed in the cells incubated in control medium.
DISCUSSION
In mammals, CNCC migrate ventrolaterally as they populate the branchial arches during craniofacial development. The ectomesenchymal cells of the first branchial arch originate from the rostral hindbrain or caudal midbrain (Osumi‐Yamashita et al. 1994; Lee et al. 1995; Imai et al. 1996) and develop into a diverse array of head and neck structures during embryogenesis. The present study has focused on proliferative potential and multilineage capacity of these ectomesenchymal cells.
Normal mouse adult cells undergo a finite number of cell divisions and ultimately enter into an arrested state called senescence. But the ectomesenchymal cells here had a high proliferative potential in monolayer culture conditions in vitro. Following several cell expansions, they still labelled extensively with BrdUrd, in the same manner as the primary cells. In situ hybridization indicated that their expression of telomerase mRNA was stable and high during culture time in the undifferentiated state. It has been proposed that telomere shortening is the molecular clock that triggers cellular senescence, so viability of these ectomesenchymal cells may have been correlated with the activity of telomerase. Previous studies have reported that telomerase is present in proliferative stem cells of renewal tissues and it is required for the maintenance of the stem cell state for cell proliferation and for differentiation. It has been suggested possibly as a common marker for stem cells (Shi et al. 2002; Simonsen et al. 2002; Lin et al. 2004). LIF is a member of the interleukin (IL)‐6 family, a multifunctional cytokine that acts on a wide range of cell types. LIF has also been identified as an inhibitor of differentiation of mouse embryonic stem (ES) cells. Mouse ES cells can be maintained without feeder layers in the presence of mouse LIF and fetal bovine serum. In previous studies, our group has isolated undifferentiated ectomesencymal cells from the first branchial arch, which express HNK‐1, in accordance with characteristic properties of migrating neural crest cells. These cells were able to renew while grown in the presence of LIF and retained the potential to differentiate into different phenotypic progeny, thereby displaying the characteristics of stem cells (Tian et al. 2004; Yan et al. 2004; Jiang et al. 2005; Xie et al. 2005). However, without the existence of LIF, the cells showed a tendency to spontaneously differentiate. These differentiated cells expressed α‐SMA or type I collagen, but not GFAP or NF in the absence of LIF, which suggested that those cells would have differentiated along the smooth muscle cell or osteoblast (although not glial or neural) lineages (Shellard et al. 1996; Wright et al. 2003; Deng et al. 2004).
Stem cells are considered to possess not only self‐replicating potential but also the ability to differentiate terminally to multiple lineages (Hall & Watt 1989). Until now, the embryonic stem cell has been the gold standard, which is capable to be induced into cells from all three embryonic germ layers (Shamblott et al. 1998; Park & Han 2000). Our findings have suggested that, like embryonic stem cells, ectomesenchymal cells isolated from the first branchial arch had a trans‐germ potential to differentiate into endothelial, odontogenic and neurogenic lineages. Traditionally, it was believed that the differentiation of adult stem cells progressed in a limited manner and their fate was restricted within a germ lineage. Recent reports, however, have shown the neurogenic potential of MSCs (Mizuno & Hyakusoku 2003), the induction of HSCs into hepatocytes (Legasse et al. 2000) and the conversion of neurogenic precursors into muscle and blood (Bjornson et al. 1999; Galli et al. 2000). In view of the plasticity observed in adult stem cells, we thought it of great value to study the differentiational potential and optimized culture conditions of ectomesenchymal cells from the mandibular arch.
This present study has significantly advanced the understanding of how this important population of pluripotent cells was initially established and the mechanisms that mediated their subsequent lineage proliferation, differentiation and final contribution to a particular cell type. Both intrinsic and extrinsic regulatory signals are critical for the proper migration and proliferation of this CNCC population (LaBonne & Bronner‐Fraser 1999). Growth and transcription factors, such as BMP, TGF‐β, FGF, Hox, Msx and Pax, have been implicated during the fate determination of neural crest cells (Garcia‐Castro & Bronner‐Fraser 1999; Christiansen et al. 2000). In early embryogenesis, ectomesenchymal cells of the mandibular arch have been described as rostral odontogenic or caudal skeletogenic cells, respectively. Teeth develop from the rostrally located ectomesenchymal cells via interactions with the overlying oral epithelial cells. It is accepted that signals from the epithelium are necessary for tooth development. More caudal ectomesenchymal cells do not form teeth in vivo but give rise to the skeletal tissues of the mandible (Tucker et al. 1999). Fibroblast growth factor‐8 (FGF‐8) has been identified as one of the key signalling molecules from oral epithelium capable of inducing and/or maintaining expression of several different mesenchymal genes in the mandibular arch (Neubüser et al. 1997; Ferguson et al. 1998; Kettunen & Thesleff 1998). In this study, the ectomesenchymal cells were seeded in medium containing dental matrix non‐collagenous protein and growth factors such as TGF‐β1, BMP‐2 and FGF‐8 during odontogenesis (Tian et al. 2004; Yan et al. 2004; Jiang et al. 2005; Xie et al. 2005). In our previous studies, we tried to investigate the effects of FGF‐8 on CNCC differentiating into ectomesenchymal cells of the first branchial arch and to determine the appropriate dose and stage of CNCC exposure to FGF‐8. Cranial neural crest explants were cultured in serum‐free medium containing modified DMEM/F12 and were supplied with varying doses of FGF‐8. The differentiation types of CNCC were determined by in situ hybridization for Hoxa2 and immunocytochemistry was for vimentin expression (Yan et al. 2004). These studies proved that the pre‐emigrating CNCC did not express Hoxa2 but did express vimentin after treatment with 100 µg of FGF‐8. Both the post‐emigration CNCC group and the control group expressed Hoxa2 and vimentin. Thus, we concluded at an early stage of CNCC emigration that the first branchial arch phenotype of CNCC could be induced by treatment with FGF‐8 and that this experiment could provide an in vitro model for study of the mechanism of tooth–jaw regeneration (Tian et al. 2004; Yan et al. 2004; Jiang et al. 2005; Xie et al. 2005).
Differentiation into various lineages was assessed using immunochemical staining and molecular analysis of specific genes or proteins. Accumulation of type I collagen and mineralization demonstrated the onset of overt odontogenesis, coincident with DSPP and DMP secreted by induced cells. Dentin is a mineralized tissue with contents similar to those of bone. The dentin extracellular matrix is composed of approximately 47% (v/v) mineral, 30% (v/v) protein and 21% (v/v) water (Legeros 1991). In dentin, type I collagen accounts for about 90% of the protein fraction and most of the dentin non‐collagenous proteins are also expressed in bone. However, some proteins appear to be more specifically expressed in dentin even though they can be found, albeit weaker, in other tissues. For instance, odontoblasts express DMP‐1 and DSPP, the latter turns into dentin sialoprotein (DSP) and dentin phosphoprotein (DPP) after proteolytic cleavage (Ritchie & Wang 1996; MacDougall et al. 1997; Gu et al. 1998). DPP is involved in matrix mineralization with a high affinity for calcium ions (Marsh 1989) and hydroxyapatite crystals (Fujisawa & Kuboki 1991).
In addition to odontogenesis, ectomesenchymal cells can be induced to differentiate into further mesodermal lineages such as to endothelial cells. Phenotypic alteration in the cells occurred when seeded into conditioned medium, paralleling with expression of factor 8 and vWF, which are essential molecules of the haemostatic mechanism (Escolar et al. 1998) and markers of endothelial differentiation (Chung‐Welch et al. 1989). Simultaneously, high expression of nestin after specific induction, rapidly verified the procedure of early neurogenic differentiation in these ectomesenchymal cells. Nestin is an intermediate filament protein expressed at high levels in neural stem cells (Sanchez‐Ramos et al. 2000). Its expression has also been observed in myogenic cells, endothelial cells and hepatic cells, indicating that it cannot be used as a marker for putative neurogenic potential. However, neurogenic induction of cells has also resulted in the assumption of neurogenic morphology and the increased expression of GFAP, the two of which bind together to form the main intermediate filament found in specialized brain cells (astrocytes). Astrocytes are star‐shaped cells that support the functions of nerve cells in the brain and spinal cord. In summary, the expression of several lineage‐specific genes and proteins revealed that these ectomesenchymal cells had both mesodermal and ectodermal capacity.
The biological function of these cranial neural crest‐derived cells has been studied in a variety of animal models. At first, it was proposed that they carried certain pre‐acquired molecular signals as they migrated into the branchial arch. Recent studies have shown that they acquire positional identity at the time they reach their final destination and contribute to the formation of various craniofacial structures. Certain growth and transcription factors have been implicated as important regulators for the critical epithelial–mesenchymal interactions through which these multipotent neural crest‐derived cells become progressively restricted to form neural crest derivatives and eventually develop into individual cell types (Anderson 1997; Thesleff & Sharpe 1997; Tucker & Sharp 1999). In agreement with these findings, we induced multiple germ layer differentiation of ectomesenchymal cells in appropriate conditions.
Thus, it is suggested that ectomesenchymal cells derived from first branchial arch might represent a novel source of multipotential stem cells capable of undergoing expansion and variant differentiation in vitro. Based on the results, we have obtained valuable information concerning the fate of the ectomesenchymal cells. It is important that we have elucidated a mechanism in which normal or abnormal orofacial development occurs. Also, we have made it clear which growth factors or cytokines play key roles during isolated cells differentiating into many kinds of committed lineages. With optimization of inductive conditions and manipulation of pivotal genes by vectors, we could improve the tissue‐engineering approaches to repair of the orofacial structures (Alsberg et al. 2001) and promote molecular research into signals that trigger periodontal (Ripamonti & Reddi 1997) or dental regeneration (Smith & Lesot 2001) in the future.
ACKNOWLEDGEMENTS
We thank Shengwei Li for excellent technical support and Lei Liu's help with manuscript preparation. This work was supported by generous grant from the Special Project of National Grand Fundamental Research Program of China (2002CCC00700) and Teaching & Research Award for Outstanding Young Teachers in Higher Education Institutions of China (2003682).
REFERENCES
- Alsberg E, Hill EE, Mooney DJ (2001) Craniofacial tissue engineering. Crit. Rev. Oral Biol. Med. 12, 64. [DOI] [PubMed] [Google Scholar]
- Anderson DJ (1997) Cellular and molecular biology of neural crest cell lineage determination. Trends Genet. 13, 276. [DOI] [PubMed] [Google Scholar]
- Bianco P, Gehron Robey P (2000) Marrow stromal stem cells. J. Clin. Invest. 105, 1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson CRR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL (1999) Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo . Science 283, 534. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life‐span by introduction of telomerase into normal human cells. Science 279, 349. [DOI] [PubMed] [Google Scholar]
- Bronner‐Fraser M (1993) Mechanisms of neural crest migration. Bioessays 15, 221. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch D, Soriano P, McMahon A, Sucov H (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127, 1671. [DOI] [PubMed] [Google Scholar]
- Christiansen JH, Coles EG, Wilkinson DG (2000) Molecular control of neural crest formation, migration and differentiation. Curr. Opin. Cell Biol. 6, 719. [DOI] [PubMed] [Google Scholar]
- Chung‐Welch N, Patton WF, Yen‐Patton GP, Hechtman HB, Shepro D (1989) Phenotypic comparison between mesothelial and microvascular endothelial cell lineages using conventional endothelial cell markers, cytoskeletal protein markers and in vitro assays of angiogenic potential. Differentiation 42, 44. [DOI] [PubMed] [Google Scholar]
- Deng MJ, Jin Y, Shi JN, Lu HB, Liu Y, He DW, Nie X, Smith AJ (2004) Multilineage differentiation of ectomesenchymal cells isolated from the first branchial arch. Tissue Eng. 10, 1597. [DOI] [PubMed] [Google Scholar]
- Drummond MW, Hoare SF, Monaghan A et al. (2005) Dysregulated expression of the major telomerase components in leukaemic stem cells. Leukemia 19, 381. [DOI] [PubMed] [Google Scholar]
- Escolar G, Carretero M, Magallon M, Quintana M, Arnau C, Castillo R, Aznar‐Salatti J (1998) von Willebrand factor contained in factor VIII concentrates of different purities supports platelet adhesion in blood samples from a heterogeneous group of patients with von Willebrand disease. Haematologica 83, 1009. [PubMed] [Google Scholar]
- Ferguson CA, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT (1998) Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev. 12, 2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa R, Kuboki Y (1991) Preferential adsorption of dentin and bone acidic proteins on the (100) face of hydroxyapatite crystals. Biochim. Biophys. Acta 1075, 56. [DOI] [PubMed] [Google Scholar]
- Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta M, Mora M, De angelis MG, Fiocco R, Cossu G, Vescovi AL (2000) Skeletal myogenic potential of human and mouse neural stem cells. Nat. Neurosci. 3, 986. [DOI] [PubMed] [Google Scholar]
- Garcia‐Castro M, Bronner‐Fraser M (1999) Induction and differentiation of the neural crest. Curr. Opin. Cell Biol. 11, 695. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Hay SJ et al. (2003) Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J. Cell Sci. 116, 1827. [DOI] [PubMed] [Google Scholar]
- Gu K, Chang SR, Slaven MS, Clarkson BH, Rutherford RB, Ritchie HH (1998) Human dentin phosphophoryn nucleotide and amino acid sequence. Eur. J. Oral Sci. 106, 1043. [DOI] [PubMed] [Google Scholar]
- Hall PA, Watt FM (1989) Stem cells: the generation and maintenance of cellular diversity. Development 106, 619. [DOI] [PubMed] [Google Scholar]
- Weissman IL (2000) Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157. [DOI] [PubMed] [Google Scholar]
- Imai H, Osumi‐Yamashita N, Ninomiya Y, Eto K (1996) Contribution of early‐emigrating midbrain crest cells to the dental mesenchyme of mandibular molar teeth in rat embryos. Dev. Biol. 176, 151. [DOI] [PubMed] [Google Scholar]
- International Guiding Principles for Animal Research (1985). Geneva: Council for International Organization of Medical Sciences. [Google Scholar]
- Jernvall J, Thesleff I (2000) Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 92, 19. [DOI] [PubMed] [Google Scholar]
- Jiang HB, Tian WD, Liu L et al. (2005) In vitro study on cranial neural crest differentiating in to ectomesenchymal cell of the first branchial arch by FGF8. Zhonghua Kou Qiang Yi Xue Za Zhi. 40, 319. [PubMed] [Google Scholar]
- Kettunen P, Thesleff I (1998) Expression and function of FGFs‐4‐8, and ‐9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev. Dyn. 211, 256. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner‐Fraser M (1999) Molecular mechanisms of neural crest formation. Annu. Rev. Cell. Dev. Biol. 15, 81. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Ziller C, Coul G (1993) Patterning of neural crest derivatives in the avian embryo: in vivo and in vitro studies. Dev. Biol. 159, 24. [DOI] [PubMed] [Google Scholar]
- Lee YM, Osumi‐Yamashita N, Ninomiya Y, Moon CK, Eriksson U, Eto K (1995) Retinoic acid stage‐dependently alters the migration pattern and identity of hindbrain neural crest cells. Development 121, 825. [DOI] [PubMed] [Google Scholar]
- Legasse E. et al. (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo . Nat. Med. 6, 1229. [DOI] [PubMed] [Google Scholar]
- Legeros RZ (1991) Calcium phosphates in enamel, dentin and bone In: Myers HM, ed. Calcium Phosphate in Oral Biology and Medicine, p. 108 New York: Kanger. [PubMed] [Google Scholar]
- Liu L, DiGirolamo CM, Navarro PAAS, Blasco MA, Keefe DL (2004) Telomerase deficiency impairs differentiation of mesenchymal stem cells. Exp. Cell Res. 294, 1. [DOI] [PubMed] [Google Scholar]
- Lumsden AGS (1988) Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth. Development 103 (Suppl.), 55. [DOI] [PubMed] [Google Scholar]
- MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT (1997) Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J. Biol. Chem. 272, 835. [DOI] [PubMed] [Google Scholar]
- Marsh ME (1989) Binding of calcium and phosphate ions to dentin phosphophoryn. Biochemistry 28, 346. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Hyakusoku H (2003) Mesengenic potential and future clinical perspective of human processed lipoaspirate cells. J. Nippon Med. Sch. 70, 300. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Prowse KR, Ho P, Weissman IL (1996) Telomerase activity in hematopoietic cells is associated with self‐renewal potential. Immunity 5, 207. [DOI] [PubMed] [Google Scholar]
- Neubüser A, Peters H, Balling R, Martin GR (1997) Antagonistic interactions between FGF and BMP signaling pathways: a mechanism for positioning the sites of tooth formation. Cell 90, 247. [DOI] [PubMed] [Google Scholar]
- Noden DM (1983) The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissue. Dev. Biol. 96, 144. [DOI] [PubMed] [Google Scholar]
- Osumi‐Yamashita N, Ninomiya Y, Doi H, Eto K (1994) The contribution of both forebrain and midbrain crest cells to the mesenchyme in the frontonasal mass of the mouse embryos. Dev. Biol. 164, 409. [DOI] [PubMed] [Google Scholar]
- Park TS, Han JY (2000) Derivation and characterization of pluripotent embryonic germ cells in chicken. Mol. Reprod. Dev. 56, 475. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, Reddi AH (1997) Tissue engineering, morphogenesis and regeneration of the periodontal tissues by bone morphogenetic proteins. Crit. Rev. Oral Biol. Med. 8, 154. [DOI] [PubMed] [Google Scholar]
- Ritchie HH, Wang LH (1996) Sequence determination of an extremely acidic rat dentin phosphoprotein. J. Biol. Chem. 271, 21695. [DOI] [PubMed] [Google Scholar]
- Romer AS (1972) The vertebrate as a dual animal‐somatic and visceral. Evol. Biol. 6, 121. [Google Scholar]
- Sadler TW (2000) Langman's Medical Embryology, 8th edn A. Lippincott Williams & Wilkins, Baltimore, Maryland, USA, p. 345. [Google Scholar]
- Sanchez‐Ramos J. et al. (2000) Adult bone marrow stromal cells differentiate into neural cells in vitro . Exp. Neurol. 164, 247. [DOI] [PubMed] [Google Scholar]
- Serakinci N, Guldberg P, Burns JS, Abdallah B, Schrodder H, Jensen T, Kassem M (2004) Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene 23, 5095. [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD (1998) Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl Acad. Sci. USA 95, 13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellard J, Perreau J, Brulet P (1996) Role of leukemia inhibitory factor during mammalian development. Eur. Cytokine Netw. 7, 699. [PubMed] [Google Scholar]
- Shi S, Gronthos S, Chen S, Reddi A, Counter CM, Robey PG, Wang CY (2002) Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat. Biotechnol. 20 587. [DOI] [PubMed] [Google Scholar]
- Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG, Kassem M (2002) Telomerase expression extends the proliferative life‐span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 20, 592. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Leaver AG (1979) Non‐collagenous components of the organic matrix of rabbit incisor dentine. Arch Oral Biol. 24, 449. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Lesot H (2001) Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair. Crit. Rev. Oral Biol. Med. 12, 425. [DOI] [PubMed] [Google Scholar]
- Tan SS, Morriss‐Kay GM (1986) The development and distribution of the cranial neural crest in the rat embryo. Cell Tissue Res. 240, 403. [DOI] [PubMed] [Google Scholar]
- Tang DG, Tokumoto YM, Apperly JA, Lloyd AC, Raff MC (2001) Lack of replicative senescence in cultured rat oligodendrocyte precursor cells. Science 291, 868. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Sharpe P (1997) Signaling networks regulating dental development. Mech. Dev. 67, 111. [DOI] [PubMed] [Google Scholar]
- Tian WD, Jiang HB, Liu L et al. (2004) In vitro culture and biological characteristics of cranial neural crest stem cell. Hua Xi Kou Qiang Yi Xue Za Zhi 22, 229. [PubMed] [Google Scholar]
- Tucker AS, Sharpe PT (1999) Molecular genetics of tooth morphogenesis and pattering: the right shape in the right place. J. Dent. Res. 78, 826. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Yamada G, Grigoriou M, Pachnis V, Sharpe PT (1999) Fgf‐8 determines rostral‐caudal polarity in the first branchial arch. Development 126, 51. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Benchimol S (1998) Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8, 279. [DOI] [PubMed] [Google Scholar]
- Wright LS, Li J, Caldwell MA et al. (2003) Gene expression in human neural stem cells: effects of leukemia inhibitory factor. J. Neurochem. 86 (1), 179. [DOI] [PubMed] [Google Scholar]
- Xie JM, Tian WD, Tang W et al. (2005) Culture and characteristics of human dental papilla cells in vitro . Hua Xi Kou Qiang Yi Xue Za Zhi 23, 187. [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK (2001) Feeder‐free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 19, 971. [DOI] [PubMed] [Google Scholar]
- Yan ZB, Tian WD, Liu L et al. (2004) Establishment of a culture system of chick embryo for mouse tooth germ development. Hua Xi Kou Qiang Yi Xue Za Zhi 22, 232. [PubMed] [Google Scholar]


