Abstract
Abstract. Apoptosis and proliferation are intimately coupled. Some cell cycle regulators can influence both cell division and programmed cell death. The linkage of cell cycle and apoptosis has been recognized for c‐Myc, p53, pRb, Ras, PKA, PKC, Bcl‐2, NF‐κB, CDK, cyclins and CKI. This review summarizes the different functions of the proteins presently known to control both apoptosis and cell cycle progression. These proteins can influence apoptosis or proliferation but different variables, including cell type, cellular environment and genetic background, make it difficult to predict the outcome of cell proliferation, cell cycle arrest or cell death. These important decisions of cell proliferation or cell death are likely to be controlled by more than one signal and are necessary to ensure a proper cellular response.
INTRODUCTION
Tissue homeostasis is dependent on the perfect balance between cell proliferation and cell death. The balance between positive and negative signals determines the decision between life or death. An imbalance can result in diseases linked with unwanted apoptosis or unwanted cell growth. A direct link between cell cycle and apoptosis may be supposed from the fact that a number of similar morphological features exist between mitosis and apoptosis. These include substrate detachment, cell rounding, cell shrinkage and chromatin condensation. (Of course important and determining differences exist, e.g. at the level of DNA, which is fragmented in apoptotic cells while it is segregated during mitosis.) Different components common to apoptosis and the cell cycle have been identified and provide a second rationale for linking cell cycle and apoptosis. Our primary focus here will be on the dual role of some proteins from cell cycle and apoptotic pathways, including c‐Myc, p53, pRb, Ras, protein kinase A (PKA), protein kinase C (PKC), Bcl‐2, NF‐κB, CDK, cyclins and CKI. Following stimulation, these proteins may induce cell proliferation, cell cycle arrest or cell death; the different outcome depends on different variables. The genetic background of the cell is important as is the cellular micro‐environment. Also, the extent of DNA damage and the level of different proteins contribute to the life or death decision‐making process.
Detailed discussion of the proteins is beyond the scope of this review and because detailed reviews are available,(Sionov & Haupt 1999; Adjei 2001; Oster et al. 2002) only some major aspects of their dual function will be described here.
c‐MYC
c‐Myc is a nuclear phosphoprotein that functions as a transcription factor stimulating both cell cycle progression and apoptosis (Facchini & Penn 1998; Penn et al. 1990). Despite the fact that c‐Myc has been widely studied since its discovery, many questions about the function and regulation of c‐Myc still exist. c‐Myc expression is regulated post‐translationally through protein phosphorylation and through interaction with other cellular proteins, primary Max (Alvarez et al. 1991; Blackwood & Eisenman 1991; Lutterbach & Hann 1994).
c‐Myc has a critical role in normal cell cycle progression, especially during transition from G0 to S phase (Spencer & Groudine 1991). c‐Myc is an early response gene, i.e. it responds directly to mitogenic signals to push cells in the G1 phase of the cell cycle (Fig. 1) (Heikkila et al. 1987; de Alboran et al. 2001). c‐Myc expression is maintained throughout the cell cycle and some observations also suggest a role for c‐Myc in G2 (Hann et al. 1985; Mateyak et al. 1997). c‐Myc can exert its effect on cell cycle progression by the transcription of genes with an important role in cell cycle control, i.e. Cdc25A, cyclin D1, cyclin D2, cyclin E, cyclin A, CDK1, CDK2, CDK4 and E2F (Born et al. 1994; Kim et al. 1994; Beier et al. 2000). Another important mechanism of the ability of c‐Myc to promote cell growth is suppression of transcription of growth arrest and the DNA damage inducible gene 45 (Gadd45), Gadd153 and of the CKI genes p15, p21, and p27 (Fig. 1) (Dang 1999; Grandori et al. 2000).
Figure 1.
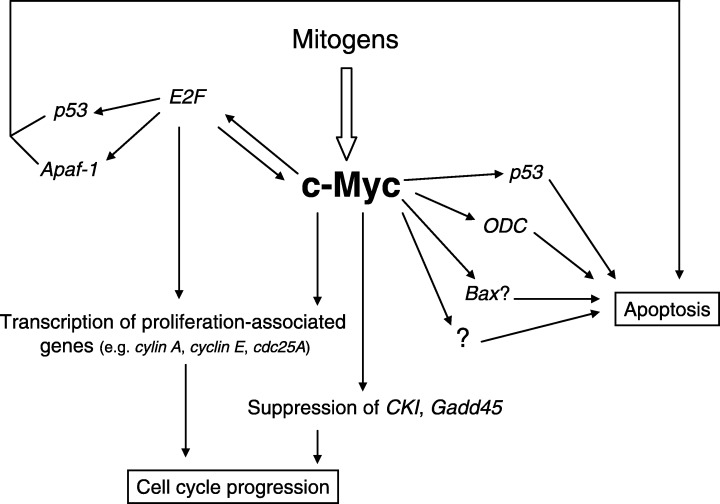
The role of c‐Myc in cell cycle and apoptosis.
Besides its role in cell cycle, c‐Myc also plays a key role in regulating apoptosis. This role was confirmed in different cell types under a wide variety of physiological conditions and both under‐ and overexpression of c‐Myc can lead to cell death (reviewed in Conzen et al. 2000 and Thompson 1998). Until now, the molecular events responsible for c‐Myc‐induced apoptosis are not well understood. Several components important for cell cycle progression, including cyclin A and Cdc25A, have been implicated in apoptotic systems associated with elevated c‐Myc. Cyclin A was elevated in rat‐1 A fibroblasts overexpressing c‐Myc and undergoing apoptosis (Hoang et al. 1994). Cdc25A is a well‐established transcriptional target of c‐Myc and can induce apoptosis in serum‐deprived fibroblasts, as does c‐Myc (Galaktionov et al. 1996). However, not all the Cdc25A substrates are yet known and its relationship with molecules participating in apoptosis remains to be elucidated (Zornig & Evan 1996; Thompson 1998). Another target for transcriptional stimulation by c‐Myc is ornithine decarboxylase (ODC) which can cause apoptosis when overexpressed (Packham & Cleveland 1994; Packham & Cleveland 1995). c‐Myc‐induced apoptosis may involve p53‐dependent and ‐independent pathways. c‐Myc transactivates the p53 gene promotor and increases the half life of p53 (Reisman et al. 1993; Hermeking & Eick 1994). However, there does not seem to be a universal requirement for p53 in c‐Myc‐mediated apoptosis (Hsu et al. 1995; Sakamuro et al. 1995). c‐Myc‐induced apoptosis has been shown to correlate with Fas ligand and Fas receptor expression (Wang et al. 1998). c‐Myc‐induced apoptosis also seems to be inhibited by Bcl‐2 and Mcl‐1 (Bissonnette et al. 1992; Fanidi et al. 1992; Reynolds et al. 1994). c‐Myc‐induced cytochrome c release involves functionally active Bax and preliminary evidence suggests that c‐Myc regulates the transcription of this pro‐apoptotic molecule (Duelli & Lazebnik 2000; Mitchell et al. 2000; Soucie et al. 2001).
A ‘dual signal’ model has been postulated were the ability of c‐Myc to drive apoptosis is distinct from its ability to drive cell division (Pucci et al. 2000; Oster et al. 2002). This model is supported by the observation that different subregions of the c‐Myc N‐terminal domain can control distinct biological functions, including apoptosis (Chang et al. 2000; Conzen et al. 2000; Nesbit et al. 2000). However, this issue cannot be directly addressed until c‐Myc target genes essential for apoptosis have been clearly identified. The factors that determine the decision of inducing either cell division or cell death needs to be further addressed. In addition, c‐Myc expression is tightly linked to the extracellular milieu and the function of c‐Myc is also probably influenced by the extracellular environment (Oster et al. 2002).
p53, pRB and E2F
The regulation and the role of the tumour suppressor proteins p53 and pRb and of the transcription factor E2F have been discussed in the previous review. p53 is widely recognized as a protein functioning during the cell cycle (i.e. an inducer of cell cycle arrest) and apoptosis (Levine 1997; Sionov & Haupt 1999). p53 regulates these processes by transactivating genes involved in different cellular functions, but p53 also activates transcription‐independent mechanisms of apoptosis (Haupt et al. 1995; Agarwal et al. 1998). pRb inhibits cell cycle progression by interacting with transcription factors such as E2F; when pRb becomes phosphorylated, E2F is released and stimulates proliferation. Besides cell cycle inhibition through E2F suppression, pRb has also been shown to suppress apoptosis. For instance, Rb‐deficient embryos show defects in fetal liver haematopoiesis, neurogenesis and lens development, and extensive apoptosis was observed in these tissues (Morgenbesser et al. 1994; Macleod et al. 1996). The mechanisms by which pRb/E2F influences apoptosis remain unknown. E2F has been shown to induce the expression of the pro‐apoptotic factor Apaf‐1 and evidence suggests a role for E2F in apoptosis following DNA damage (Blattner et al. 1999; Moroni et al. 2001). E2F cannot induce apoptosis when pRb is co‐expressed and pRb possibly has an anti‐apoptotic effect through the inhibition of E2F (Fan et al. 1996; Pucci et al. 2000).
p53 and pRb/E2F may be directly linked in cell proliferation and apoptosis. Activated p53 causes a G1 arrest by inducing p21, followed by an inhibition of cyclin/CDK. In these conditions, pRB is not phosphorylated and cells do not progress through the cell cycle. In contrast, free E2F directly induces p53 transcription, thus connecting the pRb/E2F pathway to p53‐dependent apoptosis (Hiebert et al. 1995). Each of both tumour suppressors (p53 and pRb) may thus be able to compensate for the loss of the other (King & Cidlowski 1998).
RAS
Ras is a membrane‐localized G protein. By activating the mitogen‐activated protein kinase (MAPK) signalling cascade and the phosphatidyl inositol 3‐kinase (PI3K) pathway, it has a role in cell proliferation as well as in inhibition and promotion of apoptosis (Adjei 2001). Upon activation by the MAPK kinase kinase (MAPKKK) Raf and the MAPK kinase (MAPKK) MEK (MAP/ERK kinase), the MAPK ERK (extracellular signal‐regulated kinase) translocates to the nucleus, where it phosphorylates transcription factors, resulting in expression of genes involved in cell cycle progression (e.g. cyclin D1) (Fig. 2) (Avruch et al. 1994; Hagemann & Rapp 1999). By phosphorylating and inactivating Bad, activated Raf, MEK and ERK contribute to the inhibition of apoptosis. Activation of PI3K by Ras also inhibits apoptosis by activating protein kinase B (PKB) (also known as Akt), which also results in phosphorylation and inactivation of Bad (Kauffmann‐Zeh et al. 1997). PKB also phosphorylates and inactivates caspase‐9 (Cardone et al. 1998). However, PKB can also affect cell survival by exerting its effect on the transcription factor NF‐κB via phosphorylation of the NF‐κB regulator IκB (Romashkova & Makarov 1999). The anti‐apoptotic effects of Ras can also be mediated by up‐regulation of Bcl‐2 and other anti‐apoptotic members of the Bcl‐2 family (Kinoshita et al. 1995).
Figure 2.
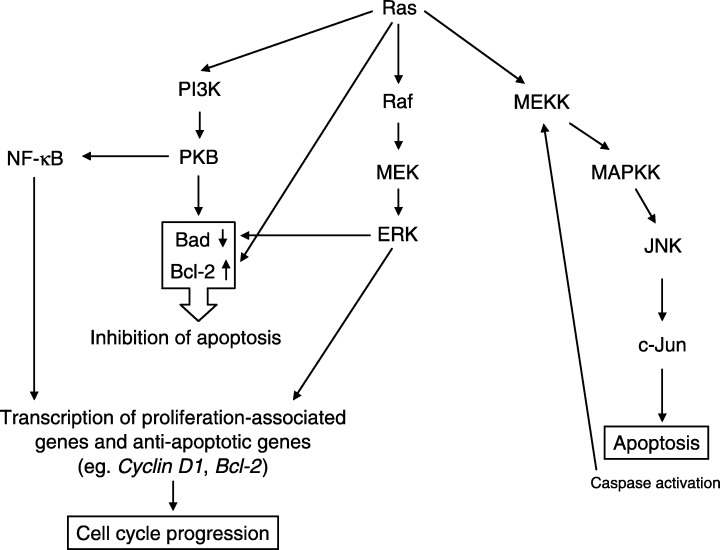
The role of Ras in cell cycle and apoptosis.
Ras‐mediated apoptosis is promoted by the c‐Jun N‐terminal protein kinase (JNK) pathway and can be brought about through the death receptor pathway or by cellular stress e.g. ultraviolet light or osmotic shock (Franklin & McCubrey 2000). In the JNK pathway, Ras activates the MAPKKK MEKK which activates different MAPKK to phosphorylate the MAPK JNK (Fig. 2). Substantial evidence has implicated JNK as an essential component of the apoptotic cascade (Tournier et al. 2000). All the elements are still not clearly defined, but active JNK phosphorylates the transcription factor c‐Jun, which is crucial for the induction of apoptosis (Leppa & Bohmann 1999). JNK activation following growth factor withdrawal results in the up‐regulation of FasL expression and apoptosis (Le Niculescu et al. 1999). MEKK can be cleaved and activated by caspases which results in JNK activation and potentiation of apoptosis (Cardone et al. 1997; Widmann et al. 1997). Under certain circumstances, JNK signalling can also promote cell survival; for instance activated c‐Jun has been suggested to have a protective role in DNA‐damage induced apoptosis in human tumour cells (Potapova et al. 2001). However, the underlying mechanisms for this effect is not yet understood (Leppa & Bohmann 1999).
PKA
PKA is a serine/threonine kinase that is activated by cyclic adenosine monophosphate (cAMP). Increased cAMP can both inhibit and promote apoptosis (Franklin & McCubrey 2000). cAMP‐induced apoptosis is mediated by inhibition of the Ras/Raf/MEK pathway (Hafner et al. 1994; Marshall 1995). In B cells, activation of PKA caused a reduced expression of the anti‐apoptotic protein Mcl‐1, associated with apoptosis (Myklebust et al. 1999). In contrast, PKA activation can result in the phosphorylation of Bad – at the same site as that induced by Raf and MEK – and is associated with the anti‐apoptotic effects of PKA (Harada et al. 1999). Effects of PKA on apoptosis are likely to be largely dependent on the cell type and the mechanisms by which apoptosis is induced (Franklin & McCubrey 2000).
BCL‐2
As discussed earlier, Bcl‐2 mainly has an anti‐apoptotic function, but this function can be lost by multi‐site phosphorylation (Haldar et al. 1995). The regulation of the function of Bcl‐2 mainly involves interactions with other proteins of the Bcl‐2 protein family, but phosphorylation may also be a crucial event in the regulation of its function (Haldar et al. 1995; Yamamoto et al. 1999). Several signal transduction pathways can be involved in Bcl‐2 phosphorylation. Bcl‐2 is phosphorylated on serine/threonine residues and the Bcl‐2 kinase(s) is/are serine/threonine kinase(s). CDK1 is a candidate Bcl‐2 kinase and has been shown to phosphorylate Bcl‐2 (Furukawa et al. 2000). However, other studies demonstrated that CDK1 did not phosphorylate Bcl‐2 (Scatena et al. 1998; Yamamoto et al. 1999). JNK was repeatedly indicated as a potential Bcl‐2 kinase (Blagosklonny 2001). It has been reported to phosphorylate Bcl‐2 at four serine/threonine sites (Maundrell et al. 1997). However, it has been suggested that several kinases may be involved in the phosphorylation of Bcl‐2 (Blagosklonny 2001).
Importantly, very high levels of Bcl‐2 can promote cell death (Shinoura et al. 1999). Bcl‐2 can also modulate the cell cycle in a way that is different from the inhibitory effect on apoptosis (Linette et al. 1996). Bcl‐2 gene expression can result in an increase of 30–60% in the length of G1 phase and under suboptimal conditions, Bcl‐2 promotes exit into quiescence and retards re‐entry into the cell cycle (Mazel et al. 1996; Adams & Cory 1998).
NF‐κB
The transcription factor NF‐κB up‐regulates several survival factors, however, it has also been associated with anti‐apoptotic activities (Baichwal & Baeuerle 1997; Hinz et al. 1999). NF‐κB up‐regulates the expression of the anti‐apoptotic Bcl‐2 family members, Bcl‐2, Bcl‐Xl and Bfl‐1/A1 (Glasgow et al. 2001). Anti‐apoptotic activity has also been shown in conjunction with certain apoptotic stimuli, e.g. TNF‐α, ionizing radiation and daunorubicin (Beg & Baltimore 1996; Wang et al. 1996). Alternatively, there is also evidence for apoptosis‐promoting functions of NF‐κB (Kaltschmidt et al. 2000). For instance, in response to anti‐cancer drugs, NF‐κB directly transactivates FasL, whose gene product contributes to cell death (Kuhnel et al. 2000).
CELL CYCLE REGULATORS
Different cell cycle regulators including Wee1, cdc27, p21, p27, pRb and CDK1 are targets for cleavage by caspases. In this way, cell cycle progression during apoptosis is stopped (Jacotot et al. 2000). Further cleavage of essential proteins will result in cell death, independently of the cell cycle. Several typical cell cycle regulators like CDK, cyclins and CKI affect apoptotic signalling, however, no clear‐cut pro‐ and anti‐apoptotic effects can be described. Little is known about the interaction of cell cycle regulatory proteins with c‐Myc and with apoptotic regulators like Bcl‐2. Neither is it known whether or how the cell cycle regulators influence mitochondrial pore opening. It will be important to answer these questions, in order to establish the link between cell cycle control and apoptosis.
CDK and cyclins
The role of CDK and cyclins in cell proliferation is widely known and was extensively discussed in the previous review. Contradictory results exist about the role of CDK in apoptosis. Some studies reported a pro‐apoptotic activity for CDK; they showed requirement of activated CDK during apoptosis of thymocytes (Gil‐Gomez et al. 1998; Hakem et al. 1999). Some apoptosis‐inducing agents (staurosporine, caffeine) can cause induction of CDK1 and CDK2 activity prior to cell death (Meikrantz et al. 1994). Dominant negative mutants of CDK1, CDK2 and CDK3 suppress apoptosis, induced by staurosporine and TNF‐α (Meikrantz & Schlegel 1996). CDK seems to be required for neuronal cell death (Rubin et al. 1994). Inhibition of CDK by flavopiridol and olomoucine protected post‐mitotic non‐dividing PC12 neuronal cells from apoptotic cell death (Park et al. 1996). Inhibition of CDK2 has also been shown to protect thymocytes from apoptosis, mitochondrial changes and caspase activation (Hakem et al. 1999). Our own data, in addition to those of others, show induction of apoptosis in haematopoietic cells in association with CDK inhibition (Arguello et al. 1998; Byrd et al. 1998; 2002a, 2002b). Cyclin D overexpression is associated with apoptosis, although this may depend on concomitant signals of cell cycle arrest (e.g. serum starvation) and cell proliferation (e.g. cyclin synthesis). Taken together, induction of apoptosis depends on the cellular context: conflicting signals for cell proliferation and cell cycle arrest may result in cell death (Kasten & Giordano 1998).
CKI
CDK inhibitors have been suggested to be indirectly involved in apoptosis through regulation of CDK. Improper regulation of CDK can send conflicting signals for cell division and cell cycle arrest. p21 is synthesized during the p53‐dependent G1 cell cycle arrest, where it can have anti or pro‐apoptotic properties. For example, overexpression of p21 inhibits radiation‐induced apoptosis in human colorectal carcinoma cells, while an inducible expression of p21 sensitizes EJ tumour cells to mitomycin C‐induced apoptosis (Lu et al. 1998; Fang et al. 1999). p27 may also have both pro‐ and anti‐apoptotic effects (Wang et al. 1997; Hiromura et al. 1999; Lloyd et al. 1999).
CONCLUSION
Several genes are common to cell cycle regulation and to apoptosis. The fate of cells is likely to be determined by their interplay. When cells are subjected to adverse (growth) conditions, complex signal transduction networks are initiated. The information received is processed and sent to subcellular organelles. For example, p53 is one protein that plays a key role in the decision to either arrest the cell cycle followed by DNA repair, or to commit cell death. The specific pathway chosen depends upon a variety of factors such as the extent of DNA damage, the presence of functional p21 and its cross‐talk with pRb and the genetic background of the cell. The important decisions of cell death or cell proliferation are likely to be controlled by more than just one signal; most likely this is a mechanism that ensures a proper cellular response.
The current understanding of the functions of cell cycle regulators in apoptosis has progressed considerably. However, many questions remain to be answered.
One can imagine catastrophic consequences for the cell, when key players in cell cycle regulation and/or apoptosis are not co‐ordinated. The knowledge of the links between cell cycle and apoptosis should be of help in understanding pathological conditions, in addition to identifying new therapeutic strategies.
REFERENCES
- Adams JM, Cory S (1998) The Bcl‐2 protein family: arbiters of cell survival. Science 281, 1322. [DOI] [PubMed] [Google Scholar]
- Adjei AA (2001) Blocking oncogenic Ras signaling for cancer therapy. J. Natl Cancer Inst 93, 1062. [DOI] [PubMed] [Google Scholar]
- Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR (1998) The p53 network. J. Biol. Chem. 273, 1. [DOI] [PubMed] [Google Scholar]
- De Alboran IM, O'Hagan RC, Gartner F, Malynn B, Davidson L, Rickert R., Rajewsky K, Depinho RA, Alt FW (2001) Analysis of C‐MYC function in normal cells via conditional gene‐targeted mutation. Immunity. 14, 45. [DOI] [PubMed] [Google Scholar]
- Alvarez. E, Northwood IC, Gonzalez. FA, Latour DA, Seth A, Abate C, Curran T, Davis RJ (1991) Pro‐Leu‐Ser/Thr‐Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c‐myc and c‐jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J. Biol. Chem. 266, 15277. [PubMed] [Google Scholar]
- Arguello F, Alexander M, Sterry JA, Tudor G, Smith EM, Kalavar NT, Greene J‐FJ, Koss W, Morgan CD, Stinson SF, Siford TJ, Alvord WG, Klabansky RL, Sausville EA (1998) Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity in vivo against human leukemia and lymphoma xenografts. Blood 91, 2482. [PubMed] [Google Scholar]
- Avruch J, Zhang XF, Kyriakis JM (1994) Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem. Sci. 19, 279. [DOI] [PubMed] [Google Scholar]
- Baichwal VR, Baeuerle PA (1997) Activate NF‐kappa B or die? Curr. Biol. 7, R94. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D (1996) An essential role for NF‐kappaB in preventing TNF‐alpha‐induced cell death. Science 274, 782. [DOI] [PubMed] [Google Scholar]
- Beier R, Burgin A, Kiermaier A, Fero M, Karsunky H, Saffrich R, Moroy T, Ansorge W, Roberts J, Eilers M (2000) Induction of cyclin E‐cdk2 kinase activity, E2F‐dependent transcription and cell growth by Myc are genetically separable events. EMBO J. 19, 5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette RP, Echeverri F, Mahboubi A, Green DR (1992) Apoptotic cell death induced by c‐myc is inhibited by bcl‐2. Nature 359, 552. [DOI] [PubMed] [Google Scholar]
- Blackwood EM, Eisenman RN (1991) Max: a helix‐loop‐helix zipper protein that forms a sequence‐specific DNA‐binding complex with Myc. Science 251, 1211. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV (2001) Unwinding the loop of Bcl‐2 phosphorylation. Leukemia 15, 869. [DOI] [PubMed] [Google Scholar]
- Blattner C, Sparks A, Lane D (1999) Transcription factor E2F‐1 is upregulated in response to DNA damage in a manner analogous to that of p53. Mol. Cell Biol. 19, 3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born TL, Frost JA, Schonthal A, Prendergast GC, Feramisco JR (1994) c‐Myc co‐operates with activated Ras to induce the cdc2 promoter. Mol. Cell Biol. 14, 5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Shinn C, Waselenko JK, Fuchs EJ, Lehman TA, Nguyen PL, Flinn IW, Diehl LF, Sausville E, Grever MR (1998) Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase‐3 without evidence of bcl‐2 modulation or dependence on functional p53. Blood 92, 3804. [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase‐9 by phosphorylation. Science 282, 1318. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Salvesen GS, Widmann C, Johnson G, Frisch SM (1997) The regulation of anoikis: MEKK‐1 activation requires cleavage by caspases. Cell 90, 315. [DOI] [PubMed] [Google Scholar]
- Chang DW, Claassen GF, Hann SR, Cole MD (2000) The c‐Myc transactivation domain is a direct modulator of apoptotic versus proliferative signals. Mol. Cell Biol. 20, 4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzen SD, Gottlob K, Kandel ES, Khanduri P, Wagner AJ, O'Leary M, Hay N (2000) Induction of cell cycle progression and acceleration of apoptosis are two separable functions of c‐Myc: transrepression correlates with acceleration of apoptosis. Mol. Cell Biol. 20, 6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV (1999) c‐Myc target genes involved in cell growth, apoptosis, and metabolism. Mol. Cell Biol. 19, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duelli DM, Lazebnik YA (2000) Primary cells suppress oncogene‐dependent apoptosis. Nat. Cell Biol. 2, 859. [DOI] [PubMed] [Google Scholar]
- Facchini LM, Penn LZ (1998) The molecular role of Myc in growth and transformation: recent discoveries lead to new insights. FASEB J. 12, 633. [PubMed] [Google Scholar]
- Fan G, Ma X, Kren BT, Steer CJ (1996) The retinoblastoma gene product inhibits TGF‐beta1 induced apoptosis in primary rat hepatocytes and human HuH‐7 hepatoma cells. Oncogene 12, 1909. [PubMed] [Google Scholar]
- Fang L, Igarashi M, Leung J, Sugrue MM, Lee SW, Aaronson SA (1999) p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene 18, 2789. [DOI] [PubMed] [Google Scholar]
- Fanidi A, Harrington EA, Evan GI (1992) Cooperative interaction between c‐myc and bcl‐2 proto‐oncogenes. Nature 359, 554. [DOI] [PubMed] [Google Scholar]
- Franklin RA, McCubrey JA (2000) Kinases: positive and negative regulators of apoptosis. Leukemia 14, 2019. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Iwase S, Kikuchi J, Terui Y, Nakamura M, Yamada H, Kano Y, Matsuda M (2000) Phosphorylation of Bcl‐2 protein by CDC2 kinase during G2/M phases and its role in cell cycle regulation. J. Biol. Chem. 275, 21661. [DOI] [PubMed] [Google Scholar]
- Galaktionov K, Chen X, Beach D (1996) Cdc25 cell‐cycle phosphatase as a target of c‐myc. Nature 382, 511. [DOI] [PubMed] [Google Scholar]
- Gil‐Gomez G, Berns A, Brady HJ (1998) A link between cell cycle and cell death: Bax and Bcl‐2 modulate Cdk2 activation during thymocyte apoptosis. EMBO J. 17, 7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow JN, Qiu J, Rassin D, Grafe M, Wood T, Perez‐Pol JR (2001) Transcriptional regulation of the Bcl‐X gene by NF‐kappaB is an element of hypoxic responses in the rat brain. Neurochem. Res. 26, 647. [DOI] [PubMed] [Google Scholar]
- Grandori C, Cowley SM, James LP, Eisenman RN (2000) The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu. Rev. Cell Dev. Biol. 16, 653. [DOI] [PubMed] [Google Scholar]
- Hafner S, Adler HS, Mischak H, Janosch P, Heidecker G, Wolfman A, Pippig S, Lohse M, Ueffing M, Kolch W (1994) Mechanism of inhibition of Raf‐1 by protein kinase A. Mol. Cell Biol. 14, 6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann C, Rapp UR (1999) Isotype‐specific functions of Raf kinases. Exp. Cell Res. 253, 34. [DOI] [PubMed] [Google Scholar]
- Hakem A, Sasaki T, Kozieradzki I, Penninger JM (1999) The cyclin‐dependent kinase Cdk2 regulates thymocyte apoptosis. J. Exp. Med. 189, 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar S, Jena N, Croce CM (1995) Inactivation of Bcl‐2 by phosphorylation. Proc. Natl Acad. Sci. USA 92, 4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann SR, Thompson CB, Eisenman RN (1985) c‐myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. Nature 314, 366. [DOI] [PubMed] [Google Scholar]
- Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, Scott JD, Korsmeyer SJ (1999) Phosphorylation and inactivation of BAD by mitochondria‐anchored protein kinase A. Mol. Cell 3, 413. [DOI] [PubMed] [Google Scholar]
- Haupt Y, Rowan S, Shaulian E, Vousden KH, Oren M (1995) Induction of apoptosis in HeLa cells by trans‐activation‐deficient p53. Genes Dev. 9, 2170. [DOI] [PubMed] [Google Scholar]
- Heikkila R., Schwab G, Wickstrom E, Loke SL, Pluznik DH, Watt R, Neckers LM (1987) A c‐myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. Nature 328, 445. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Eick D (1994) Mediation of c‐Myc‐induced apoptosis by p53. Science 265, 2091. [DOI] [PubMed] [Google Scholar]
- Hiebert SW, Packham G, Strom DK, Haffner R, Oren M, Zambetti G, Cleveland JL (1995) E2F‐1: DP‐1 induces p53 and overrides survival factors to trigger apoptosis. Mol. Cell Biol. 15, 6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M (1999) NF‐kappaB function in growth control: regulation of cyclin D1 expression and G0/G1‐to‐S‐phase transition. Mol. Cell Biol. 19, 2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromura K, Pippin JW, Fero ML, Roberts JM, Shankland SJ (1999) Modulation of apoptosis by the cyclin‐dependent kinase inhibitor p27 (Kip1). J. Clin. Invest 103, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang AT, Cohen KJ, Barrett JF, Bergstrom DA, Dang CV (1994) Participation of cyclin A in Myc‐induced apoptosis. Proc. Natl Acad. Sci. USA 91, 6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu B, Marin MC, El Naggar AK, Stephens LC, Brisbay S, McDonnell TJ (1995) Evidence that c‐myc mediated apoptosis does not require wild‐type p53 during lymphomagenesis. Oncogene 11, 175. [PubMed] [Google Scholar]
- Jacotot E, Ferri KF, Kroemer G (2000) Apoptosis and cell cycle: distinct checkpoints with overlapping upstream control. Pathol. Biol. 48, 271. [PubMed] [Google Scholar]
- Kaltschmidt B, Kaltschmidt C, Hofmann TG, Hehner SP, Droge W, Schmitz. ML (2000) The pro‐ or anti‐apoptotic function of NF‐kappaB is determined by the nature of the apoptotic stimulus. Eur. J. Biochem. 267, 3828. [DOI] [PubMed] [Google Scholar]
- Kasten MM, Giordano A (1998) pRb and the cdks in apoptosis and the cell cycle. Cell Death Differ. 5, 132. [DOI] [PubMed] [Google Scholar]
- Kauffmann‐Zeh A, Rodriguez‐Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G (1997) Suppression of c‐Myc‐induced apoptosis by Ras signaling through PI(3)K and PKB. Nature 385, 544. [DOI] [PubMed] [Google Scholar]
- Kim YH, Buchholz. MA, Chrest FJ, Nordin AA (1994) Up‐regulation of c‐myc induces the gene expression of the murine homologues of p34cdc2 and cyclin‐dependent kinase‐2 in T lymphocytes. J. Immunol. 152, 4328. [PubMed] [Google Scholar]
- King KL, Cidlowski JA (1998) Cell cycle regulation and apoptosis. Annu. Rev. Physiol. 60, 601. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Yokota T, Arai K, Miyajima A (1995) Regulation of Bcl‐2 expression by oncogenic Ras protein in hematopoietic cells. Oncogene 10, 2207. [PubMed] [Google Scholar]
- Kuhnel F, Zender L, Paul Y, Tietze MK, Trautwein C, Manns M, Kubicka S (2000) NFkappaB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J. Biol. Chem. 275, 6421. [DOI] [PubMed] [Google Scholar]
- Le Niculescu H, Bonfoco E, Kasuya Y, Claret FX, Green DR, Karin M (1999) Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol. Cell Biol. 19, 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppa S, Bohmann D (1999) Diverse functions of JNK signaling and c‐Jun in stress response and apoptosis. Oncogene 18, 6158. [DOI] [PubMed] [Google Scholar]
- Levine AJ (1997) p53, the cellular gatekeeper for growth and division. Cell 88, 323. [DOI] [PubMed] [Google Scholar]
- Linette GP, Li Y, Roth K, Korsmeyer SJ (1996) Cross talk between cell death and cell cycle progression: Bcl‐2 regulates NFAT‐mediated activation. Proc. Natl Acad. Sci. USA 93, 9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW (1999) p27kip1: a multifunctional cyclin‐dependent kinase inhibitor with prognostic significance in human cancers. Am. J. Pathol. 154, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Yamagishi N, Yagi T, Takebe H (1998) Mutated p21 (WAF1/CIP1/SDI1) lacking CDK‐inhibitory activity fails to prevent apoptosis in human colorectal carcinoma cells. Oncogene 16, 705. [DOI] [PubMed] [Google Scholar]
- Lutterbach B, Hann SR (1994) Hierarchical phosphorylation at N‐terminal transformation‐sensitive sites in c‐Myc protein is regulated by mitogens and in mitosis. Mol. Cell Biol. 14, 5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod KF, Hu Y, Jacks T (1996) Loss of Rb activates both p53‐dependent and independent cell death pathways in the developing mouse nervous system. EMBO J. 15, 6178. [PMC free article] [PubMed] [Google Scholar]
- Marshall M (1995) Interactions between Ras and Raf: key regulatory proteins in cellular transformation. Mol. Reprod. Dev. 42, 493. [DOI] [PubMed] [Google Scholar]
- Mateyak MK, Obaya AJ, Adachi S, Sedivy JM (1997) Phenotypes of c‐Myc‐deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 8, 1039. [PubMed] [Google Scholar]
- Maundrell K, Antonsson B, Magnenat E, Camps M, Muda M, Chabert C, Gillieron C, Boschert U, Vial‐Knecht E, Martinou JC, Arkinstall S (1997) Bcl‐2 undergoes phosphorylation by c‐Jun N‐terminal kinase/stress‐activated protein kinases in the presence of the constitutively active GTP‐binding protein Rac1. J. Biol. Chem. 272, 25238. [DOI] [PubMed] [Google Scholar]
- Mazel S, Burtrum D, Petrie HT (1996) Regulation of cell division cycle progression by bcl‐2 expression: a potential mechanism for inhibition of programmed cell death. J. Exp. Med. 183, 2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikrantz W, Gisselbrecht S, Tam SW, Schlegel R. (1994) Activation of cyclin A‐dependent protein kinases during apoptosis. Proc. Natl Acad. Sci. USA 91, 3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikrantz W, Schlegel R (1996) Suppression of apoptosis by dominant negative mutants of cyclin‐dependent protein kinases. J. Biol. Chem. 271, 10205. [DOI] [PubMed] [Google Scholar]
- Mitchell KO, Ricci MS, Miyashita T, Dicker DT, Jin Z, Reed JC, El Deiry WS (2000) Bax is a transcriptional target and mediator of c‐myc‐induced apoptosis. Cancer Res. 60, 6318. [PubMed] [Google Scholar]
- Morgenbesser SD, Williams BO, Jacks T, Depinho RA (1994) p53‐dependent apoptosis produced by Rb‐deficiency in the developing mouse lens. Nature 371, 72. [DOI] [PubMed] [Google Scholar]
- Moroni MC, Hickman ES, Denchi EL, Caprara G, Colli E, Cecconi F, Muller H, Helin K (2001) Apaf‐1 is a transcriptional target for E2F and p53. Nat. Cell Biol. 3, 552. [DOI] [PubMed] [Google Scholar]
- Myklebust JH, Josefsen D, Blomhoff HK, Levy FO, Naderi S, Reed JC, Smeland EB (1999) Activation of the cAMP signaling pathway increases apoptosis in human B‐precursor cells and is associated with downregulation of Mcl‐1 expression. J. Cell Physiol. 180, 71. [DOI] [PubMed] [Google Scholar]
- Nesbit CE, Tersak JM, Grove LE, Drzal A, Choi H, Prochownik EV (2000) Genetic dissection of c‐myc apoptotic pathways. Oncogene 19, 3200. [DOI] [PubMed] [Google Scholar]
- Oster SK, Ho CSW, Soucie EL, Penn LZ (2002) The myc oncogene: Marvelously Complex. Adv. Cancer Res. 84, 81. [DOI] [PubMed] [Google Scholar]
- Packham G, Cleveland JL (1994) Ornithine decarboxylase is a mediator of c‐Myc‐induced apoptosis. Mol. Cell Biol. 14, 5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packham G, Cleveland JL (1995) The role of ornithine decarboxylase in c‐Myc‐induced apoptosis. Curr. Top. Microbiol. Immunol. 194, 283. [DOI] [PubMed] [Google Scholar]
- Park DS, Farinelli SE, Greene LA (1996) Inhibitors of cyclin‐dependent kinases promote survival of post‐mitotic neuronally differentiated PC12 cells and sympathetic neurons. J. Biol. Chem. 271, 8161. [DOI] [PubMed] [Google Scholar]
- Penn LJ, Laufer EM, Land H (1990) C‐MYC: evidence for multiple regulatory functions. Semin. Cancer Biol. 1, 69. [PubMed] [Google Scholar]
- Potapova O, Basu S, Mercola D, Holbrook NJ (2001) Protective role for c‐Jun in the cellular response to DNA damage. J. Biol. Chem. 276, 28546. [DOI] [PubMed] [Google Scholar]
- Pucci B, Kasten M, Giordano A (2000) Cell cycle and apoptosis. Neoplasia 2, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D, Elkind NB, Roy B, Beamon J, Rotter V (1993) c‐Myc trans‐activates the p53 promoter through a required downstream CACGTG motif. Cell Growth Differ. 4, 57. [PubMed] [Google Scholar]
- Reynolds JE, Yang T, Qian L, Jenkinson JD, Zhou P, Eastman A, Craig RW (1994) Mcl‐1, a member of the Bcl‐2 family, delays apoptosis induced by c‐Myc overexpression in Chinese hamster ovary cells. Cancer Res. 54, 6348. [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS (1999) NF‐kappaB is a target of AKT in anti‐apoptotic PDGF signaling. Nature 401, 86. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Gatchalian CL, Rimon G, Brooks SF (1994) The molecular mechanisms of neuronal apoptosis. Curr. Opin. Neurobiol. 4, 696. [DOI] [PubMed] [Google Scholar]
- Sakamuro D, Eviner V, Elliott KJ, Showe L, White E, Prendergast GC (1995) c‐Myc induces apoptosis in epithelial cells by both p53‐dependent and p53‐independent mechanisms. Oncogene 11, 2411. [PubMed] [Google Scholar]
- Scatena CD, Stewart ZA, Mays D, Tang LJ, Keefer CJ, Leach SD, Pietenpol JA (1998) Mitotic phosphorylation of Bcl‐2 during normal cell cycle progression and Taxol‐induced growth arrest. J. Biol. Chem. 273, 30777. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Yoshida Y, Nishimura M, Muramatsu Y, Asai A, Kirino T, Hamada H (1999) Expression level of Bcl‐2 determines anti‐ or proapoptotic function. Cancer Res. 59, 4119. [PubMed] [Google Scholar]
- Sionov RV, Haupt Y (1999) The cellular response to p53: the decision between life and death. Oncogene 18, 6145. [DOI] [PubMed] [Google Scholar]
- Soucie EL, Annis MG, Sedivy J, Filmus J, Leber B, Andrews DW, Penn LZ (2001) Myc potentiates apoptosis by stimulating Bax activity at the mitochondria. Mol. Cell Biol. 21, 4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CA, Groudine M (1991) Control of c‐myc regulation in normal and neoplastic cells. Adv. Cancer Res. 56, 1. [DOI] [PubMed] [Google Scholar]
- Thompson EB (1998) The many roles of c‐Myc in apoptosis. Annu. Rev. Physiol. 60, 575. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar‐Sagi D, Jones SN, Flavell RA, Davis RJ (2000) Requirement of JNK for stress‐induced activation of the cytochrome c‐mediated death pathway. Science 288, 870. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Strnad M, Krystof V, Havlicek L, Van der Aa A, Lenjou M, Nijs G, Rodrigus I, Stockman B, Van Onckelen H, Van Bockstaele D, Berneman ZN (2002a) Antiproliferative effect on plant cytokinin analogues with an inhibitory activity on cyclin‐dependent kinases. Leukemia 16, 299. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Strnad M, Havlicek L, Van Onckelen H, Lenjou M, Nijs G, Van Bockstaele D, Berneman ZN (2002b) Plant cytokinin analogues with inhibitory activity on cyclin dependent kinases (CDK) exert their antiproliferative effect through induction of apoptosis initiated by the mitochondrial pathway: determination by a multiparametric flow cytometric analysis. Exp. Hematol. 30, 1107. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin A‐SJ (1996) TNF‐ and cancer therapy‐induced apoptosis: potentiation by inhibition of NF‐kappaB. Science 274, 784. [DOI] [PubMed] [Google Scholar]
- Wang X, Gorospe M, Huang Y, Holbrook NJ (1997) p27Kip1 overexpression causes apoptotic death of mammalian cells. Oncogene 15, 2991. [DOI] [PubMed] [Google Scholar]
- Wang R, Brunner T, Zhang L, Shi Y (1998) Fungal metabolite FR901228 inhibits c‐Myc and Fas ligand expression. Oncogene 17, 1503. [DOI] [PubMed] [Google Scholar]
- Widmann C, Johnson NL, Gardner AM, Smith RJ, Johnson GL (1997) Potentiation of apoptosis by low dose stress stimuli in cells expressing activated MEK kinase 1. Oncogene 15, 2439. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ichijo H, Korsmeyer SJ (1999) Bcl‐2 is phosphorylated and inactivated by an ASK1/Jun N‐terminal protein kinase pathway normally activated at G2/M. Mol. Cell Biol. 19, 8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornig M, Evan GI (1996) Cell cycle: on target with Myc. Curr. Biol. 6, 1553. [DOI] [PubMed] [Google Scholar]


