Abstract
The effects of myenteric denervation on the cell kinetics of the intestinal epithelium of suckling and weanling rats were investigated. The myenteric plexus of an ileal segment was partially ablated by serosal application of benzalkonium chloride (BAC) in three groups of rats: those that underwent surgery at 13 days and were killed 15 (13/28‐day‐old) or 23 (13/36‐day‐old) days after treatment, and those that were operated at 21 days (21/36‐day‐old) and were killed 15 days after treatment. The extent of denervation was assessed in whole‐mount preparations. The cell bodies of myenteric neurones were stained by NADH‐diaphorase histochemical technique. Cell proliferation was estimated by the mitotic index (MI) and morphometric analysis of villus and crypt lengths using an image analysis system. Thickness of the muscle layers was also assessed by morphometry. Cell migration on the villi was estimated by the position of the leading labelled cell 24 h after tritiated thymidine injection. The number of neurones was reduced by around 80% in rats operated at 13 days, and reduced by 98% in those operated at 21 days. The thickness of the muscle layers was increased in all groups of treated animals. MI was significantly higher 15 days after BAC‐treatment in the 13/28 group. Morphological changes in the intestinal mucosa were observed 15 days after BAC‐treatment, when there was an increase in villus height (13/28 group) and crypt depth (13/28 and 21/36 groups). Cell migration rate was accelerated in the 21/36 group. No differences where found in the 13/36 group. These results show the strong effect of myenteric ablation on cell proliferation and migration in the ileal epithelium in the first 15 days of treatment in suckling and in weanling rats, and the subsequent recovery of intestinal mucosa homeostasis later on.
INTRODUCTION
The epithelium covering the intestinal mucosa undergoes constant renewal; cells are produced in the crypts, migrate up the sides of the villi, and are extruded into the lumen. This is a continuous process that maintains the cell population in an equilibrium, in which the renewal is equal to the extrusion rate.
The processes that control epithelial cell proliferation and migration in intestine are largely unknown. Nutrients, hormones and growth factors are some of the elements that influence the proliferative activity of intestinal crypts and the velocity of cell migration along the crypt–villus axis. It has also been demonstrated that the autonomic nervous system plays a role in the cellular turnover of the intestinal epithelium and this influence can be studied through the elimination of the parasympathetic, sympathetic or myenteric innervation. This ablation is achieved by chemical or surgical methods. However, the results are contradictory. Vagotomy (Silen, Peloso & Jaffe 1966) or elimination of sympathetic neural input (Holle et al. 1989) induced mucosal cell proliferation. In contrast, other studies have reported that sympathectomy (Tutton & Helme 1974; Lachat & Gonçalves 1978; Klein 1979) or parasympathectomy (Musso et al. 1975) inhibited mitotic activity and produced a delay in migration of cells from crypts to the villi (Lachat & Gonçalves 1978). The control of cell proliferation by the myenteric plexus has been demonstrated mainly using BAC serosal application (Zucoloto et al. 1988; See et al. 1990; Holle 1991; Zucoloto et al. 1991; Hadzijahic et al. 1993). This substance is a cationic surfactant that selectively destroys most ganglion cells of the myenteric plexus (Sakata et al. 1979; Fox, Epstein & Bass 1983; Ramalho et al. 1993). After myenteric denervation, significant increases in mitotic rate of the crypt‐epithelial cells (See et al. 1990), villus height and crypt depth were observed (See et al. 1990; Holle 1991; Hadzijahic et al. 1993). The BAC treatment promoted acceleration in cell migration in the villus, estimated by the labelled cell displacement after tritiated thymidine injection (Holle 1991). On the other hand, Zucoloto et al. (1991) showed that proliferation was not altered 15, 30, 45 and 60 days after BAC treatment.
Neural control of cellular proliferation and migration in the intestinal epithelia has been less studied in neonatal animals. This may be explained by the complexity of the gastrointestinal tract during the first month of postnatal development. Besides all other controlling factors, the feeding pattern changes from suckling to weaning. During suckling, cell division in the intestinal crypt occurs at a low rate and migration is one‐quarter of that estimated in 28‐day‐old weaned rats (Koldovski, Sunshine & Kretchmer 1966). The comparison of ileal epithelial cell proliferation in suckling (8‐day‐old) and weaned rats (28‐day‐old) indicates a longer generation cycle time, lower mitotic index and slower migration rate in suckling rats (Klein 1977). By this time, if a mild fasting condition is applied, cell migration is impaired without change in cell proliferation, and that result is not observed in adult rats (Gomes & Alvares 1998). Thus, cell proliferation and migration change during development.
The evaluation of sympathectomy effects on pre‐closure and post‐closure of ileum showed that epithelial cell proliferation, in general, was inhibited, though the mitotic index increases in the post‐closure period, when the absorption of macromolecules ceases (Klein & McKenzie 1980). Furthermore, in sympathectomized rats, there was a delay in epithelial closure (Klein & Torres 1978).
More recently, it was demonstrated that the differentiation of the enteric nervous system (ENS) is not accomplished at birth, but later, during the weaning period (Faussone‐Pellegrini, Matini & Stach 1996; Matini, Mayer & Faussone‐Pellegrini 1997). Immunostaining of neuropeptides such as vasoactive intestinal peptide (VIP), was detected in the myenteric plexus in 14‐day‐old suckling rats, whereas in the submucosal plexus only in 30‐day‐old weaned rats (Matini et al. 1997). Expression of neuronal markers during postnatal development permitted the suggestion of a two step differentiation for the enteric plexuses: a morphological one, achieved during suckling and a functional one, related to the weaning period (Faussone‐Pellegrini et al. 1996).
As the myenteric ablation model has successfully demonstrated the relationship between the ENS and the kinetics of the intestinal epithelial cell population of adult rats, it was our goal to explore the effect of such denervation during the maturation of the intestine, in the first month of post‐natal life. This study investigates cell proliferation and migration in the ileum of suckling and weanling rats submitted to myenteric denervation after BAC treatment.
MATERIALS AND METHODS
Animal treatment
All procedures in this study that involve the use of animals are in accordance with ethical principles and were approved by the Ethical Committee of Animal Experimentation of the Institute of Biomedical Sciences of the University of São Paulo.
Suckling Wistar rats, of both sexes, were anaesthetized at 13 and 21 days with a solution of 2% rompum (Bayer, São Paulo, Brazil) and 5.8% ketalar (Parke‐Davis, São Paulo, Brazil) intraperitoneally (0.12 ml/100 g body wt). Rats were placed on a warm plate at 37°C, a mid‐line abdominal incision was made and a segment of the ileum of 3–4 cm near the ileumcaecal junction was exteriorized from the peritoneal cavity. The segment was treated with serosal application of the cationic surfactant, benzalkonium chloride (BAC) (Sigma, St Louis, MO, USA) in a 0.081% solution, every 5 min over 30 min, or with 0.9% saline in the controls, as described by Fox et al. (1983). After treatment, the ileal segment was thoroughly rinsed with 0.9% saline and returned to the abdominal cavity. The midline incision was closed and the animals were allowed to recover. The 13‐day‐old rats were placed with their mothers after recovery. The animals were maintained on a 12‐h light/dark cycle. All groups were weaned at 21 days of age.
Three groups of rats were studied: those that underwent surgery at 13 days and were killed 15 (13/28‐day‐old) or 23 (13/36‐day‐old) days after treatment and those that were operated at 21 days and were killed 15 days after treatment (21/36‐day‐old).
The samples taken from the ileum were used for neuronal counting, radioautography, cell kinetic and morphometric studies of the epithelium.
Neuronal counting
Each 5 cm segment of intestine was obtained from the animal immediately after death and flushed with Kreb’s solution to clean the lumen. Cotton thread ligatures were placed at the end of one side and the muscle coat was then distended by injecting Kreb’s solution into the lumen, while the other side was tied up to maintain the distention. The segments were stained by a histochemical technique to detect NADH‐diaphorase (Gabella 1987). The development of the reaction was monitored under a dissecting microscope and was stopped when the stain was intense enough (60–90 min) by immersing the intestine in 10% buffered‐formalin. After a fixation period of at least 24 h, the intestine was trimmed in small segments with the aid of a metallic circular sectioner. Under the dissecting microscope the mucosal and submucosal layers were separated from the muscle coat, which was dehydrated, diaphanized and mounted for microscopic examination. The neuronal counting was performed in six rats from each group (three controls and three BAC‐treated). The cell bodies of myenteric neurones stained by NADH‐diaphorase were counted under the light microscope (Nikon), using a Zeiss No. 2 (×8) integrative eyepiece and ×40 objective. Thirty fields of 0.09 mm2 each were quantified per animal. Results are expressed as neurones/mm2.
Radioautography
Before sacrifice, rats were weighed and injected i.p. with tritiated thymidine ([3H]dT) at a dose of 37 kBq/g body wt (specific activity 1.48 MBq/mmol, Amersham, Little Chalfont, UK) at 14.00 h. The animals were killed by ether inhalation, 24 h after injection. The treated segment of the ileum was removed and flushed with saline. The samples were opened laterally on small cards, fixed in Carnoy’s solution for 6 h and embedded in hydroxyethyl methacrylate (LKB‐Technovit 7100; Kulzer, Wehrheim, Germany). Sections of 2µm thickness were obtained. Slides processed for the radioautographic technique were covered with photographic emulsion (Ilford K5), stored in light‐proof boxes at 4°C for 20 days, developed with Kodak D19b, fixed and rinsed in distilled water. The slides were then stained with haematoxylin and eosin.
Estimation of cell migration in the villus
The leading edge of labelled cells (containing five or more grains) from one side of the villus and the total number of villus cells at the same side were determined in control and treated rats for each group. For each rat, analysis was carried out on 20 villi, each showing a continuous epithelium from villus tip to the base of the crypt. Cell migration is reported as the percentage of villus height occupied by labelled cells 24 h after [3H]dT injection. The cell migration rate was calculated from the position of the leading labelled cell in the villus divided by the time elapsed after labelling (Yeh, Yeh & Holt 1991).
Mitotic index (MI)
Another series of rats was used to obtain the MI. Intestinal samples were histologically processed as described above. The MI was obtained in the proliferative compartment that coincided with the whole crypt. For each animal used, approximately 2500 mitotic and interphase epithelial cells were counted in longitudinal sections of crypts showing a lumen, in slides stained with haematoxylin and eosin. The MI was expressed as the percentage of mitotic cells divided by the total number of cells counted.
Morphometric analysis
The crypt depth and villus height were determined in longitudinal sections by the image analysis system (Mini‐MOP; Kontron, Germany) using the same samples processed for the radioautographic technique. The measurements were made in 20 villi and 20 crypts for each animal.
Measurements of muscle thickness were performed in 10 sections from each animal. Results were expressed in micrometres.
Statistical analyses
Before statistical analysis, the indices obtained as percentages were submitted to arcsin transformation. The other data were submitted to logarithmic transformation when the variances were different. All parameters were compared by the Student’s t‐test. The significance level was set at P < 0.05.
RESULTS
Macroscopic and microscopic changes
In BAC‐treated segments, the macroscopic observation showed some degree of adherence of intestinal loops either among themselves or to the abdominal wall. Microscopically an enlargement of the muscle layer was seen in treated rats, but there was no evidence of inflammation in the mucosa. No other gross alterations or differences from the control were seen.
The BAC‐treated animals of 21/36 group presented a significant loss of body weight (Figure 1).
Figure 1.
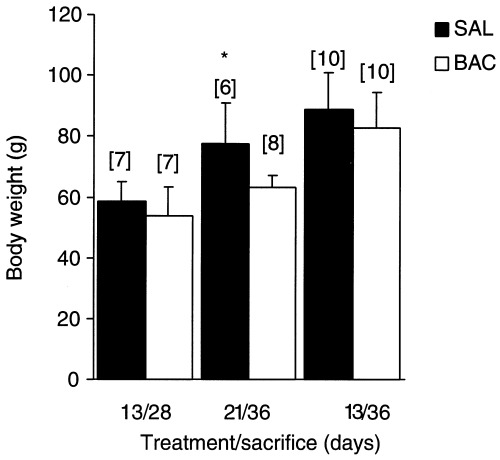
Effect of BAC‐treatment on body weight (g). *P < 0.05 compared to control, using Student's t‐test for paired observations); n = number of animals.
Neuronal quantification
The histochemical method stained selectively the myenteric neurones in the whole‐mount preparations. The other cell types from the intestinal wall were either unstained or very lightly stained. The reaction showed a sharp outline of the neuronal perikarya while their processes remained unstained. The neuronal nuclei were unstained and they were also sharply outlined by the neuronal cytoplasm (Figure 2a).
Figure 2.
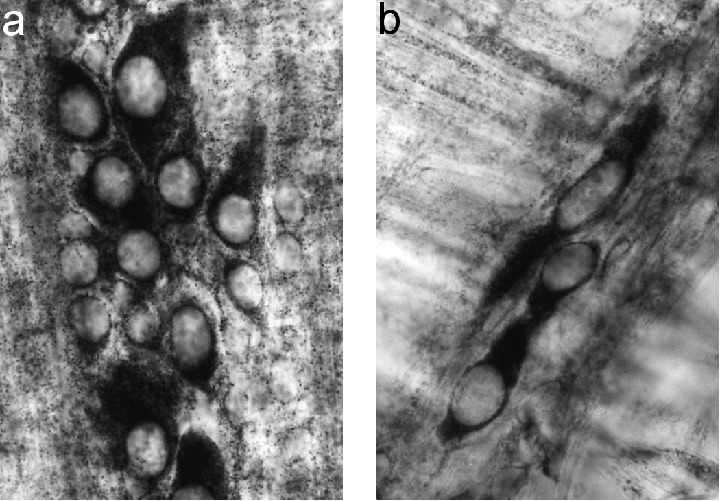
Photomicrographs of whole‐mount preparations of the muscle layer of rat ileum stained by NADH‐diaphorase to detect myenteric neurones.(a) Control rat (13/36‐day‐old), note the sharp outline of the neuronal perikarya and the unstained nuclei (×900) and(b) BAC‐treated rat (13/36 group), an area with few perikarya (×900).
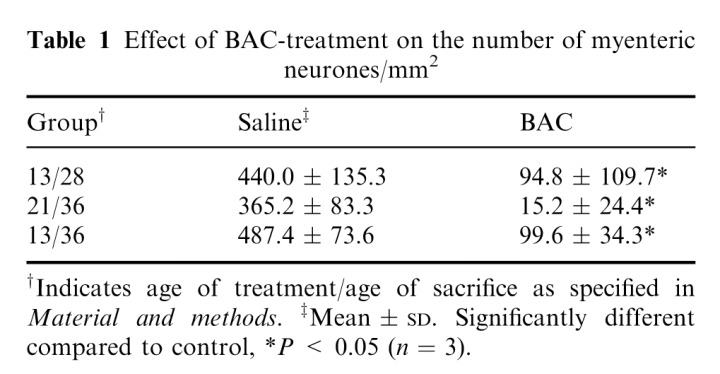
In BAC‐treated animals there were fields completely devoid of stained neurones, while some NADH‐diaphorase positive neurones where present in other areas (Figure 2b). By this technique, it is not possible to determine if there were alterations in the remaining neurones. The decrease of myenteric neurones in BAC‐treated rats of all groups was significant when compared to controls (Table 1).
Table 1.
Effect of BAC‐treatment on the number of myenteric neurones/mm2
Mitotic index
The MI was significantly higher only in denervated animals from 13/28 group (Figure 3).
Figure 3.
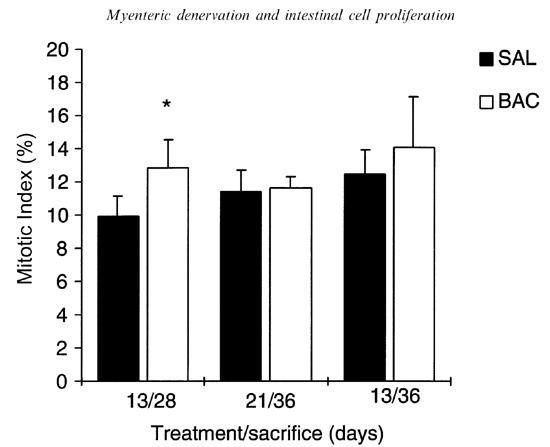
Mitotic index (%) of the ileum in saline and BAC‐treated rats; n = 5; *P < 0.05 compared to control, using Student's t‐test for paired observations.
Morphometric analysis
All BAC‐treated groups showed taller villi and deeper crypts than controls. There was an increase of 54.6% in the villus height in the BAC‐treated ileum of the 13/28 group compared to the control group (Figure 4).
Figure 4.
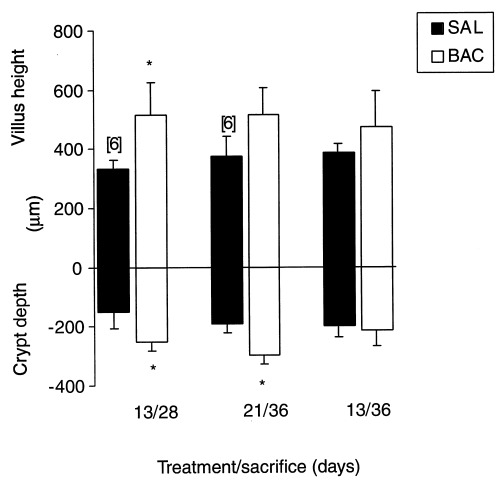
Villus height (µm) and crypt depth (µm) of the ileum in saline and BAC‐treated rats. *P < 0.05 compared to control, using Student's t‐test for paired observations; n = 5 or 6 when indicated.
Crypt depths were significantly higher in BAC‐treated rats from 13/28 and 21/36 groups; they increased 65.35% and 56.3%, respectively (Figures 4 and 5).
Figure 5.
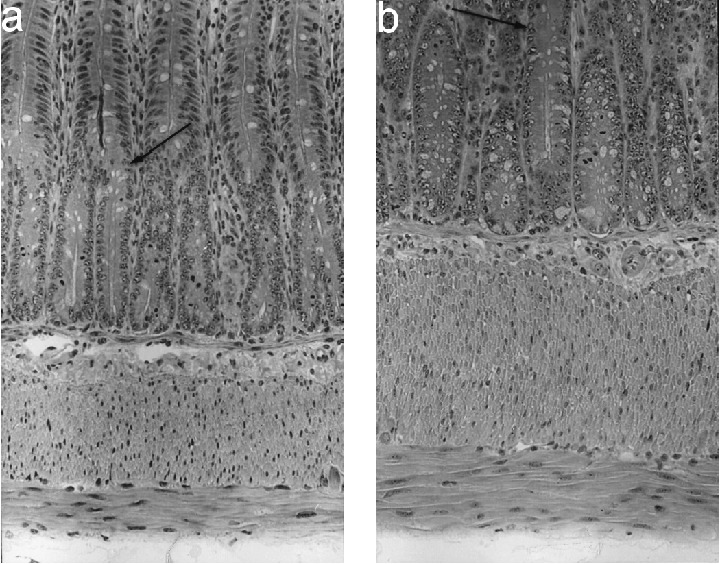
Photomicrographs of longitudinal sections of ileal crypts and muscle layers of (a) control rats (13/28‐day‐old) and (b) BAC‐treated rats (13/28 group). Enlarged crypts and muscle layers in treated rats. Arrows indicate limit between crypt and villi. haematoxylin and eosin, ×150.
The thickness of muscle layers increased significantly in all groups. The thickness was increased by 211.4% in 13/28 group, 108.6% in 21/36 group and 117.1% in 13/36 group. (Figures 5 and 6).
Figure 6.
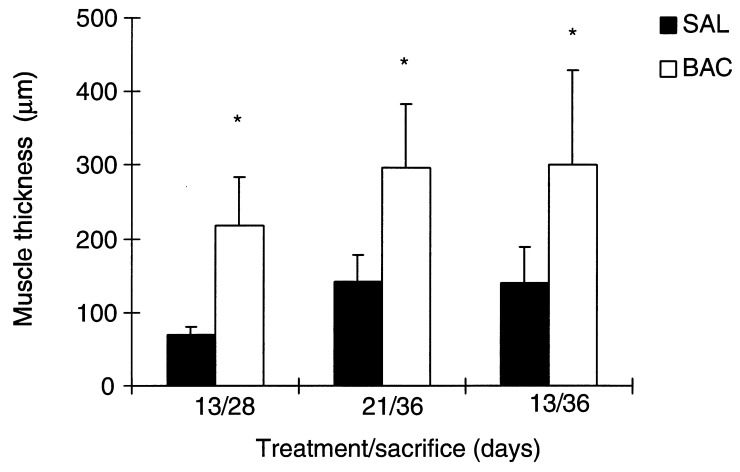
Muscular layer thickness (µm) in the ileum in saline and BAC‐treated rats; n = 5. *P < 0.05 compared to control, using Student's t‐test for paired observations.
Migration in the villus
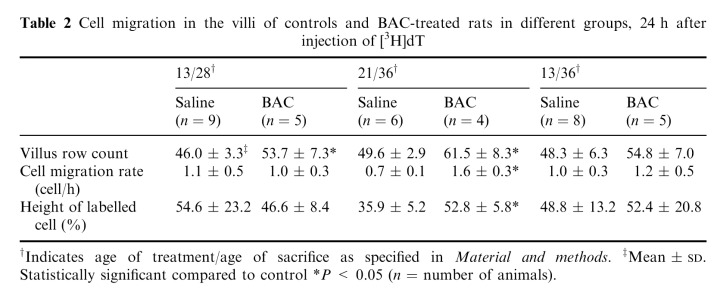
The cell migration rate in the villus, as the number of cells per hour, was significantly increased in the BAC‐treated group (21/36). Cell migration in the villus is shown in Table 2.
Table 2.
Cell migration in the villi of controls and BAC‐treated rats in different groups, 24 h after injection of [3H]dT
DISCUSSION
The results presented here demonstrate that the application of a 0.081% BAC solution to the ileum reduced the number of myenteric neurones 15 and 23 days after treatment in suckling and weaning rats and promoted important alterations in intestinal epithelial kinetics.
The selected ages for the denervation (13 and 21 days) are of utmost importance, regarding the maturation of intestine, the modification of feeding pattern and the correlated changing of the intestinal enzymes (Henning 1981). It is also during this period that ENS completes its maturation (Faussone‐Pellegrini et al. 1996).
The myenteric neurone‐ablation method, by BAC, has proven to be useful in the study of the ENS and intestinal function (Fox & Bass 1986; Dahl et al. 1987; Herman & Bass 1987; See et al. 1988; See et al. 1990; Holle 1991; Hadzijahic et al. 1993; Zucoloto et al. 1997). Serosal application of the cationic surfactant (BAC) on small and large intestine selectively destroys the myenteric neurones. The neuronal damage induced by BAC was attributed to the positive charges on this agent (Sato et al. 1978). Since the membrane of the nervous tissue is more negative than muscle membrane, the nerve fibres would be more susceptible to damage by a cationic agent (Sato et al. 1978). Nevertheless, Fox et al. (1983) obtained a reduction in the number of ganglion cells in the myenteric plexus using anionic surfactants as well. They support the notion that surfactant‐induced enteric neurotoxicity can be attributed to a generalized property of surfactants rather than to a charge specificity. More recently, Parr & Sharkey (1997) studied the role of immune cells in the neuronal loss mediated by BAC and observed increased numbers of macrophages, T and B cells, suggesting that an immune response may actively contribute to the neural tissue destruction, together with other factors.
In the present work, the major neuronal loss was shown in rats operated on at weaning, in which 98% of myenteric neurones were eliminated 15 days after BAC‐treatment. In rats operated on at suckling, the extension of denervation reached 80%, regardless of the treatment period (15 or 23 days). The lesser degree of neuronal loss may be associated with the suckling phase, owing to a late differentiation of enteric neurones (Faussone‐Pellegrini et al. 1996).
The reduction in the neurone number in adult rats is subject to great variation, from 40% to 100% denervation (Sakata et al. 1979; Fox et al. 1983; Zucoloto et al. 1988; Holle 1991; Zucoloto et al. 1991; Hadzijahic et al. 1993; Ramalho et al. 1993; Zucoloto et al. 1997). As the BAC‐treatment was similar in these studies (30 min, with an every 5 min‐application), other factors such as the selected treated segment of intestine, the period of post‐treatment, and the detergent concentration may have contributed to the denervation rate. The age when treatment was performed and consequently the age of sacrifice are also very important, as is shown in the current report.
In the present study, all groups of rats lost weight after being treated with BAC. The young animals seem to be more susceptible to the surgical procedure than adults, that gained weight after BAC treatment (Holle & Forth 1990; Holle 1991). Body weight was significantly smaller in the 21/36 group when compared to controls. The recovery after surgery may have been prevented at weaning, owing to the further stress promoted by the separation of pups from the dam (Gama & Alvares 1998).
The thickening of muscle layers after BAC treatment occurred in the same way as in adult rats (Sakata et al. 1979; Dahl et al. 1987; See et al. 1988; Holle 1991; Zucoloto et al. 1991; Parr & Sharkey 1997).
Chemical denervation of the ileum produced changes in the mucosa, and the magnitude of the effects was different in the three groups studied. The changes were seen in (a) MI; (b) cellular migration in the villus; and (c) morphometry of villi, crypts and smooth muscle layers.
Cell kinetic parameters were markedly affected 15 days after denervation in 13‐day‐old rats. Higher mitotic index, higher crypt depth and villus height were observed when compared to the controls. The cell migration in the villus was the only non‐significant parameter. It is interesting that at this same age of denervation, a longer period of post‐treatment than 23 days, resulted in the lack of modification in cell kinetic parameters, even if the degree of denervation remained unchanged. Whatever the controlling factors were in the first 15 days of treatment, they do not remain 23 days after.
Denervation at 21 days produced almost a total ablation of the myenteric plexus (96%), and the cell kinetics parameters have been equally affected. Crypt depth was also increased which reflects a previous increased MI. Cell migration was faster than in control and the final result points to a strong effect of denervation over cell proliferation also in weaning rats.
In adult rats, See et al. (1990) observed an increase in the mitotic rate of the jejunum 15 days after BAC‐treatment and an increase in mucosal DNA level after 15 and 45 days of treatment, suggesting a persistent effect on proliferation. In the duodenum, jejunum and ileum of adult rats, the labelling index also increased from 7 to 21 days after BAC treatment, and cell migration was also faster in treated segments 21 days later (Holle 1991). The continuous effect of denervation over longer periods in adult rats is a noteworthy difference to suckling or weaning rats, and must be a result of special features at these developmental ages.
The mechanisms regulating proliferation and growth of intestine are unclear. Growth factors are involved in gastrointestinal cell proliferation and migration (Alison & Sarraf 1994), though their role in the present observations is unclear and their connection to the neural system remains to be elucidated.
The neural control may be exerted by a complex interaction between submucosal and myenteric plexuses. High levels of VIP are detected after myenteric ablation (Dahl et al. 1987). In addition, See et al. (1990) have made a correlation between enlarged VIP‐submucosal neurones under myenteric denervation and the growth changes in the intestinal mucosa of adult rats. Another important connection between the enteric nervous plexuses is seen during the suckling period, when differentiation of neurones is still occurring (Pham, Gershon & Rothman 1991; Matini et al. 1997). Pham et al. (1991) followed the differentiation of labelled enteric neuronal precursors and showed that submucosal neurones tended to be formed later than their myenteric counterparts, even after birth. It is suggested that mature myenteric neurones influence the population of submucosal neurones during differentiation, in the suckling period.
In conclusion, the present findings show that there was more destruction of myenteric neurones in weaning rats than in suckling ones. This plexus destruction is followed by enhanced epithelial cell proliferation 15 days after BAC treatment in suckling and weaning rats. The mucosal changes are not seen 1 week later, i.e. 23 days after BAC, when the kinetic parameters tend to return to control values. Therefore, it is confirmed that ENS plays a role in the control of intestinal cell kinetics during the suckling and weaning periods, but in contrast to the adults, this action is not exerted in the long‐term, where it must have been overcome by other controlling factors.
Acknowledgements
The authors would like to thank Dr Patrícia Gama for correcting the English text, and Cruz Alberto Rigonati and José Rosa Gomes for technical assistance. Financial support was provided by CAPES, CNPq and FAPESP.
REFERENCES
- Alison MR, Sarraf CE.(1994). The role of growth factors in gastrointestinal cell proliferation. Cell. Biol. Intl. 18,1. [DOI] [PubMed] [Google Scholar]
- Dahl JL, Bloom DD, Epstein ML, Fox DA, Bass P. (1987). Effect of chemical ablation of myenteric neurons on neurotransmitter levels in the rat jejunum. Gastroenterology 92,338. [DOI] [PubMed] [Google Scholar]
- Faussone‐Pellegrini MS, Matini P, Stach W.(1996). Differentiation of enteric plexuses and interstitial cell of Cajal in the rat gut during pre‐ and postnatal life. Acta Anat. 155,113. [DOI] [PubMed] [Google Scholar]
- Fox DA, Bass P.(1986). Pharmacological characterization of rat jejunal contractility after chronic ablation of the myenteric plexus. J. Pharmacol. Exp. Ther. 238,372. [PubMed] [Google Scholar]
- Fox DA, Epstein ML, Bass P.(1983). Surfactants selectively ablate enteric neurons of the rat jejunum. J. Pharmacol. Exp. Ther. 227,538. [PubMed] [Google Scholar]
- Gabella G.(1987). The number of neurons in the small intestine of mice, guinea‐pigs and sheep. Neuroscience 22,737. [DOI] [PubMed] [Google Scholar]
- Gama P, Alvares EP.(1998). Corticosterone treatment inhibits cell proliferation of the gastric epithelium of suckling rats. J. Gastroenterology 33,32. [DOI] [PubMed] [Google Scholar]
- Gomes JR, Alvares EP.(1998). Cell proliferation and migration in the jejunum of suckling rats submitted to progressive fasting. Braz. J. Med. Biol. Res. 31,281. [DOI] [PubMed] [Google Scholar]
- Hadzijahic N, Renehan WE, Ma CK, Zhang X, Fogel R.(1993). Myenteric plexus destruction alters morphology of rat intestine. Gastroenterology 105,1017. [DOI] [PubMed] [Google Scholar]
- Henning SJ.(1981). Postnatal development: coordination of feeding, digestion, and metabolism. Am. J. Physiol. 241,G199. [DOI] [PubMed] [Google Scholar]
- Herman JR, Bass P.(1987). Temporal changes in mechanical properties of rat jejunal smooth muscle after myenteric plexus ablation. Am. J. Physiol. 253,G745. [DOI] [PubMed] [Google Scholar]
- Holle GE.(1991). Changes in the structure and regeneration mode of the rat small intestinal mucosa following benzalkonium chloride treatment. Gastroenterology 101,1264. [DOI] [PubMed] [Google Scholar]
- Holle GE, Forth W.(1990). Myoelectric activity of small intestine after chemical ablation of myenteric neurons. Am. J. Physiol. 258,G519. [DOI] [PubMed] [Google Scholar]
- Holle GE, Granat T, Reiser SB, Holle F.(1989). Effects of superior mesenteric and coeliac ganglionectomy on the small intestine mucosa in the Hanford mini pig. I. Histological and enzyme‐histochemical study. J. Auton. Nerv Sys. 26,135. [DOI] [PubMed] [Google Scholar]
- Klein RM.(1977). Alteration of cellular proliferation in the ileal epithelium of suckling and weaned rats: the effects of isoproterenol. Cell. Tissue Kinet. 10,353. [DOI] [PubMed] [Google Scholar]
- Klein RM.(1979). Analysis of intestinal cell proliferation after guanethidine induced sympathectomy. II. Percentage labelled mitoses studies. Cell Tissue Kinet. 12,649. [DOI] [PubMed] [Google Scholar]
- Klein RM, Mckenzie JC.(1980). Pattern of crypt cell proliferation in the pre‐ and post‐closure ileum of the neonatal rat: effects of sympathectomy. Cell. Tissue Res. 206,387. [DOI] [PubMed] [Google Scholar]
- Klein RM, Torres JJ.(1978). Analysis of intestinal cell proliferation after guanethidine‐induced sympathectomy. Cell Tissue Res. 195,239. [DOI] [PubMed] [Google Scholar]
- Koldovski O, Sunshine P, Kretchmer N.(1966). Cellular migration of intestinal epithelia in suckling and weaned rats. Nature 212,1389. [DOI] [PubMed] [Google Scholar]
- Lachat JJ, Gonçalves RP.(1978). Influence of autonomic denervation upon the kinetics of the ileal epithelium of the rat. Cell Tissue Res. 192,285. [DOI] [PubMed] [Google Scholar]
- Matini P, Mayer B, Faussone‐Pellegrini MS.(1997). Neurochemical differentiation of rat enteric neurons during pre‐ and postnatal life. Cell Tissue Res. 288,11. [DOI] [PubMed] [Google Scholar]
- Musso F, Lachat JJ, Cruz AR, Gonçalves RP.(1975). Effect of denervation on the mitotic index of the intestinal epithelium of the rat. Cell. Tissue Res. 163,395. [DOI] [PubMed] [Google Scholar]
- Parr EJ, Sharkey KA.(1997). Multiple mechanisms contribute to myenteric plexus ablation induced by benzalkonium chloride in the guinea‐pig ileum. Cell Tissue Res. 289,253. [DOI] [PubMed] [Google Scholar]
- Pham TD, Gershon MD, Rothman TP.(1991). Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J. Comp. Neurol. 314,879. [DOI] [PubMed] [Google Scholar]
- Ramalho FS, Santos GC, Ramalho LNZ, Kajiwara JK, Zucoloto S.(1993). Myenteric neuron number after acute and chronic denervation of the proximal jejunum induced by benzalkonium chloride. Neuroscience Lett 163,74. [DOI] [PubMed] [Google Scholar]
- Sakata K, Kunieda T, Furuta T, Sato A.(1979). Selective destruction of intestinal nervous elements by local application of benzalkonium solution in the rat. Experientia 35,1611. [DOI] [PubMed] [Google Scholar]
- Sato A, Yamamoto M, Iamamura K, Kashiki Y, Kunieda T, Sakata K.(1978). Pathophysiology of aganglionic colon and anorectum: an experimental study on aganglionosis produced by a new method in the rat. J. Ped. Surg. 13,399. [DOI] [PubMed] [Google Scholar]
- See NA, Epstein ML, Dahl JL, Bass P.(1990). The myenteric plexus regulates cell growth in rat jejunum. J. Aut. Nerv. Sys. 31,219. [DOI] [PubMed] [Google Scholar]
- See NA, Epstein ML, Schultz E, Pienkowski TP, Bass P.(1988). Hyperplasia of jejunal smooth muscle in the myenterically denervated rat. Cell Tissue Res. 253,609. [DOI] [PubMed] [Google Scholar]
- Silen W, Peloso O, Jaffe BF.(1966). Kinetics of intestinal epithelial proliferation: effect of vagotomy. Surgery 60,127. [Google Scholar]
- Tutton PJM, Helme RD.(1974). The influence of adrenoreceptor activity on crypt cell proliferation in the rat jejunum. Cell Tissue Kinet. 7,125. [DOI] [PubMed] [Google Scholar]
- Yeh KY, Yeh M, Holt PR.(1991). Intestinal lactase expression and epithelial cell transit in hormone‐treated suckling rats. Am. J. Physiol. 260,G379. [DOI] [PubMed] [Google Scholar]
- Zucoloto S, Deus DA, Martins AA, Muglia VF, Kajiwara JK, Garcia SB.(1997). The relationship between myenteric neuronal denervation, smooth muscle thickening and epithelial cell proliferation in the rat colon. Res. Exp. Med. 197,117. [DOI] [PubMed] [Google Scholar]
- Zucoloto S, Diaz JA, Oliveira JSM et al. (1988). Effect of chemical ablation of myenteric neurons on intestinal cell proliferation. Cell. Tissue Kinet. 21,213. [DOI] [PubMed] [Google Scholar]
- Zucoloto S, Silva JC, Oliveira JSM, Muccillo G.(1991). The chronological relationship between the thickening of smooth muscle, epithelial cell proliferation and myenteric neural denervation in the rat jejunum. Cell Prolif. 24,15. [DOI] [PubMed] [Google Scholar]


