Abstract
Objectives
Icariin, a flavonoid isolated from Epimedium pubescens, has previously been identified to exert beneficial effects on preventing bone loss and promoting bone regeneration. However, molecular mechanisms for its anabolic action have, up to now, remained largely unknown.
Materials and methods
Effects of icariin on cell proliferation and osteogenic differentiation of rat bone mesenchymal stem cells (BMSCs) were systematically evaluated. To characterize underlying mechanisms, its effects on mitogen‐activated protein kinase (MAPK) signalling pathways were determined.
Results
Results showed that icariin might not have enhanced effects on cell proliferation. However, it seemed to significantly enhance osteogenic differentiation of BMSCs, demonstrated by increasing alkaline phosphatase (ALP) activity and gene expression of collagen type I (Col I), osteocalcin (OCN) and osteopotin (OPN). It was demonstrated that icariin rapidly phosphorylated extracellular signal‐regulated kinase (ERK), p38 kinase and c‐Jun N terminal kinase (JNK). Furthermore, icariin‐stimulated osteogenic effects on BMSCs were dramatically attenuated by treatment with either specific ERK inhibitor of PD98059, p38 inhibitor of SB202190 or JNK inhibitor SP600125.
Conclusions
These results provide a potential mechanism of anabolic activity of icariin on BMSCs involving ERK, p38 and JNK MAPK pathways.
Introduction
Bone fractures or defects resulting from, for example, ageing, traffic accidents or bone cancer, have become increasingly prevalent and remained a challenge for surgeons. As growth factors play an important role in the process of bone formation, a range of exogenous osteoinductive growth factors has already been successfully applied in surgery to promote bone repair. However, with consideration to high costs of production, high quality preservation conditions as well as limited active periods, clinical application of exogenous growth factors has been limited. Over recent years, traditional Chinese medicine, an empirical system of multicomponent therapeutics 1, can potentially meet the demands of treating bone fractures and bone defects, and provide a remedy with exogenous growth factors.
The herb Epimedium pubescens, recorded in the Chinese pharmacopoeia as Chinese medicine “yinyanghuo”, has been used in Chinese traditional medicine for treatment of osteoporosis, kidney, joint and liver disorders. Icariin, the main active flavonoid glucoside isolated from the herb, has been identified to exert beneficial effects on preventing postmenopausal bone loss 2. Studies on rat models have indicated that icariin could prevent ovariectomy (OVX)‐induced bone loss, and reduction in femoral and tibial strength 3, 4. Recent studies have demonstrated that icariin can improve maturation and mineralization of osteoblasts, and enhance osteoblastic differentiation of bone mesenchymal stem cells (BMSCs) in vitro 5, 6, 7. However, systematic effects and underlying mechanisms of icariin on BMSCs have, up to now, largely been unknown.
Mitogen‐activated protein kinases (MAPKs) compose the family of messengers that convey signals from the cell surface to nucleus, in response to a wide range of stimuli, including hormones, chemicals and stress 8. MAPK signalling cascades modulate gene expression to regulate proliferation, differentiation and apoptosis 9. Extracellular signal‐regulated kinase (ERK), p38 kinase and c‐Jun N terminal kinase (JNK) form the three major families of MAPKs and each of these has its own subfamilies. The ERK signalling pathway has been intensively investigated in the regulation of osteogenic differentiation of BMSCs. Previous study has shown that commitment of hBMSCs to osteogenic or adipogenic lineages is governed by activation or inhibition, respectively, of ERK 10. Meanwhile, inhibiting or activating p38 in turn inhibits or promotes osteogenic differentiation in vitro, making it plausible that the p38 signalling pathway is a mediator in the process of osteogenic differentiation 11. Previous study has also revealed that the p38 signalling pathway contributes to osteoblast functions in vitro and corresponding bone mineralization in vivo 12. Different MAP kinases are activated by phosphorylation, stimulated by upstream kinases activated by further extracellular stimuli. Also, it has been shown that numerous bone‐active agents, including platelet‐derived growth factor (PDGF), fibroblast growth factor (FGF) and insulin‐like growth factor‐I (IGF‐I) can induce osteogenic differentiation via the ERK signalling pathway 13, 14. Other studies demonstrated that a number of cytokines can activate the p38 signalling pathway in osteoblasts, and subsequently promote osteogenic proliferation and differentiation 15, 16. Additionally, JNK signalling has been reported to play an important role in effects of osteoblast differentiation of human periosteal‐derived cells 17.
In the present study, our hypothesis was that icariin could induce osteogenic differentiation of BMSCs in a dose‐dependent manner, related to MAPK signalling pathways. To verify this, MTT assay, cell cycle analysis, assay of apoptosis, alkaline phosphatase (ALP) activity and real‐time PCR analysis for osteogenesis‐related markers, were performed to evaluate cell proliferation and osteogenic differentiation of rat BMSCs, treated with different concentrations of icariin. Furthermore, ERK, p38 and JNK signalling pathways were explored to discover whether the MAPK signalling pathway could be activated by icariin.
Methods and materials
Animal and cell culture
Tibiae and femurs were dissected from euthanised 160 ± 10 g, male Sprague–Dawley rats (Shanghai SLAC Experimental Animal Center, Shanghai, China) and bone marrow was flushed out using Dulbecco's modified Eagle's medium (DMEM; Hyclone, Waltham, MA, USA) supplemented with 100 unit/ml penicillin and 100 μg/ml streptomycin (Hyclone). To remove blood cells, washouts were collected and centrifuged at 500 g for 10 min. The precipitate was then mixed with complete DMEM supplemented with 10% foetal bovine serum (FBS; Hyclone), and plated into culture flasks maintained at 37 °C in 5% CO2. Non‐adherent cells were removed by changing medium every 3 days. When large colonies formed and became confluent, primary rat BMSCs were trypsinized with 10% trypsin–ethylene diamine tetraacetic acid (EDTA; Hyclone) and passaged. BMSCs of passage 2–3 were used for the experiments.
Cytotoxicity evaluation (LD50)
BMSCs were seeded in 96‐well plates at 5 × 103cells/well. After 24‐h incubation, cells were treated with 0, 10, 20, 40, 80 and 160 μm icariin respectively. At 24 h, cytotoxicity evaluation was carried out using the MTT assay. According to the manufacturer's instructions, 20 μl 5 mg/ml MTT (Amresco, Solon, OH, USA) solution was added and incubated at 37 °C for 4 h to form MTT formazan. Then, medium was replaced with 200 μl dimethyl sulphoxide (DMSO; Sigma, St. Louis, MO, USA) to dissolve the formazan, and absorbance was measured at 590 nm, using an ELX Ultra Microplate Reader (Bio‐tek, Burleigh, QLD, Australia). Percentage of viable cells was obtained by comparing absorbances of samples with and without icariin.
Cell proliferation
For the cell proliferation assay, BMSCs were seeded in 96‐well plates at 5 × 103 cells/well. After 24‐h incubation, they were treated with icariin at concentrations of 5, 10, 20 and 40 μm for 1, 3, 5 and 7 days respectively. Equal volume of vehicle alone (1 μL DMSO/ml medium) was added to form the control group (0 μm). According to the manufacturer's instructions, cell proliferation was assessed using the MTT assay as described above. All experiments were performed in triplicate.
Cell cycle analysis
BMSCs were plated into 6‐well plates at 2 × 105 cells/well and incubated for 24 h, followed by being incubated with icariin at concentrations of 0, 10, 20 and 40 μm respectively. After incubation for 3 days, both adherent and floating cells were harvested and then fixed overnight in cold 70% ethanol in phosphate‐buffered saline (PBS). Cells were then treated with RNase A (Beyotime, Suzhou, China) for 30 min prior to nucleic acid staining with propidium iodide (PI, Sigma) for 5 min. Cell cycles were then analysed using a BD Calibur flow cytometer (Becton Dickinson, Franklin Lake, NJ, USA).
Assay of apoptosis
An annexin V‐FITC kit (Beyotime) was used to quantify apoptosis. After being treated with the range of chosen concentrations of icariin, for 3 days, BMSCs cultured in 6‐well plates were harvested, washed in PBS at 4 °C, then resuspended in 300 μl binding buffer containing 5 μl annexin V‐FITC and 10 μl PI. After 15 min incubation at room temperature, stained cells were analysed by flow cytometry (Becton Dickinson).
Real‐time PCR assay
BMSCs were plated on 12‐well plates at 1 × 105 cells/well, and incubated for 24 h, followed by incubation with icariin at concentrations of 0, 10, 20 and 40 μm respectively. Total RNA was isolated after icariin treatment for 3, 6, 12 and 24 h, using Trizol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's recommended protocol. RNA concentrations were determined using a NanoDrop spectrophotometer (Thermo, Logan, UT, USA). Complimentary DNA (cDNA) was synthesized by means of cDNA Synthesis Reverse Transcription Kit (Fermentas; Thermo). Real‐time PCR assay for runt‐related transcription factor 2 (Runx2), Collagen I (Col I), osteopontin (OPN) and osteocalcin (OCN) was performed using a Light‐Cycler system with SYBR Premix Ex Taq™ (Takara, Japan), according to the manufacturer's instructions. Conditions of real‐time PCR were as follows: denaturation at 95 °C for 10 s; 50 cycles at 95 °C for 10 s and 60 °C for 30 s; and a final dissociation stage (95 °C for 5 min) was added at the end of the amplification procedure. β‐actin was used as internal control. Data were analysed using the comparative Ct (2−ΔΔCt) method and expressed as fold change respective to control. Each sample was analysed in triplicate. Primer sequences used are listed in Table 1.
Table 1.
List of primers used and respective forward and reverse sequences
| Gene | Forward sequence Reverse sequence |
|---|---|
| β‐actin |
5′‐GTAAAGACCTCTATGCCAACA‐3′ 5′‐GGACTCATCGTACTCCTGCT‐3′ |
| Runx2 |
5′‐ATCCAGCCACCTTCACTTACACC‐3′ 5′‐GGGACCATTGGGAACTGATAGG‐3′ |
| Collagen type I(COL I) |
5′‐CTGCCCAGAAGAATATGTATCACC‐3′ 5′‐GAAGCAAAGTTTCCTCCAAGACC‐3′ |
| Osteopontin(OPN) |
5′‐CCAAGCGTGGAAACACACAGCC‐3′ 5′‐GGCTTTGGAACTCGCCTGACTG‐3′ |
| Osteocalcin(OCN) |
5′‐GCCCTGACTGCATTCTGCCTCT‐3′ 5′‐TCACCACCTTACTGCCCTCCTG‐3′ |
Alkaline phosphatase activity
Alkaline phosphatase (ALP) activity, quantitation and staining assays were performed at day 7 after BMSC treatment with icariin at concentrations of 0, 10, 20 and 40 μm respectively. Samples of all groups were incubated in p‐nitrophenyl phosphate (pNPP) (Sigma) at 37 °C for 30 min. Absorbance values (OD) were recorded at 405 nm to determine ALP activity. Total protein contents were assessed using a Bio‐Rad protein assay kit (Bio‐Rad, Hercules, CA, USA), and OD values were normalized to bovine serum albumin (BSA; Sigma) standard curve, at 590 nm. ALP activity was accessed as OD value at 405 nm per milligram of total protein. Meanwhile, ALP staining was also performed according to the manufacturer's instructions (Beyotime). Each sample was rinsed three times in PBS and fixed in 4% paraformaldehyde for 15 min. Samples were soaked in 0.1% naphthol AS‐MX phosphate and 0.1% fast red violet LB salt, in 56 mm 2‐amino‐2‐methyl‐1,3‐propanediol, for 45 min at 37 °C, then observed using a digital camera (ECLIPSETS 100, NIKON, Tokyo, Japan). All experiments were performed in triplicate.
Western blotting
For the MAPK signalling pathway, BMSCs were cultured in medium of 20 μm icariin for 0, 15, 30, 60 and 120 min. For protein expression of Runx2 and OPN, BMSCs were cultured in 20 μm icariin medium for 0, 3, 6, 12 and 24 h. Cells were lysed on ice for 30 min in RIPA lysis buffer (Thermo, DE) supplemented with protease inhibitor cocktail, phosphatase inhibitor cocktail and phenylmethanesulphonyl fluoride (PMSF) (Kangchen, Shanghai, China). Protein concentration was measured using a BSA protein assay kit. Of the sample, 20 μg was resolved on 10% SDS‐PAGE gel and electro‐transferred to polyvinylidene difluoride membrane (PVDF, Pall, New York, USA). Membranes were blocked and incubated with appropriate primary antibodies including rabbit anti‐rat ERK, p38, JNK, phosphorylated‐ERK (p‐ERK), phosphorylated‐p38 (p‐p38), phosphorylated‐JNK (p‐JNK) (CST, USA), Runx2 (ablcam, USA) and OPN (CST, USA) at dilution of 1:1000. For normalization of protein loading, mouse anti‐rat β‐actin (Sigma) antibody was used at 1:10000 dilution. Finally, membrane reactions were visualized using horseradish peroxidase (HRP)‐conjugated secondary antibodies (Beyotime, dilution, 1:1000) with ECL plus reagents (Amersham Pharmacia Biotech, Buckinghamshire, England) by UVItec ALLIANCE 4.7 gel imaging system. Protein band intensities on scanned films were compared to their respective controls using Quantity One Image software. Bands were first rounded up using a volume rect tool, then target area intensity was calculated. Density of β‐actin was used as control for protein expression of Runx2 and OPN. Densities of ERK, p38 and JNK were quantified as control groups for protein expression of p‐ERK, p‐p38 and p‐JNK respectively.
ERK, p38 and JNK inhibitor treatment analysis
BMSCs treated with icariin at concentrations of 0 and 20 μm were cultured in medium supplemented with ERK signalling pathway inhibitor PD98059 (CST), p38 signalling pathway inhibitor SB202190 (CST) or JNK signalling pathway inhibitor SP600125 (CST), final concentration 20 μm, for 7 days respectively. Then, total RNA was isolated and cDNA was synthesized, and real‐time PCR was performed on Collagen I, OCN, OPN as specified above. In addition, ALP activity was measured by ALP staining assay as described above. Meanwhile, BMSCs treated with icariin at concentrations of 0 and 20 μm, were cultured in medium without ERK, p38 or JNK inhibitor, identified as control groups respectively.
Statistical analysis
All experiments were performed a minimum of three times. All measurements are expressed as mean ± SD. Significant differences between groups were determined using ANOVA (SPSS, v.17.5; Chicago, IL, USA), while P < 0.05 denotes statistical significance.
Results
Icariin exerted no enhanced effect on proliferation of BMSCs
LD50 assay was applied to explore appropriate concentration of icariin for this in vitro study and concentrations of 80 and 160 μm led to more than half BMSCs succumbing to apoptosis (Fig. 1a). MTT assay had negative effect on BMSC proliferation in icariin‐treated groups from day 1 to day 7 (Fig. 1b), particularly at 40 μm concentration. There were no signs of cell proliferation of BMSCs treated with icariin at 5–40 μm with cell cycle detection (Fig. 1c). As shown in Fig. 1d and 1e, icariin at 40 μm concentration induced significantly higher levels BMSC apoptosis. Levels of BMSC apoptosis treated with icariin at 40 μm were 9.87 ± 0.18%, while the control group was 6.935 ± 0.94% (P < 0.05). However, icariin at other concentrations had no significant effect on level of apoptosis compared to the control group. These results indicate that icariin had no effect on enhancing cell proliferation of BMSCs, and little effect on their apoptosis. Proliferation‐inhibition effect of icariin was in a concentration‐dependent manner, identified by cytotoxicity of BMSCs induced by icariin at concentration of more than 40 μm.
Figure 1.
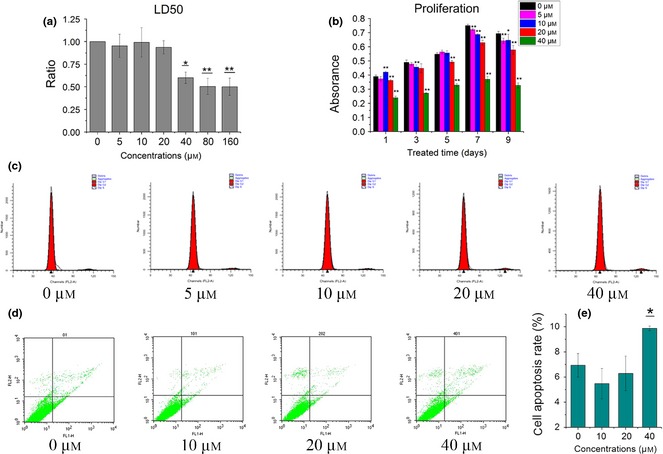
Proliferation and apoptosis of BMSC s treated with icariin. (a) Cytotoxicity evaluation with different concentrations of icariin (μm). (b) Proliferation of BMSCs after treatment of icariin by MTT assay. (c) Cell cycle assay of BMSCs after treatment of icariin. (d, e) Apoptosis assay of BMSCs (*as compared to 0 μm group at each time point, *P < 0.05, ** P < 0.01; n = 3).
Icariin enhanced osteogenic differentiation of BMSCs
Osteogenic differentiation of BMSCs was evaluated by real‐time PCR and ALP activity. Over the range of 5–40 μm, icariin enhanced mRNA expression of Runx2, Col I, OCN and OPN of BMSCs, in a dose‐dependent manner, at concentrations of 20 and 40 μm it achieved higher values (Fig. 2a–d). mRNA expression of Runx2 was promoted by almost eight times over early stages of icariin treatment at concentrations of 20 and 40 μm; then this increasing tendency slowed down as treatment time extended. In contrast, mRNA expression of OCN of icariin‐treated groups increased over the whole culture time, and icariin of 20 μm group reached 6.36 ± 0.78 times compared to the control group after 24‐h treatment (P < 0.01). Similar to Runx2, mRNA expression of Col I was promoted significantly by icariin at concentrations from 10 to 40 μm after 3‐h treatment. This was specially obvious for 20 μm concentration, at which Col I mRNA expression was induced more than 10 times compared to the control group. Then, Col I mRNA expression fell back to normal levels after 12‐h treatment. OPN mRNA expression was also significantly promoted by icariin at concentrations of 20 and 40 μm. In contrast to the others, increase in OPN mRNA expression at concentrations of 20 and 40 μm remained stable from 3 to 12 h, whereas they all fell back to normal levels after 24‐h treatment. Similar to mRNA expression, Runx2 protein was up‐regulated at 6 h after treatment of 20 μm icariin, and then fell back to normal levels at 24 h. OPN protein increased over the first 3 h, and remained at similar levels from 6 to 24 h.
Figure 2.
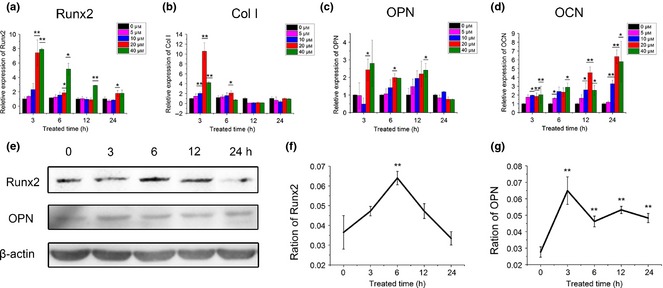
Protein and gene expression of osteoblastic markers in BMSC s treated with icariin. (a–d) Real‐time PCR analysis of Runx2, Collagen I, OCN and OPN mRNA in BMSCs treated with icariin (*compared to 0 μm at each time point). (e) Protein expression of Runx2 and OPN at concentration of 20 μm by western blotting assay. (f, g) Densitometric analysis of Runx2 and OPN expression; β‐actin density was used as control (*compared to ratio at 0 min group) (*P < 0.05, **P < 0.01; n = 3).
As shown in Fig. 3a, ALP staining at 7 days indicated that icariin significantly increased ALP activity, with its peak level at 20 μm concentration. However, staining showed ALP activity seemed to be depressed by icariin at 40 μm, while its activity quantitative assay did not demonstrate any reduction (Fig. 3b). In combination with MTT assay, it may be inferred that light staining of the 40 μm group was given rise to by lower numbers of BMSCs.
Figure 3.
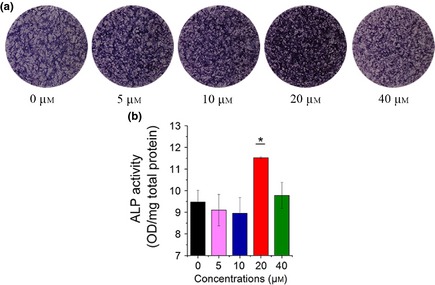
ALP activity of icariin‐treated BMSCs on day 7. (a) ALP staining of BMSCs after treatment with icariin. (b) ALP activity quantitative assay of icariin‐treated BMSCs measured by pNPP assay (*compared to 0 μm group, *P < 0.05, n = 3).
Icariin affected phosphorylation in MAPK signalling pathways
To address the role of the MAPK cascade in induction of osteogenic differentiation by icariin, we investigated protein levels of p‐ERK, ERK, p‐p38, p38, p‐JNK and JNK under icariin‐stimulated conditions at 0, 15, 30, 60 and 120 min. Dose‐dependent studies revealed that optimal concentration of icariin was 20 μm, and hence this concentration was adopted in the following studies. Results of western blotting showed that icariin (20 μm) phosphorylated ERK (p‐ERK) during the first 15 and 30 min (P < 0.01, Fig. 4a, b), while ERK level itself, seemed to be down‐regulated at 15 and 30 min (Fig. 4a). p38 was phosphorylated by icariin from 15 to 120 min (P < 0.05, Fig. 4a, c), while p38 level was also promoted over the first 15 and 30 min (Fig. 4a). Though JNK expression had no significant change, its phosphorylated level (p‐JNK) was promoted over the first 15 and 30 min (Fig. 4a, d).
Figure 4.
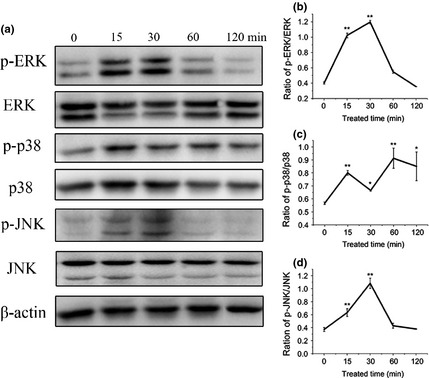
Effect of icariin on MAPK signalling pathways. (a) Western blotting of total and phosphorylated ERK, p38 and JNK following icariin treatment at 0, 15, 30, 60 and 120 min. (b–d) Densitometric analysis of p‐ERK/ERK, p‐p38/p38 and p‐JNK/JNK expression by western blot analysis (*P < 0.05, **P < 0.01; *compared to phosphorylated protein expression at 0 min).
ERK, p38 and JNK pathways involved in icariin‐stimulated osteogenic differentiation
To further investigate the role of MAPK pathways in icariin‐stimulated osteogenesis, BMSCs treated with icariin were cultured in medium supplemented with ERK signalling pathway inhibitor PD98059, p38 signalling pathway inhibitor SB202190 and JNK signalling pathway inhibitor SP600125, for 7 days, separately. Results of real‐time PCR showed that except OCN mRNA, ERK inhibitor PD98059 did not show high inhibition of expression of osteogenic genes for BMSCs cultured without icariin. Similar to PD98059, p38 inhibitor SB202190 did not show high inhibition of expression of osteogenic genes, except OPN mRNA of BMSCs cultured without icariin. However, enhanced mRNA expression of osteogenic genes Collagen I, OPN and OCN induced by icariin, was significantly inhibited by PD98059 and SB202190 respectively. JNK inhibitor SP600125 down‐regulated expression of OPN and OCN mRNA cultured without icariin, and also inhibited enhanced mRNA expression of osteogenic genes Collagen I, OPN and OCN induced by icariin (Fig. 5a–c). ALP staining showed that ALP activity in BMSCs treated with or without icariin was inhibited after PD98059, SB202190 and SP600125 treatment (Fig. 5d). These results strongly suggest that icariin promoted osteogenic differentiation of rat BMSCs, at least in part, via activation of ERK, p38 and JNK signalling pathways.
Figure 5.
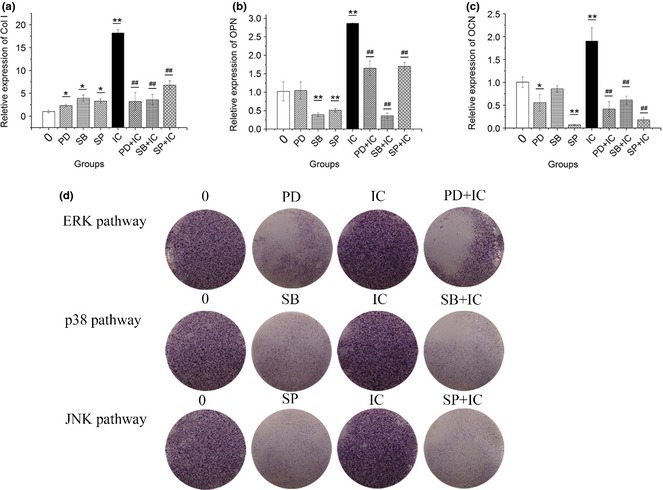
Effect of ERK , p38 and JNK signalling pathways on osteogenic differentiation of BMSC s treated with or without icariin. (a–c) Effect of PD98059 (20 μm, PD in short), SB202190 (20 μm, SB in short) and SP600125 (20 μm, SP in short) on osteogenic gene expression of BMSCs without or with icariin (0, IC in short respectively) treatment. (d) ALP staining assay. (*P < 0.05, **,## P < 0.01; *compared to 0 μm group, #compared to icariin group).
Discussion
Exogenous cytokines or growth factors, essential for bone development, can effect different cell responses, such as promoting proliferation, migration and osteogenic differentiation. Some growth factors have been used in bone regeneration, such as bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs) and platelet‐derived growth factor (PDGF) 18, 19, 20. However, due to the high quality conditions for preservation and production costs, it is difficult to further promote clinical application of growth factors. Recently, specific traditional Chinese medicine, that can promote osteogenesis (with a high levels of production and low cost), has been explored extensively.
Icariin, a major active constituent in Epimedium, has been shown to prevent osteoporosis, and promote bone formation during mandibular distraction osteogenesis 21, 22. Effects of icariin on osteoblasts are similar to its anabolic action, including induction and promotion of osteoblastic differentiation 7, 23. Appropriate drug concentration in vitro plays a highly important role in research on its effects and mechanisms. The present study was first designed to investigate optimal concentration of icariin, to evaluate osteogenesis and its underlying mechanisms. Though there was cytotoxicity when concentration of icariin was higher than 10−5 M (10 μm), as reported in some studies 24, other investigations have demonstrated that icariin at 5, 10 and 50 μm did not cause any significant microglial cell viability change compared to control groups 25, 26. To screen optimized concentration of icariin for inducing proliferation or osteogenic differentiation of rat BMSCs, concentrations from 5 to 160 μm were selected to test its median lethal dose in the present study. It was found that concentration higher than 80 μm induced more than half BMSC apoptosis. Moreover, both MTT assay and cell cycle analysis showed that icariin had no positive effect on proliferation of rat BMSCs, in contrast to results on osteoblasts reported by Song et al. 27. This may be related to the different cell type tested in the present study.
Here, icariin was found to have excellent ability to improve osteogenic differentiation and osteogenic function, as demonstrated by ALP activity and mRNA expression of Runx2, Collagen I, OCN and OPN at concentrations of 20 and 40 μm, as well as Runx2 and OPN protein levels. ALP staining and quantitative analysis demonstrated ALP activity was induced by icariin, particularly at 20 μm (Fig. 3). Runx2, an osteoblast transcriptional activator, has been reported to play an important role in regulating expression of osteoblast genes at early stages 28. Collagen I, which provides the structural framework for inorganic molecule deposition, has an apparent effect on biomechanical strength of bone tissue. In the present study, mRNA expression of Runx2 and Col I in 20 and 40 μm icariin‐treated groups peaked at 3 h, of the time points examined, then fell back from 6 to 24 h (Fig. 2a, b). Similar to mRNA expression, protein expression of Runx2 was shown to be up‐regulated at early stages of icariin treatment. OPN, as an intermediate or relatively earlier marker of osteogenic differentiation, is associated with the maturation stage of osteoblasts during attachment, and matrix synthesis before mineralization. The present study showed that OPN mRNA expression in icariin‐treated groups at 20 and 40 μm was promoted from 3 to 12 h, while it almost maintained at the same level for the control group at 24 h (Fig. 2c). OPN protein expression was also up‐regulated in the 20 μm group. As late marker of osteogenic differentiation, OCN is related to matrix deposition and mineralization. In the present study, icariin caused increase in OCN mRNA expression as extension of icariin‐treatment time, and peaked at 24 h (Fig. 2d). Its time course expression seemed to closely follow that of Runx2, which was similar to the result previously reported by Ma et al. 25. Though Runx2 mRNA expression at 40 μm was higher than that at 20 μm at 6 and 12 h, there was no significant difference for mRNA expression of OPN and OCN between 20 and 40 μm icariin‐treated groups. Considering cytotoxicity at concentration of 40 μm, as shown by apoptosis assay, optimal concentration of icariin in the present study was 20 μm (Fig. 1, 2).
MAPK kinases, including ERK, p38 and JNK pathways, are secondary messengers that convey signals from the cell surface to the nucleus in response to a wide range of stimuli, and then regulate multiple cellular activities in osteoblasts. ERK has been reported to regulate osteoblast proliferation, apoptosis and differentiation by regulating expression of cell cycle regulators as well as activity of the skeletal‐specific transcription factor Runx2 29, 30, 31, 32. Previous studies have shown that the ERK signalling pathway is involved in osteogenesis, by enhanced ALP activity, in osteoblast progenitor cells 10, 33, 34, 35. The p38 signalling pathway has been reported to involve phosphorylation of smad‐1, thus resulting in ALP expression and activation of osteoblasts 36. Activation of the JNK pathway is commonly linked to promoting apoptosis and cell death 37, 38, 39. For instance, activation of the JNK signalling pathway leads to permeabilization of outer mitochondrial membranes resulting in mitochondrial cytochrome release into the cytosol allowing apoptosis to progress 40. Recently, the JNK pathway was found to be involved in osteogenic differentiation of BMSCs or periodontal ligament stem cells 41, 42. In the present study, we examined the effects of icariin on ERK, p38 and JNK MAPK signalling pathways. In contrast to the Song et al. report that icariin induces osteoblast proliferation, differentiation and mineralization through ERK and JNK signal activation 27, the present study showed that ERK, p38 and JNK signalling pathways were all phosphorylated in BMSCs treated with icariin, while total JNK expression did not show any significant changes (Fig. 4a, b). Furthermore, we blocked ERK, p38 and JNK signalling pathways for BMSCs treated with icariin, and then ALP activity and expression of Collagen I, OPN and OCN were significantly inhibited respectively. These results suggest that ERK, p38 and JNK signalling pathways are involved in the process of icariin‐induced osteogenesis. Moreover, icariin has also been reported to stimulate osteogenic differentiation of BMSCs via activating the PI3K–AKT–eNOS–NO–sGC–cGMP–PKG signalling pathway 43. Previous studies have also shown that there is crosstalk between MAPK and AKT signalling pathways 44. However, whether this may occur for enhanced effects of icariin on BMSCs needs to be explored in the future.
In conclusion, in the present study, it has been demonstrated that the optimal concentration of icariin to promote osteogenic differentiation of BMSCs, was 20 μm. Furthermore, the ERK, p38 and JNK MAPK signalling pathways were involved in this process. This investigation has provided scientific evidence and support for the belief that icariin as traditional Chinese medicine, could be applied for bone regeneration.
Acknowledgements
This project was supported by National Research Program of China (973 Program, 2012CB933604), National Outstanding Youth Foundation (81225006), National Natural Science Foundation of China (81170939), the Shanghai Science and Technology Development Fund (12nm0501600), Specialized Research Fund for the Doctoral Program of Higher Education (20110073110076); Biomedical Engineering Cross Research Foundation of Shanghai Jiao Tong University School (YG2012MS29) and Shanghai Jiao Tong University Doctor Innovation Program (BXJ201226).
Yuqiong Wu and Lunguo Xia have contributed equally to this work.
References
- 1. Cheng KF, Leung KS, Leung PC (2003) Interactions between modern and Chinese medicinal drugs: a general review. Am. J. Chin. Med. 31, 163–169. [DOI] [PubMed] [Google Scholar]
- 2. Guo BL, Wang CL, Xiao PG (1996) Determination of flavonoids and quality evaluation of Sagittate Epimedium (Epimedium sagittatum). China Trad. Herb Drugs 27, 524–526. [Google Scholar]
- 3. Nan H, Ma MH, Nan SS, Xu LL (2009) Antiosteoporotic activity of icariin in ovariectomized rats. Phytomedicine 16, 320–326. [DOI] [PubMed] [Google Scholar]
- 4. Mok SK, Chen WF, Lai WP, Leung PC, Wang XL, Yao XS et al (2010) Icariin protects against bone loss induced by oestrogen deficiency and activates oestrogen receptor‐dependent osteoblastic functions in UMR 106 cells. Br. J. Pharmacol. 159, 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen KM, Ge BF, Ma HP, Liu XY, Bai MH, Wang Y (2005) Icariin, a flavonoid from the herb Epimedium enhances the osteogenic differentiation of rat primary bone marrow stromal cells. Pharmazie 60, 939–942. [PubMed] [Google Scholar]
- 6. Huang J, Yuan L, Wang X, Zhang TL, Wang K (2007) Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 81, 832–840. [DOI] [PubMed] [Google Scholar]
- 7. Hsieh TP, Sheu SY, Sun JS, Chen MH (2010) Icariin inhibits osteoclast differentiation and bone resorption by suppression of MAPKs/NF‐kappaB regulated HIF‐1alpha and PGE(2) synthesis. Phytomedicine 18, 176–185. [DOI] [PubMed] [Google Scholar]
- 8. Wu Y, Zhang X, Zhang P, Fang B, Jiang L (2012) Intermittent traction stretch promotes the osteoblastic differentiation of bone mesenchymal stem cells by the ERK1/2‐activated Cbfa1 pathway. Connect. Tissue Res. 53, 451–459. [DOI] [PubMed] [Google Scholar]
- 9. Yang SH, Sharrocks AD, Whitmarsh AJ (2013) MAP kinase signaling cascades and transcriptional regulation. Gene 513, 1–13. [DOI] [PubMed] [Google Scholar]
- 10. Jaiswal RK, Jaiswal N, Bruder SP et al (2000) Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen‐activated protein kinase. J. Biol. Chem. 275, 9645–9652. [DOI] [PubMed] [Google Scholar]
- 11. Kim do Y, Kim GW, Chung SH (2013) Nectandrin A Enhances the BMP‐Induced Osteoblastic Differentiation and Mineralization by Activation of p38 MAPK‐Smad Signaling Pathway. Korean J. Physiol. Pharmacol. 17, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D et al (2010) The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J. Clin. Invest. 120, 2457–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pratsinis H, Kletsas D (2007) PDGF, bFGF and IGF‐I stimulate the proliferation of intervertebral disc cell vitro via the activation of the ERK and AKT signaling pathways. Eur. Spine J. 16, 1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yokota J, Chosa N, Sawada S, Okubo N, Takahashi N, Haseqawa T et al (2014) PDGF‐induced PI3K‐mediated signaling enhances the TGF‐β‐induced osteogenic differentiation of human mesenchymal stem cells in a TGF‐β‐activated MEK‐dependent manner. Int. J. Mol. Med. 33, 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pantouli E, Boehm MM, Koka S (2005) Inflammatory cytokines activate p38 MAPK to induce osteoprotegerin synthesis by MG‐63 cells. Biochem. Biophys. Res. Commun. 329, 224–229. [DOI] [PubMed] [Google Scholar]
- 16. Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J (2003) Activation of p38 mitogen‐activated protein kinase and c‐Jun‐NH2‐terminal kinase by BMP‐2 and their implication in the stimulation of osteoblastic cell differentiation. J. Bone Miner. Res. 18, 2060–2068. [DOI] [PubMed] [Google Scholar]
- 17. Hah YS, Kang HG, Cho HY, Shin SH, Kim UK, Park BW et al (2013) JNK signaling plays an important role in the effects of TNF‐α and IL‐1β on in vitro osteoblastic differentiation of cultured human periosteal‐derived cells. Mol. Biol. Rep. 40, 4869–4881. [DOI] [PubMed] [Google Scholar]
- 18. Kim JS, Cha JK, Cho AR, Kim MS, Lee JS, Hong JY et al (2014) Acceleration of Bone Regeneration by BMP‐2‐Loaded Collagenated Biphasic Calcium Phosphate in Rabbit Sinus. Clin. Implant Dent. Relat. Res. 28 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19. Behr B, Leucht P, Longaker MT, Quarto N (2010) Fgf‐9 is required for angiogenesis and osteogenesis in long bone repair. Proc. Natl Acad. Sci. USA 107, 11853–11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SE, Yun YP, Lee JY, Shim JS, Park K, Huh JB (2013) Co‐delivery of platelet‐derived growth factor (PDGF‐BB) and bone morphogenic protein (BMP‐2) coated onto heparinized titanium for improving osteoblast function and osteointegration. J. Tissue Eng. Regen. Med. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Zhang G, Qin L, Shi Y (2007) Epimedium‐derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24‐month randomized, double‐blind and placebo‐controlled trial. J. Bone Miner. Res. 22, 1072–1079. [DOI] [PubMed] [Google Scholar]
- 22. Wei H, Zili L, Yuanlu C, Biao Y, Cheng L Xiaoxia W et al (2011) Effect of icariin on bone formation during distraction osteogenesis in the rabbit mandibule. Int. J. Oral Maxillofac. Surg. 40, 413–418. [DOI] [PubMed] [Google Scholar]
- 23. Xiao Q, Chen A, Guo F (2005) Effects of icariin on expression of OPN mRNA and type I collagen in rat osteoblasts in vitro. J. Huazhong Univ. Sci. Technolog. Med. Sci. 25, 690–692. [DOI] [PubMed] [Google Scholar]
- 24. Fan JJ, Cao LG, Wu T, Wang DX, Jin D, Jiang S et al (2011) The dose‐effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules 16, 10123–10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma HP, Ming LG, Ge BF, Zhai YK, Song P, Xian CJ et al (2011) Icariin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro. J. Cell. Biochem. 112, 916–923. [DOI] [PubMed] [Google Scholar]
- 26. Zeng KW, Fu H, Liu GX, Wang XM (2010) Icariin attenuates lipopolysaccharide‐ induced microglial activation and resultant death of neurons by inhibiting TAK1/IKK/NF‐kappaB and JNK/p38 MAPK pathways. Int. Immunopharmacol. 10, 668–678. [DOI] [PubMed] [Google Scholar]
- 27. Song L, Zhao J, Zhang X, Li H, Zhou Y et al (2013) Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor‐mediated ERK and JNK signal activation. Eur. J. Pharmacol. 714, 15–22. [DOI] [PubMed] [Google Scholar]
- 28. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754. [DOI] [PubMed] [Google Scholar]
- 29. Tanaka Y, Nakayamada S, Fujimoto H, Okada Y, Umehara H, Kataoka T et al (2002) H‐Ras/mitogen‐activated protein kinase pathway inhibits integrin‐mediated adhesion and induces apoptosis in osteoblasts. J. Biol. Chem. 277, 21446–21452. [DOI] [PubMed] [Google Scholar]
- 30. Datta NS, Chen C, Berry JE, McCauley LK et al (2005) PTHrP signaling targets cyclin D1 and induces osteoblastic cell growth arrest. J. Bone Miner. Res. 20, 1051–1064. [DOI] [PubMed] [Google Scholar]
- 31. Qin L, Li X, Ko JK, Partridge NC (2005) Parathyroid hormone uses multiple mechanisms to arrest the cell cycle progression of osteoblastic cells from G1 to S phase. J. Biol. Chem. 280, 3104–3111. [DOI] [PubMed] [Google Scholar]
- 32. Ge C, Xiao G, Jiang D, Franceschi RT. (2007) Critical role of the extracellular signal‐regulated kinase‐MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 176, 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T (2007) Mechanical stress‐mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J. Cell. Biochem. 101, 1266–1277. [DOI] [PubMed] [Google Scholar]
- 34. Lou J, Tu Y, Li S, Manske PR (2000) Involvement of ERK in BMP‐2 induced osteoblastic differentiation of mesenchymal progenitor cell line C3H10T1/2. Biochem. Biophys. Res. Commun. 268, 757–762. [DOI] [PubMed] [Google Scholar]
- 35. Celil AB, Campbell PG (2005) BMP‐2 and insulin‐like growth factor‐I mediate Osterix (Osx) expression in human mesenchymal stem cells via the MAPK and protein kinase D signaling pathways. J. Biol. Chem. 280, 31353–31359. [DOI] [PubMed] [Google Scholar]
- 36. Noth U, Tuli R, Seghatoleslami R, Howard M, Shah A, Hall DJ et al (2003) Activation of p38 and Smads mediates BMP‐2 effects on human trabecular bone‐derived osteoblasts. Exp. Cell Res. 291, 201–211. [DOI] [PubMed] [Google Scholar]
- 37. Chen L, Liu L, Yin J, Luo Y, Huang S (2009) Hydrogen peroxide‐induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int. J. Biochem. Cell Biol. 41, 1284–1295. [DOI] [PubMed] [Google Scholar]
- 38. Cho ES, Lee KW, Lee HJ (2008) Cocoa procyanidins protect PC12 cells from hydrogen‐peroxide‐induced apoptosis by inhibiting activation of p38 MAPK and JNK. Mutat. Res. 640, 123–130. [DOI] [PubMed] [Google Scholar]
- 39. Nakano H, Nakajima A, Sakon‐Komazawa S, Piao JH, Xue X, Okumura K (2006) Reactive oxygen species mediate crosstalk between NF‐kappaB and JNK. Cell Death Differ. 13, 730–737. [DOI] [PubMed] [Google Scholar]
- 40. Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A et al (2000) Requirement of JNK for stress‐induced activation of the cytochrome c‐mediated death pathway. Science 288, 870–874. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Li J, Song W, Yu J et al (2014) Mineral trioxide aggregate upregulates odonto/osteogenic capacity of bone marrow stromal cells from craniofacial bones via JNK and ERK MAPK signalling pathways. Cell Prolif. 47, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S et al (2012) Insulin‐like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem. Cell Biol. 137, 513–525. [DOI] [PubMed] [Google Scholar]
- 43. Zhai YK, Guo XY, Ge BF, Zhen P, Ma XN, Zhou J et al (2014) Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K‐AKT‐eNOS‐ NO‐cGMP‐PKG. Bone 66, 189–198. [DOI] [PubMed] [Google Scholar]
- 44. EI‐Habr EA, Levidou G, Triqka EA, Sakalidou J, Piperi C, Chatziandreou I et al (2014) Complex interactions between the components of the PI3K/AKT/mTOR pathway, and with components of MAPK, JAK/STAT and Notch‐1 pathways, indicate their involvement in meningioma development. Virchows Arch. 465, 473–485. [DOI] [PubMed] [Google Scholar]


