Abstract
Objectives: Although emerging data suggest that zoledronic acid (Zol) may have different anti‐tumour activities against a broad range of cancers, its effects on lung cancer remain largely unknown. The aim of this study was to evaluate in vitro the anti‐tumoural and anti‐angiogenetic effect of zoledronic acid in non‐small‐cell lung cancer (NSCLC) cells.
Material and methods: We treated A549 NSCLC cells with zoledronic acid to investigate survival, cell cycle activity, anti‐angiogenic activity and apoptotic responses to it.
Results: We observed that highest Zol concentration (100 μm) caused arrest in G1 phase of the cell cycle and also induced different percentages of apoptosis in presence (0.9% versus 4.4%) or absence (2.4% versus 28.5%) of serum (P = 0.0001). Zol concentration from 5 to 100 μm for 2 days induced significant concentration‐dependent cell death in adherent cells. Furthermore, Zol (10–100 μm) induced dose‐dependent reduction both of mRNA and protein expression of VEGF associated with parallel decrease in VEGF secretion in the culture medium.
Conclusion: Taken together, these results support a possible anti‐cancer and anti‐angiogenetic activity of Zol. Our data may not only provide a basis for the clinical use of this drug as preventive agent of bone metastases but also suggest that Zol deserves attention as an anti‐cancer agent in non‐small‐cell lung cancer.
Introduction
Zoledronic acid (Zol) is a third‐generation bisphosphonate used as therapy for bone metastasis in malignancy and for metabolic disorders such as bone pain, fractures and hypercalcaemia. This drug acts through induction of osteoclast apoptosis, probably due to inhibition of isoprenilation of proteins required for osteoclast survival. Prenylated proteins are important intermediates of cell signalling and cytoskeletal organization. Activated ras proteins indeed trigger cascades of phosphorylation events through sequential activation both of PI3 kinase/AKT pathway, which is critical for cell survival, and of Raf/Mek/Erk kinase pathway that is implicated in cell proliferation as well as in survival (1).
Emerging data suggest that Zol has anti‐tumour activity against cells of lines derived from a broad range of tumours including breast, prostate and pancreatic cancers, through a variety of mechanisms including induction of apoptosis, and inhibition of invasion, adhesion and tumour angiogenesis. An anti‐angiogenic effect has been suggested by Wood et al. who found dose‐dependent inhibition of proliferation of human umbilical vein endothelial cells in vitro, following exposure to Zol (2). In two small‐sized series of patients with bone metastases from different tumours, Santini et al. showed significant reduction in circulating VEGF levels after single bisphosphonate administration and the effect was longer lasting after Zol than with pamidronate (3). However mechanisms of this effect have not been fully explained and it is not clear whether Zol may target endothelial and/or malignant cells.
Large phase III clinical trials have demonstrated that VEGF‐targeted therapies may improve clinical outcome in a number of tumours, including colon, breast, lung, renal and probably, liver carcinomas (4, 5, 6, 7, 8). If this strategy were meaningful, then assessment of anti‐angiogenic effects of a generally well‐tolerated drug such as Zol might be useful, specially in those tumours which remain intrinsically chemoresistant; this may be the case for non‐small‐cell lung cancer (NSCLC). In a mouse model of NSCLC which has high‐potential for bone metastases, Lu et al. have demonstrated that Zol was synergistic with paclitaxel in reducing bone metastases and prolonging survival (9). Based on a randomized phase III trial showing clinical benefit in management of a subgroup of patients with bone metastases from lung cancer, a role for Zol in preventing skeletal metastases from NSCLC has been recently suggested (10). However, in vitro studies have not adequately addressed the activity of Zol in lung cancer and hence its effect remains largely unknown (11, 12, 13).
Thus, we have studied and evaluated effects of Zol on survival, cell cycle activity and apoptotic response as well as on expression and secretion of VEGF in A549 and 95D cells, both human lung adenocarcinoma‐derived lines.
Materials and methods
Cell lines
A549 NSCLC cells were obtained from ATCC (Rockville, MD, USA) and maintained in DMEM supplemented with 10% foetal bovine serum, 2 mm l‐glutamine, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulphate. 95D NSCLC cells were obtained from ATCC (Rockville) and maintained in completed RPMI 1640 (Gibco, Grand Land, NY, USA) containing 10% heat‐inactivated foetal bovine serum and supplemented 2 mm l‐glutamine, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulphate. Cells were cultured at 37 °C in a humidified 5% CO2 incubator and routinely transferred when 90–95% confluent. Cell viability was determined by the trypan blue exclusion test. Cells were regularly observed for mycoplasma pollution with mycoplasma detection reagents provided by Roche (Mannheim, Germany).
Reagents
Zoledronic acid was kindly supplied as the hydrated disodium salt by Novartis, Pharma AG (Basel, Switzerland). Stock solution of zoledronic acid was prepared at concentration 10 mm in 0.9% saline; aliquots were stored at −20 °C and diluted in culture medium (10–100 μm) immediately before use.
Treatments
A549 and 95 D lung cancer cells were resuspended in medium, in serum and under serum‐free conditions (starved) at 1 × 105 cells/ml, and 100 μl of 105 cells/ml cell suspension were distributed to each well (Costar, Cambridge, MA, USA) and allowed to adhere for 24 h. Cell suspensions were thereafter treated with increasing concentrations of zoledronic acid (10–100 μm) and were incubated for 48 h.
MTT assay
3‐(4,5‐dimethyl‐thiazoyl)‐2,5‐diphenyl‐SH‐tetrazolium bromide (MTT) assay was performed on both A549 and 95D cell lines. Target cells were resuspended in medium at 1 × 105 cells/ml, and 100 μl of 105 cells/ml cell suspension was distributed to each well of 96‐well plates (Costar) and allowed to adhere for 24 hours. Wells containing 200 μl medium alone (without cells) and reagents were used as negative control. After treatment with incubation doses of zoledronic acid (10–100 μm) for 48 h, 20 μl MTT solution (5 mg/ml) was added to each well, and microplates were further incubated at 37 °C for 4 h. Unreacted supernatants in wells were discarded, and 100 μl acidified isopropanol (0.04 N HCl in isopropanol) was added to the cultures and mixed thoroughly to dissolve the dark‐blue crystals of formazan. Absorbance values of each well were determined using microplate enzyme‐linked immunoassay reader equipped with a 570 nm filter. Negative control well was used for baseline zero absorbance. Results are presented as percentage viability, determined as (1 − absorbance of experimental well/absorbance of positive control well) × 100. Each experiment was repeated three times.
RNA isolation and cDNA synthesis
Total RNA of A549 cells was isolated from cell lysate with QIAzol (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Possible genomic DNA contamination was removed by on‐column DNase treatment with RNase‐free DNase set (Qiagen). Purity and integrity of RNA were checked by gel electrophoresis according to standard procedures. One microgram of total RNA was incubated for 5 min at 70 °C, and cDNA synthesis was performed for 1.5 h at 42 °C with ImProm‐II (Promega, Leiden, Netherlands) according to the manufacturer’s instructions. Two microlitres of cDNAs obtained were used for amplification with AmpliTaq Gold (Perkin‐Elmer Corp., Norwalk, CT, USA) using the following primers: VEGF for 5′‐GGATGTCTATCAGCGCAGCTAG‐3′, VEGF rev 5′‐TCACCGCCTCGGCTTGTCACAT‐3′ and as internal control GADPH‐for 5′‐GTGAAGGTCGGTGTGAACGGATTTGGCCGT‐3′ and GADPH rev 5′‐CCACCACCCTGTTGCTGTAG‐3′.
Real‐time quantitative reverse transcription‐polymerase chain reaction (RT‐PCR)
For real‐time reaction, 2 ml total cDNA diluted 1:20 was mixed with DNA Master Mix SYBR Green I (Roche) and with the specific primers: VEGF for 5′‐TGTCTTGCTCTATCTTTCTT‐3′, VEGF rev 5′‐CTTGCCTTGCTGCTCTACCT‐3′, Cyclin B1 for 5′‐TGACTTTGCTTTTGTGACTG‐3′ and rev 5′‐GTGTCCATTCACCATTATCC‐3′, Cyclin D1 for 5′‐GAAGATCGTCGCCACCTG‐3′ and rev 5′‐GAAGCGGTCCAGGTAGTTCA‐3′, Survivin for 5′‐TGCCCCGACGTTGCC‐3′ and rev 5′‐CAGTTCTTGAATGTAGAGATGCGGT‐3′. PCR profile was as follows: 10 min at 95 °C, followed by 45 cycles of 10 s at 95 °C and 8 s at 60 °C and 20 s at 72 °C. Data were analysed using Sequence Detection System Software (Applied Biosystems, Foster City, CA, USA). Parameter C t (threshold cycle) was defined as cycle number at which the fluorescent signal passed a fixed value (threshold) above baseline. Relative mRNA copy numbers were calculated from standard curves generated with 10‐fold dilution series of template DNA. Copy number of each target gene was normalized to housekeeping gene RNA polymerase 18S: for 5′‐CGGCTACCACATCCAAGGAA‐3′; and rev‐5′‐GCTGGAATTACCGCGGCT‐3′.
Western blotting
A549 cells were plated on six‐well plates in normal culture medium up to 50% confluence. Cells were then rinsed in sterile phosphate‐buffered saline (PBS) and cultured for further 48–72 h in medium with or without serum, in the presence or absence of different concentrations of zoledronic acid (10–100 μm). Culture medium was discarded and cells were harvested in lysis buffer [20 mmol/l Tris (pH 7.4), 150 mmol/l NaCl, 1 mmol/l EDTA, 1 mmol/l EGTA, 1% Triton, 2.5 mmol/l sodium PPi, 1 mmol/l h‐glycerolphosphate, 1 mmol/l Na3VO4, 1 Ag/ml leupeptin; Cell Signaling, Beverly, MA, USA] and clarified by centrifugation. After boiling supernatants in reducing SDS sample buffer, equal amounts of protein (80 ng) were loaded per lane and samples were electrophoresed on 15% polyacrylamide SDS gel and transferred to nitrocellulose membranes. VEGF was detected with antibody SC‐507 and actin was detected with antibody SC‐8432 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), according to manufacturers’ recommendations. Phosphorylation status of p38 was studied with anti‐phospho‐p38 and anti‐total p38 antibodies (Cell Signaling), as recommended by the manufacturer. Protein bands were visualized by chemiluminescence using an enhanced chemiluminescence kit (Amersham Europe GMBH, Cologno Monzese, Italy).
Cell cycle analysis
Sub‐confluent A549 cells (treated with increasing concentrations of Zol for 48 and 72 h) were removed from culture dishes by trypsinization, washed twice in PBS and incubated in PBS containing 0.12% Triton X‐100, 0.12 mm EDTA, and 100 μg/ml DNase‐free ribonuclease A (Sigma Chemical, St Louis, MO, USA). Then, 50 μg/ml propidium iodide (Sigma) was added to each sample for 20 min at 4 °C, in the dark. Stained nuclei were analysed by flow cytometry (FACScan; BD Biosciences, Franklin Lake, NJ, USA) using cellquest Software. Cell cycle distribution was based on 2N and 4N DNA content.
Annexin V flow cytometric analysis of apoptosis
To assess the effect of Zol on apoptosis, A549 cells were analysed by flow cytometric detection of fluorescein isothiocyanate (FITC)‐labelled annexin‐V. Annexin‐V‐FITC binds to phosphatidylserine residues, which are translocated from the inner to the outer leaflet of plasma membranes during early stages of apoptosis. Briefly, adherent cells of A549 were plated at final concentration of 3 × 105 cells. After 2–3 days exposure to Zol (10–100 μm), cells were detached using trypsin/ethylenediaminetetraacetic acid solution. Then, cells were collected and washed in PBS. A549 cells (approximately 1 × 105 cells per sample) were stained with annexin V‐FITC (Molecular Probes, Poortgebouw, Netherlands) for 15 min at room temperature in the dark. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, Buccinasco, Italy) and 5000–10 000 events were acquired for each sample. Apoptosis was evaluated measuring percentage of mean fluorescence intensity (%MFI) using FLI‐H (Log scale). Data were collected and analysed with cellquest 3.1 Software (Becton Dickinson) equipped with an MC100 automatic photomicrograph camera. Analysis was carried out by triplicate determination on at least three separate experiments.
Statistical analysis
Statistical analyses were performed using chi‐square test with epi‐info Software (Ver. 3.5.1). P‐value <0.05 was considered statistically significant.
Results
Zoledronic acid decreased cell viability by apoptotic cell death
Effects of Zol on viability, both of A549 and 95D cells, was analysed using the MTT cytotoxicity assay, in presence or absence of the drug. After 48‐h incubation, with or without 10% foetal bovine serum, cell survival was determined by ability of viable cells to reduce MTT dye to formazan. Zol induced significant (P = 0.0001) concentration‐dependent cell death of adherent cells exposed to 10–100 μm of drug for 2 days (Fig. 1a,b). Cytotoxic effects became more evident after exposure to ≥50 μm Zol concentration. 10 μm Zoledronic acid had little effect on viability of A549 and 95D cells after 48‐h incubation. In contrast, Zol at concentrations higher than 10 μm caused sharp dose‐dependent reduction in viability of serum‐incubated A549 and 95D cells compared to control cells (31% and 30% reduction at 10 μm; 62% and 64% reduction at 100 μm, P < 0.0001); similar reduction was seen in starved A549 and 95D cells (30% and 37% decrease with 10 μm; 73% and 74% decrease at 100 μm Zol concentration, respectively, P < 0.0001).
Figure 1.
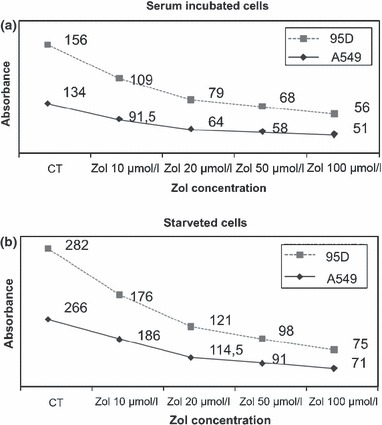
Zol induces dose‐dependent reduction of viability of A549 and 95D cells. (a) MTT cytotoxic assay of serum‐incubated A549 and 95D cells. (b) MTT cytotoxic assay of starved A549 and 95D cells.
Zoledronic acid induced cell cycle arrest and apoptosis
Flow cytometry analysis of DNA content was performed on A549 cells to identify cell cycle perturbations after Zol treatment for 48 h. A clear effect of Zol was seen only at highest concentration (100 μm). Figure 2a shows increase in number of cells arrested in G1‐phase after Zol treatment (from 76.1% in control cells to 83.6% in presence of 100 μm Zol, respectively). This observation was concomitant with reduction in cells in S‐phase: 10.1% for 100 μm Zol versus 20.1% in untreated A549 cells. Zol‐induced apoptosis was lower in presence (0.9% control versus 4.4% Zol‐treated) (Fig. 2a) than in absence (2.4% control versus 28.5% Zol‐treated) of serum (Fig. 2b) (P =0.0001). To investigate this result further, percentages of apoptotic cells were evaluated by flow cytometry, through detection of fluorescein‐labelled annexin V, which recognizes inverted phosphatidylserine on exterior surfaces of plasma membranes – an early stage apoptotic marker. Using this method, we confirmed that 100 μm zoledronic acid determined a statistically significant increase in apoptosis (43%) versus untreated cells [1.52; CI (1.35–1.72); P = <0.0001] in serum‐free condition (Fig. 2c).
Figure 2.
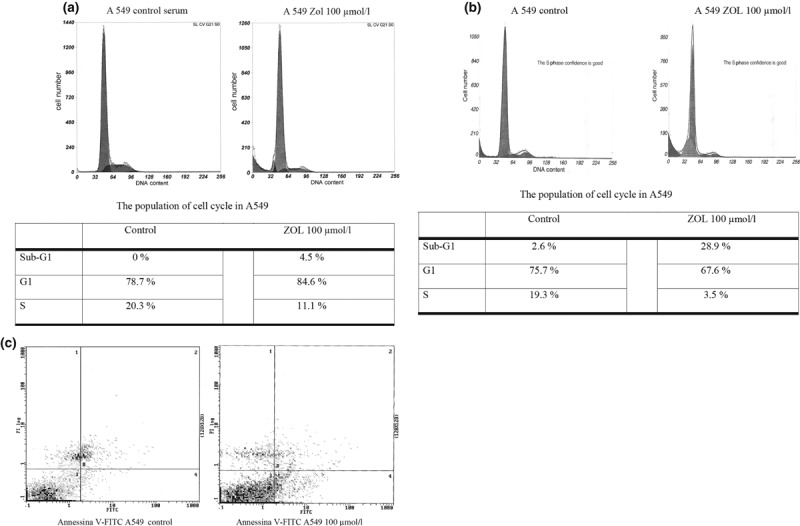
Zol induces cell cycle arrest and apoptosis. (a) Cell cycle analysis of A549 cells treated with Zol in presence of serum. (b) Cell cycle analysis of A549 cells starved and treated with Zol. (c) Apoptosis of A549 cells evaluated by annexin V flow cytometric analysis.
Zoledronic acid down‐regulated cyclins and survivin in A549 lung cancer cells
To identify molecules involved in Zol‐induced cell cycle arrest, we examined mRNA expression status of a selection of proteins involved in cell cycle regulation. At 50 and 100 μm, Zol significantly down‐regulated cyclin D1, cyclin B1 and survivin (Fig. 3a–c), whereas no differences were seen at lower doses of Zol (10–20 μm). The effect was striking for these three proteins and was dose‐dependent. mRNA decrease was of similar magnitude for all the three proteins; indeed, percentage of relative units ranged between 20% and 30% with Zol 50 μm, <5% with 100 μm Zol.
Figure 3.
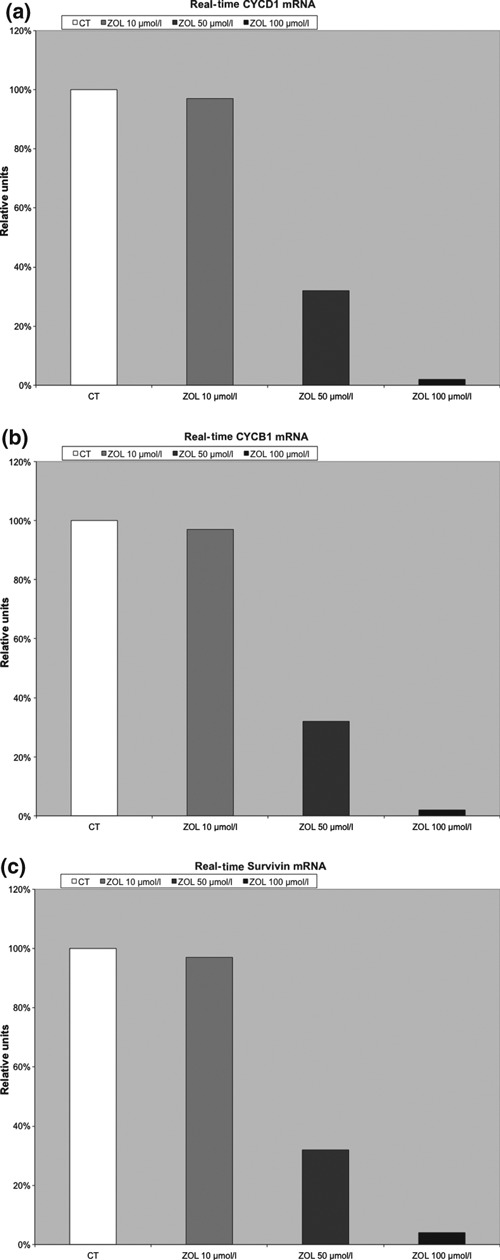
Zol effect on cyclins and survivin. (a) Zol significant down‐regulation of Cyclin D1. (b) Zol significant down‐regulation of cyclin B1. (c) Zol significant down‐regulation of survivin.
Zoledronic acid reduced VEGF expression and secretion
Effects of Zol on angiogenesis were studied by evaluating VEGF expression and secretion. After 48 h exposure to different concentrations of Zol (10–100 μm), dose‐dependent reduction of VEGF mRNA was observed, as shown by real time‐PCR (Fig. 4a). Close relationship to protein expression of VEGF was suggested by western blot analysis. Indeed, Fig. 4b shows clear reduction in VEGF protein after 50 μm Zol concentration. Accordingly, reduced VEGF secretion was seen in culture medium, as shown by ELISA. This effect was evident with concentrations of 50 μm or more, but was not detected with lower concentrations of Zol (10 μm) (Fig. 4c).
Figure 4.
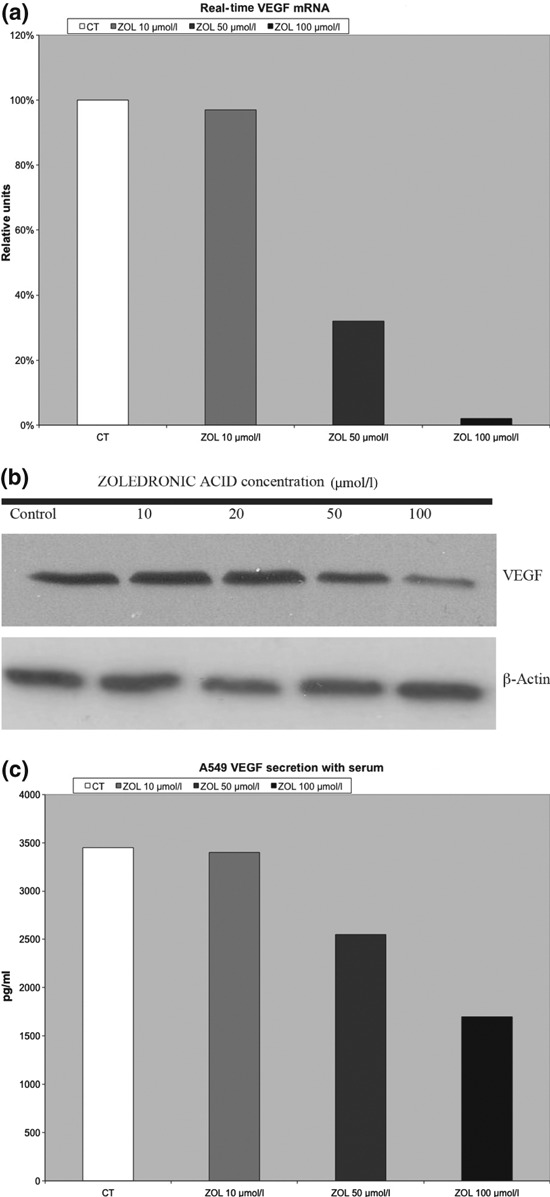
Zol reduces expression and secretion of VEGF. (a) Zol significant down‐regulation of VEGF mRNA. (b) Concentration‐course changes of VEGF in serum incubated A549 cells treated with Zoledronic acid by using western blot analysis. Equal amounts of protein were loaded in each lane. β‐actin was used as internal control. (c) Zol reduced VEGF secretion in culture medium.
Discussion
Over the last two decades, bisphosponates (Zol in particular), have been widely used for prevention of skeletal complications in patients with bone metastases of breast, lung and prostate cancers. Zol causes 50% reduction in risk of complications, which is significantly higher in comparison to non‐such treated patients, and it also seems to be at least 20% more efficient than pamidronate (14). In a small scale study, prophylactic administration of Zol to patients with a broad spectrum of tumours without skeletal involvement induced significant reductions in bone metastases in comparison to controls (15). Moreover, in NSCLC patients with bone metastases and high baseline levels of serum N‐telopeptide of type I collagen (NTX), Zol has been shown to reduce risk of death by 35% versus placebo treated (16). Clinical effects of Zol on survival have also been suggested in a randomized, open‐label study in previously untreated patients with multiple myeloma (17). Recently, three randomized studies of early breast cancer have demonstrated that Zol added to hormonal therapy after surgery, significantly improved not only bone mineral density but also DFS (18, 19, 20). In breast cancer, addition of Zol to neoadjuvant therapy also increased rate of pathologically complete responses in comparison to chemotherapy alone (21).
Preclinical experimental data suggest both direct and indirect anti‐cancer effects of bisphosponates (22). In murine models of myeloma, Zol has been shown to prevent development of bone involvement and tumour growth by increased survival (23). Moreover, in murine models of breast cancer, Zol seems to inhibit not only occurrence of bone metastases but also lung and liver metastases (24). Among possible anti‐tumoural mechanisms of Zol, the effect on angiogenesis has aroused the greatest interest. Previous studies suggested that Zol may inhibit angiogenesis through an apoptotic effect on endothelial cells and tumour microenvironment (25, 26). In our study, the MTT‐assay showed that Zol had a direct dose‐dependent cytotoxic effect on human lung cancer cell lines. We observed that Zol may have affected angiogenesis by direct down‐regulation of mRNA and protein expression of VEGF, derived by autocrine production of tumoural cells, not by paracrine secretion of endothelial cells and or from the microenvironment. These results are in concordance with the clinical observation of reduction in circulating VEGF levels after a single dose of biphosphonate, in patients with bone metastases from different tumours (3). Despite that VEGF serum levels rapidly rise with drug clearance, Zol activity in preventing skeletal complication was not affected. This might imply that not the sustained reduction in circulating VEGF, but events at tumour tissue level are related to the Zol effect.
In the present study, we also found that Zol was able to induce apoptosis in cancer cells of lines of human non‐small‐cell lung tumours. Exact mechanisms of the apoptotic signal of Zol remain unknown. The signal may involve alteration in Bcl‐2 expression and seems p53‐unrelated (27). In vitro reduction in cyclin D levels and G1 arrest of cells in our study are in agreement with a p53‐independent mechanism. This original observation contrasts with a recent finding of Li et al. who did not observe any apoptotic effect in Line‐1 lung murine adenocarcinoma cells (12). This discrepancy might be due to them being different models, but proapoptotic effects of Zol suggested by our experiments are more in agreement with results obtained in cell lines of other human cancers. Dose‐dependent down‐regulation of survivin in our experiments is in accord with pro‐apoptotic effects of Zol and deserves more attention, given the emerging role of survivin as a target in lung cancer (28). On the other hand, G1‐phase arrest of cycling cells that we have observed might be also consistent with reports that Zol enhances cisplatin cytotoxicity (13).
A major concern with preclinical data is that maximum detectable serum concentration after single administration of Zol is 10–100 times lower than that required to induce apoptosis and growth inhibition in vitro (29). Drug concentrations and exposure times used in our experiments are commonly reported and are in agreement with further preliminary assays in our laboratory. Furthermore, other authors have found that the apoptotic effect of Zol on breast cancer cells was greater with drug concentration of 100 μmol in the medium (11, 12, 13, 30). It should be considered, however, that around 55% total dose of Zol after administration is diverted to the skeleton from which location it is slowly released into the circulation (final half‐life 7 days) (31). Binding to bone matrix allows Zol to reach high concentration gradient and to carry out its anti‐cancer effects selectively in this tissue, rather than in other organs. However, real concentration of Zol in the tumour microenvironment in vivo is unknown. Results of the above‐mentioned clinical trials in breast cancer suggest that Zol concentration in target tissues is metabolically active and that slow release from bone or other storing tissues might have a role.
Overall, our study suggests that Zol may have an anti‐cancer and anti‐angiogenic effect through its ability to inhibit both tumour cell population growth and VEGF release in cell lines of human lung cancer. Ability to target tumour cell division was also shown when Zol was combined with several agents used in therapy for lung cancer (13, 30, 32). On the other hand, convergence of Zol effect on an endothelial target seems clinically confirmed by higher rate of jaw osteonecrosis when Zol is combined with bevacizumab (33). Taken together with our study results, these observations support the use of Zol in combination therapy for lung cancer as has been done for breast cancer. Continued inhibition of VEGF, however, might promote emergence of resistance through angiogenic pathways independent of VEGF; furthermore, the possibility of rapid vascular regrowth after reversal of VEGF inhibition has been shown in some cases (34, 35). Indications on this matter might be derived by NCT00172042 study, which is evaluating Zol in prevention of bone metastases in patients with stage III non‐small cell lung cancer.
References
- 1. Yuasa T, Kimura S, Ashihara E, Habuchi T, Maekawa T (2007) Zoledronic acid – a multiplicity of anti‐cancer action. Curr. Med. Chem. 14, 2126–2135. [DOI] [PubMed] [Google Scholar]
- 2. Wood J, Bonjean K, Ruetz S, Bellahcène A, Devy L, Foidart JM et al. (2002) Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J. Pharmacol. Exp. Ther. 302, 1055–1061. [DOI] [PubMed] [Google Scholar]
- 3. Santini D, Vincenzi B, Avvisati G, Dicuonzo G, Battistoni F, Gavasci M et al. (2002) Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin. Cancer Res. 8, 1080–1084. [PubMed] [Google Scholar]
- 4. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA et al. (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Eng. J. Med. 357, 2666–2676. [DOI] [PubMed] [Google Scholar]
- 5. Ramalingam SS et al. (2008) Outcomes for elderly, advanced‐stage non‐small‐cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J. Clin. Oncol. 26, 60. [DOI] [PubMed] [Google Scholar]
- 6. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342. [DOI] [PubMed] [Google Scholar]
- 7. Escudier B et al. (2007) Bevacizumab plus interferon alfa‐2a for treatment of metastatic renal cell carcinoma: a randomised, double‐blind phase III trial. Lancet 370, 2103. [DOI] [PubMed] [Google Scholar]
- 8. Llovet JM et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378. [DOI] [PubMed] [Google Scholar]
- 9. Lu S, Zhang J, Zhou Z, Liao ML, He WZ, Zhou XY et al. (2008) Synergistic inhibitory activity of zoledronate and paclitaxel on bone metastasis in nude mice. Oncol. Rep. 20, 581–587. [PubMed] [Google Scholar]
- 10. Al Husaini H, Wheatley‐Price P, Clemons M, Shepherd FA (2009) Prevention and management of bone metastases in lung cancer: a review. J. Thorac. Oncol. 4, 251–259. [DOI] [PubMed] [Google Scholar]
- 11. Matsumoto S, Kimura S, Segawa H, Kuroda J, Yuasa T, Sato K et al. (2005) Efficacy of the third‐generation bisphosphonate, zoledronic acid alone and combined with anti‐cancer agents against small cell lung cancer cell lines. Lung Cancer 47, 31–39. [DOI] [PubMed] [Google Scholar]
- 12. Li Y‐Y, Chang JW‐C, Chou W‐C, Liaw C‐C, Wang H‐M, Huang J‐S et al. (2007) Zoledronic acid is unable to induce apoptosis, but slows tumor growth and prolongs survival for non‐small‐cell lung cancers. Lung Cancer 59, 180–191. [DOI] [PubMed] [Google Scholar]
- 13. Ozturk OH, Bozcuk H, Burgucu D, Eckincl D, Ozdogan M, Akca S et al. (2007) Cisplatin cytotoxicity is enhanced with zoledronic acid in A 549 lung cancer cell line: preliminary results of an in vitro study. Cell Biol. Int. 31, 1069–1071. [DOI] [PubMed] [Google Scholar]
- 14. Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M et al. (2003) Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double‐blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J. Clin. Oncol. 21, 3150–3157. [DOI] [PubMed] [Google Scholar]
- 15. Coleman R (2001) The role of zoledronic acid in cancer: clinical studies in the treatment and prevention of bone metastases. Semin. Oncol. 28, 11–16. [DOI] [PubMed] [Google Scholar]
- 16. Hirsh V, Major PP, Lipton A et al. (2008) Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J. Thorac. Oncol. 3, 228–236. [DOI] [PubMed] [Google Scholar]
- 17. Avilés A, Nambo MJ, Neri N, Castañeda C, Cleto S, Huerta‐Guzmán J (2007) Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Med. Oncol. 24, 227–230. [DOI] [PubMed] [Google Scholar]
- 18. Gnant M, Mlineritsch B, Luschin‐Ebengreuth G, Kainberger F, Kässmann H, Piswanger‐Sölkner JC et al. (2008) Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early‐stage breast cancer: 5‐year follow‐up of the ABCSG‐12 bone‐mineral density substudy. Lancet Oncol. 9, 840–849, Epub 2008 Aug 19. [DOI] [PubMed] [Google Scholar]
- 19. Brufsky AM, Bosserman LD, Caradonna RR, Haley BB, Jones CM, Moore HC et al. (2009) Zoledronic acid effectively prevents aromatase inhibitor‐associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: Z‐FAST study 36‐month follow‐up results. Clin. Breast Cancer 9, 77–85. [DOI] [PubMed] [Google Scholar]
- 20. Eidtmann H, de BoerR, Bundred N, Llombart‐Cussac A, Davidson N, Neven P et al. (2010) Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36‐month results of the ZO‐FAST Study. Ann. Oncol. 21, 2188–2194. [DOI] [PubMed] [Google Scholar]
- 21. Coleman RE, Winter MC, Cameron D, Bell R, Dodwell D, Keane MM et al. (2010) The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti‐tumour activity in breast cancer. Br. J. Cancer 102, 1099–1105, Epub 2010 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clezardin P (2005) Anti‐tumour activity of zoledronic acid. Cancer Treat. Rev. 31(Suppl. 13), 1–8. [DOI] [PubMed] [Google Scholar]
- 23. Croucher PI, De Hendrik R, Perry MJ, Hijzen A, Shipman CM, Lippitt J et al. (2003) Zoledronic acid treatment of 5T2MM‐bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J. Bone Miner. Res. 18, 482–492. [DOI] [PubMed] [Google Scholar]
- 24. Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda T (2004) Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin. Cancer Res. 10, 4559–4567. [DOI] [PubMed] [Google Scholar]
- 25. Yamada J et al. (2009) Anti‐angiogenic property of zoledronic acid by inhibition of endothelial progenitor cell differentiation. J. Surg. Res. 151, 115–120. [DOI] [PubMed] [Google Scholar]
- 26. Corso A, Ferretti E, Lazzarino M (2005) Zoledronic acid exerts its antitumor effect in multiple myeloma interfering with the bone marrow microenvironment. Hematology 10, 215–224, Review. [DOI] [PubMed] [Google Scholar]
- 27. Kuroda J, Kimura S, Segawa H, Sato K, Matsumoto S, Nogawa M et al. (2004) P5‐indipendent anti‐tumor effects of the nitrogen‐containing bisphosphonate zoledronic acid. Cancer Sci. 95, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Falleni M, Pellegrini C, Marchetti A, Oprandi B, Buttitta F, Barassi F et al. (2003) Survivin in lung cancer. J. Pathol. 200, 620–626. [DOI] [PubMed] [Google Scholar]
- 29. Skerjanec A, Berenson J, Hsu C, Major P, Miller WH Jr, Ravera C et al. (2003) The pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with varying degrees of renal function. J. Clin. Pharmacol. 43, 154–162. [DOI] [PubMed] [Google Scholar]
- 30. Jagdev SP, Coleman RE, Shipman CM, Rostami‐H A, Croucher PI (2001) The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br. J. Cancer 84, 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S et al. (2002) Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J. Clin. Pharmacol. 42, 1228. [DOI] [PubMed] [Google Scholar]
- 32. Yildiz M, Celik‐Ozenci C, Akan S, Akan I, Sati L, Demir R et al. (2006) Zoledronic acid is synergic with vinblastine to induce apoptosis in a multidrug resistance protein‐1 dependent way: an in vitro study. Cell Biol. Int. 30, 278–282. [DOI] [PubMed] [Google Scholar]
- 33. Christodoulou C, Pervena A, Klouvas G, Galani E, Falagas ME, Tsakalos G et al. (2009) Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology 76, 209–211. [DOI] [PubMed] [Google Scholar]
- 34. Mitchell DC, Bryan BA (2010) Anti‐angiogenic therapy: adapting strategies to overcome resistant tumors. J. Cell. Biochem. 111, 543–553. [DOI] [PubMed] [Google Scholar]
- 35. Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T et al. (2006) Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Invest. 116, 2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]


