INTRODUCTION
Generation and regeneration of the various tissues of the body depends ultimately on a subset of cells described as stem cells, but the criteria used to define a ‘stem cell’ vary quite widely. At least some of this difficulty arises because of real biological differences between different types of stem cells. For example, embryonic stem cells and somatic stem cells appear to differ in their proliferative potentials and in the plasticity that enables entry into multiple pathways of differentiation (Orkin & Morrison 2002). However, even the properties of the somatic stem cells renewing a single tissue type, which might be expected to have essentially similar properties, may also vary quite widely. For example, the patterns of distribution, behaviour, and marker expression of epithelial stem cells differ depending on whether they are found in skin, oral mucosa, cornea, intestine or glands (Cotsarelis et al. 1999; Lavker & Sun 2000; Pellegrini et al. 2001; Marshman et al. 2002; Alonso & Fuchs 2003). The basic defining features common to somatic stem cells have been proposed to be first, a high capacity for self‐renewal and second, the ability to produce cells that differentiate to maintain tissue structure and function (Lajtha 1979). Many continuously renewing tissues, including blood and epithelia, have an additional proliferative phase where the cells generated by stem cell divisions enter the differentiation pathway but divide several times to amplify the maturing population before terminally differentiating (Potten 1981; Morrison et al. 1997). Such tissues therefore consist of a hierarchy of at least three cell types (Fig. 1): (i) stem cells which typically divide infrequently but retain an extensive self‐renewal capacity; (ii) amplifying cells that have a limited capability for proliferation; and (iii) post‐mitotic differentiating or differentiated cells (Tudor et al. 2004). Individual haematopoeitic stem cells normally generate several phenotypically different cell lineages and have also been shown to have extensive developmental plasticity, at least experimentally. This has led to the inclusion of pluripotentiality among some defininitions of stem cells (Huntly & Gilliland 2005). The generation of a multilineage pattern is a normal characteristic of epithelial stem cells in hair follicles, the gut, and glands (Oshima et al. 2001; Marshman et al. 2002) but whether this is a universal property of epithelial stem cells has yet to be established.
Figure 1.
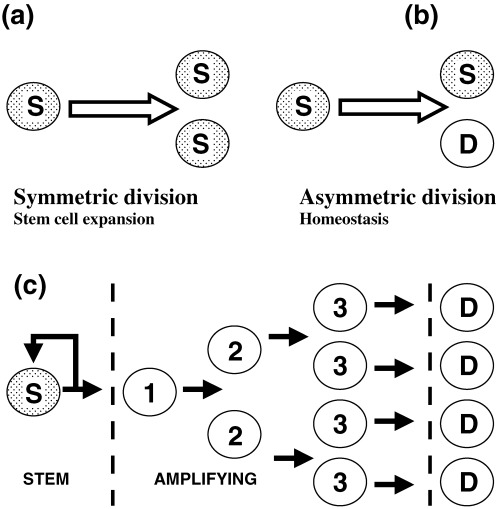
Stem cell division patterns in epithelia. (a, b) Illustrate two different stem cell division patterns. (a) A stem cell divides to produce two daughter cells identical with itself. This symmetric division pattern produces an expanding stem cell population and is characteristic of embryonic stem cells. (b) A stem cell divides to produce two disimilar cells, one of which remains identical to the parent stem cell and the second which has lost stem cell properties. This asymmetric division pattern is associated with the steady state renewal of tissues where the number of stem cells remains constant while feeding cells into a differentiation pathway. In epithelia and most other tissues there is typically also an amplification phase as shown in (c). Here, the non‐stem cell daughter and its progeny, described as amplifying cells, undergo a series of symetrical divisions to expand the differentiating cell population. As illustrated, the broken lines divide the cell population into three categories of stem, amplifying and differentiated cells. It is assumed that cells normally progress from left to right but it is uncertain whether there are sharp transitions between these compartments. Experimental evidence indicates that amplifying cells can regain stem cell properties if exposed to viral or developmental influences (Barrandon et al. 1989; Pearton et al. 2005). Factors that influence the fraction of total cells that are stem cells include the rate of stem cell division and the time cells take to differentiate. However, the number of amplification divisions has a major effect and adding or removing one amplification tier approximately halves or doubles the proportion of stem cells.
The concept that the growth of tumours, like the growth of normal tissues, depends on a subpopulation of stem cells was proposed many years ago (Hamburger & Salmon 1977) but until relatively recently questions about the presence of malignant stem cells in tumours were the source of much controversy (Denekamp 1994; Kummermehr 2001). This disagreement often seems to have been largely a question of semantics or to have arisen because of differing expectations about the criteria required to define a stem cell. For example, it is apparent that tumours are capable of continued growth and thus contain cells with a high capacity for self‐renewal, the first of Lajtha's stem cell criteria. Concerning Lajtha's second criterion, many tumours show patterns of differentiation which, although more or less abnormal, can be seen as an attempt to generate the structure of the tissues of origin. Disagreement thus seems to have focused, not on whether tumours contain indefinitely proliferating malignant cells that give rise to differentiating cells, i.e. contain cells with basic stem cell properties, but whether these cells have other stem cell properties such as generation of hierachies of proliferative cells with different regenerative abilities (Denekamp 1994; Kummermehr 2001). Many reports have indicated that both in vitro clonogenicity and in vivo re‐initiation of tumours is restricted to a small fraction of the total tumour cells. This could be explained by either a stem cell hierarchy or by stochastic mechanisms (Reya et al. 2001), but the presence of hierarchical proliferative patterns in leukaemias and in breast cancers has since been shown by prospective identification of ‘tumour initiating’ subpopulations with unique cell surface marker patterns (Bonnet & Dick 1997; Al Hajj et al. 2003). These observations are particularly important because they indicate that malignant stem cells form only a small fraction of the total cell population but, as the cells driving tumour growth, are the cells that need to be targeted for therapeutic elimination (Behbod & Rosen 2005). Further, normal stem and amplifying cells differ in a range of properties, including unique symmetric and asymmetric division patterns (Sherley 2002) which, if retained in malignancy, would be likely to produce differential effects on the responses of stem and amplifying cells to therapy, possibly with selective stem cell survival (Al Hajj & Clarke 2004; Jones et al. 2004). Stem cell self‐renewal probabilities are changed fundamentally in malignancy (Sherley 2002; Al Hajj & Clarke 2004) and malignant stem cells need to be identified and their division mechanisms better characterized to enable methods for manipulation of their growth to be developed.
EPITHELIAL STEM CELLS
The general properties of normal mammalian stem cells were first established by studies of haematopoiesis which demonstrated the existence of haematopoietic precursors that are able reconstitute all haematopoietic lineages (Morrison et al. 1995). As the most extensively studied stem cell system, information derived from haematopoietic stem cells has tended to provide a basic conceptual framework expected to fit other types of stem cell systems (Reya et al. 2001). In the haematopoietic system, stem cells form only a small subpopulation of the precursor cells, they divide infrequently to generate several phenotypic lineages, and the lineage‐committed cells show extensive amplification before terminal differentiation (Morrison et al. 1995; Sherley 2002; Al Hajj & Clarke 2004). Epithelial stem cells similarly divide relatively slowly to generate amplification hierarchies and form only part of the total proliferative population, although apparently a much larger fraction than in the haematopoietic system (Potten 1981; Tudor et al. 2004). Haematopoietic stem cells depend for their maintenance on ‘stem cell niches’ that provide supportive influences derived from secondary non‐haematopoietic cell populations (Spradling et al. 2001). Epithelial stem cells in structures such as intestinal villi and hair follicles also generate several phenotypic lineages and may be related to connective tissue niches generated by non‐epithelial cells (Oshima et al. 2001; Marshman et al. 2002). However, epithelia such as the interfollicular epidermis and oral mucosa have a less complex structure with simpler stem cell patterns that generate single phenotypic lineages, and apparently have more autonomy with less dependence on extra‐epithelial influences for their patterning and survival (Tudor et al. 2004). Intra‐epithelial patterning mechanisms, for example by delta/notch signalling (Lowell et al. 2000), may be of more importance in setting up the spacing of stem cell territories within such epithelia.
As a consequence of the hierarchical proliferative patterns present in epithelial tissues in vivo (Potten 1981), stem cells typically divide infrequently and can therefore be localized by their retention of an incorporated DNA label (Bickenbach 1981). In mice, label‐retaining stem cells are precisely distributed in relation to units of epithelial structure such as the small columnar units present in the epidermis, the rete of the oral mucosa, the bulge region of hair follicles, the limbal region of cornea, and crypts of the gut (Cotsarelis et al. 1999; Lavker & Sun 2000; Pellegrini et al. 2001; Marshman et al. 2002; Tudor et al. 2004). Stem cells can also be localized by examining the lineages arising from individual stem cells transduced with marker genes such as Lac‐Z and this has confirmed distribution patterns derived from label retention (Mackenzie 1997). Although the search for markers expressed exclusively by epithelial stem cells has been unsuccessful, it has identified some molecules, such as integrins, keratins and p63, that are expressed at higher levels by some human stem cells and can be used to identify ‘stem cell zones’ (Jones & Watt 1993; Cotsarelis et al. 1999). Staining for such markers suggests that the distribution of stem cells in human cornea and hair follicles corresponds to the equivalent murine tissues (Jones & Watt 1993; Cotsarelis et al. 1999). However, the stem cell positions in human epidermis determined by lineage marking do not correspond to the in vivo distribution of molecules such as β1‐integrin, whose expression has been functionally correlated with stem cell properties (Jones & Watt 1993; Ghazizadeh & Taichman 2005).
Structures such as glands and intestinal villi consist largely of simple epithelial cells but often contain a range of differing cellular phenotypes. Studies of mammary and prostate glands indicate differential expression of a range of markers such as keratins and integrins that appear to relate to the presence of stem, amplifying and differentiated populations (Clark 2005; Hudson 2005). Breast epithelia contain a ‘side population’ with the high ABC transporter function characteristic of stem cells (Welm et al. 2003) and the structure of both breast and prostate suggests a distribution of stem cells in relation to supportive mesenchymal niches. Intestinal crypts and breast (Potten et al. 2002; Smith 2005) both show the presence of cells that distribute newly synthesized chromosomes asymmetrically in keeping with the ‘immortal strand’ concept proposed as an anticancer mechanism (1978, 2002).
Despite these regionally specific patterns of stem cell distribution and behaviour, and the in vivo association of stem cells with stem cell niches, when epithelial cells are isolated and grown in vitro they show common patterns of clonogenicity that are largely independent of their tissue of origin. Human epithelial cells generate a range of differing colony types (Barrandon & Green 1985; Barrandon & Green 1987). Some individual founder cells generate compact colonies of small cells, most of which can be repeatedly passaged, others generate irregular colonies containing fewer cells capable of extensive growth, and yet others form colonies of large flattened cells that fail to proliferate after passage. These colony forms, referred to as holoclones, meroclones and paraclones, are considered to be derived from stem cells and early and late amplifying cells, respectively (Barrandon & Green 1987). Label‐retaining cells isolated from murine epithelia have been shown to be more clonogenic than other epithelial cells, a finding that supports a relationship between in vitro clonogenicity and in vivo stem‐like properties (Morris & Potten 1994). Micro‐dissection of the bulge region of hair follicles and of the limbal region of the cornea shows the presence of highly clonogenic cells localized to regions identified as stem‐cell‐rich zones by other methods such as label retention (Oshima et al. 2001; Pellegrini et al. 2001). The repeated regeneration of clonal heterogeneity as keratinocytes are passaged in vitro suggests that the generation of stem and amplifying cell hierarchies is largely an intrinsic ability of epithelial cells (Tudor et al. 2004). Although extra‐epithelial influences from stem cell ‘niches’ (Spradling et al. 2001) may be necessary to generate complex epithelial structure, they do not appear necessary to determine all aspects of basic stem cell behaviour.
CANCER STEM CELLS
Following earlier suggestions about the existence of stem cells in carcinomas (Hamburger & Salmon 1977; Pierce & Speers 1988), the development of immune deficient mice, and particularly the NOD‐SCID transplant model, enabled assessment of functional differences within cancer cell populations. The first study definitively to identify malignant stem cells did so by showing that the subpopulation of cells able to initiate acute human myeloid leukaemia in a SCID mouse model could be prospectively identified by its CD34+, CD38− phenotype (Bonnet & Dick 1997). Thus, the tumour‐initiating cells had a phenotype similar to normal haematopoietic stem cells and they were similarly able to generate hierarchical clones of differentiating cells. Further studies have since shown stem cell patterns in a range of other haematological tumours (Reya et al. 2001). The presence of stem cell hierarchies in solid tumours had been suggested by the pattern of re‐growth in irradiated murine epithelial tumours (Kummermehr 2001) and definitive evidence for their presence was provided when it was shown that cells able to re‐initiate human breast cancer can be prospectively identified as a CD44+, ESA+, CD24−/low subpopulation (Al Hajj et al. 2003). In the same year, several other reports described stem cell patterns in tumours of the central nervous system and showed that neural tumours were initiated by cells with a CD133+ phenotype (Ignatova et al. 2002; Singh et al. 2003; Welm et al. 2003) and recently a stem cell pattern has been shown within lung cancer (Kim et al. 2005).
STEM CELL PATTERNS IN CANCER CELL LINES
Key stem cell properties such as asymmetric division are extremely difficult to study in vivo and it would therefore be useful to have appropriate in vitro systems that retain stem cell patterns for such investigations (Sherley 2002). One of the first modern studies of tumour stem cells showed that only a small fraction of the cells isolated from a wide range of human tumours is clonogenic under defined in vitro conditions and pointed out the value of in vitro methods for studying the differential sensitivities of this putative malignant stem cell population (Hamburger & Salmon 1977). However, whether stem cell patterns persist in cell lines derived from tumours has been questioned, as has the value of information that might be derived from them (Pardal, Clarke & Morrison 2003). These questions seem to arise again as a result of differences of opinion about what a stem cell is expected to be. As reviewed above, epithelial stem cells in normal tissues have well‐ordered, region‐specific patterns of distribution and proliferation, and may generate several phenotypic lineages. This behaviour appears to result from cellular interactions occurring both between epithelial cells and between epithelial cells and matrix cells. In tumours, order is lost and it is uncertain how extensively stem cell behaviour is consequently disturbed. As malignant stem cells cannot yet be identified in tumours in situ, it remains to be elucidated how their behaviour, numbers and distribution in tumours differ from the normal tissue of origin. Similar questions also remain concerning stem cells in cancer cell lines; changes that may have occurred during adaptation to conditions in vivo, and the in vitro growth conditions themselves, are clearly likely to result in altered properties. Yet cell lines clearly contain cells with the basic stem cell property of unlimited proliferation and there is increasing evidence for their retention of a proliferative hierarchy.
Evidence that even malignant cell lines retain stem and amplifying cell patterns has come from several sources. The behaviourally different subpopulations of cells isolated from the MCF7 breast cell line by density sedimentation have a range of differing proliferative capabilities (Resnicoff et al. 1987) and, in the same cell line, some cells have the ‘side population’ characteristics of stem cells (Welm et al. 2003). Malignant cell lines derived from various other tissues also display similar ‘side populations’ (Hirschmann‐Jax et al. 2004; Setoguchi et al. 2004). While not corresponding fully to in vivo conditions, ‘organotypic’ cultures provide a more normal environment than standard cultures and cell lines derived from head and neck carcinomas contain only small subpopulations of cells with clonogenic characteristics under these conditions (Mackenzie 2003).
It is a feature of malignant cell lines that they characteristically show marked morphological heterogeneity and this has been associated with cellular diversity generated by genetic instability (Weiss 2000). This, together with uncertainty about the effects of cell adaptation to in vitro conditions (Burdall et al. 2003), has cast doubt on the value of cell lines for studies of malignant stem cell properties (Pardal et al. 2003). Definitive studies of malignant stem cells have therefore used freshly isolated tumour cells transplanted to immune deficient recipients, a system expected to mimic more closely the in vivo situation (Al Hajj et al. 2003). However, the morphological heterogeneity displayed by malignant epithelial cells has not been related to the fact that even normal keratinocytes are markedly heterogeneous in vitro. Normal keratinocytes generate a range of different colony forms that are classified morphologically as holoclones, meroclones and paraclones, and are considered to be generated, respectively, by stem cells and by early and late amplifying cells (Barrandon & Green 1987). Holoclones take the form of compact round colonies, paraclones form loose irregular colonies and meroclones have intermediate features.
Using essentially similar clonal assays, we have examined 15 cell lines derived from oral carcinomas, and the DU145 and MCF7 cell lines derived from prostate and breast tumours (Locke et al. 2005). Each of these malignant cell lines developed a range of colony morphologies paralleling the holoclone, meroclone and paraclone morphologies developed by normal keratinocytes (Fig. 2). The cells of malignant holoclones were found to be small, rapidly adhesive and highly clonogenic. They showed higher levels of expression of molecules such as β1‐integrin, β‐catenin, e‐cadherin, and cytokeratin 15 that are expressed more strongly by some epithelial stem cells (Cotsarelis et al. 1999; Tudor et al. 2004). Paraclones express some markers of early differentiation and meroclone and early paraclone colonies have relatively high rates of cell proliferation, suggesting their correspondence to early and late amplifying cells. Plating cells isolated from holoclone and paraclone colonies showed that the ability to generate new cell lines was restricted to holoclone cells and the new cell lines produced the full range of colony morphologies characteristic of the parental lines. Holoclones also showed stronger staining than paraclones for CD44, a molecule expressed by haematopoietic stem cells and the major distinguishing marker for tumour initiating cells in breast cancers (Al Hajj et al. 2003). The behaviour and patterns of marker expression of malignant holoclone cells were thus similar both to normal epithelial stem cells and to tumour‐initiating cells. These cells therefore appear to possess the essential defining properties of malignant stem cells.
Figure 2.
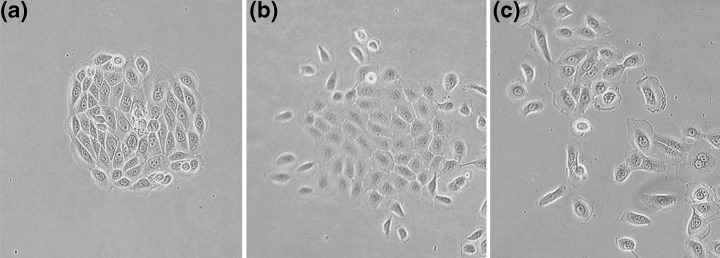
Clonal morphologies in malignant cell lines. Colony morphologies formed by the CA1 cell line which was derived from an oral carcinoma. (a) A holoclone characterized by its round colony outline and small, closely packed and slightly spindle‐shaped cells. (b) A meroclone with larger, more flattened, central cells that remain in contact with each other unlike cells at the periphery of the colony which have separated and acquired an ovoid outline. (c) A paraclone in which few flattened cells remain in contact and the colony consists largely of scattered ovoid cells. Differences in colony morphologies are readily distinguishable but the continuous gradient of change from one colony form to the next makes classification somewhat arbitrary.
CANCER STEM CELLS AS THE TARGETS OF THERAPY
Solid epithelial tumours are the major cause of cancer deaths and it has now been demonstrated that breast and lung cancers, like haematological malignancies, are driven by a small population of malignant stem cells (Al Hajj et al. 2003). In normal epithelia, stem and amplifying cells divide in different ways, have different apoptotic sensitivities, and different levels of expression of multidrug resistance transporters (Potten 2001; Hirschmann‐Jax et al. 2004; Kondo et al. 2004; Tsai 2004). Persistence of similar differences between malignant stem and amplifying cells could influence therapeutic outcomes. Stem cell resistance to drug effects, as a result, for example of proliferative quiescence or higher expression of ABC transporters, may result in survival of malignant stem cells. Identification of differential properties of stem and amplifying cells is therefore required to investigate the possibility of stem cell targeting, or at least to permit monitoring of the effects of therapeutic interventions on stem cells.
Some years ago the concept of ‘directed differentiation’ was raised as a potential way of producing tumour atrophy (Pierce & Speers 1988). The presence of hierarchical stem cell patterns in tumours suggests the possible effectiveness of a related concept based on shifting asymmetric division to produce stem cell loss. As illustrated in Fig. 1, normal tissue homeostasis results from a controlled pattern of asymmetric division and a shift away from this pattern is necessary for tumours to expand and metastasize. This results in changes that raise the probability of self‐maintenance to a value higher than the 0.5 value required to maintain a steady state. If, however, the system could be manipulated to reduce the probability of stem cell self‐renewal to below 0.5, tumour atrophy would effectively follow. How self‐renewal mechanisms can be translated therapeutically for the control of malignancy is unclear but stem cell division patterns can be manipulated in vitro by mechanisms related to p53 (Sherley 2002) and the Notch, Sonic hedgehog and Wnt signal‐transduction pathways have also been associated both with self‐renewal and with cancer (Molofsky et al. 2004; Tsai 2004). It has been suggested that similar self‐renewal mechanisms function in normal and malignant cells (Reya et al. 2001). Progress has been made investigating self‐renewal mechanisms in lower organisms (Faubert et al. 2004) but in vivo investigation of mechanisms controlling normal or malignant human stem cell division patterns is extremely difficult. If the mechanisms controlling asymmetric division are similar in vitro and in vivo, as the work reviewed above suggests, the presence of stem cell subpopulations in cell lines should assist molecular analyses of differences between stem and amplifying cells. The extensive information currently available about normal cell cycle control mechanisms has largely been generated using cell lines and presumably, for cancer cell lines, such data relate mainly to the majority non‐stem cell population. Analysis of holoclone and paraclone cells by RT‐PCR has identified a range of differentially expressed genes that may provide new markers to aid malignant stem cell identification (Locke et al. 2005). Further focus on mechanisms controlling asymmetric division in malignant stem cells might assist the development of new strategies for their therapeutic manipulation, based, for example on methods for inducing their exit from the stem cell compartment. The ability to identify molecular differences between holoclone and paraclone cells within malignant cell lines provides new opportunities, both for their identification and for the initial in vitro monitoring of the effectiveness of methods developed to target malignant stem cell populations.
ACKNOWLEDGEMENT
This work was supported in part by a grant from the Biotechnology and Biological Sciences Research Council.
REFERENCES
- Al Hajj M, Clarke MF (2004) Self‐renewal and solid tumor stem cells. Oncogene 23, 7274. [DOI] [PubMed] [Google Scholar]
- Al Hajj M, Wicha MS, Benito‐Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso L, Fuchs E (2003) Stem cells of the skin epithelium. Proc. Natl Acad. Sci. USA 100, 11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Green H (1985) Cell size as a determinant of the clone‐forming ability of human keratinocytes. Proc. Natl Acad. Sci. USA 82, 5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Green H (1987) Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl Acad. Sci. USA 84, 2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Morgan JR, Mulligan RC, Green H (1989) Restoration of growth potential in paraclones of human keratinocytes by a viral oncogene. Proc. Natl Acad. Sci. USA 86, 4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbod F, Rosen JM (2005) Will cancer stem cells provide new therapeutic targets? Carcinogenesis 26, 703. [DOI] [PubMed] [Google Scholar]
- Bickenbach JR (1981) Identification and behavior of label‐retaining cells in oral mucosa and skin. J. Dent. Res. 60, 1611. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3, 730. [DOI] [PubMed] [Google Scholar]
- Burdall SE, Hanby AM, Lansdown MR, Speirs V (2003) Breast cancer cell lines: friend or foe? Breast Cancer Res. 5, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R (2005) Isolation and characterization of human mammary stem cells. Cell Prolif. 38, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Kaur P, Dhouailly D, Hengge U, Bickenbach J (1999) Epithelial stem cells in the skin: definition, markers, localization and functions. Exp. Dermatol. 8, 80. [DOI] [PubMed] [Google Scholar]
- Denekamp J (1994) Tumour stem cells: facts, interpretation and consequences. Radiother. Oncol. 30, 6. [DOI] [PubMed] [Google Scholar]
- Faubert A, Lessard J, Sauvageau G (2004) Are genetic determinants of asymmetric stem cell division active in hematopoietic stem cells? Oncogene 23, 7247. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S, Taichman LB (2005) Organization of stem cells and their progeny in human epidermis. J. Invest. Dermatol. 124, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger AW, Salmon SE (1977) Primary bioassay of human tumor stem cells. Science 197, 461. [DOI] [PubMed] [Google Scholar]
- Hirschmann‐Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK (2004) A distinct ‘side population’ of cells with high drug efflux capacity in human tumor cells. Proc. Natl Acad. Sci. USA 101, 14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson D (2005) Epithelial stem cells in prostate growth and disease. Cell Prolif. 38, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntly BJ, Gilliland DG (2005) Leukaemia stem cells and the evolution of cancer‐stem‐cell research. Nat. Rev. Cancer 5, 311. [DOI] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA (2002) Human cortical glial tumors contain neural stem‐like cells expressing astroglial and neuronal markers in vitro . Glia 39, 193. [DOI] [PubMed] [Google Scholar]
- Jones PH, Watt FM (1993) Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Matsui WH, Smith BD (2004) Cancer stem cells: are we missing the target? J. Natl Cancer Inst. 96, 583–585. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T (2005) Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121, 823. [DOI] [PubMed] [Google Scholar]
- Kondo T, Setoguchi T, Taga T (2004) Persistence of a small subpopulation of cancer stem‐like cells in the C6 glioma cell line. Proc. Natl Acad. Sci. USA 101, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummermehr JC (2001) Tumour stem cells – the evidence and the ambiguity. Acta Oncol. 40, 981. [DOI] [PubMed] [Google Scholar]
- Lajtha LG (1979) Stem cell concepts. Differentiation 14, 23. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Sun TT (2000) Epidermal stem cells: properties, markers, and location. Proc. Natl Acad. Sci. USA 97, 13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M, Heydon M, Fawell S, Mackenzie IC (2005) Retention of intrinsic stem cell hierarchies in carcinoma‐derived cell lines. Cancer Res. 65, 8944. [DOI] [PubMed] [Google Scholar]
- Lowell S, Jones P, Le RI, Dunne J, Watt FM (2000) Stimulation of human epidermal differentiation by delta‐notch signalling at the boundaries of stem‐cell clusters. Curr. Biol. 10, 491. [DOI] [PubMed] [Google Scholar]
- Mackenzie IC (1997) Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J. Invest. Dermatol. 109, 377. [DOI] [PubMed] [Google Scholar]
- Mackenzie IC (2003) Growth of malignant oral epithelial stem cells after seeding into organotypical cultures of normal mucosa. J.Oral Path. Med. 33, 71. [DOI] [PubMed] [Google Scholar]
- Marshman E, Booth C, Potten CS (2002) The intestinal epithelial stem cell. Bioessays 24, 91. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Morrison SJ (2004) Diverse mechanisms regulate stem cell self‐renewal. Curr. Opin. Cell Biol. 16, 700. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Potten CS (1994) Slowly cycling (label‐retaining) epidermal cells behave like clonogenic stem cells in vitro . Cell Prolif. 27, 279. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Uchida N, Weissman IL (1995) The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 11, 35. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Wright DE, Cheshier SH, Weissman IL (1997) Hematopoietic stem cells: challenges to expectations. Curr. Opin. Immunol. 9, 216. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Morrison SJ (2002) Stem‐cell competition. Nature 418, 25. [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y (2001) Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233. [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ (2003) Applying the principles of stem‐cell biology to cancer. Nat. Rev. Cancer 3, 895. [DOI] [PubMed] [Google Scholar]
- Pearton DJ, Yang Y, Dhouailly D (2005) Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc. Natl Acad. Sci. USA 102, 3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, De McKeon F, , LM (2001) p63 identifies keratinocyte stem cells. Proc. Natl Acad. Sci. USA 98, 3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GB, Speers WC (1988) Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 48, 1996. [PubMed] [Google Scholar]
- Potten CS (1981) Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int. Rev. Cytol. 69, 271. [DOI] [PubMed] [Google Scholar]
- Potten CS (2001) Apoptosis in oral mucosa: lessons from the crypt. A commentary. Oral Dis. 7, 81. [PubMed] [Google Scholar]
- Potten CS, Hume WJ, Reid P, Cairns J (1978) The segregation of DNA in epithelial stem cells. Cell 15, 899. [DOI] [PubMed] [Google Scholar]
- Potten CS, Owen G, Booth D (2002) Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 115, 2381. [DOI] [PubMed] [Google Scholar]
- Resnicoff M, Medrano EE, Podhajcer OL, Bravo AI, Bover L, Mordoh J (1987) Subpopulations of MCF7 cells separated by Percoll gradient centrifugation: a model to analyze the heterogeneity of human breast cancer. Proc. Natl Acad. Sci. USA 84, 7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414, 105. [DOI] [PubMed] [Google Scholar]
- Setoguchi T, Taga T, Kondo T (2004) Cancer stem cells persist in many cancer cell lines. Cell Cycle 3, 414. [DOI] [PubMed] [Google Scholar]
- Sherley JL (2002) Asymmetric cell kinetics genes: the key to expansion of adult stem cells in culture. Stem Cells 20, 561. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821. [PubMed] [Google Scholar]
- Smith GH (2005) Label‐retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development 132, 681. [DOI] [PubMed] [Google Scholar]
- Spradling A, Drummond‐Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414, 98. [DOI] [PubMed] [Google Scholar]
- Tsai RY (2004) A molecular view of stem cell and cancer cell self‐renewal. Int. J. Biochem. Cell Biol. 36, 684. [DOI] [PubMed] [Google Scholar]
- Tudor D, Locke M, Owen‐Jones E, Mackenzie IC (2004) Intrinsic patterns of behavior of epithelial stem cells. J. Invest. Dermatol. Symp. Proc. 9, 208. [DOI] [PubMed] [Google Scholar]
- Weiss L (2000) Cancer cell heterogeneity. Cancer Metastasis Rev. 19, 345. [Google Scholar]
- Welm B, Behbod F, Goodell MA, Rosen JM (2003) Isolation and characterization of functional mammary gland stem cells. Cell Prolif. 36, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]


