Abstract
Abstract. Objective: Recent studies have demonstrated the potential of bone marrow‐derived cells (BMDC) to differentiate into cardiomyocytes. Up‐regulation of stromal cell‐derived factor‐1 (SDF‐1), a member of the chemokine CXC subfamily, mediating recruitment of BMDC has been documented in infarcted myocardium; however, it remains unknown whether SDF‐1 plays a role in cardiomyogenesis of BMDC. Materials and methods: Adherent BMDCs were cultured with SDF‐1, or specific inhibitor for PI3K, CXCR4 or Akt with SDF‐1, respectively. After 2 weeks, mRNAs and proteins from BMDCs were examined. Results: Two weeks after supplementation with SDF‐1, either murine or human adherent BMDC cultured in vitro expressed cardiac specific mRNAs (NKX2.5, atrial natriuretic factor and heavy chain β‐myosin) and proteins (troponin I and heavy chain cardiac myosin), and expression levels were partly decreased by combined treatment of CXCR4, PI3K or Akt inhibitor, with SDF‐1. Conclusions: The novel findings suggest that beyond its role in mobilization and homing of BMDC, SDF‐1 can promote BMDC to give rise to cardiomyocyte phenotypes in vitro, and the SDF‐1/CXCR4/PI3K/Akt pathway may be one of the molecular mechanisms regulating cardiomyogenesis.
INTRODUCTION
Recent studies have shown the potential of bone marrow‐derived cells (BMDC) to differentiate to cardiomyocytes in vitro and in vivo (Makino et al. 1999; Hakuno et al. 2002; Chen et al. 2006; Eisenberga et al. 2006), and pilot clinical trials have also demonstrated improvement of heart function led by BMDC infusion into patients with myocardial infarction (Schächinger et al. 2006; Assmus et al. 2006). Optimization strategies developed to enhance generation of BMDC into cardiomyocytes may augment effectiveness of the technology for treatment of myocardial infarction; however, this development will be hindered by current limited knowledge of mechanisms involved in differentiation of BMDC.
After transfusion into the infarcted heart, BMDCs encounter specific local environmental signals, including presence of chemokines, with capability to promote cardiac regeneration. Identification of these chemokines serves as base to unravel details of how BMDC give rise to cardiomyocytes, and raises the opportunity to develop new agents able to enhance efficacy of BMDC to differentiate into cardiomyocytes.
Stromal cell‐derived factor‐1 (SDF‐1) is a member of the chemokine CXC subfamily, and CXCR4 and CXCR7, two 7‐transmembrane receptors, are the known receptors for SDF‐1 (Nagasawa et al. 1996; Kucia et al. 2005; Burns et al. 2006). SDF‐1 is up‐regulated in the heart after myocardial infarction and plays a critical role in BMDC recruitment to the heart (Abbott et al. 2004). Recently, Kucia and Wojakowski demonstrated a small proportion of non‐adherent CXCR4+ BMDC expressing early cardiac markers (Kucia et al. 2004; Wojakowski et al. 2004). Based on these observational findings, we hypothesized that SDF‐1 could have a positive role to play in generation of cardiomyocytes from BMDC, and the SDF‐1/CXCR4/PI3K/Akt pathway could be involved in this process.
MATERIALS AND METHODS
The investigation conforms with the ‘Guide for the Care and Use of Laboratory Animals’ published by the US National Institutes of Health (NIH Publication no. 85‐23, revised 1996), and with principles outlined in the Declaration of Helsinki for use of human tissue or subjects.
Reagents and antibodies
Recombinant murine and human SDF‐1 were purchased from Biovision (Mountain View, CA, USA) and Cytolab (Rehovot, Israel). LY294002 (a specific inhibitor for PI3K) and AMD3100 (a specific inhibitor for CXCR4) were from Sigma (St. Louis, MO, USA), and SH‐5 (a specific inhibitor for Akt) was from Alexis (San Diego, CA, USA). Antibodies against Akt, phospho‐Akt (Thr‐308), troponin I and connexin 43 were obtained from Cell Signalling (Danvers, MA, USA). The monoclonal antibody to cardiac myosin heavy chain (MHC) was from Abcam (Cambridge, UK) and 4′,6‐diamidino‐2‐phenylindole (DAPI) was purchased from Roche (Mannheim, Germany).
Murine bone marrow‐derived cells
Murine BMDCs were isolated from 20‐ to 30‐day‐old Balb/c mice, as previously described (Edelberg et al. 2002). In brief, the mice were euthanized and femurs removed and cut proximally and distally. Bone marrow was flushed from the interior and plated on a culture vessel in low‐glucose Dulbecco's modified Eagle's medium (DMEM) with 10% foetal bovine serum (FBS). Adherent primary BMDCs were kept after 48 h, and the culture medium (low‐glucose DMEM + 10% FBS) was supplemented with recombinant murine SDF‐1 only (50 ng/mL), SDF‐1 (50 ng/mL) and LY294002 (10 µm), SDF‐1 (50 ng/mL) and AMD3100 (5 µg/mL), SDF‐1 (50 ng/mL) and SH‐5 (5 µm), or without additives and was changed every 2 days with the same formulation.
Human bone marrow‐derived cells
Fresh bone marrow, donated by two healthy men, was directly plated on a culture vessel in low‐glucose DMEM, supplemented with 10% FBS, and adherent BMDCs were kept after 48 h. The 5th‐passage adherent BMDCs were used in the following study, and the culture medium (low‐glucose DMEM + 10% FBS) was supplemented with or without recombinant human SDF‐1 (50 ng/mL) and was changed every 2 days.
Immunostaining
Murine BMDCs were plated on cover glasses in 6‐well tissue culture plates in culture medium (low‐glucose DMEM + 10% FBS) supplemented with or without SDF‐1. Medium was changed every 2 days, and cover glasses were prepared for immunostaining after 2 weeks. As previously described (Chen et al. 2006), BMDCs were stained for troponin I, connexin 43 and cardiac MHC, visualized with FITC‐ or TRITC‐conjugated secondary antibody and nuclei were stained with DAPI counterstain. All studies were performed in triplicate using samples from different culture preparations.
Reverse transcription‐polymerase chain reaction
Two weeks after replacement of medium supplemented with the different agents, RNA was isolated. After reverse transcription (50 °C for 50 min), Polymerase chain reaction (PCR) was performed using Premix Ex Taq (Takara, Dalian, China) with the use of primers showed in Table 1. The PCR programme consisted of a denaturation step for 2 min at 95 °C, followed by 35 cycles of 30 s of denaturation at 95 °C, 30 s of annealing at 55–60 °C depending on the primer combination, and 1 min of extension at 72 °C. Reactions were completed by a final extension step for 7 min at 72 °C. PCR reaction products were separated by agarose gel electrophoresis and analysed by densitometry using Quantity‐1 (Bio‐Rad, Hercules, CA, USA). Each sample was assessed in triplicate, and the mean density ratio of target mRNA to glyceraldehyde‐3‐phosphate dehydrogenase in each sample was used for statistical analysis.
Table 1.
Primers for reverse transcription‐polymerase chain reaction
| Product (bp) | Forward primer | Reverse primer |
|---|---|---|
| Murine ANF (282 bp) | 5′‐TTGGCTTCCAGGCCATATTG‐3′ | 5′‐AAGAGGGCAGATCTATCGGA‐3′ |
| Murine connexin 43 (243 bp) | 5′‐ACTTCAGCCTCCAAGGAGTTC‐3′ | 5′‐GTGTTACAGCGAAAGGCAGAC‐3′ |
| Murine β‐MHC (203 bp) | 5′‐GCCAACACCAACCTGTCCAAGTTC‐3′ | 5′‐TGCAAAGGCTCCAGGTCTGAGGGC‐3′ |
| Murine NKX2.5 (217 bp) | 5′‐CAGTGGAGCTGGACAAAGCC‐3′ | 5′‐TAGCGACGGTTCTGGAACCA‐3′ |
| Murine GAPD (156 bp) | 5′‐TTCCAGTATGACTCCACTCACG‐3′ | 5′‐AGACTCCACGACATACTCAGCA‐3′ |
| Human ANF (456 bp) | 5′‐ATGAGCTCCTTCTCCACCAC‐3′ | 5′‐TCAGTACCGGAAGCTGTTAC‐3′ |
| Human connexin 43 (232 bp) | 5′‐AGGCGTGAGGAAAGTACCAA‐3′ | 5′‐ACACCTTCCCTCCAGCAGTT‐3′ |
| Human β‐MHC (335 bp) | 5′‐ATCAAGGAGCTCACCTACCAG‐3′ | 5′‐AGCTGTTACACAGGCTCCAG‐3′ |
| Human NKX2.5 (205 bp) | 5′‐GAGAGTTTGTGGCGGCGATT‐3′ | 5′‐CGACGGCGAGATAGCAAAGG‐3′ |
| Human β‐actin (587 bp) | 5′‐CCAAGGCCAACCGCGAGAAGATGAC‐3′ | 5′‐AGGGTACATGGTGGTGCCGCCAGAC‐3′ |
ANF, atrial natriuretic factor; GAPD, glyceraldehyde‐3‐phosphate dehydrogenase; MHC, myosin heavy chain.
Western blotting
Two weeks after replacement of medium supplemented with the different reagents, levels of phospho‐Akt (Thr‐308) and total Akt and expression of troponin I, connexin 43 and cardiac MHC were analysed by Western blotting. Cell lysates were separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and were electrically transferred to polyvinylidene fluoride membranes. After incubation with primary antibody followed by secondary antibody conjugated to horseradish peroxidase, protein bands in Western blots were visualized by chemiluminescence, recorded on X‐ray films, and analysed by densitometry using Quantity‐1. Each sample was performed in triplicate, and the mean density ratio of target protein to β‐actin in each sample was used for statistical analysis.
Statistical analysis
Data are expressed as mean ± SE. Significance of differences among means was evaluated using analysis of variance (anova), followed by the Turkey's test for multiple comparisons. Statistical analysis was performed using SPSS (version 10.0, SPSS Inc., Chicago, IL, USA). Significant differences were defined as P < 0.05.
RESULTS
SDF‐1 promoting BMDC differentiation to cardiomyocyte phenotypes
In order to test the role of SDF‐1 in the differentiation of BMDC‐derived cardiomyocytes, murine and human BMDCs had been cultured in the medium with or without SDF‐1 for 2 weeks, then subjected to RT‐PCR analysis with primers specific for cardiomyocytes. Expression of several cardiac specific genes was assessed using RNA from murine ventricle and human atrium as positive controls. Figure 1 shows that in the presence of SDF‐1, both murine and human BMDCs express cardiac–specific mRNA such as NKX2.5, β‐MHC and a secretion product, atrial natriuretic factor (ANF). These data indicate a cardiomyocyte nature of BMDC‐derived cells.
Figure 1.

Representative RT‐PCR gene expression profiles of cultured BMDCs. Two weeks after supplemented with SDF‐1, either adherent primary murine (a) or 5th‐passage human (b) BMDCs, expressed cardiac–specific mRNAs encoding NKX2.5, β‐MHC and atrial natriuretic factor (ANF). Four samples were tested for the target mRNA, and three RT‐PCRs were separately performed for each sample. Murine ventricle and human atrial cells were used as positive controls (control (P)) and phosphate‐buffered saline as negative control (control (N)).
To further characterize the BMDC‐derived cells, Western blotting and immunostaining were performed; murine ventricle and human atrium were used as positive controls. As shown in Fig. 2, supplementation of the culture medium with SDF‐1 led both murine and human BMDC to express cardiac–specific proteins, cardiac MHC and troponin I. Consistent with the findings of RT‐PCR studies, these results support that SDF‐1 promotes BMDC differentiation into cardiomyocyte phenotypes in vitro.
Figure 2.
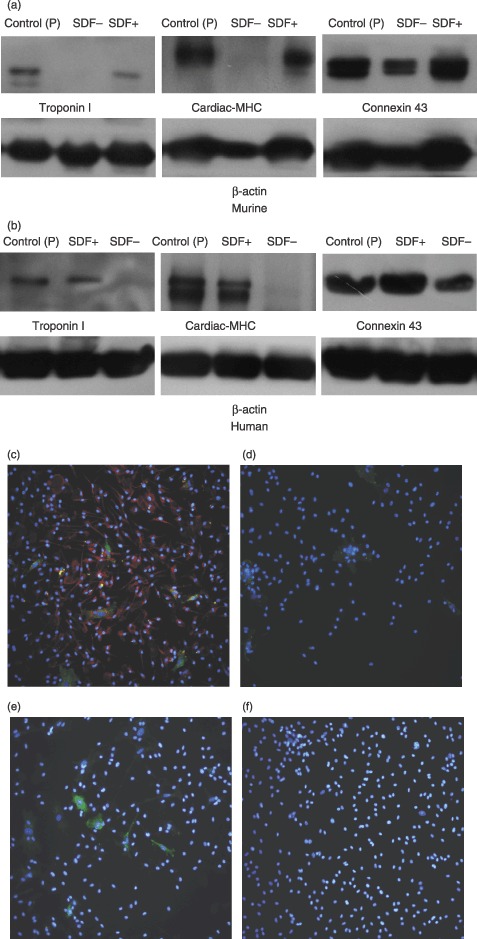
Western blotting and immunostaining study to show cardiomyogenesis of BMDCs. Two weeks after supplementation with SDF‐1, troponin I and cardiac MHC were observed in either adherent primary murine (a) or 5th‐passage human (b) BMDC, via Western blotting. Four samples were tested for the target protein, and three Western blots were separately performed for each sample. Murine ventricle and human atrial cells were used as positive controls (control (P)). (c) Immunostaining study to demonstrate cardiac MHC (red) and connexin 43 (green) with nuclear counterstain (blue) in murine cultured BMDC supplemented with SDF‐1. (d) In murine BMDCs supplemented without SDF‐1, only less connexin 43 (green) was found. (e, f) Compared to without SDF‐1 (f), treatment with SDF‐1 (e) induced murine BMDCs to express troponin I (green) (magnification ×400).
The observation of positive connexin 43 irrespective of SDF‐1 supplementation suggests that BMDC possess the capability to express connexin 43, in accordance with previous reports (Valiunas et al. 2004; Chang et al. 2006).
Contribution of SDF‐1/CXCR4/PI3K/Akt to differentiation of BMDC‐derived cardiomyocytes
To elucidate the molecular mechanisms of how SDF‐1 promotes BMDC differentiation into cardiomyocyte phenotypes, the potential functions of SDF‐1/CXCR4/PI3K/Akt pathway in the process of differentiation were investigated. As shown in 3, 4, although no statistically significant change was observed in quantitative analysis, there are trends towards down‐regulation of levels of mRNAs encoding ANF and β‐MHC and levels of proteins including cardiac MHC and troponin I after combination treatment with SDF‐1 and the specific inhibitors of CXCR4, PI3K and Akt, AMD3100, LY294002 and SH‐5, respectively.
Figure 3.
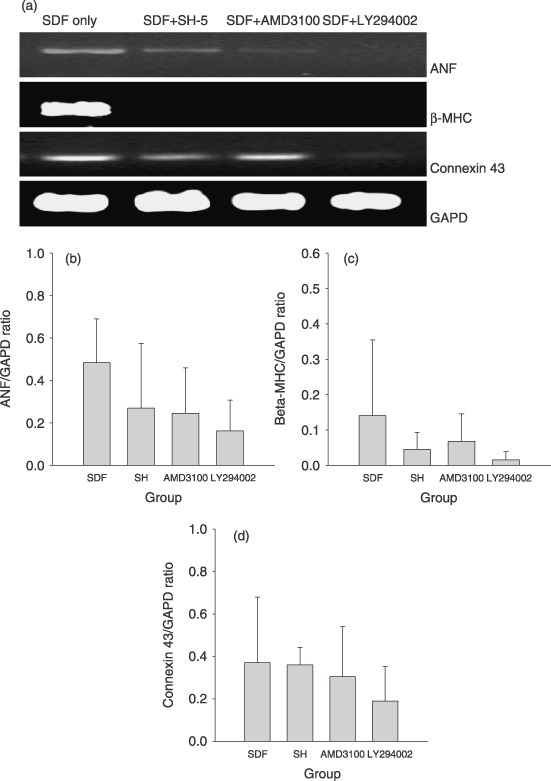
Combined treatment of the inhibitors of CXCR4 (AMD3100), PI3K (LY294002) or Akt (SH‐5), partly antagonized the effective of SDF‐1 on cardiomyogenesis. (a) Representative RT‐PCR gene expression profiles of murine BMDCs supplemented with SDF‐1 or mixture of SDF‐1 and different inhibitors. In the presence of any inhibitor, atrial natriuretic factor (ANF) and β‐MHC were obviously down‐regulated. (b–d) Quantification of density ratio of mRNA encoding ANF (b), β‐MHC (c) and connexin 43 (d) to glyceraldehyde‐3‐phosphate dehydrogenase (GAPD). After treatment with each inhibitor, a trend towards down‐regulation of ANF or β‐MHC mRNA was observed, even though the difference was not statistically significant, whereas no obvious change was found in connexin 43 mRNA. Five samples were tested for the target mRNA, and three RT‐PCRs were separately performed for each sample. Mean density ratio of target mRNA to GAPD in each sample was used for statistical analysis.
Figure 4.
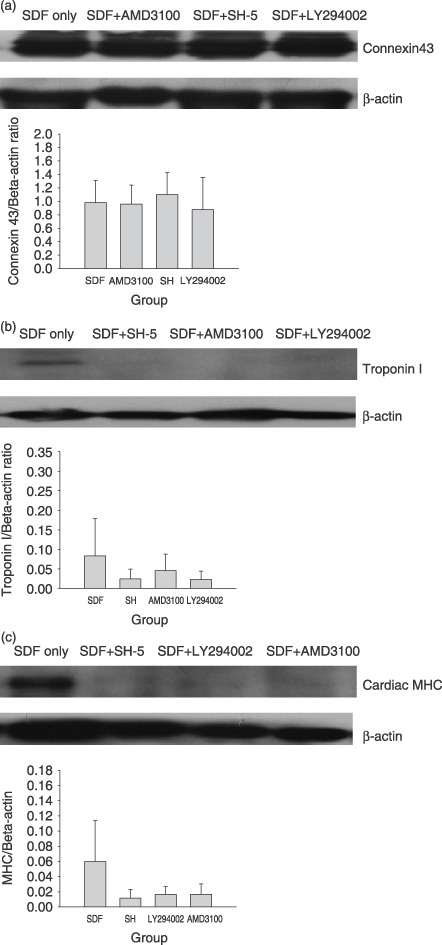
Western blotting and quantification analysis showed the antagonism of CXCR4, PI3K or Akt inhibitor to the effect of SDF‐1 on cardiomyogenesis. (a) No change of connexin 43 expression was found. (b, c) Compared to SDF‐1 alone, each inhibitor tended to decrease the expression of troponin I and cardiac MHC, although the decrease was not statistically significant. Five samples were tested for the target protein, and three Western blots were separately performed for each sample. Mean density ratio of target protein to β‐actin in each sample was used for statistical analysis.
In order to detect the effects of different reagents on phosphorylation of Akt, levels of phospho‐Akt (Thr‐308) and pan‐Akt were measured. As shown in Fig. 5, density ratio of phosphoryled Akt to total Akt was enhanced by the treatment of SDF‐1 from baseline 0.73 ± 0.07 to 1.01 ± 0.35, the ratio was reduced to 0.76 ± 0.12 after combination treatment with SDF‐1 and AMD3100, and phosphorylation of Akt was further inhibited after combination treatment with SDF‐1 and LY294002 (0.37 ± 0.11) or SH‐5 (0.68 ± 0.43). These findings indicate that the inhibitor of CXCR4, AMD3100, can antagonize up‐regulation of phosphoryled Akt associated with SDF‐1, and that the inhibitors of PI3K and Akt, LY294002 or SH‐5 not only offset the effect of SDF‐1 but also excessively down‐regulate the baseline of Akt phosphorylation of BMDC.
Figure 5.
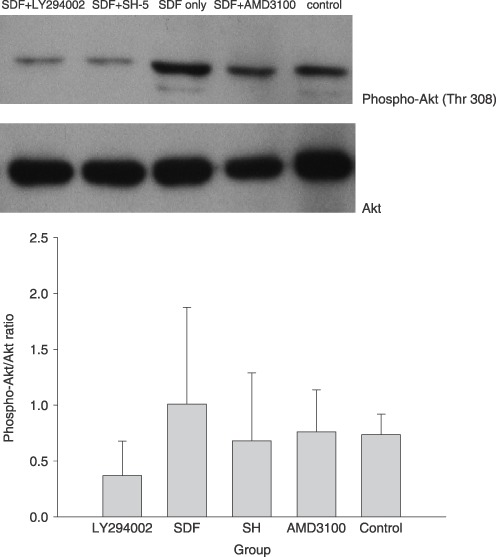
Phosphorylation of Akt was measured by the ratio of phospho‐Akt to total Akt. The phospho‐Akt/Akt ratio was lowered when BMDCs were cultured in medium supplemented with SDF‐1 and LY294002, a specific PI3K inhibitor, or SH‐5, a specific Akt inhibitor. Four samples were tested for phospho‐Akt and total Akt, and three Western blots were separately performed for each sample. Mean density ratio in each sample was used for statistical analysis.
These data suggest that the SDF‐1/CXCR4/PI3K/Akt pathway could play a role in SDF‐1‐associated differentiation of BMDC‐derived cardiomyocytes; however, inhibition of this pathway can not completely terminate the progression of differentiation.
DISCUSSION
There are two major findings from this study.
-
1
SDF‐1 promotes BMDC differentiation into cardiomyocyte phenotypes in vitro.
-
2
Activation of SDF‐1/CXCR4/PI3K/Akt partly contributes to this differentiation.
Up‐regulation of SDF‐1 mediating the recruitment of BMDCs has been previously documented in infarcted myocardium (Abbott et al. 2004); however, the function of SDF‐1 on BMDC differentiation into cardiomyocytes is uncertain. In this study, we reveal the potential of both murine and human BMDC cultured in the presence of SDF‐1 to give rise to cardiomyocyte‐like cells in vitro. Results of RT‐PCR for cardiac–specific mRNA (NKX2.5, β‐MHC and ANF) and Western blotting and immunostaining for cardiac MHC and troponin I confirmed the generation of BMDC‐derived cardiomyocytes. For the first time, our findings suggest that, beyond its role in mobilization and homing of BMDC, SDF‐1 may contribute to establishing a microenvironment supporting BMDC differentiation. However, as shown by the immunostaining study, not all BMDCs expressed cardiac markers, and spontaneous beating was not found after induction of SDF‐1. These data imply that SDF‐1 may be a participant, not exclusive factor, involved in the complex process of initiating and advancing differentiation from BMDCs into cardiomyocytes.
After binding to CXCR4, the SDF‐1/CXCR4 axis activates several intracellular signalling pathways, including PI3K/Akt pathway, crucial for both cell trafficking and interaction with the intercellular environment (Wang et al. 2000; Kucia et al. 2005; Alsayed et al. 2007). Recently, activation of PI3K/Akt has been proposed to play a crucial role in early cardiomyogenesis by crosstalk with the canonical Wnt pathway (Naito et al. 2003, 2005). To verify whether SDF‐1/CXCR4/PI3K/Akt is involved in SDF‐1‐associated differentiation, inhibitors specific to CXCR4, PI3K and Akt, respectively, were supplemented to culture media with SDF‐1. The results showed that these inhibitors antagonized the positive effects of SDF‐1 on BMDC differentiation, which supports a critical role for SDF‐1/CXCR4/PI3K/Akt in the process of SDF‐1 promoting cardiomyogenesis. However, cardiomyogenesis was not completely depressed after treatment of these inhibitors. Although the exact mechanisms are not determined by this study, we hypothesize that observation of incomplete depression can be attributed to the following possibilities:
-
1
In addition to PI3K/Akt, there are other important pathways involved in signalling from the SDF‐1/CXCR4 axis, such as protein kinase C (PKC) (Wang et al. 2000; Kucia et al. 2005), which likely promotes cardiac differentiation via the noncanonical protein kinase C‐dependent signalling pathway (Koyanagi et al. 2005).
-
2
Although CXCR4 is the predominant receptor, non‐CXCR4 receptors (e.g. CXCR7) reacting with SDF‐1, have been reported (Rubbert et al. 1998; Burns et al. 2006). If non‐CXCR4 receptors are expressed on the cell membrane of BMDCs, the incomplete antagonism of AMD3100, a CXCR4 inhibitor, to SDF‐1 observed in this study, can be explained.
-
3
The inhibitors cannot totally block activation of their target kinases.
Kucia and Wojakowski have demonstrated that a small proportion of non‐adherent CXCR4+‐BMDC express the early cardiac markers (Kucia et al. 2004; Wojakowski et al. 2004). However, this cell population would be irrelevant to the adherent BMDC differentiation associated with SDF‐1 in our study. Previous work has revealed cardiomyocytes derived from mesenchymal stem cells (MSC) (Makino et al. 1999) and endothelial progenitor cells (EPC) (Badorff et al. 2003), the subpopulations of adherent BMDCs, and CXCR4 expression in a percentage of these cells (Yamaguchi et al. 2003; Wynn et al. 2004). Thus, it is reasonable to speculate that part of CXCR4+‐MSC or CXCR4+‐EPC may be the main cells responsible for stimulation of SDF‐1, and once identified and purified, these cells could be used to further enhance the efficacy of stem cell transplantation, and reduce the occurrence of side effects related to other cell types.
It should be noted that verification of our findings in an in vivo trial is very important, because other various biochemical stimuli constituting the local milieu around an infracted zone is likely to complicate the influences of SDF‐1. In addition, it is prudent to validate the present observations using inhibitory treatment mediated by gene regulation before final conclusions are drawn. It has recently been reported that bone marrow MSC constitutively express SDF‐1 (Nakayama et al. 2007), and it appears that spontaneous differentiation from BMDCs into cardiomyocytes should be observed, according to our hypothesis. However, expression of SDF‐1 in culture medium 1–3 days after replacement was not found by our enzyme‐linked immunosorbent assay study (data not shown). Therefore, it is reasonable that specific cardiac makers were not demonstrated in BMDCs without SDF‐1 here.
In summary, the findings of our present study provide, for the first time, a novel insight into the role of SDF‐1 in cardiomyogenesis of BMDCs, and raise the possibility of the SDF‐1/CXCR4/PI3K/Akt pathway as a molecular mechanism for cardiomyocyte differentiation.
ACKNOWLEDGEMENTS
This work was supported by grants from the Chinese National Nature Science Foundation (30600607, 30770877), China Postdoctoral Science Foundation (20060400305) and Chinese Medicine Board.
REFERENCES
- Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ (2004) Stromal cell‐derived factor‐1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 110, 3300–3305. [DOI] [PubMed] [Google Scholar]
- Alsayed Y, Ngo H, Runnels J, Leleu X, Singha UK, Pitsillides CM, Spencer JA, Kimlinger T, Ghobrial JM, Jia X, Lu G, Timm M, Kumar A, Cote D, Veilleux I, Hedin KE, Roodman GD, Witzig TE, Kung AL, Hideshima T, Anderson KC, Lin CP, Ghobrial IM (2007) Mechanisms of regulation of CXCR4/SDF‐1 (CXCL12)‐dependent migration and homing in multiple myeloma. Blood 109, 2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmus B, Honold J, Schächinger V, Britten MB, Fischer‐Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM (2006) Transcoronary transplantation of progenitor cells after myocardial infarction. N. Engl. J. Med. 355, 1222–1232. [DOI] [PubMed] [Google Scholar]
- Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S (2003) Transdifferentiation of blood‐derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation 107, 1024–1032. [DOI] [PubMed] [Google Scholar]
- Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold MET, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ (2006) A novel chemokine receptor for SDF‐1 and I‐TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 203, 2201–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, Marbán E, Abraham R (2006) Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation 113, 1832–1841. [DOI] [PubMed] [Google Scholar]
- Chen M, Fan ZC, Liu XJ, Deng JL, Zhang L, Rao L, Yang Q, Huang DJ (2006) Effects of autologous stem cell transplantation on ventricular electrophysiology in doxorubicin‐induced heart failure. Cell Biol. Int. 30, 576–582. [DOI] [PubMed] [Google Scholar]
- Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S (2002) Young adult bone marrow‐derived endothelial precursor cells restore aging‐impaired cardiac angiogenic function. Circ. Res. 90, E89–E93. [DOI] [PubMed] [Google Scholar]
- Eisenberga CA, Burchb JBE, Eisenberga LM (2006) Bone marrow cells transdifferentiate to cardiomyocytes when introduced into the embryonic heart. Stem Cells 24, 1236–1245. [DOI] [PubMed] [Google Scholar]
- Hakuno D, Fukuda K, Makino S, Konishi F, Tomita Y, Manabe T, Suzuki Y, Umezawa A, Ogawa S (2002) Bone marrow‐derived regenerated cardiomyocytes (CMG Cells) express functional adrenergic and muscarinic receptors. Circulation 105, 380–386. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Haendeler J, Badorff C, Brandes RP, Hoffmann J, Pandur P, Zeiher AM, Kuhl M, Dimmeler S (2005) Non‐canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J. Biol. Chem. 280, 16838–16842. [DOI] [PubMed] [Google Scholar]
- Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ (2004) Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ. Res. 95, 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska‐Wieczorek A, Ratajczak J, Ratajczak MZ (2005) Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF‐1‐CXCR4 axis. Stem Cells 23, 879–894. [DOI] [PubMed] [Google Scholar]
- Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S (1999) Cardiomyocytes can be generated from marrow stromal cells in vitro . J. Clin. Invest. 103, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T (1996) Defects of B‐cell lymphopoiesis and bone‐marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF‐1. Nature 382, 635–638. [DOI] [PubMed] [Google Scholar]
- Naito AT, Akazawa H, Takano HT, Nagai MT, Aburatani H, Komuro I (2005) Phosphatidylinositol 3‐Kinase‐Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt Signaling. Circ. Res. 97, 144–151. [DOI] [PubMed] [Google Scholar]
- Naito AT, Tominaga A, Oyamada M, Oyamada Y, Shiraishi I, Monzen K, Komuro I, Takamatsu T (2003) Early stage‐specific inhibitions of cardiomyocyte differentiation and expression of Csx/Nkx‐2.5 and GATA‐4 by phosphatidylinositol 3‐kinase inhibitor LY294002. Exp. Cell. Res. 291, 56–69. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Mutsuga N, Tosato G (2007) FGF2 posttranscriptionally down‐regulates expression of SDF1 in bone marrow stromal cells through FGFR1 IIIc. Blood 109, 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubbert A, Combadiere C, Ostrowski M, Arthos J, Dybul M, Machado E, Cohn MA, Hoxie JA, Murphy PM, Fauci AS, Weissman D (1998) Dendritic cells express multiple chemokine receptors used as coreceptors for HIV entry. J. Immunol. 160, 3933–3941. [PubMed] [Google Scholar]
- Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann HYuJT, Corti R, Mathey DG, Hamm CW, Sûselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM (2006) Intracoronary bone marrow‐derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 355, 1210–1221. [DOI] [PubMed] [Google Scholar]
- Valiunas V, Doronin S, Valiuniene L, Potapova I, Zuckerman J, Walcott B, Robinson RB, Rosen MR, Brink PR, Cohen IS (2004) Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J. Physiol. 555, 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JF, Park IW, Groopman JE (2000) Stromal cell‐derived factor‐1alpha stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide‐3 kinase and protein kinase C. Blood 95, 2505–2513. [PubMed] [Google Scholar]
- Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ (2004) Mobilization of CD34/CXCR4+, CD34/CD117+, c‐met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation 110, 3213–3220. [DOI] [PubMed] [Google Scholar]
- Wynn RF, Hart CA, Corradi‐Perini C, O’Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I (2004) A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104, 2643–2645. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch‐Marce M, Masuda H, Losordo DW, Isner JM, Asahara T (2003) Stromal cell‐derived factor‐1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 107, 1322–1328. [DOI] [PubMed] [Google Scholar]


