Abstract
Abstract. Many peptides, hormones and growth factors have been implicated in the control of cell renewal in the gastrointestinal epithelium. Leptin is present in the stomach and salivary glands and leptin receptors are seen throughout the gut. Leptin can stimulate mitogen‐activated protein kinase activity in vitro and short‐term infusion has been reported to have a proliferative action on the colon in vivo, suggesting a biological link between obesity, physical activity and colon cancer. Food intake is one of the most important determinants of intestinal mucosal cell renewal, thus any direct effects of leptin on the gut may be hidden. This problem has been avoided experimentally by maintaining animals on total parenteral nutrition (TPN). Male Wistar rats were anaesthetized and cannulae were inserted into the jugular vein to deliver the TPN diet to which had been added 0, 0.5, 2.5, or 10 mg/kg of recombinant murine leptin. Orally fed rats were also studied. After 6 days of treatment, all animals were injected with vincristine and killed 2 h later. Tissue weight was recorded and crypt cell proliferation (arrested metaphases) and crypt fission were scored in ‘microdissected’ crypts. Leptin infusion led to a small decrease in body weight and in the weight of the caecum. Intestinal cell proliferation was significantly reduced by TPN when compared to the orally fed rats, but the addition of leptin had no effect on the small intestine or colon. Crypt fission was also significantly lowered in the TPN group. Fission was slightly but significantly increased in the proximal and mid‐colon of the leptin‐treated rats, but was decreased in the distal colon. Although leptin did not significantly alter cell proliferation, it had significant effects on the process of crypt fission in the colon, which varied according to the exact locality.
INTRODUCTION
Several peptide hormones and growth factors have been implicated in the control of gastrointestinal epithelial cell renewal (Goodlad et al. 2001), and the adipocyte‐derived hormone leptin has recently been added to this list (Schneider et al. 2001).
Leptin is the product of the ob gene and is produced by fat cells. It is released into the circulation to signal fat status, modify food intake and energy expenditure, and also to signal and modify reproductive status. Leptin is the signal that informs the brain that energy stores are sufficient to support the high energy demands of reproduction, and may be a major determinant of the timing of puberty (Ahima et al. 1997).
Leptin is also found in the gastrointestinal juice, where it is stored in the stomach and salivary glands; moreover, the leptin receptor is seen throughout the gut (Attele et al. 2002) suggesting a physiological role in the regulation of nutrient absorption (Barrenetxe et al. 2002). Leptin can stimulate mitogen‐activated protein kinase (MAPK) activity in vitro (Takahashi et al. 1997) and increase the proliferation of gastric mucosa cell lines (Schneider et al. 2001). The leptin receptor is expressed in human colon cancer cell lines and human colonic tissue. Short‐term leptin treatment has been reported to exert a proliferative effect on cells of the colon in vivo (Hardwick et al. 2001). Leptin is also produced in the mammary glands and secreted into the colostrum; leptin treatment can increase the length of the small intestine and of intestinal villi and can increase the crypt mitotic index in piglets (Wolinski et al. 2003).
There are many pathologies associated with obesity, including colorectal cancer (Steinbach et al. 1994), and obesity is associated with elevated serum levels of leptin and insulin. Examination of cryopreserved pre‐diagnostic sera from men with cancer of the colon has shown a 3‐fold increase in colon cancer risk with increasing concentrations of leptin (Stattin et al. 2003) [however, colon cancer patients without weight loss had low leptin levels (Arpaci et al. 2002)]. There is a significant correlation between serum concentration of leptin and colon cell proliferation and aberrant crypt foci in carcinogen‐treated rats, indicating that the enhancement of colon cell proliferation and carcinogenesis by high‐fat diets is mediated through elevated serum leptin (Liu et al. 2001). Thus, if leptin is a growth factor for the colon epithelium this could provide a biological explanation for the observed associations between obesity and colon cancer (Hardwick et al. 2001).
Leptin also has specific effects on T‐lymphocyte responses and administration of leptin to mice reversed the immunosuppressive effects of acute starvation (Lord et al. 1998). Moreover, leptin deficiency can alter the immune response in colitis (Siegmund et al. 2002) and colitis is generally considered to be a predisposing factor for colorectal cancer (Levin 1992; Cooper et al. 2001; Rutter et al. 2004). Overweight and obesity have been shown to be associated with elevated markers of low‐grade systemic inflammation (Visser et al. 1999).
Studies on the effects of leptin are complicated by its influence on food intake, as food intake is probably the most important determinant of intestinal mucosal cell renewal (Goodlad et al. 2001). In the present study, we used the intravenous feeding (total parenteral nutrition; TPN) model (Fitzgerald et al. 2002) to circumvent the problem, as all the nutrients are delivered by a multi‐channel pump.
MATERIALS AND METHODS
Wistar rats (six per group) were anaesthetized with hypnorm and valium and intravenous cannulae were inserted into the jugular vein to deliver the TPN diet + 0, 0.5, 2.5, or 10 mg/kg r‐murine leptin (courtesy of Amgen Inc., Thousand Oaks, CA, USA). Orally fed rats were also studied.
Male rats, aged 9–10 weeks, were housed individually in wire‐bottomed cages and water was provided ad libitum. The TPN diet was delivered at a rate of 60 mL/rat per day, giving 1.8 g nitrogen, 6.0 g lipid, 8.5 g glucose and 1047 kJ/kg per day, this was originally designed to be isocaloric with the intake of the orally fed rats (Goodlad et al. 1987). After 6 days of treatment, the animals were injected with vincristine sulphate then killed 2 h later and autopsies were performed. The rats were all killed within a 3‐h period at the same time of day. Tissue weight was recorded and sections of intestine were fixed in Carnoy's solution for 1–3 h then stored in 70% ethanol.
All procedures were approved by the Cancer Research UK animal ethics committee and covered by the Home Office Animal Procedures Act, 1986.
Cell proliferation and crypt fission were assessed using previously validated methods (Goodlad et al. 1991; Goodlad 1994). Representative samples of tissue from the proximal, mid and distal small intestine and colon (taken from positions 10%, 50% and 90% of the total length of the small bowel or colon) were hydrated, hydrolysed and stained with the Feulgen reaction. The mucosal crypts were gently teased apart under a dissection microscope and the numbers of mitoses per crypt (mean of 20 crypts) and crypt fission events per 200 crypts were then determined in this ‘microdissected’ tissue. All samples were counted blind, without knowing the category identification.
Statistics
Results are presented here as the mean ± standard error of the mean. Data were tested by a two‐way analysis of variance using a general linear model followed by Dunnett's test using minitab statistical software (release 10.5 Xtra Minitab Ltd, Coventry, UK). The effect of site in this sector was included as a third variable for a three‐way analysis. If the presence of one factor changes the result of the other, this is indicated by an interaction effect.
RESULTS
Wet weights of tissues
During the investigation the TPN‐fed rats lost 2.6% of their body weight whilst the orally fed rats gained 22% (Fig. 1). Weight loss was significantly greater in the leptin‐infused rats (P = 0.003), with the animals given a high dosage losing 6.6% of their body weight. The weights of the various regions of the gut were significantly lower in the TPN‐fed rats than in the rats that were fed orally (P = 0.05 to P = 0.001, Fig. 1). No effect of leptin infusion was seen on the relative weights of the stomach, small intestine, or colon, but leptin was associated with a 13% decrease in weight of the caecum (P = 0.045, which was significant whether expressed as gross weight or as percentage of total body weight).
Figure 1.
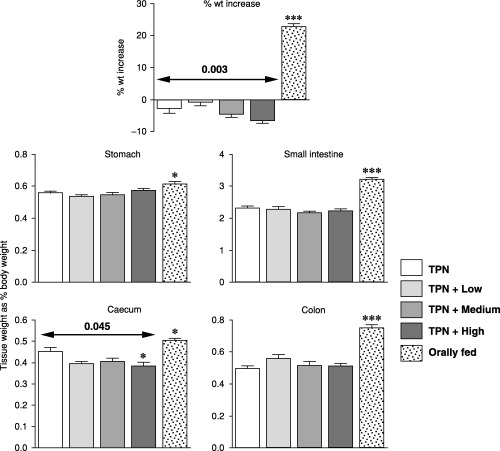
Effects of the various treatments on tissue weights. Arrows show results of analysis of variance for the TPN and leptin groups and Dunnett's tests. Results from orally fed rats were compared to the TPN results (t‐test); *P < 0.05, **P < 0.01 and ***P < 0.001.
Small intestine
Cell proliferation in the small intestine of TPN‐fed rats was significantly reduced to 36% of that in the orally fed rats (P < 0.001; Fig. 2), but leptin had no effect on proliferation. Crypt fission was also significantly reduced (by 50%) in the TPN rats when compared to the orally fed animals but leptin had no effect on small bowel crypt fission.
Figure 2.
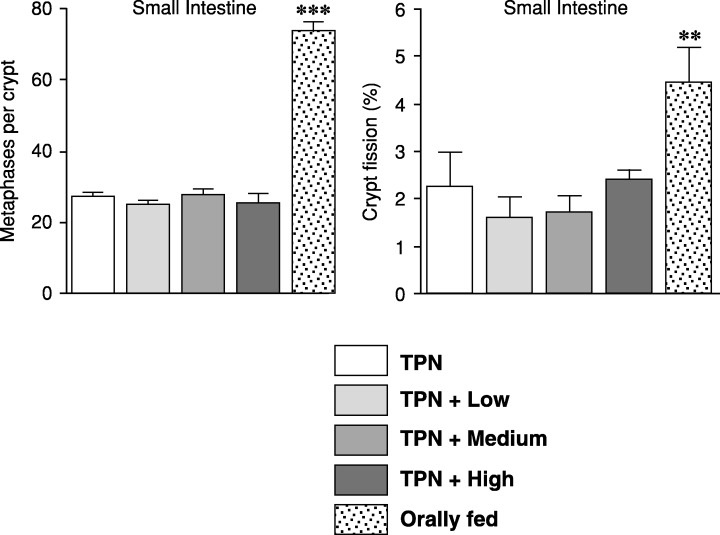
Effects of the various treatments on cell proliferation and crypt fission in the mid small bowel (50% of the total length of the small bowel). Results from orally fed rats were compared to the TPN results (t‐test); **P < 0.01 and ***P < 0.001.
Colon
Cell proliferation in the colon was reduced by TPN to 46%, 42% and 66% of the values in the orally fed animals (P < 0.001, P < 0.01 and P < 0.05, respectively, Fig. 3). Leptin infusion however, had no effect on cell proliferation in the colon.
Figure 3.
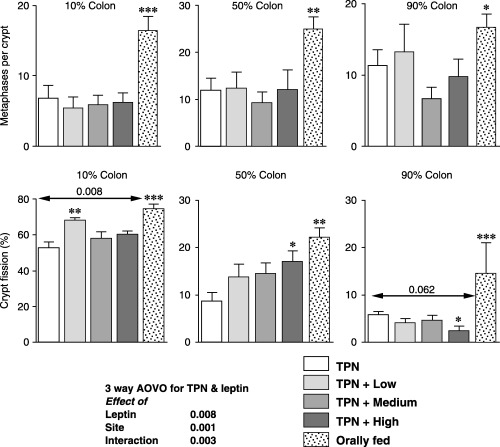
Effects of the various treatments on cell proliferation and crypt fission in the colon. The percentage lengths of the total colon defined the sites. Arrows indicate results of analysis of variance for the TPN and leptin groups and Dunnett's tests. Orally fed rat results compared with the TPN results by t‐test; *P < 0.05, **P < 0.01 and ***P < 0.001.
Crypt fission was also significantly reduced by TPN to 70%, 38% and 41% of the values seen in the orally fed group (P < 0.001, P < 0.01 and P < 0.001, respectively, Fig. 3).
In the proximal colon, leptin was associated with a significant rise in crypt fission (P = 0.008) with the effect of the low dose, at 29%, being the most prominent; the increase was only 10–12% for the other doses. In the mid colon, the fission values were 59%, 67% and 97% greater for the low, medium and high doses; however, this was only statistically significant for the high dose.
Crypt fission values in the distal colon were reduced by leptin to 69%, 80% and 42% of the TPN control values; however, only the effect of the high dose was statistically significant (at P < 0.05). When all the colon fission results were incorporated into a three‐way analysis the effects of leptin were significant at P = 0.008, the effects of site were also significant (P < 0.001) and the different responses to leptin were demonstrated by the interaction effect where P = 0.003.
DISCUSSION
The rats given TPN without the addition of leptin lost a little body weight and the rats given leptin TPN lost significantly more. Weight loss, or failure to gain weight, is often seen in TPN‐fed rats, and may in part reflect the problems of getting sufficient amounts of diet into the animals, as the calorific density is usually about 4.18 kJ or 1 kcal/mL. However, some of this weight loss is the result of the loss of gut tissue and of gut contents. The weight loss was greater in the leptin‐infused rats, confirming that the leptin also had an effect on energy balance. The rats were aged 9–10 weeks and weighed c. 200 g, which is a good size for the TPN cages; however, this is also in the middle of the period of maximum weight gain, which explains the large increase in body weight seen in the orally fed group.
Profound atrophy, associated with removal of the luminal contents from the intestine in the TPN model, was again dramatically demonstrated in this study. This atrophy should help make any proliferative actions more prominent, however, no sign of increased proliferation was observed, in contrast to the report of Hardwick et al. (2001). Their in vivo study, however, involved only a single time‐point (16‐h single injection of leptin). We studied the effects of leptin 6 days post‐treatment, by which time the gut often shows adaptive responses, but it is possible that leptin only has a transient action on proliferation. In contrast to the Hardwick study, Aparicio et al. (2004) did not find that leptin had a major effect on cell proliferation, and we have previously found a paradoxical inhibitory action for leptin on the caecum and colon [in a starved mouse model (Chaudhary et al. 2000)].
While not influencing cell proliferation here, leptin did appear to have effects on crypt fission. These consequences may be linked to the results of Aparicio et al. (2004) who found reduced formation of aberrant crypt foci in leptin‐treated rats in the chemical carcinogen, azoxymethane (AOM) model. Most colonic aberrant crypt foci (and tumours) are located distally and this was the site in which crypt fission appeared to be reduced.
Several studies have found diverse responses in the various regions of the colon. The colon is comprised from two embryological sources namely the midgut (ascending and transverse colon) and the hindgut (descending and sigmoid colon), and thus have separate blood supplies. In humans and in rodents the distal colon is the site of most aberrant crypt foci and tumours. However, the histology of rodent tumours is biphasic. In the proximal colon poorly differentiated mucin‐secreting carcinomas developed de novo while well‐differentiated tubular adenocarcinomas and adenomas follow the adenoma–carcinoma sequence in the distal colon, explaining why aberrant crypt foci are mainly found there (Sunter et al. 1978; Park et al. 1997). In the human, there is also evidence for different mechanisms in the tumorigenesis of proximal and distal colon cancers with sporadic microsatellite instability tumours occurring in the proximal colon, and most sporadic chromosomal instability tumours being distributed in the distal colon (Lindblom 2001).
While some of these differences are anatomical, others may reflect changes in the luminal milieu, as there is a marked proximo–distal gradient in release of fermentation products, with most short‐chain fatty acid production occurring proximally. Diurnal variations in proliferation also mirror the intake of food several hours previously (Goodlad & Wright 1984) suggesting that luminal nutrition determines cell renewal at the various sites in the colon. However, systemic infusion of peptide hormones has shown that the distal colon may be generally less responsive (Goodlad et al. 1987).
Leptin is a hormone with many properties and while it may not be mitogenic to the gut in vivo, it may act directly on cell lines. One of the problems of studies of intestinal cell proliferation is the different response to various agents in vitro and in vivo (Lupton 2004). For example, the effect of short‐chain fatty acids in vitro is to stimulate apoptosis and differentiation (Mathers 1998), however, in vivo the short‐chain fatty acids stimulate cell proliferation (Goodlad 2001). While cell lines may respond to growth factors and cytokines in the same way as in vivo, because they are using the same molecular mechanisms, immortalization means that they may have altered receptor function or expression. Notwithstanding this, it is unlikely that they will respond in the same way to some dietary factors because the enterocyte is normally part of a complex, multi‐layered defensive structure that is quite different from being naked and vulnerable immersed in a culture solution.
Leptin has effects on a variety of tissues, including vascular endothelial cells, and it can induce angiogenesis, aberrant vasculature (Park et al. 2001) and haematopoiesis (Umemoto et al. 1997), suggesting other ways by which leptin could modify the processes central to carcinogenesis. The development of premalignant lesions of the colon is enhanced in leptin receptor deficient (db/db) mice with hyperleptinaemia and hyperinsulinemia (Hirose et al. 2004), again suggesting that leptin may be anti‐carcinogenic.
The initial excitement following the discovery of leptin has not been sustained because humans do not lose weight following leptin administration (unless they are the very rare human equivalent of the ob/ob mouse), suggesting that most humans are leptin resistant. However, a recent report has found that adenovirus‐induced hyperleptinaemia rapidly depletes body fat, with the adipocytes becoming shrunken, fatless and encased in a thick basement‐membrane‐like matrix, implying that the fat‐storing adipocytes are able to oxidize fat; this ability to transform adipocytes into unique fat‐burning cells suggests a novel therapeutic strategy to combat obesity (Orci et al. 2004).
Leptin may also function as a neuromodulator of the growth‐hormone‐releasing factor–growth hormone–insulin‐like growth factor axis, communicating to this hormonal system the nutritional status of the animal (Lapaglia et al. 1998). It may be that decreased leptin concentrations are the primary defect responsible for the altered growth hormone secretion in food‐deprived rats (Carro et al. 2000).
Leptin is acutely regulated by fasting and re‐feeding and administration of the diet intravenously here may have altered this. However, feeding rats similar numbers of calories with standard food or infusion of TPN diets into the duodenum or intravenously have demonstrated that intravenous glucose infusion was responsible for stimulation of the leptin gene and hormone secretion (Levy et al. 1997).
In conclusion, our results contradict claims that leptin can stimulate intestinal cell proliferation. However, leptin would appear to have a paradoxical inhibitory action on the caecum and colon (Chaudhary et al. 2000) and some of these effects may be the result of altered crypt fission. Moreover, reduction in fission in the distal colon would indicate that leptin might be anti‐carcinogenic.
ACKNOWLEDGEMENTS
The work of A. J. FitzGerald has been funded by the Biology and Biotechnology Science Research Council (BBSRC).
REFERENCES
- Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS (1997) Leptin accelerates the onset of puberty in normal female mice. J. Clin. Invest. 99, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio T, Guilmeau S, Goiot H, Tsocas A, Laigneau JP, Bado A, Sobhani I, Lehy T (2004) Leptin reduces the development of the initial precancerous lesions induced by azoxymethane in the rat colonic mucosa. Gastroenterology, 126, 499–510. [DOI] [PubMed] [Google Scholar]
- Arpaci F, Yilmaz MI, Ozet A, Ayta H, Ozturk B, Komurcu S, Ozata M (2002) Low serum leptin level in colon cancer patients without significant weight loss. Tumori 88, 147. [DOI] [PubMed] [Google Scholar]
- Attele AS, Shi ZQ, Yuan CS, Casanova ML, Larcher F, Casanova B, Murillas R, Fernandez‐Acenero MJ, Villanueva C, Martinez‐Palacio J, Ullrich A, Conti CJ, Jorcano JL (2002) Leptin, gut, and food intake. Biochem. Pharmacol. 63, 1579. [DOI] [PubMed] [Google Scholar]
- Barrenetxe J, Villaro AC, Guembe L, Pascual I, Munoz‐Navas M, Barber A, Lostao MP (2002) Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut 50, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Seoane LM, Senaris R, Casanueva FF, Dieguez C (2000) Leptin increases in vivo GH responses to GHRH and GH‐releasing peptide‐6 in food‐deprived rats. Eur J. Endocrinol. 142, 66. [DOI] [PubMed] [Google Scholar]
- Chaudhary M, Mandir N, Fitzgerald AJ, Howard JK, Lord GM, Ghatei MA, Bloom SR, Goodlad RA (2000) Starvation, leptin and epithelial cell proliferation in the gastrointestinal tract of the mouse. Digestion 61, 223. [DOI] [PubMed] [Google Scholar]
- Cooper HS, Everley L, Chang WC, Pfeiffer G, Lee B, Murthy S, Clapper ML (2001) The role of mutant Apc in the development of dysplasia and cancer in the mouse model of dextran sulfate sodium‐induced colitis. Gastroenterology 121, 1407. [DOI] [PubMed] [Google Scholar]
- Fitzgerald AJ, Ghatei MA, Mandir N, Bloom SR, Iversen L, Goodlad RA (2002) Effects of amidated gastrin and glycine‐extended gastrin on cell proliferation and crypt fission in parenterally and orally fed rats. Digestion 66, 58. [DOI] [PubMed] [Google Scholar]
- Goodlad RA (1994) Microdissection‐based techniques for the determination of cell proliferation in gastrointestinal epithelium: application to animal and human studies In: Celis JE, ed. Cell Biology: a Laboratory Handbook, p. 205 San Diego & London: Academic Press. [Google Scholar]
- Goodlad RA (2001) Dietary fibre and the risk of colorectal cancer. Gut 48, 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad RA, Levi S, Lee CY, Mandir N, Hodgson H, Wright NA (1991) Morphometry and cell proliferation in endoscopic biopsies: evaluation of a technique. Gastroenterology 101, 1235. [DOI] [PubMed] [Google Scholar]
- Goodlad RA, Nightingale JMD, Playford RJ (2001) Intestinal adaptation In: Nightingale J, ed. Intestinal Failure, p. 243 London: Greenwich Medical Media. [Google Scholar]
- Goodlad RA, Wilson TJ, Lenton W, Gregory H, McCullagh KG, Wright NA (1987) Intravenous but not intragastric urogastrone‐EGF is trophic to the intestine of parenterally fed rats. Gut 28, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad RA, Wright NA (1984) The effects of starvation and refeeding on intestinal cell proliferation in the mouse. Virchows Arch. B Cell. Pathol. Incl. Mol. Pathol. 45, 63. [DOI] [PubMed] [Google Scholar]
- Hardwick JC, Van Den Brink GR, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP (2001) Leptin is a growth factor for colonic epithelial cells. Gastroenterology 121, 79. [DOI] [PubMed] [Google Scholar]
- Hirose Y, Hata K, Kuno T, Yoshida K, Sakata K, Yamada Y, Tanaka T, Reddy BS, Mori H (2004) Enhancement of development of azoxymethane‐induced colonic premalignant lesions in C57BL/KsJ‐db/db mice. Carcinogenesis 25, 821. [DOI] [PubMed] [Google Scholar]
- Lapaglia N, Steiner J, Kirsteins L, Emanuele M, Emanuele N (1998) Leptin alters the response of the growth hormone releasing factor–growth hormone–insulin‐like growth factor‐I axis to fasting. J. Endocrinol. 159, 79. [DOI] [PubMed] [Google Scholar]
- Levin B (1992) Ulcerative‐colitis and colon cancer – biology and surveillance. J. Cellular Biochem. 1992, 47. [DOI] [PubMed] [Google Scholar]
- Levy JR, Legall‐Salmon E, Santos M, Pandak WM, Stevens W (1997) Effect of enteral versus parenteral nutrition on leptin gene expression and release into the circulation. Biochem. Biophys. Res. Commun. 237, 98. [DOI] [PubMed] [Google Scholar]
- Lindblom A (2001) Different mechanisms in the tumorigenesis of proximal and distal colon cancers. Curr. Opin. Oncol. 13, 63–69. [DOI] [PubMed] [Google Scholar]
- Liu Z, Uesaka T, Watanabe H, Kato N (2001) High fat diet enhances colonic cell proliferation and carcinogenesis in rats by elevating serum leptin. Int. J. Oncol. 19, 1009. [DOI] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI (1998) Leptin modulates the T‐cell immune response and reverses starvation‐induced immunosuppression. Nature 394, 897. [DOI] [PubMed] [Google Scholar]
- Lupton JR (2004) Microbial degradation products influence colon cancer risk: the butyrate controversy. J. Nutr. 134, 479. [DOI] [PubMed] [Google Scholar]
- Mathers JC (1998) Nutrient regulation of intestinal proliferation and apoptosis. Proc. Nutr. Soc. 57, 219. [DOI] [PubMed] [Google Scholar]
- Orci L, Cook WS, Ravazzola M, Wang MY, Park BH, Montesano R, Unger RH (2004) Rapid transformation of white adipocytes into fat‐oxidizing machines. Proc. Natl Acad. Sci. USA 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Goodlad RA, Wright NA (1997) The incidence of aberrant crypt foci and colonic carcinoma in dimethylhydrazine‐treated rats varies in a site‐specific manner and depends on tumor histology. Cancer Res. 57, 4507. [PubMed] [Google Scholar]
- Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, Jang Y, Cho SY, Kim HS (2001) Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro . Exp. Mol. Med. 33, 95. [DOI] [PubMed] [Google Scholar]
- Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A (2004) Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 126, 451. [DOI] [PubMed] [Google Scholar]
- Schneider R, Bornstein SR, Chrousos GP, Boxberger S, Ehninger G, Breidert M (2001) Leptin mediates a proliferative response in human gastric mucosa cells with functional receptor. Horm. Metab. Res. 33, 1. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Lehr HA, Fantuzzi G (2002) Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology 122, 2011. [DOI] [PubMed] [Google Scholar]
- Stattin P, Palmqvist R, Soderberg S, Biessy C, Ardnor B, Hallmans G, Kaaks R, Olsson T (2003) Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol. Rep. 10, 2015. [PubMed] [Google Scholar]
- Steinbach G, Heymsfield S, Olansen NE, Tighe A, Holt PR (1994) Effect of caloric restriction on colonic proliferation in obese persons: implications for colon cancer prevention. Cancer Res. 54, 1194. [PubMed] [Google Scholar]
- Sunter JP, Appleton DR, Wright NA, Watson AJ (1978) Pathological features of the colonic tumours induced in rats by the administration of 1,2‐dimethylhydrazine. Virchows Arch. B Cell Pathol. 29, 211. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K (1997) Leptin induces mitogen‐activated protein kinase‐dependent proliferation of C3H10T1/2 cells. J. Biol. Chem. 272, 12897. [DOI] [PubMed] [Google Scholar]
- Umemoto Y, Tsuji K, Yang FC, Ebihara Y, Kaneko A, Furukawa S, Nakahata T (1997) Leptin stimulates the proliferation of murine myelocytic and primitive hematopoietic progenitor cells. Blood 90, 3438. [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB (1999) Elevated C‐reactive protein levels in overweight and obese adults. JAMA 282, 2131. [DOI] [PubMed] [Google Scholar]
- Wolinski J, Biernat M, Guilloteau P, Westrom BR, Zabielski R (2003) Exogenous leptin controls the development of the small intestine in neonatal piglets. J. Endocrinol. 177, 215. [DOI] [PubMed] [Google Scholar]


