Abstract
Abstract. Objective: It is not known whether or not epithelial progenitors of the pyloric antrum are involved in gastric carcinogenesis. Normally, these progenitors give rise to two main cell lineages: pit and gland mucous cells. This study was designed to examine the changes that occur in pyloric antral mucous cell lineages and their progenitors during development of gastric adenoma and carcinoma in trefoil factor 1 (TFF1) knockout mice. Materials and methods: Pyloric antral mucosal tissues of TFF1 knockout mice at ages from 3 days to 17 months were processed for histochemical analysis using Ulex europaeus and Grifforia simplifolica lectins as markers for pit and gland mucous cells, respectively. The dividing epithelial progenitors were identified by using immunohistochemical and electron microscopy techniques. Results: TFF1 loss was associated with amplification of both mucus‐secreting pit and gland cells. Both lectins examined bound not only to mature mucous cells, but also to most of epithelial progenitors which gradually amplified with age and frequently were seen in mitosis. Analysis of 12‐ to 17‐month‐old TFF1‐deficient stomachs revealed occasional groups of poorly differentiated mucosal cells with features similar to those of epithelial progenitors (or stem cells), in the basal portion of the antral mucosa. These cells eventually invaded the muscularis mucosa while maintaining some capacity to differentiate. Conclusion: This study shows that the progenitors of pit and gland mucous cells contribute to gastric carcinogenesis in the pyloric antrum of TFF1 knockout mice, strongly supporting the concept of stem cell origin of cancer.
INTRODUCTION
Mouse stomach has three main regions: fundus, corpus and pyloric antrum. The mucosa of the pyloric antrum is lined by a single layer of epithelial cells which invaginates into numerous long pits ending in short glands (Fig. 1). These cells of the luminal surface and pits are mucus‐secreting, and are characterized by spherical electron dense granules in their apical cytoplasm. Mucous cells of the glands have large spherical electron‐lucent granules scattered in their cytoplasm. Because the pits and glands of the pyloric antrum are mostly made‐up of mucus‐secreting cells, they are referred to as mucous pit‐gland units (Lee et al. 1982). In the gland, next to its border with the pit, there is a narrow region called the isthmus; this region is packed with small columnar cells characterized by high nucleus‐to‐cytoplasm ratio. Electron microscope examination of them has revealed the presence of some undifferentiated granule‐free cells anchored in a specific stem cell niche. By using 3H‐thymidine autoradiography, Leblond and colleagues characterized the dynamics of these undifferentiated cells. It was estimated that their cell cycle takes 8.4 h and their S phase lasts for only 5.8 h. The overall turnover time of these isthmal progenitor cells was estimated at about 1 day (Lee & Leblond 1985; El‐Alfy & Leblond 1987).
Figure 1.
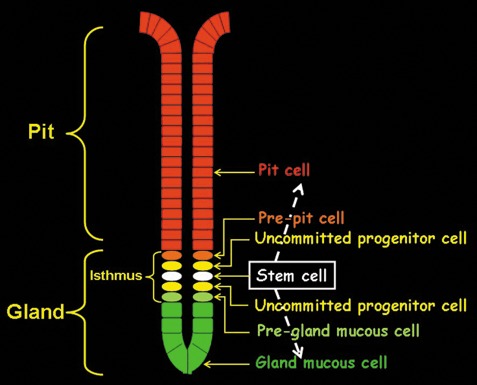
Schematic representation of organization of epithelial cells in the antrum of mouse stomach. The cells form units in gastric pits and glands. In the isthmus region of the gland, multipotential stem cells give rise to uncommitted progenitors which include mottled‐ and mixed‐granule cells. These poorly differentiated cells undergo proliferation and differentiation to give rise to committed progenitors namely, pre‐pit and pre‐gland cells. The pre‐pit cells mature while migrating upwards to form pit cells and pre‐gland cells mature while migrating downwards to form gland mucous cells.
Detailed analysis using tritiated thymidine labelling combined with electron microscopy (EM) revealed that these isthmus stem cells are responsible for production of transitional progenitor cells with dual features. These progenitors maintain high capacity for cell division and contain mottled or mixed granules, similar to but smaller than those of mucous pit‐gland unit cells. These transitional cells are uncommitted progenitors that undergo further proliferation and differentiation to give rise to two committed progenitor cell types, pre‐pit and pre‐gland mucous cells (Fig. 1). Maturation of these progenitors is associated with their migration in one of two directions: upward to the pit where they produce only the small dense granules and thus become pit cells and downward to the gland base where they maintain the capacity to produce only the large pale granules and thus become gland mucous cells (Lee & Leblond 1985; Karam 1999).
Proliferation of gastric stem cells and migration‐associated differentiation of their progeny are regarded as important factors involved in repair of gastric mucosal injury (Tarnawski 2005). Furthermore, maintaining control of the normal dynamic features of these stem cells and their progeny is necessary to avoid enhanced cell proliferation that may lead to hyperplasia and eventually gastric adenoma and/or carcinoma (Burkert et al. 2006). Little, however, is known regarding factors that regulate dynamism of stem cells in the isthmus region of the pyloric antral glands.
Trefoil factors (TFFs) are a group of small peptides which have been found to be implicated in a number of gastrointestinal cell biological processes: motility, secretion, protection, restitution/repair, and cell proliferation/migration/differentiation programmes (Ribieras et al. 1998). TFF1 is synthesized and secreted by the mucus‐secreting pit cells of the corpus and pyloric antral regions of the stomach (Rio et al. 1988). TFF2 is a product of the mucus‐secreting neck cells of oxyntic glands (Lefebvre et al. 1993). TFF3 was initially discovered in mucus‐secreting goblet cells of the intestine and recently also has been found to be a product of some gastric pit cells and pepsinogen‐secreting zymogenic cells, too (Suemori et al. 1991; Karam et al. 2004).
Lack of TFF1 in a knockout mouse model has been associated with: 5‐fold increase of mitotic figures in the pyloric antrum, elongation of pit regions of the mucosa and finally loss of the tubular appearance of pit‐gland units and then development of adenomas in all TFF1‐deficient mice. When these animals attained 5 months of age, 30% of them developed a more dramatic situation; cells of adenomas exhibited malignant changes and localized carcinomas (in situ) developed (Lefebvre et al. 1996; Tomasetto & Rio 2005).
The study described in this article was performed to address three main questions concerning alterations in the pyloric antrum of TFF1 knockout mice. (i) What happens to isthmal progenitor cells during the morphological changes in the mucous pit‐gland units of the pyloric antrum? (ii) Which epithelial cell lineage is affected by TFF1 deficiency? (iii) What is the evolution of pyloric antrum alteration in old mice?
MATERIALS AND METHODS
Animals
C57BL/6 J/129/Svj mixed genetic background normal and TFF1 knockout mice generated by Lefebvre et al. (1996) were used in this study. Mice of both sexes were used at 3 and 21 postnatal days, and at 1, 2, 3, 6, 12 and 17 months (n = 3–7 for each age group). Mice were maintained in a pathogen‐free state and were provided with standard chow diet ad libitum.
Light microscope and lectin/immunohistochemical studies
Pyloric antra of stomachs cut along the longitudinal axis were fixed in Bouin's solution and were embedded in paraffin wax. A portion of sections (cut at 5 µm) was stained with haematoxylin and eosin or periodic acid Schiff (PAS) for general histology and mucus staining, respectively. Other sections were used for demonstration of lectin binding and immunohistochemistry using methods as described previously (Falk et al. 1994; Karam et al. 1997, 2005, 2004). Sections were examined with a bright field or fluorescence microscope.
Mucus‐secreting pit and gland cells of the pyloric antrum were labelled by using two lectins, tetramethylrhodamine isothiocyanate (TRITC)‐labelled Ulex europaeus type I agglutinin (UEA‐I) and fluorescein isothiocyanate (FITC)‐labelled Grifforia simplifolica II (GSII). These lectins were obtained from Sigma (St. Louis, MO, USA) and have been previously identified and regularly used as reliable lineage‐specific markers for pit and neck cells, respectively, of the oxyntic mucosa of developing and adult mice (Falk et al. 1994; Karam et al. 1997, 2005, 2004). Sections of tissue blocks containing both control and TFF1‐deficient gastric antral mucosae were deparaffinized, rehydrated, blocked in buffer containing 1% bovine serum albumin, 0.5% Tween‐20 in phosphate‐buffered saline and finally were incubated for 1 h in UEA‐I and GSII lectins at 1 : 600 and 1 : 200 dilutions, respectively. To label mitotic isthmal progenitor/stem cells of normal pyloric antral glands and dividing tumour cells, tissue sections of pyloric antrum of control and TFF1 knockout mice were processed for overnight incubation with mouse monoclonal antibody specific for proliferating cell nuclear antigen (PCNA; Medical & Biological Laboratories, Woburn, MA, USA) at 1 : 100 dilution. Antigen‐antibody binding sites were identified by using peroxidase‐labelled goat anti‐mouse immunoglobulin G and 3,3′‐diaminobenzidine (Sigma) as a colour reagent. Then, tissue sections were counterstained with PAS. Antibody raised against the synthetic peptide corresponding to the carboxy‐terminal end of TFF1 was used to examine expression of TFF1 as mentioned previously (Karam et al. 2004).
EM studies
Individual old age TFF1 knockout mice and normal mice (n = 3, each) were perfused with 0.1 m Na‐cacodylate buffer containing 2% paraformaldehyde and 2.5% glutaraldehyde. Pyloric antra of the stomachs were dissected and were immersed in the same fixative for 2 h. Following osmication, tissues were dehydrated and processed for infiltration and embedding in Araldite (Karam et al. 2003). Ultrathin sections were stained with uranyl acetate and lead citrate and were examined using a Philips EM.
RESULTS
Both pit and gland mucous cell lineages were amplified in TFF1‐deficient mice
By comparing mucosae of the pyloric antrum of control and TFF1‐deficient mice during their postnatal development, it appeared that TFF1 deficiency was associated with an increase in thickness of the mucosa as early as 3 days postnatally (Fig. 2a,b). Mucosal thickening continued with advancing age and became more apparent in 2‐month‐old mice (Fig. 2g,h).
Figure 2.
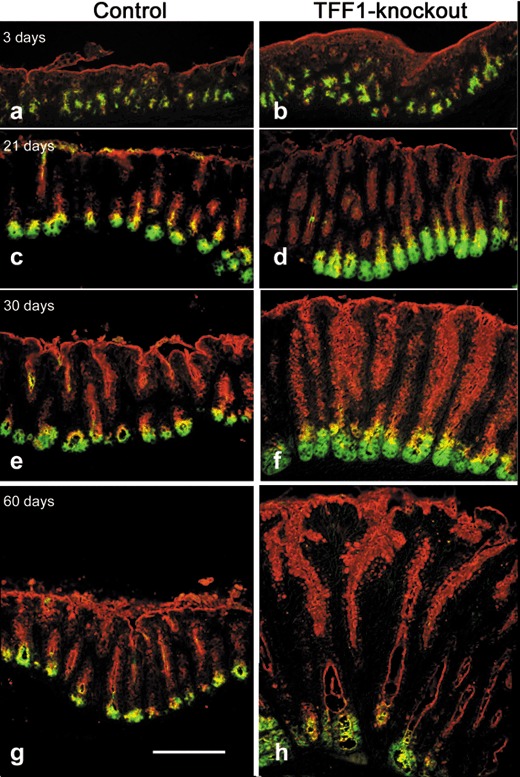
Lectin histochemical analysis of the antral mucosa of normal (a, c, e, g) and TFF1 knockout mice (b, d, f, h) at the ages of 3 (a, b), 21 (c, d), 30 (e, f), and 60 (g, h) days. Tissue sections were deparaffinized, hydrated and subsequently incubated with TRITC‐conjugated UEA‐I lectin (red) and FITC‐conjugated GSII lectin (green). In all tissues, pit cells along the luminal surface and pit walls are labelled with UEA‐I, and gland mucous cells are labelled with GSII. By comparing each of the control tissues on the left with their age‐matched TFF1 knockout mouse tissues on the right, note that in all age groups, regions of labelled pit and gland mucous cells expand in TFF1 deficiency. Also note that in all tissues an intermediate region between each pit and gland is labelled with both lectins. These are probably the isthmus uncommitted progenitors with mottled and mixed granules. Double labelled progenitors appear to be more prominent in TFF1 knockout tissues than in control ones. Bar = 90 µm (a–h).
Lectin probing of tissue sections of these mice, at ages of 3 days up to 2 months, was performed to determine whether UEA‐I and GSII lectins, known as markers for mucous cells in the oxyntic mucosa, could also be used to label mucous cells in the pyloric antrum of C57BL/6 J/129/Svj mice. The results showed that while UEA‐I lectin could be used to label mucus‐secreting cells lining the luminal surface and the long tubular pits of the pyloric antrum, as well as their isthmal progenitors, GSII lectin was a good marker for mucus‐secreting gland cells and their progenitors (Fig. 2a,c,e,g). Thus, in control mice, numerous cells lining the luminal surface and the pit regions were stained only with one lectin, UEA‐I. Blind glandular ends of epithelial units were labelled with the second lectin, GSII (Fig. 2a,c,e,g). Then, a region intermediate between the pit and gland mucous cells appeared to be double labelled with both lectins. Apices of the progenitor cells in the isthmus region appeared orange or yellow due to overlapping of red and green of UEA‐I and GSII lectin staining. These cells were most probably uncommitted mixed granule progenitors, previously described by Lee & Leblond (1985).
In TFF1‐deficient mice, we observed an increase in number of cells binding UEA‐I (red) and GSII (green) lectins. Moreover, comparing double‐labelled isthmus progenitor cell regions in control and TFF1 knockout mice revealed their expansion with TFF1 deficiency in the various age groups examined (Fig. 2b,d,f,h), indicating that thickening of the mucosa of TFF1 knockout mice resulted from amplification of pit and gland mucous cell lineages and most probably of their progenitors.
Amplification mainly concerned proliferating epithelial progenitors
Isthmus cells, including stem cells and epithelial progenitors, are able to proliferate while differentiated pit and gland mucous cells lose this capacity. Thus, in order to determine whether amplified cells were indeed proliferative progenitors, sections of pyloric antral mucosal control and TFF1 knockout mice were probed with anti‐PCNA antibody. PCNA is expressed during G1–S phases of the cell cycle and, therefore, its positive immunohistochemical staining is a marker of cell proliferation (Matsumoto et al. 1987). As expected, PCNA‐labelled dividing cells were located in the narrow isthmus region of the pit‐gland units of control mice (Fig. 3a). In contrast, dividing cells in the TFF1 knockout mice were scattered over large areas of the thickened tissue. Moreover, PCNA‐positive cells correlated with the areas double labelled with both UEA‐I and GSII lectins and showed very little or no PAS staining (Fig. 3b,c). This indicated that most of the amplified cells were proliferating and producing very little or no mucus and, therefore, corresponded to epithelial progenitors.
Figure 3.
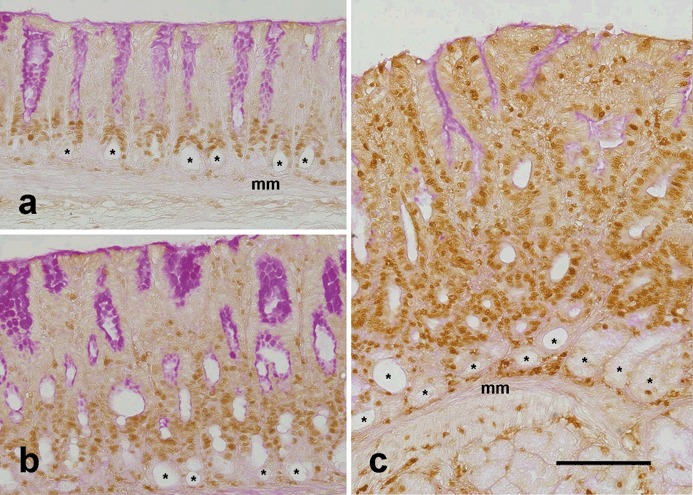
Labelling of dividing cells in control (a) and TFF1‐deficient (b, c) mice at ages of 1 month (b) and 2 months (a, c). Tissue sections were probed with polyclonal anti‐proliferating cell nuclear antigen (PCNA) antibody and then were incubated with biotinylated anti‐rabbit immunoglobulin G. Following incubation with peroxidase‐conjugated avidin, antigen‐antibody binding sites were visualized by adding diaminobenzidine colouring agent. Nuclei of proliferating cells in the G1 or S phase of the cell cycle appear brown because they express a high level of PCNA. Note that in control tissue (a), proliferating cells occur in isthmus regions at the pit‐gland borders. Also note the round lumina (asterisks) of the glands at the bottom of the mucosa, next to the muscularis mucosa (mm). These lumina are lined with terminally differentiated post‐mitotic gland mucous cells which are not labelled with PCNA. In TFF1 knockout tissues (b, c), the mucosa is thick and PCNA‐labelled proliferating cells are numerous, as compared to those of control tissue in (a). The three tissue sections were counterstained with periodic acid Schiff (PAS). PAS‐positive cells are seen at the luminal surface and along the pit walls, especially in (a) and (b). Note that PCNA‐labelled cells in control and knockout tissues show little or no PAS‐positive staining. Bar = 90 µm (a–c).
Histological evidence of benign and malignant tumours in TFF1 knockout mice depending on mouse age
A sequence of morphological changes that included hyperplasia, dysplasia, localized neoplasia (carcinoma in situ), and finally invasive neoplasia were observed in pyloric antra of TFF1 knockout mice. Hyperplasia was observed very early, at postnatal day 3 and continued throughout the life of the mice; by 6–12 months, dysplasia was evident. The long tubular epithelial pit‐gland units characteristic of the pyloric antrum of control mice (Fig. 4a,c) was replaced in TFF1 knockout mice by round or ovoid acinar epithelial profiles of varying sizes (Fig. 4b). However, no ulcerative lesions were observed here. These dysplastic changes were associated with decrease in the amount of PAS‐stained mucus (compare a with b). These data confirm that the type of amplified cells was not differentiated cells capable of producing mucus, but progenitors that produced no or few mucous granules. These progenitor cells maintained some capacity to differentiate and occasionally formed mucus‐secreting pit‐like cells, deep in the gastric mucosa, next to the muscularis mucosa (PAS‐positive cells, right side of Fig. 4b,d).
Figure 4.
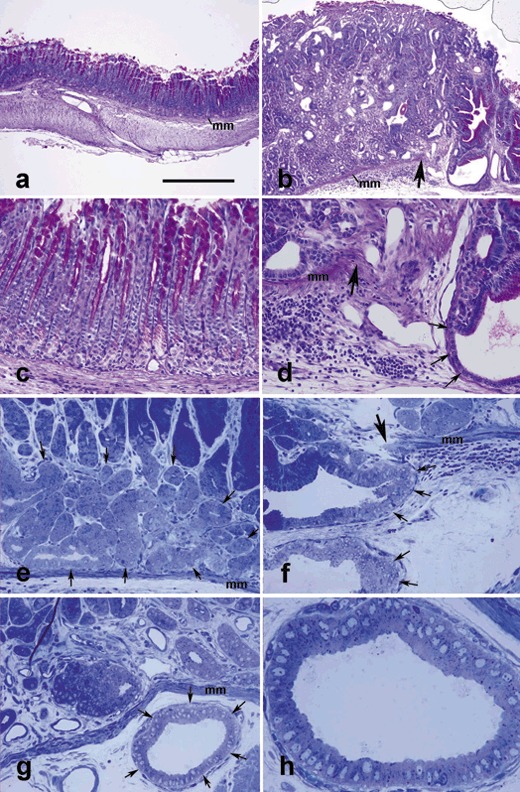
Antral mucosal tissue sections of control (a, c) and TFF1 knockout (b, d–h) mice at 12 (a–d) and 17 (e–h) months of age, stained with periodic acid Schiff (PAS) and haematoxylin (a–d) or toluidine blue (e–h). In control tissue (a), the muscularis mucosa (mm) separates the epithelial units above from submucosa and muscularis externa below. In (b), note that the epithelial units are simple and tubular and apices of cells lining pit regions are PAS‐positive (purple). In TFF1 knockout tissue, note increased thickness of the mucosa (b versus a) and decreased amount of PAS‐stained mucus, as compared to control tissue in (a). Also note invasive epithelial profiles (b, d, f–h), penetrating through the muscularis mucosa (mm) which is lacking at the right (in b, d) or left (in f) of the large arrow. In 0.5‐µm‐thick plastic sections of the basal portion of the pyloric antral mucosal tissues, amplified progenitor cells form a large group of pale cells (surrounded by arrows in e) which appear similar to a carcinoma in situ, or they cross the muscularis mucosa and invade the submucosa to form multiple cyst‐like profiles (arrows in f and g). High magnification of the invasive profile of (g) is shown in (h). It illustrates the small size of the invasive cells with little or no visible secretory dense granules. Bar = 300 (a, b), 90 (c–g), and 35 (h) µm.
In 12‐ to 17‐month‐old mice, increase in mucosal thickness was associated with massive amplification in numbers of some epithelial progenitors and their expansion near the muscularis mucosa. These groups of cells which exemplified carcinoma in situ were observed in the pyloric antrum (Fig. 4e). In addition, a sign of submucosal invasion was frequently detected (Fig. 4f–h) – some of the pyloric antral tissues of the TFF1 knockout mice showed discontinuity of the muscularis mucosae (Fig. 4b,d,f). Groups of expanding numbers of glandular epithelial cells progressively found their way into the submucosa through gaps in the muscularis mucosa (Fig. 4b,d,f,g,h). These invading cells usually formed small submucosal cyst‐like structures lined by a single layer of cells. The epithelial lining of these invasive glandular profiles was occasionally stratified. Regardless of the single or multilayered epithelial lining, a large amount of extruded cell debris was also found in dilated central lumina of these invasive profiles (4, 5). Identification of glandular cells within the submucosa was a sign of early invasion.
Figure 5.
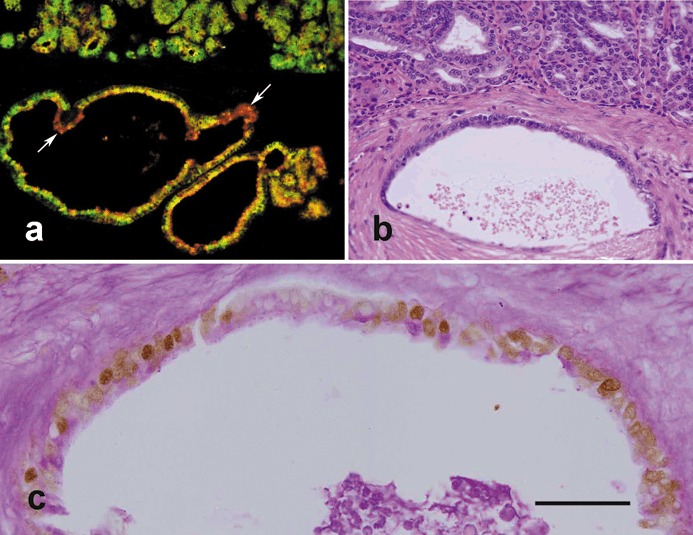
Analysis of invasive cells in the pyloric antral mucosae of 12‐month‐old TFF1 knockout mice. (a) Tissue was probed with both UEA‐I (red) and GSII (green) lectins and shows numerous double labelled cells (yellow) suggesting that uncommitted progenitors with dual features of pre‐pit and pre‐gland mucous cells are amplified and have invaded the muscularis mucosa to form variable profiles, mainly cyst‐like, in the submucosa. Note that a few cells appear mostly green and some appear mostly red (arrows) indicating the capacity of invading progenitors to differentiate. (b) A further pyloric antral mucosal tissue section stained with haematoxylin and eosin shows the basal portion of the antral mucosa with numerous small epithelial cells forming variable profiles, some of them having invaded the underlying submucosa to form a relatively large cyst‐like structure seen in the lower half of the figure. (c) Tissue section adjacent to (b), probed with anti‐proliferating cell nuclear antigen (PCNA) antibody and counterstained with periodic acid Schiff (PAS) as in Figure 3. Note nuclei of many of the invading cells lining the cyst‐like structure appear brownish and hence, PCNA‐positive. However, apical cytoplasm of the lining cells contains little or no mucus (PAS‐negative). Bar = 90 (a, b); 35 (c) µm.
Invasive glandular cells found in the submucosa of old TFF1‐deficient mice were mainly progenitor cell types
To characterize the nature of the invasive glandular cells found in the submucosa, pyloric antral tissue sections of old control and of TFF1 knockout mice had been processed for lectin labelling. Control tissue showed the usual pattern of double UEA‐I and GSII labelling, similar to that shown in Fig. 2g. In the TFF1 knockout tissue, as seen previously at younger ages, there was dramatic expansion in the areas labelled with UEA‐I and GSII lectins. In addition, although some cells appeared to be positive for only one lectin, most of the invasive epithelial cells located in the submucosa were double labelled with UEA‐I and GSII (Fig. 5a), a feature similar to that of the uncommitted isthmus progenitor cells, with dual feature – a mix of mucous granules similar to but smaller than those of pit and gland mucous cells.
Invasive cells also appeared to be small and similar in morphology to amplified type of cells in the base of the mucosa (Fig. 5b). PAS staining showed very little or no reaction (Fig. 5c). We also performed PCNA immunoprobing and found that these invasive cyst‐like structures were composed mostly of highly proliferating cells (Fig. 5c). Thus, these findings are consistent with the fact that the invasive cells were mostly progenitor cells.
Immature nature of the submucosal invading cells of old TFF1‐deficient mice
Ultrastructural features of the invading cells were determined by using EM. In addition to their immature epithelial cell features (high nucleus‐to‐cytoplasm ratio, many free ribosomes and few membrane‐bound organelles), the cells were frequently seen to be undergoing mitosis. Some of them appeared similar to pre‐pit cells with a few dense secretory granules, a few others had no secretory granules and, hence, were similar to undifferentiated stem cells and still others were similar to mixed granule cells with both spherical electron dense granules and electron lucent granules in their apical cytoplasm (Fig. 6). Thus, these EM features showed that the invading cells comprised a mixed population of amplified isthmus progenitors and stem cells. Moreover, some mature mucous and enteroendocrine cells were occasionally observed among these progenitors. As gastric cell turnover is known to be extremely rapid, these data suggest that the invading progenitors/stem cells were able to self‐renew and differentiate in this ectopic submucosal cell niche.
Figure 6.
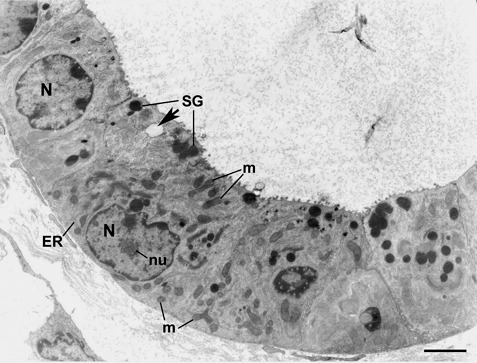
Electron micrograph showing ultrastructure of the invading cells. The cells have features of immature epithelial progenitors: cytoplasm with relatively few small organelles: mitochondria (m), cisternae of rough endoplasmic reticulum (ER), secretory granules (SG), nucleus (N) containing much diffuse chromatin and reticulate nucleous (nu). The cell at the upper left appears to be the least differentiated in comparison to the other cells. The neighbouring cell has mixed granules: spherical dense granules (SG) and pale electron lucent granule (arrow). Connective tissue elements of the submucosa are seen at the lower left. Bar = 4 µm.
DISCUSSION
Gastric cancer accounts for around 10% of newly diagnosed cancers in some areas of the world and, therefore, may be regarded as the fourth most common newly diagnosed cancer (Roder 2002). Moreover, gastric cancer may be the second leading cause of cancer death worldwide (Parkin 2001). The reason for high frequency and fatality of gastric cancer is mainly due to late diagnosis and lack of programs for its early detection (Hohenberger & Gretschel 2003); thus, identification of early events in gastric carcinogenesis is a challenging task. Mouse models of cancer are useful to dissect the early events of carcinogenesis that are difficult to identify in humans.
In 1996, the TFF1 knockout mouse was found to be a good model for spontaneous development of gastric adenoma and cancer (Lefebvre et al. 1996) and recent experimental studies support the notion that TFF1 acts as a tumour suppressor gene that may be involved in development and/or progression of gastric cancer (Calnan et al. 1999; Bossenmeyer‐Pourie et al. 2002; Carvalho et al. 2002; Beckler et al. 2003). Strongly supporting this TFF1 function, screening of different types of human gastric cancer has revealed remarkable down‐regulation of TFF1 expression due to either allelic loss at the TFF1 gene locus, TFF1 promoter methylation, or TFF1 gene single point mutation (Calnan et al. 1999; Beckler et al. 2003; Yio et al. 2006). Thus, the TFF1 knockout mouse provides an excellent model to look at alterations that are associated with precancerous lesions and to understand the development of gastric adenocarcinoma (Tomasetto & Rio 2005).
In the present study, we have shown that from birth to more than 1 years of age, TFF1 knockout mice show a sequence from pre‐neoplastic to benign and then malignant neoplasms. Thus, from birth to 6 months, TFF1 deficiency was associated with a gradual increase in mucosal thickness leading to development of adenomas in the pyloric antrum. In a previous study, we have shown that changes in the commitment program of epithelial progenitors of the oxyntic region of TFF1 knockout mouse stomachs were associated with their amplification in numbers (Karam et al. 2004). The situation was more pronounced in the pyloric antrum where nodular lesions and even carcinoma in situ in the basal portion of the mucosa were observed at around 6 months of age (Lefebvre et al. 1996). Finally, in the 12‐month‐old knockout mice, some glandular cells had found their way through the muscularis mucosa and had invaded the submucosa. Thus, the TFF1 knockout mouse model recapitulates the classical chronological scheme of multistage carcinogenesis, including initiation (due to TFF1 deficiency), promotion and progression of the malignancy.
To date, the nature of cells involved in the origin of gastric cancer is still debatable. Some studies suggest that carcinogenesis might derive from stem cells (Potter 1978; Hirata & Hirata 2002; Burkert et al. 2006; Sell 2006; Trosko and Tai 2006). Collectively, our results support this hypothesis because in the pyloric antrum of TFF1‐deficient mice, the cells, involved in early mucosal thickening, carcinoma in situ, as well as the submucosal invasion with cyst‐like structures, were mainly of the epithelial progenitor type. That gastric progenitors, including undifferentiated granule‐free stem cells, were amplified in early stages of gastric tumorigenesis and formed the invasive cells of the gastric adenocarcinomas raises a potential biological role of stem cells in the tumorigenic cascade. Thus, this study also can be taken as one further piece of evidence for the stem cell origin of gastric cancer (Sell 2006; Trosko & Tai 2006; Alison et al. 2008). Finally, the invading cells crossed the muscularis mucosa, grew in the connective tissue of the submucosa and maintained some capacity to differentiate. This finding supports the idea of autocrine control of gastric stem cells and their capacity to differentiate outside their niche; thus, they are the source of instruction for their own commitment programme (Mills et al. 2002).
Thus, our results together with growing data concerning the stem cell origin of cancer strongly suggest that some members of isthmus progenitor cells are involved in epithelial tumorigenesis and knowledge of this may have an early diagnostic, therapeutic, and/or prognostic clinical value.
ACKNOWLEDGEMENTS
We thank Rony John and Saeed Tariq in UAE University and members of the animal and polyclonal antibody facilities in the Institute of Genetics and Cellular and Molecular Biology for their excellent technical assistance. This work was supported by Terry Fox Foundation for Cancer Research (to SMK) and funds from the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, the Association pour la Recherche sur le Cancer, the Ligue Nationale Française Contre le Cancer and the Comités du Haut‐Rhin et du Bas‐Rhin, and the «Ruban Rose» Price (to MCR).
REFERENCES
- Alison MR, Murphy G, Leedham S (2008) Stem cells and cancer: a deadly mix. Cell Tissue Res. 331, 109–124. [DOI] [PubMed] [Google Scholar]
- Beckler AD, Roche JK, Harper JC, Petroni G, Frierson HF Jr, Moskaluk CA, El‐Rifai W, Powell SM (2003) Decreased abundance of trefoil factor 1 transcript in the majority of gastric carcinomas. Cancer 98, 2184–2191. [DOI] [PubMed] [Google Scholar]
- Bossenmeyer‐Pourie C, Kannan R, Ribieras S, Wendling C, Stoll I, Thim L, Tomasetto C, Rio MC (2002) The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1‐S phase transition and reducing apoptosis. J. Cell Biol. 157, 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkert J, Wright NA, Alison MR (2006) Stem cells and cancer: an intimate relationship. J. Pathol. 6, 4235–4245. [DOI] [PubMed] [Google Scholar]
- Calnan DP, Westley BR, May FE, Floyd DN, Marchbank T, Playford RJ (1999) The trefoil peptide TFF1 inhibits the growth of the human gastric adenocarcinoma cell line AGS. J. Pathol. 188, 312–317. [DOI] [PubMed] [Google Scholar]
- Carvalho R, Kayademir T, Soares P, Canedo P, Sousa S, Oliveira C, Leistenschneider P, Seruca R, Gött P, Blin N, Carneiro F, Machado JC (2002) Loss of heterozygosity and promoter methylation, but not mutation, may underlie loss of TFF1 in gastric carcinoma. Lab. Invest. 82, 1319–1326. [DOI] [PubMed] [Google Scholar]
- El‐Alfy M, Leblond CP (1987) Long duration of mitosis and consequences for the cell cycle concept, as seen in the isthmal cells of the mouse pyloric antrum. II. Duration of mitotic phases and cycle stages, and their relation to one another. Cell Tissue Kinet. 20, 215–226. [DOI] [PubMed] [Google Scholar]
- Falk P, Roth KA, Gordon JI (1994) Lectins are sensitive tools for defining the differentiation programs of mouse gut epithelial cell lineages. Am. J. Physiol. 266 (6 Part 1), G987–G1003. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Hirata S (2002) Physio‐mitotic theory and a new concept of cancer development. Med. Hypotheses 58, 361–364. [DOI] [PubMed] [Google Scholar]
- Hohenberger P, Gretschel S (2003) Gastric cancer. Lancet 362, 305–315. [DOI] [PubMed] [Google Scholar]
- Karam SM (1999) Lineage commitment and maturation of epithelial cells in the gut. Front. Biosci. 4, D286–D298. [DOI] [PubMed] [Google Scholar]
- Karam SM, John R, Alpers DH, Ponery AS (2005) Retinoic acid stimulates the dynamics of mouse gastric epithelial progenitors. Stem Cells 23, 433–441. [DOI] [PubMed] [Google Scholar]
- Karam SM, Li Q, Gordon JI (1997) Gastric epithelial morphogenesis in normal and transgenic mice. Am. J. Physiol. 272 (5 Part 1), G1209–G1220. [DOI] [PubMed] [Google Scholar]
- Karam SM, Straiton T, Hassan WM, Leblond CP (2003) Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells 21, 322–336. [DOI] [PubMed] [Google Scholar]
- Karam SM, Tomasetto C, Rio MC (2004) Trefoil factor 1 is required for the commitment programme of mouse oxyntic epithelial progenitors. Gut 53, 1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ER, Leblond CP (1985) Dynamic histology of the antral epithelium in the mouse stomach. II. Ultrastructure and renewal of isthmal cells. Am. J. Anat. 172, 205–224. [DOI] [PubMed] [Google Scholar]
- Lee ER, Trasler J, Dwivedi S, Leblond CP (1982) Division of the mouse gastric mucosa into zymogenic and mucous regions on the basis of gland features. Am. J. Anat. 164, 187–207. [DOI] [PubMed] [Google Scholar]
- Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, Lemeur M, Wendling C, Tomasetto C, Chambon P, Rio MC (1996) Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 274, 259–262. [DOI] [PubMed] [Google Scholar]
- Lefebvre O, Wolf C, Kedinger M, Chenard MP, Tomasetto C, Chambon P, Rio MC (1993) The mouse one P‐domain (pS2) and two P‐domain (mSP) genes exhibit distinct pattern of expression. J. Cell Biol. 122, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Moriuchi T, Koji T, Nakane PK (1987) Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin. EMBO J. 6, 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JC, Andersson N, Hong CV, Stappenbeck TS, Gordon JI (2002) Molecular characterization of mouse gastric epithelial progenitor cells. Proc. Natl. Acad. Sci. USA 99, 14819–14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM (2001) Global cancer statistics in the year. Lancet Oncol. 2, 533–543. [DOI] [PubMed] [Google Scholar]
- Potter VR (1978) Phenotypic diversity in experimental hepatomas: the concept of partially blocked ontogeny. The 10th Walter Hubert Lecture. Br. J. Cancer 38, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribieras S, Tomasetto C, Rio MC (1998) The pS2/TFF1 trefoil factor, from basic research to clinical applications. Biochim. Biophys. Acta 1378, F61–F77. [DOI] [PubMed] [Google Scholar]
- Rio MC, Bellocq JP, Daniel JY, Tomasetto C, Lathe R, Chenard MP, Batzenschlager A, Chambon P (1988) Breast cancer‐associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science 241, 705–708. [DOI] [PubMed] [Google Scholar]
- Roder DM (2002) The epidemiology of gastric cancer. Gastric Cancer 5 (Suppl. 1), 5–11. [DOI] [PubMed] [Google Scholar]
- Sell S (2006) Cancer stem cells and differentiation therapy. Tumour Biol. 27, 59–70. [DOI] [PubMed] [Google Scholar]
- Suemori S, Lynch‐Devaney K, Podolsky DK (1991) Identification and characterization of rat intestinal trefoil factor: tissue‐ and cell‐specific member of the trefoil protein family. Proc. Natl. Acad. Sci. USA 88, 11017–11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnawski AS (2005) Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig. Dis. Sci. 50, S24–S33. [DOI] [PubMed] [Google Scholar]
- Tomasetto C, Rio MC (2005) Pleiotropic effects of trefoil factor 1 deficiency. Cell. Mol. Life Sci. 62, 2916–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trosko E, Tai MH (2006) Adult stem cell theory of the multi‐stage multi‐mechanism theory of carcinogenesis: role of inflammation on the promotion of initiated cells In: Dittmar T, Zaenkar KS, Schmidt A, eds. Infections and Inflammation: Impacts on Oncogenesis. Contribution to Microbiology. S. Karger AG; Publishers, Basel, pp 45–65. [DOI] [PubMed] [Google Scholar]
- Yio X, Diamond M, Zhang JY, Weinstein H, Wang LH, Werther L, Itzkowitz S (2006) Trefoil factor family‐1 mutations enhance gastric cancer cell invasion through distinct signaling pathways. Gastroenterology 130, 1696–1706. [DOI] [PubMed] [Google Scholar]


