Abstract
Abstract. Stress‐induced effects in human acute leukaemia cells (HL‐60) were studied by flow cytometry using the fluorescent dye carboxyfluorescein succinimidyl ester which allows the analysis of several successive cell generations for up to 10 days. Asynchronously cycling cells subjected to heat shock (30 min at 41 °C) responded in two distinct ways: while one fraction of the cell population (about 15%) re‐entered the cell cycle after a short delay, other cells became arrested at different phases of the cell cycle and remained arrested for up to several days and finally underwent apoptosis. Weak electromagnetic fields (60 µT, 50 Hz) alleviated the heat‐induced block and the fraction of arrested cells was significantly smaller.
INTRODUCTION
Cells are well prepared to cope with adverse conditions. The stress response ensures that vital cellular functions are maintained as long as possible while other functions are suspended during the period of stress. Cell division is one of the most easily disrupted cellular functions. This effect has been studied in detail using heat shock as a stressor (Rice et al. 1986; Hang & Fox 1996; Kuhl et al. 2000) and cell cycle arrest has been described as a typical cellular response to thermal stress (Kuhl & Rensing 2000). However, the observed cell‐cycle arrest may differ qualitatively and quantitatively depending on the cell type studied and the conditions of thermal stress (Coss 1986; Rice et al. 1986; Higashikubo et al. 1993). Above a certain and cell‐type specific stress level cells are no longer viable and undergo apoptosis or necrosis (Van der Waal et al. 1997).
One of the major gene products induced by thermal stress is the heat shock protein 70 (HSP70). This protein plays important roles in the folding of damaged proteins and in the control of a variety of cellular functions including the cell cycle. Elevated levels of HSP70 prior to a stress improves the cellular stress resistance, a phenomenon that is referred to as cytoprotection (or thermoprotection). Recent studies leave no doubt that the HSP70 level is critical for cytoprotection (see Discussion for details).
The report that ELF‐EMF exposure (0.8–300 µT, 60 Hz) leads to the stress‐typical induction of the HSP70 gene (Jin et al. 2000) prompted us to study this interesting effect in more detail. We found that pulsed magnetic fields (50 Hz, sinusoidal) with a flux density of 10–140 µT is sufficient to induce a strong expression of the three human HSP70 genes (A, B, and C) in human HL‐60 cells (Tokalov & Gutzeit, unpublished data). The biological implications of the ELF‐EMF induced gene expression have not been explored, but in view of the cytoprotective effect of HSP70 and other important functions for cellular homeostasis, further studies are called for.
The analysis of cell proliferation is usually carried out by studying the incorporation of BrdU or radioactive thymidine. The technique of flow cytometry has been very useful for analysing the cell cycle but the quantification of the DNA content does not allow the analysis of different cell generations in one experiment.
However, when cells are labelled with the dye carboxyfluorescein diacetate succinimidyl ester (CFSE) the fluorescence intensity decreases predictably during successive cell cycles such that different cell generations can be distinguished (Lyons & Parish 1994). By quantification of the fluorescence signal of each cell, eight to ten successive cell divisions can be resolved by flow cytometry (Glimm & Eaves 1999; Lyons 1999; Parish 1999). CFSE does not seem to be toxic and lymphocyte differentiation does not seem to be affected by the compound (Hasbold et al. 1999). The results presented in this communication illustrate the power of this technique in particular for the analysis of mixed populations of cycling and arrested cells.
MATERIALS AND METHODS
Cell culture
Acute myeloid leukaemia cells (HL‐60, DSMZ, Heidelberg, Germany) were maintained in RPMI 1640 medium with 10% heat‐inactivated foetal calf serum (Gibco, Cergy, France). Cells were grown at 37 °C in a humidified 5% CO2 atmosphere and maintained at a density of 2 × 105 − 1 × 106 cells/ml by resuspending the cells in fresh culture medium every 2 days.
Exposure to ELF‐EMF and heat shock
Cell cultures (106 cells/ml, 15 ml per flask) were exposed to sinusoidal ELF‐EMF (50 Hz, 60 ± 0.2 µT) and/or thermal stress at 39, 41 or 43 °C for 30 min. Control cultures were maintained at 37 °C. ELF‐EMF was generated by a set of Merritt coils (Merritt et al. 1983; Kirschvink 1991) as described before (Junkersdorf et al. 2000). The correlation between coil current and the magnetic field was experimentally determined and was found to be linear in the range of 1–150 µT with a precision of ±2%. The harmonic distortion was determined to be smaller than 1%. During the exposure, the magnetic flux density was controlled by adjusting the coil current with a precision of ±1.5% using a Digital‐Multimeter (m 3860‐M, Conrad Electronic, Hirschau, Germany). For the experimental set‐up, a location in the laboratory was chosen in which stray field sources could produce a magnetic field of 1 µT at most.
The temperature of the cell cultures was controlled using a specially designed plastic chamber with the dimensions 1800 × 1400 × 60 mm. Water of desired temperature (±0.1 °C; F15 waterbath, Julabo Labortechnik, Seelbach, Germany) was pumped through cavities drilled in a serpentine way into the bottom plate of the chamber. One hour before the exposure of the cells to thermal stress, the desired temperature in the chamber was reached and did not change until the end of the experiment. The temperature was controlled using a GTH 175/MO digital thermometer (Greisinger Electronic, Regenstauf, Germany) with a precision of ±0.1 °C. The plastic chamber was placed in the centre of the Merritt coils so that the two stressors (thermal stress and ELF‐EMF) could easily be applied alone or simultaneously. After the exposure to the stressor(s), the cells were cultured under standard conditions at 37 °C and analysed as described below.
Analysis of cell proliferation
Cells were stained with carboxyfluorescein succinimidyl ester (CFSE, Molecular Probes, Eugene, OR, USA) as described by Lyons (1999). The cells were labelled with 10 µm CFSE in phosphate‐buffered saline (PBS) for 10 min at 37 °C, washed and suspended in culture medium (approximately 2 × 105 cells per ml). One hour before the exposure to the respective stressors, the culture was split into the required number of samples with equal volumes for parallel experiments. After 2, 4, 6, 8, and 10 days a defined volume of the cell suspension of each sample was fixed in 70% ethanol and stored overnight at −20 °C. The cells were centrifuged for 5 min at 300 g, and the pellet was resuspended in PBS containing 50 µg/ml propidium iodide (PI) and 0.2 mg/ml RNase (Sigma, Taufkirchen, Germany), incubated for at least 45 min. Between 1 and 5 × 105 cells per sample were analysed by flow cytometry (CyFlow, Partec, Muenster, Germany). For each variable (exposure conditions, culture periods, etc.) a minimum of 10 samples were quantified. The fraction of cells present in different cell generations and their representation in the respective cell cycle phases was calculated using the CyFlow software (Partec).
Statistical analysis
Statistical analysis was performed using Student's t‐test. Significance levels were set at P < 0.05.
RESULTS
We chose the human leukaemia cell line HL‐60 for the experiments because these cells are known to respond to stressors, heat shock and ELF‐EMF, with the stress‐typical gene expression which can be studied both on the mRNA and the protein level.
We analysed the effects of the stressors on the cell cycle over several cell divisions by making use of the fluorescent dye CFSE. Immediately after CFSE staining, the cells form a rather homogeneous population as shown in two‐dimensional plots (Fig. 1a) using the parameters CFSE staining and side scattering (SSC). Additional staining with PI allows the cell cycle distribution to be analysed based on DNA quantification (Fig. 1b). When cells have passed through one or more cell cycles after CFSE labelling, the cycling cells can be distinguished from non‐cycling cells due to the successive loss of fluorescence with each cell division (Fig. 1c). More information is obtained when CFSE‐labelled cells are stained with PI after the incubation period (2 days in Fig. 1d) as, in this case, the cell cycles of successive cell generations can be analysed at the same time. In the example shown in Fig. 1(d) the cells had been stressed for 30 min before being cultured for 2 days. At the end of the incubation many cells did not divide and remained mostly in the G1 or S stage (labelled ‘0’ division) or underwent apoptosis while cells that divided once were present mostly in the G2/M stage. Another cell population was already in the second round of cell division and the fastest dividing cells had reached the G1 stage of the third round of division. The pattern of the cell‐cycle phases in the three cell generations reflect the fact that cells were essentially pulse‐labelled by CFSE at the beginning of the experiment.
Figure 1.
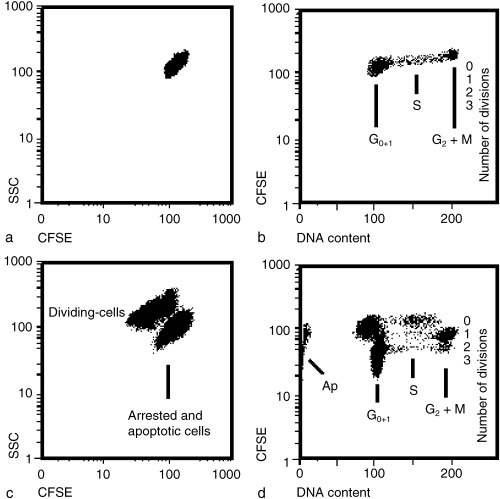
Separation of different HL‐60 cell populations by CFSE and/or PI staining. The cell cultures were CFSE‐stained and analysed immediately (a, b) or exposed for 30 min to both thermal stress (41 °C) and ELF‐EMF (60 µT) and cultured for 2 days (c, d). (a) The double parameter analysis of CFSE fluorescence versus side scattering (SSC) shows that the control cells comprise a homogeneous cell population before the exposure to stressors. (b) PI staining in addition to CFSE allows analysis of the cell cycle distribution of the control cells (marked ‘0’ division). (c) Stressed cells cultured for 2 days consist of proliferating cells and arrested or apoptotic cells. These two cell populations can be separated by SSC and CFSE quantification. (d) By plotting CFSE versus PI fluorescence intensity, cell‐cycle arrested (‘0’ division) and apoptotic (Ap) cells can be distinguished from cycling cell populations. Due to reduction in CFSE fluorescence with each division three generations of cells can be distinguished (labelled 1–3). Furthermore, this allows analysis of the cell cycles of each cell generation separately.
To characterize the cycling pattern of HL‐60 cells over several generations in response to heat shock and/or ELF‐EMF, cells were stained with CFSE 1 hour before exposure to the stressor(s) for 10 min. In 2‐day intervals for up to 10 days cells were collected, fixed, stained with PI and examined by flow cytometry. Firstly, the proliferation of control cultures was analysed. In Fig. 2 the proliferation for up to seven cell generations over a period of 6 days is illustrated. After 2 days of culture, the unstressed cells were in the second round of cell division (69 ± 3%) while some cells apparently cycled more slowly (11 ± 2% in the first division) and some cells divided faster (14 ± 3% in the third division) and the remaining cells (6 ± 4%) were apoptotic. As we used asynchronously proliferating cell cultures in all experiments, a difference of one cell cycle is to be expected when analysing proliferation during several rounds of division. However, during the course of the experiment it became apparent that cells cycle at different speeds. After 4 days of culture, the cells had passed through a minimum of three and a maximum of six rounds of cell divisions. At this time, only a small fraction of the cells did not divide or was apoptotic (5 ± 3%) while the remaining cells (95 ± 3%) proliferated and this percentage remained nearly constant for up to 10 days (Fig. 3).
Figure 2.
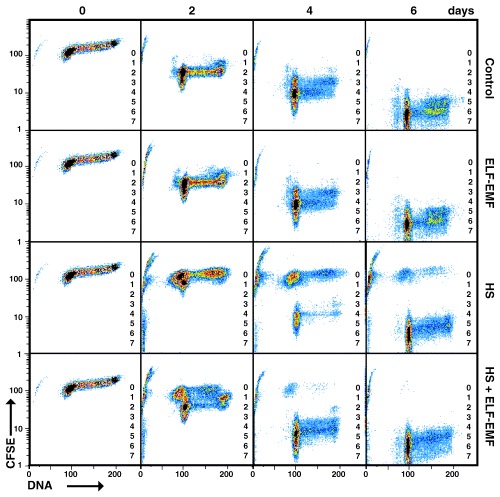
Effect of stressors on cell proliferation. Asynchronously proliferating HL‐60 cells were stained with CFSE (day 0) and divided up into four parallel cultures. One culture was maintained in stress‐free conditions as the control while the other three cultures were incubated for 1 h and then exposed to the stressor(s) as indicated (ELF‐EMF 60 µT, 50 Hz and/or heat shock at 41 °C, both for 30 min). The analysis of cell proliferation was carried out by flow cytometry with additional PI staining after 2, 4 and 6 days of incubation. The different cell generations are indicated (1–7). Cells remaining at cycle 0 did not proliferate during the period of culture.
Figure 3.
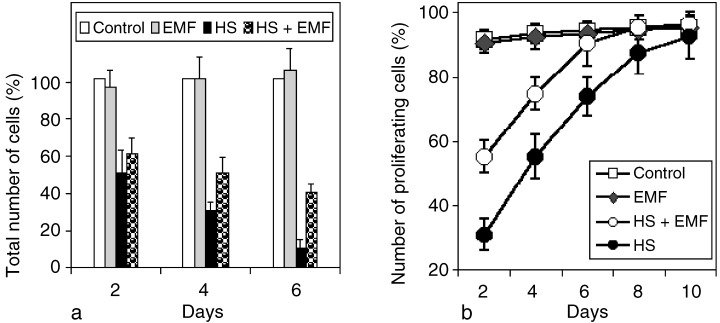
Cytoprotective effect of ELF‐EMF (60 µT, 50 Hz).Total cell number in controls (100%) and heat shocked, ELF‐EMF‐exposed, and heat shocked and ELF‐EMF‐treated cells after 2, 4 and 6 days of culture. Note that the reduction in cell number after heat shock is greater compared with samples that were, in addition, exposed to ELF‐EMF. Percentage of proliferating HL‐60 cell cultures analysed for a period of 10 days. The cell‐cycle arrest induced by HS is reduced by ELF‐EMF. Arrested cells are continuously removed from the cultures by apoptosis and the fraction of cells that are proliferating increases continuously during the course of the experiment. The exposure conditions were identical to the experiments illustrated in Figure 2.
Compared with control cultures, ELF‐EMF exposure alone (50 Hz, 60 µT) neither changed the rate of cell division (Fig. 2) nor the total number of cells (Fig. 3a) or the percentage of dividing cells (Fig. 3b). From these data it can be concluded that ELF‐EMF did not affect proliferation of asynchronously dividing HL‐60 cells under the chosen experimental conditions.
As it was our intention to analyse the effects of ELF‐EMF in the presence of thermal stress, the heat shock conditions had to be optimized first. In preliminary experiments the cells were exposed to different temperatures for 30 min and the cell‐cycle analysis carried out as described above. A rise of the temperature by 2 °C (39 °C) above the normal culture temperature (37 °C) only had a minor effect with respect to cell division as most cells (80 ± 8% on the second day) continued cycling. However, when exposed to 41 °C, the percentage of proliferating cells decreased to 32 ± 4%. At 43 °C only a small fraction of cells (4 ± 3%) proliferated. Based on these data it seemed appropriate to carry out all further experiments with thermal stress at a temperature of 41 °C.
It had been noticed before by several authors (Nitta et al. 1997; Kuhl et al. 2000) that heat shock halts the cell cycle and under certain conditions an increased fraction of cells accumulates at the G1 or G2/M cell cycle checkpoints. The CFSE‐labelling technique reveals an interesting differential effect on the exposed cells. A large fraction of the exposed cells (68 ± 5%) lost the ability to proliferate and never regained it. Such cells whose cell cycle was blocked by heat shock finally became apoptotic (Fig. 2). The two cell populations, i.e. cycling and arrested cells, could be distinguished during the entire experiment and a widening gap between these populations in the two‐dimensional plot became apparent. For example, 4 days after the treatment 44 ± 7% of the cells had remained arrested while others had undergone three to five rounds of division. No cells were present in the first or second division. From the arrested cells, no new cell generations arose for up to 10 days (data not shown). The number of arrested cells decreases as a result of apoptosis and the cycling cells become more numerous, so that the fraction of proliferating cells increases continuously. Within 10 days the level of the control is reached (94 ± 7%).
When cells were exposed to both stressors, i.e. elevated temperatures (41 °C) and ELF‐EMF (60 µT, 50 Hz) at the same time for 30 min, the cell cycle analysis showed a surprising difference to heat shock treatment alone. Two days after the treatment a much smaller fraction of cells (44 ± 5%) showed cell cycle arrest compared with cells that were only heat shocked (68 ± 5%). This strong effect was consistently observed and is highly significant (P < 0.05). The protective effect of ELF‐EMF was reflected in the increased total number of cells compared with cultures that were only heat shocked (Fig. 3a), and also in the higher percentage of proliferating cells which was noticeable for up to 6 days (Fig. 3b) when the control level was finally reached (92 ± 7%).
The effect of ELF‐EMF on heat stressed cells was 2‐fold. Not only was the fraction of cycling cells increased but also cells initiated cycling faster than cells only exposed to thermal stress. Precisely 100 000 cells for each experimental condition were analysed 2 days after exposure to the stressor(s) in 11 independent experiments, and the presence of cells in the first three cell cycles was compared (Fig. 4). The differences are particularly obvious when the number of cells in the second cell cycle are compared. Twice as many cells were in the second cell cycle after cells had been exposed to ELF‐EMF (in addition to heat shock) compared with cells that were only heat shocked. This difference was significant (P < 0.05) and was also apparent after 4 days of culture (not shown).
Figure 4.
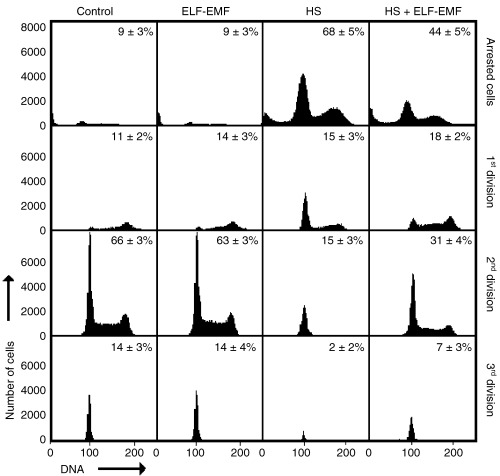
Percentage of CFSE‐labelled cells in different cell generations 2 days after the HL‐60 cells were stressed as indicated in Figure 2. This graphical representation illustrates the different dynamics of cell division in the stressed and unstressed (control) cell cultures. The different cell generations (from no division to third division) were quantified by gating using the FlowMax software.
DISCUSSION
The effects of heat shock on gene expression and the cell cycle has been studied in a number of different cell lines (Higashikubo et al. 1993; Nitta et al. 1997; Kuhl et al. 2000). For technical reasons, the analysis of the cell cycle in asynchronously dividing cells could not be followed up during consecutive divisions and the fate of those cells that were arrested at a specific point in the cell cycle by the heat treatment (Kuhl et al. 2000) could not be studied for prolonged periods of time. In the present study, we were able to analyse cell divisions by CFSE staining for several cell generations after heat shock, ELF‐EMF and simultaneous exposure to both stressors. With this method up to six to eight (Glimm & Eaves 1999; Oostendorp et al. 2000) successive generations of cell can be resolved. One of the major advantages of CFSE staining is that relatively high intracellular concentration of the dye can be achieved at low extracellular dye concentration. This is a crucial point, as the major reason for cellular toxicity appears to be excessive binding to cell surface proteins (Parish 1999). For the first few days after labelling the cells of each divisions can be separated with reasonable precision but later (6–10 days) the CFSE fluorescence intensity differences are so small that the identification of each cell generation is difficult and has not been attempted in this study. The fractions of dividing and cell‐cycle arrested cells can, however, easily be quantified (Fig. 2).
ELF‐EMF has been shown to induce significant biological alterations in a variety of cells and tissues (Hong 1995; Goodman & Blank 1998; Gutzeit 2001). These changes include the induction of several early response genes, including c‐myc (Jin et al. 2000), c‐fos (Rao & Henderson 1996) and HSP70 mRNA (Goodman & Blank 1998) and resulted in an elevated level of stress inducible HSP70 protein production (Goodman & Henderson 1988). Our recent results (Tokalov & Gutzeit, unpublished) clearly show that in HL‐60 cells heat shock genes, in particular the three HSP70 genes (A, B, and C) are induced by ELF‐EMF (50 Hz, 10–140 µT), a reaction that is enhanced by simultaneous exposure to heat shock.
What is the biological significance of the ELF‐EMF induced elevated HSP70 level? Apart from the well‐studied chaperone function (Morimoto 1998; Feber & Hofmann 1999) and the role in the control of the cell cycle and of apoptosis (Gabai & Sherman 2002), the HSP70 are known to fulfil crucial roles in cellular protection and repair and were suggested to mediate the well‐known effect of acquired thermotolerance (Laszlo 1992; Chen et al. 1999; De Maio 1999; Barnes et al. 2001). When transfected Rat‐1 fibroblasts overexpressed the HSP70 gene, they became more thermotolerant than control cells (Li et al. 1996). The most compelling evidence comes from studies with transgenic mice. It was shown that constitutive expression of human inducible HSP70 protects the myocardium from ischemia and reperfusion injury (Marber et al. 1995; Plumier et al. 1995). Inactivation of HSP70 resulted in deficient maintenance of acquired thermotolerance and increased sensitivity to heat stress‐induced apoptosis (Huang et al. 2001).
As ELF‐EMF induces the expression of HSP70, and the function of HSP70 in cytoprotection is well documented, it is not surprising that ELF‐EMF may show cytoprotective effects in suitable cell or animal test systems. This effect was demonstrated in several human cancer cell lines which were exposed to ELF‐EMF and acquired increased resistance to heat‐induced apoptosis (Han et al. 1998; Carmody et al. 2000; Robison et al. 2002). For example, exposure to 150 µT (60 Hz) ELF‐EMF protected HL‐60, HL‐60R, and Raji cell lines against apoptosis induced by heat shock for up to 48 h (Robison et al. 2002). Furthermore, the effect of cytoprotection was also observed in animal test systems. For example, exposure to ELF‐EMF protected fertilized Sciara coprophila eggs from lethal hyperthermia (Goodman & Blank 1998). ELF‐EMF (like heat stress) reduced anoxia‐induced mortality in chick embryos. According to Dicarlo et al. (1999) this effect can be used to test for the existence of weak ELF‐EMF. Exposure to a magnetic field (60 Hz) with different field strengths for 20 min was suggested as an alternative to hyperthermia for the induction of HSP70 for pre‐surgical cytoprotection of normal human cells (Han et al. 1998). However, the cell type specific response to HSP70 induction (see, for example Huang et al. 2001) suggests that the degree of cytoprotection by ELF‐EMF may vary in a wide range depending on the system studied.
The observed ELF‐EMF induced re‐entry of heat shocked arrested cells may be interpreted as a cytoprotective effect of ELF‐EMF. The previously demonstrated ELF‐EMF induced elevated HSP70 levels under the same stress conditions (Tokalov & Gutzeit, unpublished results) lends support to this interpretation. While the phenomenon of thermoprotection is well documented, the molecular mechanisms that lead to the observed effects are not fully understood. It seems plausible that the HSP70 mediated repair processes (chaperone function) of stress‐induced damage allows cells to re‐enter the cell cycle faster than cells in which the HSP70 level is lower in comparison. Specific interactions of HSP70 with molecules regulating cell cycle, apoptosis and other vital functions are, however, likely to exist. A possible target molecule is the c‐jun N‐terminal kinase (JNK) which is activated by a number of protein‐damaging stressors and promotes apoptosis, but HSP70 inhibits its activation (Gabai & Sherman 2002). This function of HSP70 is not dependent on its chaperone function because mutations of the ATPase‐ and substrate‐binding domain do not inhibit HSP70‐mediated kinase inhibition. A role of c‐Jun in promoting cell division was suggested by studies using microinjection of neutralizing antibodies or antisense RNA, which cause a partial G1 arrest and block entry into S phase (Riabowol et al. 1992). Conversely, cell‐cycle distribution in cells overexpressing c‐Jun is shifted toward S phase (Pfarr et al. 1994). The c‐Jun proto‐oncogene encodes a component of the mitogen‐inducible immediate early‐transcription factor AP‐1 and has been implicated as a positive regulator of cell proliferation and G1 to S‐phase progression by induction of cyclin D1 expression (Herber et al. 1994; Sherr 1996) and by attenuation of p21 accumulation (Schreiber et al. 1999). In the light of these findings, the observed effects of ELF‐EMF on apoptosis and cell proliferation may, at least in part, be explained by elevated HSP70 levels resulting in JNK suppression. Details of the regulation of c‐jun under the stress conditions remain to be elucidated.
The interaction of stressors and the physiological consequences on the cellular and molecular level have not been explored, although the importance of stress‐induced reactions for a number of medical questions is apparent (Gutzeit 2001). A substitution of current hyperthermia treatment by ELF‐EMF exposure appears to be a strategy worth pursuing. Another facet of stress interaction is the treatment of patients with elevated levels of HSP due to, for example, fever, inflammation, ischemia, reperfusion damage or infection. There is good reason to assume that the physiological response of such patients to, for example, radiation stress, cytostatics or hyperthermia will significantly differ from that of healthy persons. There is no doubt, that both basic and applied research in this area is called for.
ACKNOWLEDGEMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (Gu 208/11–1). The flow cytometer used in this study was purchased with a grant of the State of Saxony.
REFERENCES
- Barnes JA, Dix DJ, Collins BW, Luft C, Allen JW (2001) Expression of inducible Hsp70 enhances the proliferation of MCF‐7 breast cancer cells and protects against the cytotoxic effects of hyperthermia. Cell Stress Chaperones 6, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody S, Wu XL, Lin H, Blank M, Skopicki H, Goodman R (2000) Cytoprotection by electromagnetic field‐induced hsp70: a model for clinical application. J. Cell Biochem. 79, 453. [DOI] [PubMed] [Google Scholar]
- Chen YC, Lin‐Shiau SY, Lin JK (1999) Involvement of heat‐shock protein 70 and P53 proteins in attenuation of UVC‐induced apoptosis by thermal stress in hepatocellular carcinoma cells. Photochem. Photobiol. 70, 78. [PubMed] [Google Scholar]
- Coss RA (1986) Decay of thermal resistence following acute heating is independent of the G1‐ to S‐phase transition. Rad. Res. 107, 143. [PubMed] [Google Scholar]
- De Maio A (1999) Heat shock proteins: facts, thoughts and dreams. Shock 11, 1. [DOI] [PubMed] [Google Scholar]
- Dicarlo AL, Farrell JM, Litovitz TA (1999) Myocardial protection conferred by electromagnetic fields. Circulation 99, 813. [DOI] [PubMed] [Google Scholar]
- Feber ME, Hofmann GE (1999) Heat‐shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Sherman MY (2002) Interplay between molecular chaperones and signalling pathways in survival of heat shock. J. Appl. Physiol. 92, 1743. [DOI] [PubMed] [Google Scholar]
- Glimm H, Eaves CJ (1999) Direct evidence for multiple self‐renewal divisions of human in vivo repopulating hematopoietic cells in short‐term culture. Blood 94, 2161. [PubMed] [Google Scholar]
- Goodman R, Blank M (1998) Magnetic field stress induces expression of hsp70. Cell Stress Chaperones 3, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Henderson A (1988) Exposure of salivary gland cells to low‐frequency electromagnetic fields alters polypeptide synthesis. Proc. Natl Acad. Sci. USA 85, 3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzeit HO (2001) Interaction of stressors and the limits of cellular homeostasis. Biochem. Biophys. Res. Commun. 283, 721. [DOI] [PubMed] [Google Scholar]
- Han L, Lin H, Head M, Jin M, Blank M, Goodman R (1998) Application of magnetic field‐induced heat shock protein 70 for presurgical cytoprotection. J. Cell. Biochem. 71, 577. [DOI] [PubMed] [Google Scholar]
- Hang H, Fox MH (1996) Levels of 70‐kDa heat shock protein through the cell cycle in several mammalian cell lines. Cytometry 25, 367. [DOI] [PubMed] [Google Scholar]
- Hasbold J, Gett AV, Rush JS, Deenick E, Avery D, Jun J, Hodgkin PD (1999) Quantitative analysis of lymphocyte differentiation and proliferation in vitro using carboxyfluorescein diacetate succinimidyl ester. Immunol. Cell Biol. 77, 516. [DOI] [PubMed] [Google Scholar]
- Herber B, Truss M, Beato M, Muller R (1994) Inducible regulatory elements in the human cyclin D1 promoter. Oncogene 9, 2105. [PubMed] [Google Scholar]
- Higashikubo R, White RA, Roti Roti JL (1993) Flow cytometric BrdUrd‐pulse‐chase study of heat‐induced cell cycle progression delays. Cell Prolif. 26, 337. [DOI] [PubMed] [Google Scholar]
- Hong FT (1995) Magnetic field effects on biomolecules, cells, and living organisms. Biosystems 36, 187. [DOI] [PubMed] [Google Scholar]
- Huang L, Mivechi NF, Moskophidis D (2001) Insights into regulation and function of the major stress‐induced hsp70 molecular chaperone in vivo: analysis of mice with targeted gene disruption of the hsp70.1 or hsp70.3 gene. Mol. Cell Biol. 21, 8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Blank M, Goodman R (2000) ERK1/2 phosphorylation, induced by electromagnetic fields, diminishes during neoplastic transformation. J. Cell. Biochem. 78, 371. [DOI] [PubMed] [Google Scholar]
- Junkersdorf B, Bauer H, Gutzeit HO (2000) Electromagnetic fields enhance the stress response at elevated temperatures in the nematode Caenorhabditis elegans . Bioelectromagnetics 21, 100–106. [DOI] [PubMed] [Google Scholar]
- Kirschvink JL (1991) Uniform magnetic fields and double‐wrapped coil systems: improved techniques for the design of bioelectromagnetic experiments. Bioelectromagnetics 13, 401–411. [DOI] [PubMed] [Google Scholar]
- Kuhl NM, Rensing L (2000) Heat shock effects on cell cycle progression. Cell Mol. Life Sci. 57, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl NM, Kunz J, Rensing L (2000) Heat shock‐induced arrests in different cell cycle phases of rat C6‐glioma cells are attenuated in heat shock‐primed thermotolerant cells. Cell Prolif. 33, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo A (1992) The thermoresistant state: protection from initial damage or better repair? Experim. Cell Res. 202, 519. [DOI] [PubMed] [Google Scholar]
- Li WX, Chen CH, Ling CC, Li GC (1996) Apoptosis in heat‐induced cell killing: the protective role of hsp‐70 and the sensitisation effect of the c‐myc gene. Radiat. Res. 145, 324. [PubMed] [Google Scholar]
- Lyons AB (1999) Divided we stand: tracking cell proliferation with carboxyfluorescein diacetate succinimidyl ester. Immunol. Cell Biol. 77, 509. [DOI] [PubMed] [Google Scholar]
- Lyons AB, Parish CR (1994) Determination of lymphocyte division by flow cytometry. J. Immunol. Meth. 171, 131. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH (1995) Overexpression of the rat inducible 70‐kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J. Clin. Invest. 95, 1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt R, Purcell C, Stroink G (1983) Uniform magnetic field produced by three, four and five square coils. Rev. Sci. Instrum. 54, 879–882. [Google Scholar]
- Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones and negative regulators. Genes Dev. 12, 3788. [DOI] [PubMed] [Google Scholar]
- Nitta M, Okamura H, Aizawa S, Yamaizumi M (1997) Heat shock induces transient p53‐dependent cell cycle arrest at G1/S. Oncogene 15, 561. [DOI] [PubMed] [Google Scholar]
- Oostendorp RA, Audet J, Eaves CJ (2000) High‐resolution tracking of cell division suggests similar cell cycle kinetics of hematopoietic stem cells stimulated in vitro and in vivo . Blood 95, 855. [PubMed] [Google Scholar]
- Parish CR (1999) Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol. Cell Biol. 77, 499. [DOI] [PubMed] [Google Scholar]
- Pfarr CM, Mechta F, Spyrou G, Lallemand L, Carillo S, Yaniv M (1994) Mouse JunD negatively regulates fibroblast growth and antagonises transformation by ras. Cell 76, 747. [DOI] [PubMed] [Google Scholar]
- Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN (1995) Transgenic mice expressing the human heat shock protein 70 have improved post‐ischemic myocardial recovery. J. Clin. Invest. 95, 1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Henderson AS (1996) Regulation of c‐fos is affected by electromagnetic fields. J. Cell Biochem. 63, 358. [DOI] [PubMed] [Google Scholar]
- Riabowol K, Schiff J, Gilman MZ (1992) Transcription factor AP‐1 is required for initiation of DNA synthesis and is lost during cellular ageing. Proc. Natl Acad. Sci. 89, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G, Laszlo A, Li G, Gray J, Dewey W (1986) Heat shock proteins within the mammalian cell cycle: relationship to thermal sensitivity, thermal tolerance, and cell cycle progression. J. Cell Physiol. 126, 291. [DOI] [PubMed] [Google Scholar]
- Robison JG, Pendleton AR, Monson KO, Murray BK, O'Neill KL (2002) Decreased DNA repair rates and protection from heat induced apoptosis mediated by electromagnetic field exposure. Bioelectromagnetics 23, 106. [DOI] [PubMed] [Google Scholar]
- Schreiber M, Kolbus A, Piu F, Szabowski A, Mohle‐Steinlein U, Tian J, Karin M, Angel P, Wagner EF (1999) Control of cell cycle progression by c‐Jun is p53 dependent. Genes Dev. 13, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ (1996) Cancer cell cycles. Science 274, 1672. [DOI] [PubMed] [Google Scholar]
- Van der Waal R, Malyapa RS, Higashikubo R, Roti Roti JL (1997) A comparison of the modes and kinetics of heat‐induced cell killing in HeLa and L5178Y cells. Radiat. Res. 148, 455. [PubMed] [Google Scholar]


