Abstract
Lithium salts are widely used for treatment of psychiatric illness. Lithium also affects cell proliferation. During investigation of the effect of lithium chloride on the central nervous system (CNS) of nephrectomized rats, we noted numerous mitotic figures in the neural lobe of the pituitary. Morphologic criteria established that the mitotic cells were astrocytes, the supporting glial cells of the CNS, also known as pituicytes. Equimolar doses of chlorides of chemically related cations (sodium, potassium, rubidium) had no such effect.
INTRODUCTION
Lithium salts are widely used for treatment of bipolar affective disorder. Animal experiments and tissue culture studies have revealed instances of either enhancement or inhibition of proliferative activity by this cation. Our earlier studies revealed that a single injection of lithium chloride, although widely distributed in rat tissues, reached highest concentration in the neural lobe of the pituitary ( Levine et al. 1993 ). Pursuing this observation, we have now prolonged the exposure to lithium by removing both kidneys and thereby preventing its excretion. Rats treated in this manner had many mitoses in the neural lobe. We present herein the morphologic aspects as well as data on the requisite dose, timing and route of the injection of lithium.
MATERIALS AND METHODS
Male Lewis rats were bred in the Nathan S. Kline Institute for Psychiatric Research. The rats were maintained in hanging shoebox type plastic cages with hardwood bedding, and with Laboratory Rodent Diet 5001 (PMI Feeds, St. Louis, MO, USA) and tap water freely available except as specified. The rats were used when 7–10 weeks old (weight 180–260 g).
Bilateral nephrectomy is usually lethal in 1 or 2 days. Therefore, we used a new procedure that allows for much longer survival ( Levine & Saltzman 1997). The rats' regular food was replaced by sucrose cubes (Domino Dots, Domino Sugar Corp., Brooklyn, NY, USA) in order to eliminate exogenous sources of potassium and ammonia nitrogen that contribute to uremic toxicity. The cubes were soaked in olive oil so as to provide lipids and additional calories without adding potassium or nitrogen. At the same time, the right kidney was removed through a dorsal midline incision under anaesthesia with ketamine/acepromazine intramuscularly. The wound was closed with sutures in the muscle and metal clips in the skin. One week later, the left kidney was removed after re‐opening the original skin wound. (The staggered nephrectomies avoided the excessive stress associated with removal of both kidneys simultaneously, and allowed time for the right adrenal to regenerate should any injury have occurred). A volatile anaesthetic, isoflurane, was used for the second nephrectomy because anephric rats might have difficulty eliminating a nonvolatile anaesthetic. Lithium chloride, 0.15 m in distilled water, was injected one or more days later, usually by intraperitoneal (IP) route.
The rats were killed at intervals by exsanguination while under CO2 anaesthesia. The brain was removed quickly and the base of the skull immersed in Bouin's fluid to fix the pituitary body in situ. The next day, the pituitary was removed, embedded in paraffin in entirety, sectioned at 5 µm at three levels and stained with haematoxylin and eosin. The three levels of pituitary were separated from each other by 300 µm. The level of pituitary which presented the largest area of neural lobe was selected for study. Coded slides were studied at 400 × magnification and mitoses in the entire area of neural lobe were counted independently by two of the authors. As stated by Chambers (1945): ‘A mitotic figure was not considered such unless the nuclear membrane was absent and multiple chromosomes present.’ These counts agreed within 1, 2, or 3 mitoses and are reported as the average, rounded to the closest whole number. The area of the neural lobe was determined with the aid of an ocular reticule, calibrated by a stage micrometer. The total number of mitoses divided by the area in mm2 constituted an index of mitotic activity. The density of parenchymal cells in the neural lobe was estimated by counting all the nonendothelial cells in 20 randomly selected high power (400 ×) microscopic fields from 10 lithium‐treated rats and 20 such fields from 10 control rats from the same experiment.
RESULTS
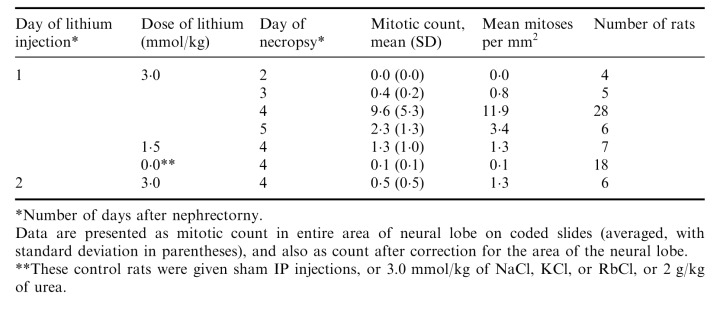
Lewis rats were injected with an isotonic solution (0.15 m) of LiCl at a dose of 3.0 mmol/kg IP, one or two days after the second nephrectomy and necropsies were performed one to four days later. The pituitary had abundant mitoses in the entire area of the neural lobe, but not in the anterior or intermediate lobes, or in the supraoptic nuclei or other brain areas. The mitoses were in astrocytes (pituicytes) as indicated by their large size and wide distribution without any relation to blood vessels ( Fig. 1). The mitotic cells had abundant cytoplasm, usually well demarcated, round or oval, but sometimes irregular, with striations. The chromosomes were widely dispersed. All these are typical features of mitotic astrocytes as described and illustrated by Cavanagh (1970) and by Kawamoto & Kawashima (1984). Astrocytes in early prophase were also numerous but were not included in mitotic counts because the nuclear envelope was still intact. Some specimens had clusters of mitoses. The neural lobe had abundant mitoses only in rats given LiCl one day after the second nephrectomy and studied three days later ( Table 1). These rats had an average of 11.9 mitoses/mm2 (n = 28) compared to the controls which had no mitoses except for two rats with a single mitosis (average 0.1 mitoses/mm2, n = 18). This difference was statistically highly significant, P < 0.001.
Figure 1.
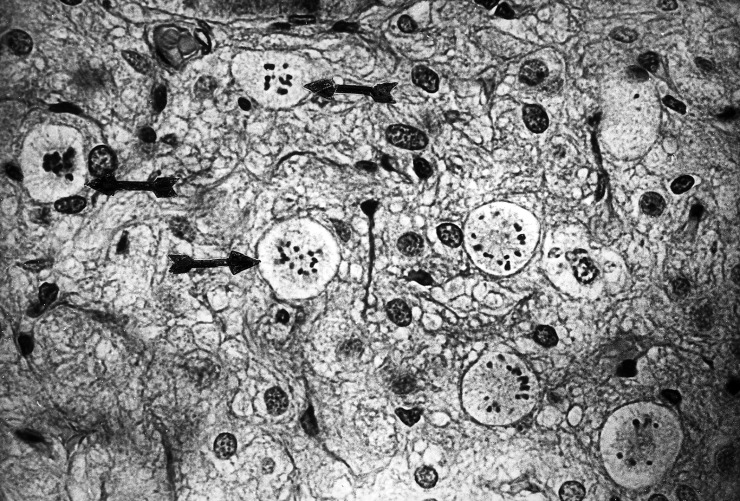
A small portion of the neural lobe with eight mitotic figures, from a nephrectomized rat treated with a single dose of LiCl. Arrows on the left side point to three mitoses. The right side has five cells in mitosis. Individual chromosomes are clearly visible. This unusual clustering of mitoses supports the suggestion that some pituicytes may divide more than once after stimulation. This single 5 µm section had 31 mitoses in all, in an area of 1.23 mm2. In contrast, neural lobes from control rats usually had no mitoses in the entire section. Hematoxylin and eosin (magnification × 400).
Table 1.
Timing and dose of lithium treatment for neural lobe mitoses in nephrectomized Lewis rats
Despite the presence of mitotic figures, there was no evidence of increased cell density in the neural lobe. The average number of nonendothelial nuclei in entire microscopic fields at 400 × magnification was 99.4 from lithium‐treated rats (range 80–118) and 100.4 from control rats (range 80–150).
A lower dose of LiCl or different timing of injection or necropsy were less effective ( Table 1). In one experiment (not included in Table) similar mitotic counts were found whether the LiCl was given IP (29, 20, 15, 14, 10, 3 mitoses) or intravenously (23, 14, 14, 12, 8, 1 mitoses in individual nephrectomized rats). Equimolar doses of isotonic NaCl, KCl, or RbCl (3.0 mmol/kg), large amounts of urea, and sham injections were ineffective ( Table 1).
Three days after the single IP dose of LiCl, serum levels were high (1.6–2.3 mEq/L) because of the inability of nephrectomized rats to excrete it. At this time the serum urea nitrogen had increased from the normal value of 15–20 mg/dl to 336 mg/dl.
Additional controls included rats that had intact kidneys and were fed either a normal diet or sucrose with oil. These rats had no mitoses or only 1 or 2 in the entire section, whether or not they had been given a single 3.0 mmol/kg dose of LiCl.
DISCUSSION
Striking mitotic activity in neural lobe astrocytes was produced by lithium. In experiments that did not involve lithium, some observers have noted mitoses in endothelial cells as well as pituicytes of the neural lobe of dehydrated rats. Duchen (1962) illustrated the small size of endothelial mitoses and their location in vessel walls. However, the large size of mitotic cells in our material, the wide dispersion of the chromosomes, and the absence of a topographic relation to capillaries indicates that most or all of the mitoses were in pituicytes, in agreement with Murray (1968) on salt‐treated rats. This conclusion is supported by the observation that cells in early prophase, before dissolution of the nuclear envelope, were numerous in many of the mitotically active neural lobes, and these cells also were clearly pituicytes and had no relation to the blood vessels. Microglial cells in the neural lobe can proliferate in response to osmotic stress, at least in the mouse ( Lawson, Perry & Gordon, 1993). However, the microglia are far fewer in number than the pituicytes, and their increased DNA synthesis in the mouse was not accompanied by detectable mitoses ( Lawson et al. 1993 ). These facts, plus the morphology of the mitotic cells in our rat specimens support our conclusion that the mitoses were in pituicytes. Finally, it should be noted that the neural lobe does not contain any neuronal nuclei.
Increased numbers of visible mitoses can, theoretically, be caused by prolonged duration of mitosis as well as by an increase in the number of mitotic cells. Also, increased cellular density and/or increased cross‐sectional area of the neural lobe could augment the number of pituicytes available to undergo mitosis. Increased cell density was excluded by our counts of nonendothelial nuclei, and increased cross‐sectional area would not affect the mitotic index (mitoses/mm2). Also, the very low or absent mitotic activity of the normal neural lobe in control rats makes it unlikely that prolonged duration of mitosis could cause a perceptible increase of mitotic figures and certainly could not approach the mitotic activity produced by lithium.
Mitotic activity in the neural lobe has been previously reported after osmotic stimulation, either hypertonic NaCl ( Selye & Hall 1943; Chambers 1945; Leveque & Small 1959; Duchen 1962; Murray 1968; Paterson & Leblond 1977; Murugaiyan & Salm 1995) or deprivation of water ( Kawamoto & Kawashima 1984; Leveque & Small 1959). Our finding of peak effects of an isotonic solution of LiCl after 3 days can be compared to results with hypertonic salt stimulation (peak at 4 days) and with water deprivation stimulation (peak at 6 days) ( Leveque & Small 1959). In all these models the number of mitoses decreased after the peak has passed despite continuation of the inciting factor.
Lithium has been previously reported to inhibit or to enhance cell proliferation in bone marrow, tumours or tissue culture cells. We have now added pituicytes to the list of cell types for which lithium is a mitogen. The mechanism may involve potassium locally in the neural lobe, inasmuch as lithium may displace some K from cells and extracellular K acts as a mitogen for cultured astrocytes ( Hertz & Richardson 1984; Canady, Ali‐Osman & Rubel, 1990). However, it is also possible that lithium may act indirectly, by stimulating vasopressin production and/or transport in the hypothalamic‐neurohypophyseal system or through second messenger systems. Inasmuch as lithium can enter cells, it might cause hypertonicity of the intracellular content of hypothalamic neurones which might be misinterpreted as a systemic osmotic signal, with vasopressin secretion and pituicyte mitosis as the result. This suggestion is supported by reports that lithium depletes vasopressin in the neural lobe and stimulates secretion of vasopressin by neurones of the supraoptic and paraventricular nuclei in the hypothalamus.
REFERENCES
- Canady KS, Ali‐Osman F, Rubel EW (1990). Extracellular potassium influences DNA and protein syntheses and glial fibrillary acidic protein expression in cultured glial cells. Glia 3,368. [DOI] [PubMed] [Google Scholar]
- Cavanagh JB (1970). The proliferation of astrocytes around a needle wound in the rat brain. J. Anat. 106,471. [PMC free article] [PubMed] [Google Scholar]
- Chambers GH (1945). Changes in the rat's posterior pituitary following sodium chloride administration. Anat. Rec. 92,391. [Google Scholar]
- Duchen LW (1962). The effects of ingestion of hypertonic saline on the pituitary gland in the rat: a morphologic study of the pars intermedia and posterior lobe. J. Endocrinol. 25,161. [Google Scholar]
- Duchen LW (1968). Changes in the volume of the lobes of the pituitary gland and in the weight and water content of organs of rats given hypertonic saline. J. Endocrinol. 41,593. [DOI] [PubMed] [Google Scholar]
- Hertz I & Richardson JS (1984). Is neuropharmacology merely the pharmacology of neurons ‐ or are astrocytes important too? Trends Pharmacol. Sci. 5,272. [Google Scholar]
- Kawamoto K & Kawashima S (1984). Ultrastructural changes and proliferation of pituicytes in mouse posterior lobe during water deprivation and rehydration. Acta Anat. 119,136. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S (1993). Microglial responses to physiological change: osmotic stress elevates DNA synthesis of neurohypophyseal microglia. Neuroscience 56,929. [DOI] [PubMed] [Google Scholar]
- Leveque TF & Small M (1959). The relationship of the pituicyte to the posterior lobe hormones. Endocrinology 65,909. [DOI] [PubMed] [Google Scholar]
- Levine S & Saltzman A (1997). Carbohydrate diet prolongs survival of rats with acute uremia after bilateral nephrectomy. Nephron 77,242. [DOI] [PubMed] [Google Scholar]
- Levine S, Saltzman A, Katof B et al. (1993). Lithium accumulation in the neurointermediate lobe of rat pituitary exceeds that in other endocrine glands. Neuroendocrinol. Lett. 15,357. [Google Scholar]
- Murray M (1968). Effects of dehydration on the rate of proliferation of hypothalamic neuroglia cells. Exp. Neurol. 20,460. [DOI] [PubMed] [Google Scholar]
- Murugaiyan P & Salm AK (1995). Dehydration‐induced proliferation of identified pituicytes in fully adult rats. Glia 15,65. [DOI] [PubMed] [Google Scholar]
- Paterson JA & Leblond CP (1977). Increased proliferation of neuroglia and endothelial cells in the supraoptic nucleus and hypophysial neural lobe of young rats drinking hypertonic sodium chloride solution. J. Comp. Neurol. 175,373. [DOI] [PubMed] [Google Scholar]
- Selye H & Hall CE (1943). Further studies concerning the action of sodium chloride on the pituitary. Anat. Rec. 86,579. [Google Scholar]


