Abstract
Objectives
Scrophularia striata Boiss (Scrophulariaceae) is a plant that grows in northeastern Iran; it has been used traditionally to treat various inflammatory disorders. This study was designed to investigate cytotoxic effects of S. striata extract, on the Jurkat human leukaemia cell line (T‐cell leukaemia).
Materials and methods
Phytochemical assay by thin layer chromatography and 2, 2 diphenyl‐1‐picryl‐hydrazyl were used to evaluate main compounds and antioxidant capacity of the plant extract, respectively. Its inhibitory effect on Jurkat cells was evaluated by MTT assay. In addition, cell cycle distribution and apoptotic cell death were evaluated by propidium iodide and annexin V‐FITC/ propidium iodide staining.
Results
These showed that the main components present in S. striata extract included flavonoids, phenolic compounds and phenyl propanoids. Treatment with extract was significantly cytotoxic to the tumour cell line. In addition, flow cytometry analysis indicated that S. striata extract induced cell cycle arrest in G2/M phase and apoptosis of tumour cells.
Conclusions
Results of the study indicated that S. striata extract could inhibit leukaemia cell proliferation by inducing G2/M phase arrest and apoptosis.
Introduction
Malignancy is one of the leading causes of death in the world. Although traditional cancer therapies, including surgery, chemotherapy and radiotherapy, are the standard methods of patient treatment, they are not fully effective. Using natural agents such as medicinal plants, in tumour therapy arouses extensive interest, aspiring to minimal side effects, better safety and efficiency. Many of the most useful anti‐cancer drugs (including vinblastin, vincristine, etoposide and taxol) that have been approved for use in anti‐cancer therapy, are plant‐derived compounds 1, 2, 3, 4, 5; in addition, during our course of study on different medicinal plants, we have found inhibitory effects of several plants on tumour cell proliferation 6, 7. Scrophularia striata Boiss (Scrophulariaceae) is a plant that grows in northeastern Iran that has been used as a traditional herb for various purposes. Several species of Scrophularia have been used in folk medicine since ancient times, as a sedative and for treatment of illnesses such as scrophula, scabies, eczema, psoriasis and tumours. Our previous in vitro studies have demonstrated inhibitory effects of S. striata extract on nitric oxide production and on pro‐inflammatory cytokine (including TNF‐α, IL‐1β and PGE2) production by macrophages 8, 9. In addition, anti‐tumour, anti‐inflammatory and immunomodulatory activities of some species of Scrophularia have been shown by other investigators 10, 11, 12, 13. Moreover, several compounds from various Scrophularia species with anti‐inflammatory and neuroprotective properties (including iridoids and phenyl propanoids) have been isolated. Further, flavonoids, phenolic compounds, quercetin and isorhamnetin 3‐O‐rutinoside, with antioxidant activity, have also been identified from S. striata 14. In the present study, we investigated cytotoxic effects and induction of apoptosis of S. striata in the Jurkat human leukaemia cell line.
Materials and methods
Plant material and extract preparation
Aerial parts of S. striata were collected from the Ruin region of northeastern Iran, in May 2010, and air dried at room temperature. A sample was authenticated by Dr Faride Attar, from Tehran University, Faculty of Sciences and a voucher specimen (Herbarium No: 36501) was preserved in the herbarium of the Tehran University Faculty of Sciences, Tehran, Iran. Aerial components of the plant were dried, powdered (20 g) and macerated in 80% ethanol solution for 3 days with three changes of solution. Resulting extract was filtered and evaporated under vacuum into the final dried powder of S. striata. Extract including phenyl propanoids, phenolic compounds and flavonoids dissolved in dimethylsulphoxide (% 0.1 v/v); this process was performed here.
Phytochemical assay
To be able to identify chemical components of the extract, thin‐layer chromatography was performed. A variety of indicators including vanillin sulphuric acid, ferric chloride and natural product polyethylene glycol were used. Indictors were sprayed on prepared thin layers of extract and were observed at 260 and 280 nm wavelengths under UV light.
DPPH assay
DPPH testing was used to evaluate antioxidant capacity of the plant extract. Briefly, one thousand microlitres of selected concentrations (250, 125, 62.5, 31.25, 15.62 and 7.81 μg/ml) of S. striata extract in ethanol was added to 4 ml of 0.004% methanolic solution of DPPH. After 60 min incubation at room temperature, absorbance was read against a blank at 517 nm. Inhibition of free radical by DPPH percent (I%) was calculated as follows:
A blank is the Absorbance of control reaction (containing all reagents except the test compound).
A sample is the Absorbance of test compound. Extract concentration providing 50% inhibition (IC50%) was calculated from the graph inhibition percentage against extract concentration. IC50% values were compared to IC50% value of a ‘standard’ antioxidant, in this case ascorbic acid (AA), obtained by the same procedure.
Measurement of total phenolic compounds
Total phenolic content of the dry herb was determined by using Folin‐Ciocalteau assay.
One aliquot (1 ml) of extract or standard solution of gallic acid (20, 40, 60, 80 and 100 mg/l) was added to 25 ml in a volumetric flask, containing 9 ml of distilled deionized water (dd H2O) and a reagent blank using dd H2O was prepared. One millilitre Folin‐Ciocalteu's phenol reagent was added to the mixture and shaken. After 5 min, 10 ml 7% Na2CO3 solution was added to the mixture, the solution was diluted to 25 ml with dd H2O and mixed. After incubation for 90 min at room temperature, absorbance against the prepared reagent blank was determined at 750 nm. Data of total phenolic contents are expressed as milligrams gallic acid equivalents (GAE) per gram dry weight (mgGAE/gDW). All samples were analysed in duplicate.
Cell culture
Jurkat tumour cell line (T cell leukaemia) prepared from the National Cell Bank of Iran and maintained by culturing in RPMI 1640 medium (Sigma, St Louis, MO, USA), was supplemented with 10% heat‐inactivated foetal calf serum (Gibco‐BRL Grand Island, NY, USA), 2 mm l‐glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin. Cell viability was determined using the trypan blue dye exclusion test.
Cell viability assay
Effects of S. striata extract on the Jurkat human tumour cell line were determined using MTT (3‐(4, 5‐dimethylthiazoyl)‐2, 5‐ diphenyltetrazolium bromide) assay. Briefly, cells were added to flat‐bottomed micro‐culture plates in the presence or absence of selected concentrations (50, 100, 200 and 400 μg/ml) of the extracts (in triplicate) and incubated at 37 °C, in 5% humidified CO2 for 48 h. Then, 10 μl of MTT (5 mg/ml, Sigma) was added to each well and incubation continued for further 4 h at 37 °C. In each well, 100 μl/well solubilization solution, containing isopropanol and 10% SDS in 0.01 m HCl, was added. After complete solubilization of formazan crystals, plates were read at 570 nm on an ELISA reader; reference wavelength was 690 nm. Mean optical density (OD) ± SD for each group of replicates was calculated. Percent inhibition of cells exposed to various treatments was obtained as follows:
Annexin V‐FITC/PI apoptosis assay
To determine apoptosis, an Annexin V–FITC apoptosis Detection Kit (Invitrogen, Carlsbad, CA, USA) was used, according to the manufacturer's protocol. Briefly, 1 × 105 cells/ml were treated with the selected concentrations (50, 100, 200 and 400 μg/ml) of S. striata extract, for 24 h at 37 °C. Cells were then harvested and re‐suspended in binding buffer. They were then stained with 10 μl AnnexinV–FITC and 5 μl propidium iodide (PI) for 15 min at room temperature in the dark. Apoptotic index was immediately determined by flow cytometry.
Cell cycle analysis
Cells (1 × 105 cells/ml) were treated with the selected concentrations (50, 100, 200 and 400 μg/ml) of the extract for 24 h; they were then centrifuged and fixed in 70% ethanol. After washing, cells were resuspended in 1 ml PBS containing 10 mg/ml RNase and 1 mg/ml PI (Sigma); they were then incubated for 1 h at 37 °C in the dark. Thereafter, cells were analysed on a FACScalibur flow cytometer (Becton‐Dickinson, San Jose, CA, USA). Taxol treatment (1.75 μg/ml) was used as positive control.
DNA fragmentation analysis
In this study, isolation of fragmented DNA from Jurkat cells cultured in 24‐well plates was carried out according to the procedure of Amirghofran et al., with modifications 7. Briefly, 2 × 106 cells/ml were treated with plants extract then collected by centrifugation (2000 g, 10 min). Pellets were resuspended in 0.5 ml DNA lysis buffer (2% SDS, 10 mm EDTA, 10 mm Tris‐Hcl, pH 8.5) and lysate was immediately incubated with 0.1 mg/ml proteinase k (Sigma) before being incubated for 3 h at 37 °C. After addition of isopropanol, DNA was precipitated with 70% ethanol. The suspension was then centrifuged, and DNA treated with 100 μl 10 mm Tris‐HCl (pH 7.5) and 0.5 mg/ml RNase A (Boehringer Mannheim, Mannheim, Germany), at 37 °C for 24 h. Samples were then loaded into 2% agarose gels containing ethidium bromide, and were electrophoresed. DNA bands was visualized under ultraviolet illumination and photographed.
Statistical analysis
Data are presented as mean ± SD. Statistical analyses were performed by one‐way analysis of variance (ANOVA) and a post–hoc Bonferroni test to express the difference among the groups. All analyses were performed using SPSS 16. Data were considered statistically significant at P < 0.05.
Results
Chemical components of the extract
Phytochemical assay by thin layer chromatography revealed S. striata extract's main components including phenyl propanoids, phenolic compounds and flavonoids (Table 1).
Table 1.
Phytochemical results of Scrophularia striata extract
| Compounds | Reagents | Standards | Results |
|---|---|---|---|
| Phenylpropanoids and terpenoids | Vanillin sulphuric acid | Cinamic acid | + |
| Phenolic compounds | Ferric chloride | Nepitrin | + |
| Flavonoids | Natural product reagent | Quercetin | + |
Measurement of total phenolic compounds and antioxidant activity
Phenolic compounds are well recognized as antioxidants which can act as free radical terminators 15 and have been known to show medicinal activity as well as to exhibit physiological functions 16. In this study, we used Folin‐Ciocalteau assay for standardizing aerial parts of S. striata and quantity of total phenolic compounds; flavonoids and phenolic compounds of dry herb were measured respectively, as shown in Table 2, with gallic acid as standard. In addition, free radical scavenging capacities of the extract were measured by DPPH assay and results are also provided in Table 2, with ratios (IC50%) AA/(IC50%) extract shown. These represent AA equivalent of the extract antioxidant capacity, that is, amount of AA in milligram equivalent to one gram of extract.
Table 2.
Measurement of phenolic compounds and antioxidant capacity of Scrophularia striata extract
| Total phenolic compounds in dry herb (mgGAE/gdw) | DPPH radical scavenging activity, IC50% (mg/l) | Ascorbic acid equivalent of the extract antioxidant capacity (mg/g) |
|---|---|---|
| 10.96 | 316.69 | 29.8 |
Effect of S. striata extract on tumour cell viability
In this study, normal human peripheral blood mononuclear cells (PBMCs) were used as normal cells compared to the test Jurkat human leukaemia cells. In a preliminary experiment on PBMCs, results indicated that extract up to 400 μg/ml had no significant toxicity over 48 h. Thus, the Jurkat cells were incubated with selected concentrations (0–400 μg/ml) of extract for 48 h. As shown Fig. 1, extract significantly (P < 0.05) inhibited Jurkat cell proliferation in a dose‐ and time‐dependent manner. For example, treatment with 400 μg/ml of extract for 48 h resulted in 58% Jurkat cells proliferation inhibition. Results indicate that this extract can induce a cytotoxic effect on Jurkat human leukaemia cells.
Figure 1.

Effect of Scrophularia striata extract on viability of Jurkat human leukaemia tumour cells. Tumour cells were incubated with selected concentrations (0–400 μg/ml) of extract for 48 h. Results indicated that the extract significantly (*P < 0.05, **P < 0.001) inhibited Jurkat cell population growth in a dose‐ and time‐dependent manner compared to non‐treated (control group) cells. Results shown are representative of three independent experiments.
Effect of S. striata extract on apoptosis of Jurkat cells
To determine whether the cytotoxic effect of S. striata extract on Jurkat cells was related to apoptotic cell death, we evaluated phosphatidylserine exposure as an early marker of apoptosis, using annexin V‐FITC/PI double staining. In control (untreated) groups, 92.2% cells were viable, 7.5% cells were in the early and late stages of apoptosis (lower right + upper right). While, after 24‐h treatment with S. striata extract, the apoptotic cell population increased from 19.2 to 53.3% when extract concentration increased from 50 to 400 μg/ml. As shown in Fig. 2, percentages of Annexin V‐positive cells increased gradually in a dose‐dependent manner after S. striata extract treatment, suggesting that the extract induced the apoptotic response.
Figure 2.

Effect of Scrophularia striata extract on induction of apoptosis in Jurkat cells. Apoptosis‐inducing effect of S. striata extract on Jurkat human leukaemia cells evaluated by the annexin V‐FITC (AV)/PI method. Dot‐plot graphs show viable cells (AV−/PI−), early phase apoptotic cells (AV+/PI−), late phase apoptotic cells (AV+/PI+) and necrotic cells (AV−/PI+).
Effect of S. striata extract on Jurkat cell cycle distribution
To determine whether anti‐tumour and proliferation‐inhibitory effects of S. striata extract on Jurkat cells was related to induction of cell cycle arrest, distribution of cells in different phases of the cell cycle was evaluated by measuring DNA content. As shown in Fig. 3, after treatment with different doses of S. striata extract for 24 h, proportions of cells in G2/M increased from 4.3 to 28.2% compared to control groups. In addition, proportion of cells in G0/G1 fell from 36.8 to 19.1% compared to control groups while percentages of S phase cells almost retained the same levels. Results indicated that S. striata extract induced G2/M cell cycle arrest in these Jurkat cells in a dose‐dependent manner.
Figure 3.

Effect of Scrophularia striata extract on cell cycle of Jurkat cells. Jurkat cell line treated with selected concentrations of S. striata extract for 24 h. Distribution of cells in different phases of the cell cycle was evaluated by measuring DNA content. Results showed that S. striata extract induced G2/M cell cycle arrest in Jurkat cells in a dose‐dependent manner. Taxol treatment was used as positive control. Results shown are representative of three independent experiments.
Effect of S. striata extract on Jurkat cell DNA fragmentation
To confirm effects of the extract on induction of apoptosis in the cell line, DNA products were examined for appearance of characteristic DNA laddering in treated cells. Ladder formation was detected after exposing the cells with 100–200 μg/mL of extract. As shown in Fig. 4, increased inter‐nucleosomal DNA fragmentation was dose dependently apparent in the cells, indicating that the extract caused the cells to undergo apoptosis.
Figure 4.
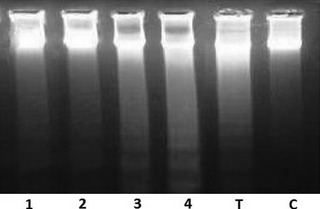
Effect of Scrophularia striata on DNA fragmentation in Jurkat cell line. Cell line after treatment with 1 = 50μg/ml, 2 = 100lg/ml, 3 = 200μg/ml, 4 = 400μg/ml, T = Taxol μg/ml, C = negative control. DNA laddering typical for apoptotic cells are visible for the cells treated with the extract.
Discussion
In this study, we evaluated cytotoxic activity of S. striata extract on Jurkat human leukaemia cells. We found that S. striata extract inhibited Jurkat cell population growth in a dose‐ and time‐dependent manner. Moreover, results indicated that extract up to 400 μg/ml had no significant toxicity to normal PBMCs. It has been shown previously that some anti‐cancer effects of medicinal plants are based on induction of apoptosis 6, 7, 17, 18, 19. Apoptosis is a normal physiological process that plays an important role in homeostasis and expansion of normal and cancer cells 20, 21; also dysregulation of apoptosis is usually considered to be a major cancer cell property 22. Also, many studies have shown that apoptosis is an important mechanism by which various anti‐cancer agents exert antitumour effects 23, 24. In previous studies, cytotoxic activity of some species of Scrophularia have been reported on cancer cell lines 12, 25. Here, to determine whether cytotoxicity of S. striata extract on Jurkat cells was related to apoptotic cell death, percentages of apoptotic cells were measured by annexin V‐FITC/PI staining. Our results indicated that proportions of apoptotic cells increased with increasing concentration of S. striata extract. These data indicated that cytotoxic effects of S. striata extract seemed to be mediated by induction of apoptosis in the Jurkat cells. In addition, one previous study has shown that a further genus of Scrophularia, S. floribunda extract, induced apoptosis in tumour cells through induction of cell cycle arrest 25. Thus, in this study, to determine whether induction of apoptosis and anti‐tumour effects of S. striata extract on Jurkat cells was associated with induction of cell cycle arrest, distribution of cells in different phases of the cell cycle was evaluated by measuring intracellular DNA content. Our finding indicate that S. striata extract induced G2/M phase cell cycle arrest in Jurkat cells in a dose‐dependent manner. On the other hand, the antioxidant, anti‐inflammatory and immunomodulatory activities of some species of Scrophularia have also been shown by several investigators 10, 12, 26; S. striata has been used to treat various inflammatory disorders 8, 26, 27. Our previous studies indicated the inhibitory effect of S. striata extract on pro‐inflammatory mediator production by macrophages including nitric oxide, TNF‐α, IL‐1β and PGE2 production and also suppressive effects on matrix metalloproteinases in the Wehi‐164 tumour cell line in vitro 8, 9, 13. In addition, antioxidant properties of compounds such as iridoide glycosides and phenylpropanoid esters isolated from S. buergeriana have been reported 28, 29, 30, 31. However, in our previous and present studies, phenolic compounds, phenyl propanoids and two flavonoids, quercetin and isorhamnetin 3‐O‐rutinoside, were identified from extracts of this plant 14. Several studies showed that quercetin is a dietary antioxidant and also it has anti‐inflammatory and anti‐tumour effects (32–36). Moreover, other studies have shown that isorhamnetin 3‐O‐rutinoside induces apoptosis in human chronic myelogenous erythroleukaemia cells (K562) 37, 38. This plant extract in the present and previous studies is indicated as having antioxidant, anti‐inflammatory and anti‐tumour properties. Whether these compounds are the main responsible agents for anti‐tumour effects of S. striata and are involved in this activity needs further investigation. In conclusion, our findings here show that S. striata has cytotoxic activity on Jurkat human leukaemia tumour cells. The ability of this plant to induce apoptosis through G2/M phase cell cycle arrest on the leukaemic cell line makes it a candidate for further studies to discover the active components involved and mechanisms by which they induce apoptosis.
Conflict of interest
None.
Acknowledgements
This study was supported by Cellular and Molecular Research Center (CMRC), Deputy for Research, Qazvin University of Medical Sciences, Qazvin, Iran and Department of Medicinal Plants Research Center, Institute of Medicinal Plants (IMP), ACECR, Karaj, Iran.
References
- 1. Cordell GA, Beecher CW, Pezzuto JM (1991) Can ethnopharmacology contribute to the development of new anticancer drugs? J. Ethnopharmacol. 32, 117–133. [DOI] [PubMed] [Google Scholar]
- 2. Ribereau‐Gayon G, Jung ML, Frantz M, Anton R (1997) Modulation of cytotoxicity and enhancement of cytokine release induced by Viscum album L. extracts or mistletoe lectins. Anticancer Drugs 8(Suppl 1), S3–S8. [DOI] [PubMed] [Google Scholar]
- 3. Taixiang W, Munroa j, Guanjian L (2005) Chinese medical herbs for chemotherapy side effects in colorectal cancer patients. Cochrane Database Syst. Rev. 25, CD004540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang SX, Bastow KF, Tachibana Y, Kuo SC, Hamel E, Mauger A et al (1999) Antitumor agents. 196. Substituted 2‐thienyl‐1, 8‐naphthyridin‐ 4‐ones: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J. Med. Chem. 42, 4081–4087. [DOI] [PubMed] [Google Scholar]
- 5. Lee KH (1999) Novel antitumor agents from higher plants. Med. Res. Rev. 19, 569–596. [DOI] [PubMed] [Google Scholar]
- 6. Amirghofran Z, Bahmani M, Azadmehr A, Javidnia K (2006) Anticancer effects of various Iranian native medicinal plants on human tumor cell lines. Neoplasma 53, 428–433. [PubMed] [Google Scholar]
- 7. Amirghofran Z, Bahmani M, Azadmehr A, Javidnia K (2006) Induction of apoptosis in leukemia cell lines by Linum persicum and Euphorbia cheiradenia . J. Cancer Res. Clin. Oncol. 132, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azadmehr A, Afshari A, Baradaran B, Hajiaghaee R, Rezazadeh S, Monsef‐Esfahani H (2009) Suppression of nitric oxide production in activated murine peritoneal macrophages in vitro and ex vivo by Scrophularia striata ethanolic extract. J. Ethnopharmacol. 124, 166–169. [DOI] [PubMed] [Google Scholar]
- 9. Azadmehr A, Maliji GH, Hajiaghaee R, Shahnazi M, Afaghi A (2012) Inhibition of pro‐inflammatory cytokines by ethyl acetate extract of Scrophularia striata . Trop. J. Pharm. Res. 11, 893–897. [Google Scholar]
- 10. Diaz AM, Abad MJ, Fernandez L, Silván AM, De Santos J, Bermejo P (2004) Phenylpropanoid glycosides from Scrophularia scorodonia: in vitro anti‐inflammatory activity. Life Sci. 74, 2515–2526. [DOI] [PubMed] [Google Scholar]
- 11. Bas E, Recio MC, Abdallah M, Máñez S, Giner RM, Cerdá‐Nicolás M et al (2007a) Inhibition of the pro‐inflammatory mediators production and anti‐inflammatory effect of the iridoid scrovalentinoside. J. Ethnopharmacol. 110, 419–427. [DOI] [PubMed] [Google Scholar]
- 12. Azadmehr A, Hajiaghaee R, Afshari A, Amirghofran Z, Refieian‐Kopaei M, yousofi Darani H et al (2011) Evaluation of in vivo immune response activity and in vitro anti‐cancer effect by Scrophularia megalantha . J. Med. Plants. Res. 5, 2365–2368. [Google Scholar]
- 13. Hajiaghaee R, Monsef‐Esfahani HR, Khorramizadeh MR, Saadat F, Shahverdi AR, Attar F (2007) Inhibitory effect of aerial parts of Scrophularia striata on matrix metalloproteinases expression. Phytother. Res. 21, 1127–1129. [DOI] [PubMed] [Google Scholar]
- 14. Monsef‐Esfahani H, Hajiaghaee R, Shahverdi AR, Khorramizadeh MR, Amini M (2010) Flavonoids, Cinnamic acid and Phenyl propanoid from aerial parts of Scrophularia striata . Pharm. Biol. 48, 333–336. [DOI] [PubMed] [Google Scholar]
- 15. Shahidi F, Wanasundara JPD (1992) Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 32, 67–103. [DOI] [PubMed] [Google Scholar]
- 16. Sofowora A (1993) Medicinal Plants and Traditional Medicine in Africa. Ibadan, Nigeria: Spectrum Books Ind, 289pp. [Google Scholar]
- 17. Cuendet M, Pezzuto JM (2004) Antitumor activity of bruceantin: an old drug with new promise. J. Nat. Prod. 67, 269–272. [DOI] [PubMed] [Google Scholar]
- 18. Li X, Ji L, Wang Z (2002) Advances in the study on the effects of Chinese herbal drugs on apoptosis. Zhong Yao Cai 25, 135–139. [PubMed] [Google Scholar]
- 19. Shahneh FZ, Valiyari S, Azadmehr A, Hajiaghaee R, Yaripour S, Bandehagh A et al (2013) Inhibition of Growth and Induction of Apoptosis in Fibrosarcoma Cell Lines by Echinophora platyloba DC: In Vitro Analysis. Adv. Pharmacol. Sci. 2013, 512931. doi: 10.1155/2013/512931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adam‐Klages S, Adam D, Janssen O, Kabelitz D (2005) Death receptors and caspases: role in lymphocyte proliferation, cell death, and autoimmunity. Immunol. Res. 33, 149–166. [DOI] [PubMed] [Google Scholar]
- 21. Liu YL, Tang LH, Liang ZQ, You BG, Yang SL (2010) Growth inhibitory and apoptosis inducing by effects of total flavonoids from Lysimachia clethroides Duby in human chronic myeloid leukemia K562 cells. J. Ethnopharmacol. 131, 1–9. [DOI] [PubMed] [Google Scholar]
- 22. Abbott RG, Forrest S, Pienta KJ (2006) Simulating the hallmarks of cancer. Artif. Life 12, 617–634. [DOI] [PubMed] [Google Scholar]
- 23. Wang J, Wu A, Xu YF, Liu JW, Qian XH (2009) M2‐A induces apoptosis and G2–M arrest via inhibiting PI3K/Akt pathway in HL60 cells. Cancer Lett. 283, 193–202. [DOI] [PubMed] [Google Scholar]
- 24. Sun B, Geng S, Huang XJ, Zhu J, Liu S, Zhang YJ et al (2011) Coleusin factor exerts cytotoxic activity by inducing G0/G1 cell cycle arrest and apoptosis in human gastric cancer BGC‐823 cells. Cancer Lett. 301, 95–105. [DOI] [PubMed] [Google Scholar]
- 25. Giessrigl B, Yazici G, Teichmann M, Kopf S, Ghassemi S, Atanasov AG et al (2012) Effects of Scrophularia extracts on tumor cell proliferation, death and intravasation through lymphoendothelial cell barriers. Int. J. Oncol. 40, 2063–2074. [DOI] [PubMed] [Google Scholar]
- 26. Bas E, Recio MC, Manez S (2007b) New insight into the inhibition of the inflammatory response to experimental delayed‐type hypersensitivity reactions in mice by scropolioside A. Eur. J. Pharmacol. 555, 199–210. [DOI] [PubMed] [Google Scholar]
- 27. Schinella GR, Tournier HA, Prieto JM (2002) Antioxidant activity of anti‐inflammatory plant extracts. Life Sci. 18, 1023–1033. [DOI] [PubMed] [Google Scholar]
- 28. Jeong EJ, Lee KY, Kim SH (2008) Cognitive‐enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine‐treated mice. Eur. J. Pharmacol. 588, 78–84. [DOI] [PubMed] [Google Scholar]
- 29. Kim SR, Lee KY, Koo KA (2002a) Four new neuroprotective iridoid glycosides from Scrophularia buergeriana roots. J. Nat. Prod. 65, 1696–1699. [DOI] [PubMed] [Google Scholar]
- 30. Kim SR, Kim YC (2002b) Neuroprotective phenylpropanoid esters of rhamnose isolated from roots of Scrophularia buergeriana . Phytochemistry 54, 503–509. [DOI] [PubMed] [Google Scholar]
- 31. Kim SR, Koo KA, Sung SH (2003) Iridoids from Scrophularia buergeriana attenuate glutamate‐induced neurotoxicity in rat cortical cultures. J. Neurosci. Res. 74, 948–955. [DOI] [PubMed] [Google Scholar]
- 32. Joskova M, Franova S, Sadlonova V (2011) Acute bronchodilator effect of quercetin in experimental allergic asthma. Bratisl. Lek. Listy 112, 9–12. [PubMed] [Google Scholar]
- 33. Matsuda H, Ando S, Morikawa T, Kataoka S, Yoshikawa M (2005) Structure‐activity relationships of 1′S‐1′‐acetoxychavicol acetate for inhibitory effect on NO production in lipopolysaccharide‐activated mouse peritoneal macrophages. Bioorg. Med. Chem. Lett. 15, 1949–1953. [DOI] [PubMed] [Google Scholar]
- 34. Jang YW, Lee JY, Kim CJ (2010) Anti‐asthmatic activity of phenolic compounds from the roots of Gastrodia elata Bl. Int. Immunopharmacol. 10, 147–154. [DOI] [PubMed] [Google Scholar]
- 35. Medeiros KC, Figueiredo CA, Figueredo TB, Freire KR, Santos FA, Alcantara‐Neves NM et al (2008) Anti‐allergic effect of bee pollen phenolic extract and myricetin in ovalbumin‐sensitized mice. J. Ethnopharmacol. 119, 41–46. [DOI] [PubMed] [Google Scholar]
- 36. Spagnuolo C, Russo M, Bilotto S, Tedesco I, Laratta B, Russo GL (2012) Dietary polyphenols in cancer prevention: the example of the flavonoid quercetin in leukemia. Ann. N Y Acad. Sci. 1259, 95–103. [DOI] [PubMed] [Google Scholar]
- 37. Boubaker J, Bhouri W, Ben Sghaier M, Ghedira K, Dijoux Franca MG, Chekir‐Ghedira L (2011) Ethyl acetate extract and its major constituent, isorhamnetin 3‐O‐rutinoside, from Nitraria retusa leaves, promote apoptosis of human myelogenous erythroleukaemia cells. Cell Prolif. 44, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tundis R, Loizzo MR, Bonesi M, Menichini F, Statti GA, Menichini F (2008) In vitro cytotoxic activity of Salsola oppositifolia Desf. (Amaranthaceae) in a panel of tumour cell lines. Z. Naturforsch. C. 63, 347–354. [DOI] [PubMed] [Google Scholar]


