Abstract
Objectives: Recently, plant lectins have attracted great interest due to their various biological activities such as anti‐cancer, anti‐fungal and anti‐viral activities. We have reported earlier concerning anti‐proliferation of human cancer cell lines by a galactose‐binding lectin (AML), from a Chinese herb, Astragalus membranaceus. In the present study, detailed investigations into the mechanism of such anti‐proliferation properties have been carried out.
Materials and methods: Mechanism of apoptosis initiation in K562 cells by AML was investigated by morphology, flow cytometry and western blot analysis.
Results: AML induced apoptosis in a caspase‐dependent manner in the chronic myeloid leukemia cell line, K562. Furthermore, we observed that cytotoxicity and apoptosis of K562 cells induced by AML were completely abolished in presence of lactose or galactose.
Conclusions: Our results suggest that AML could act as a potential anti‐cancer drug.
Introduction
Plant lectins are carbohydrate‐binding proteins of non‐immune origin with cell agglutinating ability, and are ubiquitous in nature (1, 2). In recent years, plant lectins have attracted increased interest due to their various biological activities, such as agglutination, toxicity, anti‐proliferation of cancer cells and immunomodulation, as well as having anti‐fungal and anti‐viral activities (3, 4, 5, 6, 7, 8, 9, 10). It is now widely accepted that plant lectins have a great potential in treating, preventing and helping to diagnose various chronic diseases including cancer (6). Some plant lectins like mistletoe lectin have been used for many years as alternative therapy for breast cancer patients (11). Apoptosis of cancer cells by mistletoe lectins (MLs) is mainly via mitochondrial and/or death‐receptor pathways (12, 13, 14). Most plant lectins induce apoptosis of cancer cells, mediated through caspase‐dependent pathways (15, 16, 17, 18, 19).
So far, some plant lectins, such as ricin (20), WGA (21, 22) have been reported (6, 14) as possessing significant anti‐cancer properties. Several studies have suggested a strong correlation between certain lectin‐binding patterns and their biological effects in various tumours (6). MLs consist of a catalytic A‐chain, which inhibits protein synthesis, and B‐chain, which facilitates binding of appropriate carbohydrate residues on cell surfaces, thereby inducing receptor‐mediated cell death (23, 24).
Previously, we have isolated and characterized a novel lectin, AML, from the dried roots of Astragalus membranaceus, which has been used in China for many centuries as a traditional herbal medicine to treat various chronic diseases (25). AML is a monomeric protein with molecular mass of 31.5 kDa and a glycoprotein with 10.7% neutral sugar. AML is a galactose‐binding lectin, which is best inhibited by D‐galactose and its derivatives, with pronounced preference for o‐nitrophenyl‐β‐D‐galactopyranoside (8.3 mm). AML belongs to a legume lectin family, and has carbohydrate‐binding specificities, and further similarities to other reported legume lectins, (6, 9, 25, 26). Furthermore, AML has been found to inhibit proliferation of HeLa cells, the chronic myeloid leukemia (CML) cell line K562 and more human cancer cell lines (25). Some other legume lectins such as concanavalin A, AMML and French bean haemagglutinin have also been reported to have apoptosis induction in various types of cancer cells via different molecular mechanisms (9, 26, 27, 28). In the present study, we have demonstrated that AML‐induced apoptosis in CML is a caspase‐dependent manner. Furthermore, we observed that cytotoxicity and apoptosis of CML cells induced by AML were completely abolished in the presence of lactose or galactose.
Materials and methods
Chemicals and reagents
Astragalus membranaceus lectin (AML) was extracted and purified as described previously by Yan et al. (2010). Annexin V/FITC (fluorescein isothiocyanate) was purchased from the Center for Human Disease Genomics, Peking University, Beijing, China. MTT (3‐(4, 5‐dimethylthiazol‐2‐yl)‐2, 5‐diphenyltetrazolium bromide) was obtained from Amresco, Solon, OH, USA. DAPI (4′, 6‐diamidino‐2‐phenylindole, dihydrochloride) stain and RPMI 1640 medium were purchased from Invitrogen, Carlsbad, CA, USA. Bovine serum albumin (BSA), Dulbecco’s modified Eagle’s medium (DMEM), propidium iodide (PI) and fluorescein isothiocyanate (FITC) were purchased from Sigma, St. Louis, MO, USA. Mouse anti‐α‐tubulin (CB100999) and mouse anti‐Bcl‐2 (sc‐7382) were obtained from California Bioscience, Inc., Coachella, CA, USA and Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA respectively. Rat anti‐caspase‐3 (M097‐3), z‐VAD‐fmk and anti‐mouse IgG coupled to horseradish peroxidase (HRP) antibodies were obtained from Medical and Biological Laboratories Company, Naka‐ku, Nagoya, Japan. All other chemicals used were of analytical grade.
Tumour cell lines and culture conditions
Human leukemia cell line, CML K562, was obtained from the Chinese Academy of Medical Sciences, Beijing, China. Culturing and maintenance of K562 cells were performed as described previously (Yan et al., 2009). For culturing K562 cells, RPMI 1640 medium was used, supplemented with 10% of FCS, 100 U/ml penicillin and 100 μg/ml streptomycin. All cells were cultured at 37 °C in a humidified atmosphere with 5% CO2. Cell viability was checked by MTT assay as described previously (Yan et al., 2010).
Cell morphology
Exponentially growing K562 cells (1 × 105 cells/ml) were treated with 20 μg/ml AML for 24 h. Apoptotic nuclear morphology was visualized after DAPI staining of AML‐treated cells. Cells were first fixed in 3.7% paraformaldehyde for 10 min at room temperature and then washed three times in PBS and 0.1% Triton X‐100 for 2 min. Fixed cells were then stained with DAPI (10 μg/ml), in the dark, for 10 min. After washing three times in PBS, cells were visualized using a fluorescence microscope (Olympus Corporation, Japan).
DNA fragmentation assay
Ladder patterns of DNA fragmentation were analysed using agarose gel electrophoresis. K562 cells were incubated with AML (20 μg/ml) for different time intervals, and their DNA (1 × 106) was isolated using the Apoptotic DNA Ladder Kit, Applygen Technologies Inc., (Beijing, China). After ethanol precipitation, DNA from each sample was subjected to electrophoresis with 1% agarose slab gel containing 0.2 μg/ml ethidium bromide (EB).
Flow cytometry
Apoptosis was analysed using annexin V labelling and PI staining followed by flow cytometry. After incubation with AML (10 μg/ml) for 0, 12, 24 and 48 h, K562 cells were harvested, washed in cold PBS and suspended in binding buffer (10 mm HEPES, 140 mm NaCl, 2.5 mm CaCl2). Cells (1 × 106 cells/ml) were then stained with 10 μl annexin V/FITC and 5 μl PI (50 μg/ml) and kept in the dark at room temperature for 15 min ahead of the flow cytometric studies (Becton‐Dickinson, Franklin, NJ, USA).
Cell surface binding assay
Binding of AML to surfaces of K562 cells was detected by fluorescence microscopy and flow cytometry using FITC as fluorescence marker. First, AML and BSA (negative control) were labelled by FITC (1 mg/ml), which was dissolved in cold PBS and dialysed against PBS at 4 °C overnight; cell agglutination activity of the lectin remained unchanged after FITC‐labelling (data not shown). The cells (4 × 105 cells/ml) were washed three times with PBS and 1 ml of cells were seeded into each well in a 24‐well plate. AML and BSA were added to each well separately at final concentration of 10 μg/ml. Cells from 24‐well plates, previously incubated for 30 min at 37 °C in CO2 incubator, were then washed three times in cold PBS, then were observed under the fluorescence microscope and subjected to flow cytometry.
Western blot analysis
K562 cells treated with AML (10 μg/ml) for 0, 12, 24 and 48 h were washed in cold PBS and re‐suspended in 200 μl of lysis buffer [50 mm Tris‐HCl (pH 7.4), 1% Triton X‐100, 150 mm NaCl] containing protease inhibitors [1 mg/ml aprotinin, 10 mg/ml leupeptin and 1 mm 4‐(2‐aminoethyl) benzenesulfonyl fluoride] and incubated for 30 min on ice. Protein extracts were collected by centrifugation (9720 g, 10 min) and mixed with equal volume of 5× sodium dodecyl sulphate (SDS) gel loading buffer (1 m pH 6.8 Tris‐HCl, 10% SDS, and 0.01% bromophenol blue and 50% glycerol) and boiled for 5 min to completely denature protein. After SDS‐PAGE, protein bands were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA), which were blocked in 0.5% fat‐free dried milk in Tris‐buffered saline (TBS) with 0.05% Tween 20 (TBST), for 1 h at room temperature, and washed in TBST three times. Subsequently, membranes were incubated overnight at 4 °C with appropriate primary antibodies at adequate dilutions, in blocking buffer (0.5% BSA in TBS). After washing in TBST, membranes were incubated with anti‐rat or anti‐mouse horseradish peroxidase‐conjugated secondary antibodies (1:2000 dilutions) in the above‐mentioned blocking buffer for 1 h at room temperature. Bands were detected using enhanced chemiluminescence kit (Applygen Technologies Inc., China).
Statistical analysis
Results are expressed as the means and standard deviations of triplicate measurements. Each experiment was performed at least three times. Statistical comparisons were made by student’s t‐test and P < 0.05 was considered statistically significant. Western blots are representative of three independent experiments.
Results
Cell morphological changes and apoptosis induced by AML
To demonstrate anti‐proliferative action of AML during apoptosis, the lectin‐treated tumour cells were stained with DAPI and observed under a fluorescence microscope, for any morphological changes in the cells. In the control cells, nuclei (with their DNA) were found to be round in shape and appeared to be homogeneously stained, whereas in AML‐treated cells, chromatin condensation was at the periphery of nuclear membranes and fragmentation of nuclear bodies were observed (Fig. 1b,c). A hallmark observation of separated nuclear DNA in most cells undergoing apoptosis, is the appearance of DNA ladders due to its inter‐nucleosomal degradation, resulting from activation of nuclear endonucleases. Such ladder‐like patterns of DNA fragments were observed in 20 μg/ml AML‐treated cells after 48 h incubation, however, during the period of 12–24 h, smear‐like DNA degradation was observed in the AML‐treated cells (Fig. 1a). Apoptosis of K562 cells by AML was occurred in a time‐dependent manner.
Figure 1.
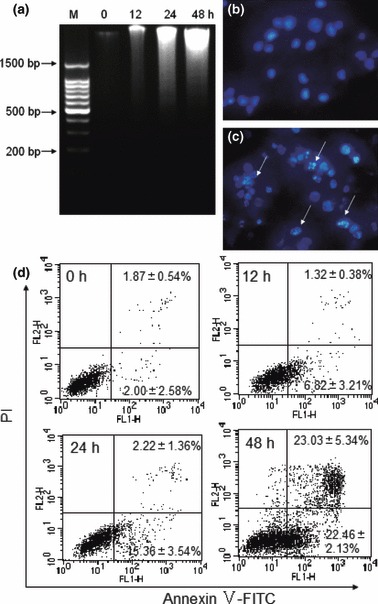
AML induced apoptosis in K562 cells. DNA fragmentation was checked by ethidium bromide staining (a). Cells were cultured with 20 μg/ml AML for 0, 12, 24 and 48 h respectively, ‘M’ represents marker. Morphological observation of nuclei was visualized using a fluorescence microscope and DAPI staining (400×). Cells were incubated in the absence (b) or presence (c) of 20 μg/ml AML for 24 h. Arrows indicate apoptotic features (condensed chromatin and nuclear fragmentation). Apoptosis of K562 cells was quantified using annexin V/PI staining and flow cytometry (d). Cells were treated with 10 μg/ml AML for 0, 12, 24 and 48 h respectively. Data expressed as mean ± SD, obtained from experiments performed in triplicate.
Furthermore, to confirm this apoptosis‐causing property of AML, the K562 cells were stained simultaneously with annexin V and PI. Early or late apoptosis was determined after a variety of time periods of AML treatment. As shown in Fig. 1d, in the region of 5% of cells which were not treated with AML were found either in early or late stage of apoptosis. Upon AML treatment, approximately 7% and 15% cells were found in early apoptosis stages after 12 and 24 h respectively, and less than 5% total cells appeared to be in late apoptosis. It is interesting to observe that after 48 h, the proportion of early and late stage of apoptotic cells increased to 23.68% and 23.03% respectively and this ratio of apoptotic to non‐apoptotic cells increased in a time‐dependent manner. The above results clearly suggest that AML induces apoptosis in K562 cells.
Effects of caspase inhibitor in AML‐induced cell death
A pan‐caspase inhibitor, z‐VAD‐fmk, was used to ascertain involvement of caspases in AML‐induced K562 cell death. Cells were treated with AML in the presence and absence of z‐VAD‐fmk. Anti‐proliferative activity of AML was found to be significantly lower in the presence of z‐VAD‐fmk. Over 48 h AML treatment in the presence of the tested inhibitor, the anti‐proliferative ratio was maintained in the region of 35%, which increased to 65% as concentration of AML increased. In particular, when AML was added at 5 μg/ml, the inhibitory ratio was less than 10% in the presence of inhibitor, whereas it was more than 30% in the absence of inhibitor (Fig. 2a). To confirm whether anti‐proliferative activity of AML was through a caspase‐mediated system of apoptosis, annexin V and PI staining was carried out with AML alone or with AML plus caspase inhibition. As shown in Fig. 2b, a significant decrease in early and late apoptosis was observed with AML and caspase inhibitor treated cells compared to only AML‐treated cells. These results indicate that AML‐induced apoptosis in our cells was mediated through caspase activation pathways.
Figure 2.
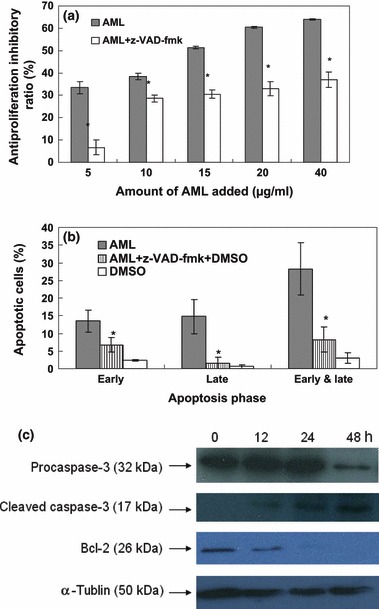
AML induced apoptosis through a mitochondria‐mediated and caspase‐dependent pathway. Cells were incubated in a variety of concentrations of AML and 40 mm z‐VAD‐fmk, for 48 h: cell viability was checked by MTT assay (a). Cells were incubated in 10 μg/ml AML and 40 mm z‐VAD‐fmk for 48 h; apoptosis was investigated using flow cytometry (b). Data expressed as mean ± SD, obtained from experiments performed in triplicate, *P < 0.05 versus AML group. The K562 cells were treated with 10 μg/ml AML for the variety of time periods, and levels of Bcl‐2 and caspase‐3 were detected by western blot analysis (c). α‐tublin was used as equal control, and results are representative of experiments performed in triplicate.
Analysis of expression of Bcl‐2 and caspase‐3 by immunoblotting
Caspase‐3 is known to play a vital role in caspase‐dependent apoptotic pathways, which can be activated by relevant modulins, like Bcl‐2, released from mitochondrial membranes. We investigated expression of caspase‐3 and Bcl‐2 in AML‐treated cells by western blot analysis. Decreased levels of pro‐caspase‐3 expression and increased concentrations of the cleavage product of caspase‐3, were observed in AML‐treated K562 cells (Fig. 2c). Simultaneously, quantities of modulin, Bcl‐2 were reduced with increase in AML treatment time. These results also clearly indicate involvement of mitochondria‐mediated caspase‐dependent apoptosis in AML‐treated cells.
Cell surface binding by AML
Lectins recognize specific carbohydrate topology by conjugation with certain glycans on cell surfaces. Thus, cell surface binding of FITC‐labelled AML to our cells was studied using fluorescence microscopy. AML was found to bind strongly to the cells compared to non‐lectin treated controls, BSA treatment only (Fig. 3).
Figure 3.
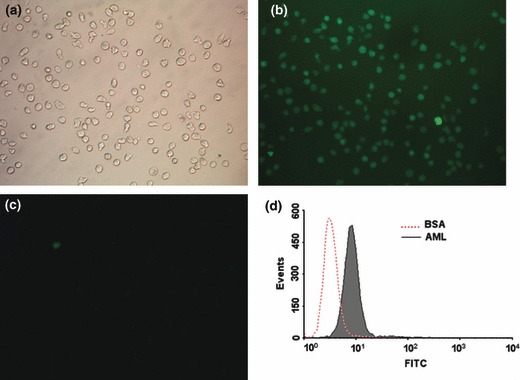
Observation of AML binding to K562 cell surface. K562 cells incubated with AML‐labelled FITC, were observed using phase contrast microscopy (a, 200×) or fluorescence microscopy (b, 200×), and BSA‐labelled FITC was used as negative control (c). Fluorescent intensity of cell surfaces was checked using flow cytometry (d); red dotted line represents cells bound with BSA as negative control. Results are representative of experiments performed in triplicate.
Effects of sugars on AML‐induced cytotoxicity and apoptosis in K562 cells
We have previously reported the selective inhibition of AML haemagglutination activity by galactose or lactose (25). To test whether these sugars have any effect on the anti‐proliferative activity of AML, experiments were carried out in the presence of free carbohydrates such lactose and D‐galactose. As negative control, cells were cultured with AML in the presence of other sugars such as glucose, mannose and fructose. Cells were cultured with sugars, without AML to discern effects of sugars on the cells. Anti‐proliferative activity of AML was abrogated in the presence of galactose‐containing sugars, lactose and D‐galactose, while the other sugars did not cause any anti‐proliferative effects of AML (Fig. 4a). Annexin V and PI staining indicated that apoptosis induced by AML was completely abrogated in the presence of galactose (Fig. 4b). These results suggest that cytotoxicity and apoptosis‐causing AML to K562 cells were abolished, which could be due to binding with lactose or galactose, blocking biological activity of AML.
Figure 4.
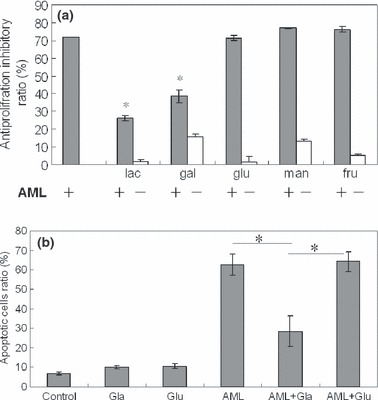
Effect of different sugars on anti‐proliferative activity of AML on K562 cells. Cells were incubated with AML (20 μg/ml) and/or sugar (50 mm) (Lac for lactose, gal for galactose, glu for glucose, man for mannose, fru for fructose; ± cells incubated with or without AML). Data expressed as mean ± SD, obtained from experiments performed in triplicate, *P < 0.05 versus control.
Discussion
Plant lectins are proteins or glycoproteins that are able to recognize and bind specific carbohydrates distributed on cell membranes. This property of lectins has been certified to trigger certain tumour cells to activate cell death (6). In our previous studies, we have found that AML was more inhibitory to K562 cells (IC50 = 15 μg/ml) compared to the HeLa cell line (25). In addition, compared to other lectins reported earlier, AML seemed to have more significant cell proliferation inhibition on CML cells (25, 29, 30). In our present investigation, results of DNA laddering and annexin V assays clearly indicated that AML can induce apoptosis in these CML cells, although apoptosis of HeLa cells by a further Astragalus lectin, AMML, has reported been (9).
Understanding intracellular mechanisms involved during human tumour cell apoptosis induced by lectins is of great importance for the development of lectin‐based anti‐cancer drugs (14). Caspases, which belong to a family of cysteine proteases, have been well established as major players in apoptosis‐causing mechanisms. Caspase‐3, a key member of the caspase family, is known to initiate the cell death cascade after its activation by stimulation from mitochondria and/or cell death receptors (31, 32). Our study using pan‐caspase inhibitor, z‐VAD‐fmk, indicated that anti‐proliferative activity of AML is mediated through a caspase‐dependent mechanism. Furthermore, lower expression levels of anti‐apoptotic protein, Bcl‐2, further support our hypothesis that anti‐proliferative activity of AML on CML cells is probably through mitochondrion‐associated apoptotic signalling pathways (32, 33). Induction of mitochondrial caspase‐dependent apoptotic signalling by ingredients derived from plants or medicinal herbs has been well demonstrated in various cancer cells (13, 14, 17, 34). Amongst reported plant lectins, some induce apoptosis in cancer cells through caspase‐independent signalling way (15, 30), but some lectins such as SNA‐I/II, POL and PCL resemble AML in their molecular mechanisms of apoptosis in their corresponding cancer cells (17, 18, 19, 35). As AML is capable of inducing apoptosis in CML cells by a traditional signalling pathway, it is reasonable to expect that AML can play an important role in treatment of cancers in the way of some other reported lectins (6, 11, 26).
Carbohydrate‐binding specificity of lectins plays an important role in their biological activities. Investigations have been carried out to demonstrate similarities in signalling pathways of apoptosis induced by galactose‐binding lectins like AML (6). Some plant lectins, such as those of mistletoe, which also bind specifically through lactose or galactose, bring about caspase‐dependent apoptosis through mitochondria and/or death receptors of human tumour cells (12, 13, 36) and apoptosis of K562 cells caused by a D‐galactose lectin (CvL) via a caspase‐independent mechanism, has been reported (30). A Galβ1‐3Gal‐NAc‐binding lectin (Jacalin) from jackfruit seeds inhibited CD45 tyrosine phosphatase activity of human B lymphocytes resulting in apoptosis (37). Thus, molecular mechanisms of lectin‐induced apoptosis of tumour cells seems to be complex and cannot be judged simply by carbohydrate‐binding property of lectins.
Lectin‐mediated cytotoxicity is a complex process, and binding of lectin to appropriate carbohydrate moieties present on the cell membrane is presumed to be an initial step (38). In our study, cell surface binding of K562 cells was demonstrated using fluorescence‐labelled AML (Fig. 3). Several other plant lectins showing cancer cell surface binding ability have been visualized with analogical lectin‐labelling methods under fluorescence microscope (15, 30, 39). Furthermore, it was observed that lactose or galactose, added simultaneously with AML protected the K562 cells from death and apoptosis. Some previous reports have indicated that the anti‐proliferative activity of plant lectins, including the well‐known legume lectin concanavalin A, is inhibited by their special binding carbohydrates, but there are few reports that show that apoptosis‐inducing ability can simultaneously be inhibited (7, 27). Even though details of lectin‐mediated cytotoxicity and apoptosis are not well known, our data clearly indicate that AML binds to appropriate galactose‐containing receptors, which are responsible for triggering the apoptotic signals in K562 cells.
In conclusion, we have found that AML can induce apoptosis through caspase‐dependent pathways in human K562 leukemia cells, and this lectin‐mediated apoptotic mechanism is considered to have strong correlation with its sugar‐binding specificity. To the best of our knowledge, this is the first time a detailed mechanism of apoptosis, induced by a plant lectin from Astragalus genus, has been reported. Our results may provide promising insights for pharmaceutical exploitation in the treatment of CML.
Acknowledgements
This work was supported by the New Century Excellent Talents in University (NCET‐08‐0534).
References
- 1. Van Damme EJ, Peumans WJ, Barre A, Rouge P (1998) Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 17, 575–692. [Google Scholar]
- 2. Van Damme EJ, Lannoo N, Peumans WJ (2008) Plant lectins. Adv. Bot. Res. 48, 107–209. [Google Scholar]
- 3. Janssen O, Scheffler A, Kabelitz D (1993) In vitro effects of mistletoe extracts and mistletoe lectins. Cytotoxicity towards tumor cells due to the induction of programmed cell death (apoptosis). Arzneimittelforschung 43, 1221–1227. [PubMed] [Google Scholar]
- 4. Kiss R, Camby I, Duckworth C, Decker RD, Salmon I, Pasteels JL et al. (1997) In vitro influence of Phaseolus vulgaris, Griffonia simplicifolia, concanavalin A, wheat germ, and peanut agglutinins on HCT‐15, LoVo, and SW837 human colorectal cancer cell growth. Gut 40, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh J, Singh J, Kamboj SS (2004) A novel mitogenic and antiproliferative lectin from a wild cobra lily, Arisaema flavum . Biochem. Biophys. Res. Commun. 318, 1057–1065. [DOI] [PubMed] [Google Scholar]
- 6. De Mejia EG, Prisecaru VI (2005) Lectins as bioactive proteins: a potential in cancer treatment. Crit. Rev. Food Sci. 45, 425–445. [DOI] [PubMed] [Google Scholar]
- 7. Peng H, Lv H, Wang Y, Liu YH, Li CY, Meng L et al. (2009) Clematis montana lectin, a novel mannose‐binding lectin from traditional Chinese medicine with antiviral and apoptosis‐inducing activities. Peptides 30, 1805–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Melo CML, Paim BA, Zecchin KG, Morari J, Chiaratti MR, Correia MTS et al. (2010) Cramoll1,4 lectin increases ROS production, calcium levels, and cytokine expression in treated spleen cells of rats. Mol. Cell. Biochem. 342, 163–169. [DOI] [PubMed] [Google Scholar]
- 9. Yan QJ, Li YX, Jiang ZQ, Sun Y, Zhu LF, Ding ZF (2009) Antiproliferation and apoptosis of human tumor cell lines by a lectin (AMML) of Astragalus mongholicus . Phytomedicine 16, 586–593. [DOI] [PubMed] [Google Scholar]
- 10. Fang EF, Lin P, Wong JH, Tsao SW, Ng TB (2010) A lectin with anti‐HIV‐1 reverse transcriptase, antitumor, and nitric oxide inducing activities from seeds of Phaseolus vulgaris cv. extralong autumn purple bean. J. Agric. Food. Chem. 58, 2221–2229. [DOI] [PubMed] [Google Scholar]
- 11. Schumacher K, Schnerder B, Reich G, Stiefel T, Stoll G, Bock PR et al. (2003) Influence of postoperative complementary treatment with lectin‐standardized mistletoe extract on breast cancer patients. A controlled epidemiological multicentric retrolective cohort study. Anticancer Res. 23, 5081–5087. [PubMed] [Google Scholar]
- 12. Büssing A, Multani AS, Pathak S, Pfuller U, Schietzel M (1998) Induction of apoptosis by the N‐acetyl‐galactosamine‐specific toxic lectin from Viscum album L. is associated with a decrease of nuclear p53 and Bcl‐2 proteins and induction of telomeric associations. Cancer Lett. 130, 57–68. [DOI] [PubMed] [Google Scholar]
- 13. Choi SH, Lyn SY, Park WB (2004) Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch. Pharm. Res. 27, 68–76. [DOI] [PubMed] [Google Scholar]
- 14. Liu B, Bian HJ, Bao JK (2010) Plant lectins: potential drugs from bench to clinic. Cancer Lett. 287, 1–12. [DOI] [PubMed] [Google Scholar]
- 15. Gastman B, Wang K, Han J, Zhu ZY, Huang XJ, Wang GQ et al. (2004) A novel apoptotic pathway as defined by lectin cellular initiation. Biochem. Biophys. Res. Commun. 316, 263–271. [DOI] [PubMed] [Google Scholar]
- 16. Lavastre V, Chiasson S, Cavalli H, Girard D (2005) Viscum album agglutinin‐I (VAA‐I) induces apoptosis and degradation of cytoskeletal proteins in human leukemia PLB‐985 and X‐CGD cells via caspases: lamin B1 is a novel target of VAA‐I. Leukemia Res. 29, 1443–1453. [DOI] [PubMed] [Google Scholar]
- 17. Liu B, Cheng Y, Zhang B, Bian HJ, Bao JK (2009) Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria‐mediated ROS–p38–p53 pathway. Cancer Lett. 275, 54–60. [DOI] [PubMed] [Google Scholar]
- 18. Shahidi‐Noghabi S, Van Damme EJ, Iga M, Smagghe G (2010) Exposure of insect midgut cells to Sambucus nigra L. agglutinins I and II causes cell death via caspase‐dependent apoptosis. J. Insect Physiol. 56, 1101–1107. [DOI] [PubMed] [Google Scholar]
- 19. Zhang ZT, Peng H, Li CH, Liu JJ, Zhou TT, Yan YF et al. (2010) Polygonatum cyrtonema lectin induces murine fibrosarcoma L929 cell apoptosis via a caspase‐dependent pathway as compared to Ophiopogon japonicus lectin. Phytomedicine 18, 25–31. [DOI] [PubMed] [Google Scholar]
- 20. Plattner VE, Wagner M, Ratzinger G, Gabor F, Wirth M (2008) Targeted drug delivery: binding and uptake of plant lectins using human 5637 bladder cancer cells. Eur. J. Pharm. Biopharm. 70, 572–576. [DOI] [PubMed] [Google Scholar]
- 21. Narayanan S, Surendranath K, Bora N, Surolia A, Karande AA (2005) Ribosome inactivating proteins and apoptosis. FEBS Lett. 579, 1324–1331. [DOI] [PubMed] [Google Scholar]
- 22. Constanze E, Barbara N, Hermann W, Heike W, Ludwig J (2009) Inhibitory effect of the lectin wheat germ agglutinin (WGA) on the proliferation of AR42J Cells. Acta Histochem. 111, 335–342. [DOI] [PubMed] [Google Scholar]
- 23. Büssing A (1996) Induction of apoptosis by the mistletoe lectins: a review on the mechanisms of cytotoxicity mediated by Viscum album L. Apoptosis 1, 25–32. [Google Scholar]
- 24. Vervecken W, Kleff S, Pfuller U, Bussing A (2000) Induction of apoptosis by mistletoe lectin land its subunits. No evidence for cytotoxic effects caused by isolated A‐ and B‐chains. Int. J. Biochem. Cell Biol. 32, 317–326. [DOI] [PubMed] [Google Scholar]
- 25. Yan QJ, Zhu LF, Kumar N, Jiang ZQ, Huang LH (2010) Characterisation of a novel monomeric lectin (AML) from Astragalus membranaceus with anti‐proliferative activity. Food Chem. 122, 589–595. [Google Scholar]
- 26. Fu LL, Zhou CC, Yao S, Yu JY, Liu B, Bao JK (2011) Plant lectins: targeting programmed cell death pathways as antitumor agents. Int. J. Biochem. Cell Biol. 43, 1442–1449. [DOI] [PubMed] [Google Scholar]
- 27. Liu B, Li CY, Bian HJ, Min MW, Chen LF, Bao JK (2009) Antiproliferative activity and apoptosis‐inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch. Biochem. Biophys. 482, 1–6. [DOI] [PubMed] [Google Scholar]
- 28. Lam SK, Ng TB (2010) First report of a haemagglutinin‐induced apoptotic pathway in breast cancer cells. Biosci. Rep. 30, 307–317. [DOI] [PubMed] [Google Scholar]
- 29. De Carvalho DD, Schmitmeier S, Novello JC, Markland FS (2001) Effect of BJcuL (a lectin from the venom of the snake Bothrops jararacussu) on adhesion and growth of tumor and endothelial cells. Toxicon 39, 1471–1476. [DOI] [PubMed] [Google Scholar]
- 30. Queiroz AFS, Silva RA, Moura RM, Dreyfuss JL, Paredes‐Gamero EJ, Souza ACS et al. (2009) Growth inhibitory activity of a novel lectin from Cliona varians against K562 human erythroleukemia cells. Cancer Chemother. Pharmacol. 63, 1023–1033. [DOI] [PubMed] [Google Scholar]
- 31. Riedl SJ, Shi YG (2004) Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 5, 897–907. [DOI] [PubMed] [Google Scholar]
- 32. Lavrik IN, Golks A, Krammer PH (2005) Caspases pharmacological manipulation. J. Clin. Invest. 115, 2665–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Desagher S, Martinou J (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol. 10, 369–377. [DOI] [PubMed] [Google Scholar]
- 34. Ramakrishnan G, Lo Muzio L, Elinos‐Báez CM, Jagan S, Augustine TA, Kamaraj S et al. (2009) Silymarin inhibited proliferation and induced apoptosis in hepatic cancer cells. Cell Prolif. 42, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu B, Zhang B, Min MW, Bian HJ, Chen LF, Liu Q et al. (2009) Induction of apoptosis by Polygonatum odoratum lectin and its molecular mechanisms in murine fibrosarcoma L929 cells. Biochim. Biophys. Acta 1790, 840–844. [DOI] [PubMed] [Google Scholar]
- 36. Khil LY, Kim W, Lyu S, Park WB, Yoon JW, Jun HS (2007) Mechanisms involved in Korean mistletoe lectin‐induced apoptosis of cancer cells. World J. Gastroenterol. 20, 2811–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma BY, Yoshida K, Baba M, Nonak M, Matsumoto S, Kawasaki N et al. (2008) The lectin Jacalin induces human B‐lymphocyte apoptosis through glycosylation‐dependent interaction with CD45. Immunology 127, 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim M, Rao MV, Tweardy D, Prakash M, Galili U, Gorelik E (2008) Lectin‐induced apoptosis of tumour cells. Glycobiology 3, 447–453. [DOI] [PubMed] [Google Scholar]
- 39. Marty‐Detraves C, Francis F, Baricault L, Fournier D, Paquereau L (2004) Inhibitory action of a new lectin from Xerocomus chrysenteron on the cell‐substrate adhesion. Mol. Cell. Biochem. 258, 49–55. [DOI] [PubMed] [Google Scholar]


