Abstract
Abstract. Objectives: Liver regeneration is attenuated in old age and is substantially slower after 90% than after 70% partial hepatectomy (PH). We have previously demonstrated that the proliferative response to a primary mitogen is intact in aged mice, indicating that impaired liver regeneration is not due to loss of proliferative capacity. Here, we have investigated whether mitogenic effects of triiodothyronine (T3) could reverse the impaired regeneration of ageing or 90% hepatectomy, in the rat. Materials and methods: T3 (20 µg/100 g body weight) was administered to 14‐month‐old rats subjected to 70% PH or to young rats subjected to 90% PH. Cell‐proliferative capacity was determined by bromodeoxyuridine incorporation and microscopy and changes of cell cycle‐related proteins were analysed by Western blot analysis. Results: Treatment of old intact rats with T3 increased cyclin D1 expression that was followed by an enhanced proliferative response, the labelling index (LI), being 7.8% versus 1.3% of controls. T3 given before 70% PH stimulated regenerative response (LI was 10.8% versus 2.28%), and expression of cyclin D1 and proliferating cell nuclear antigen (PCNA) 24 h after PH. Pre‐treatment with T3 also improved the regenerative response of the liver after 90% hepatectomy (LI was 27.9% versus 14.2%). Conclusions: These findings show in principle that mitogen‐induced hyperplasia could be applied to human therapy in patients with reduced regenerative capacity or massive loss of hepatocytes.
INTRODUCTION
Liver regeneration after partial hepatectomy has been the target of many studies that have probed control mechanisms of liver growth (Higgins & Anderson 1931; Akerman et al. 1992; Cressmann et al. 1996; Michalopoulos & DeFrances 1997; Yamada et al. 1997 and many more). This type of regenerative liver growth, termed compensatory regeneration or compensatory hyperplasia, also occurs after liver cell necrosis due to chemical, nutritional, vascular or viral injury. Several studies on the regenerative response of liver that follows 2/3 partial hepatectomy (70% PH) have shown a striking difference in both magnitude of the peak response in DNA synthesis and time at which maximal DNA synthesis occurs, between young‐ (4–8 weeks) and old‐aged (12–15 months) rats and mice (Bucher et al. 1964; Stocker & Heine 1971; Fry et al. 1984). The initial response to PH was both delayed and reduced in older animals; while 99% of hepatocytes entered DNA synthesis in young ones (2–3 months of age) following PH, only 30% entered into S phase in aged ones.
In recent years, an increasing number of agents (primary mitogens) capable of inducing hepatocyte proliferation without causing liver injury (direct hyperplasia), has been identified (Columbano & Shinozuka 1996). Most of these agents (e.g. peroxisome proliferators, retinoids, thyroid hormone and the halogenated hydrocarbon TCPOBOP), are ligands of various nuclear receptor transcription factors. These agents activate peroxisome proliferator‐activated receptor‐α, retinoic acid receptor, thyroid hormone receptor and constitutive androstane receptor (CAR), respectively, which regulate genes involved in lipid metabolism, adipogenesis, xenobiotic detoxification, and differentiation (Schultz et al. 2000; Qatanani & Moore 2005). These ligand‐activated nuclear receptors also stimulate hepatocyte proliferation (Ledda‐Columbano & Columbano 2003). Notably, numerous early changes considered to be essential for liver regeneration after PH are not observed in nuclear receptor‐mediated hepatocyte proliferation (Ledda‐Columbano et al. 1998; Ledda‐Columbano et al. 2000a; Pibiri et al. 2001): activation of transcription factors nuclear factor‐kappa B (NF‐κB), STAT3 and CCAAT/enhancer binding protein; increased expression of immediate early genes coding for c‐fos, c‐myc, LRF‐1 and egr‐1; and release of cytokines (tumour necrosis factor‐α and interleukin‐6). The early signal transduction pathways involved in nuclear receptor‐mediated hepatocyte proliferation are therefore quite different from those of liver regeneration.
Recently, we have found that hepatocyte proliferation, induced by ligands of the CAR is age‐independent, in contrast to liver regeneration. This suggests that regenerative failure occurs by a mechanism upstream of proliferation, as hepatocytes retain their proliferative capacity when stimulated by a direct mitogen (Ledda‐Columbano et al. 2004).
In the present study, we focused on two questions: (i) can a direct mitogen, namely T3, be used to stimulate liver growth in situations where liver regeneration is attenuated, such as in the elderly and (ii) can a direct mitogen accelerate liver regeneration after massive hepatic injury? To address the latter questions, we exploited the rat model of 90% partial hepatectomy (Gaub & Iversen 1984; Zieve et al. 1985).
MATERIALS AND METHODS
Animals
Wistar rats purchased from Charles River (Milano, Italy) were used in these experiments. The animals were fed a laboratory chow diet provided by Ditta Mucedola (Settimo Milanese, Italy) and had free access to food and water. We followed Italian ‘Guidelines for the Care and Use of Laboratory Animals’ during the investigation. All experiments were performed in a temperature‐controlled room with alternating 12 h dark/light cycles. T3 was purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Experimental protocol 1
Hepatocyte proliferation was induced in 14‐month‐old Wistar rats, by a single intraperitoneal injection of T3 (Sigma Chem. Co.), at a dose of 20 µg/100 g body weight, dissolved in NaOH 0.001N. For determination of total hepatocyte proliferation, bromodeoxyuridine (BrdUrd, Sigma Chemical Co.) was provided continuously in drinking water (1 mg/mL).
Experimental protocol 2
Fourteen‐month‐old Wistar rats were subjected to 70% PH with or without pre‐treatment with a single dose of T3 (20 µg/100 g body weight), 5 h prior to surgery. For determination of total hepatocyte proliferation, BrdUrd was provided continuously in drinking water (1 mg/mL) starting 4 h after surgery, and the animals were sacrificed 24 h after PH.
Experimental protocol 3
Ten‐week‐old Wistar rats were subjected to 90% PH. Briefly, this procedure consisted of resecting the left lateral, median and both right liver lobes, leaving only the superior and inferior caudate lobes. The left lateral and median lobes were first removed as in the conventional 70% PH; afterwards, the right superior and inferior lobes were separately resected by using a Meltzer's slip knot ligation passing through the liver parenchyma and encompassing the vascular pedicle of each right lobe along the portal vein. Immediately after surgery, rats were given a subcutaneous injection of 10% glucose solution and then were given a 20% glucose solution in the drinking water. One group of animals received a single dose of T3 (20 µg/100 g body weight), 5 h prior to surgery. BrdUrd was provided continuously in drinking water (1 mg/mL) starting 4 h after surgery, and the animals were sacrificed 24 h after PH. Food was withdrawn after T3 administration.
Histology and immunohistochemistry
Immediately after death, liver sections were fixed in 10% buffered formalin and were processed for staining with haematoxylin and eosin or for immunohistochemistry. The remaining liver was snap‐frozen in liquid nitrogen and kept at –80 °C until further use. For determination of hepatocyte proliferation, mouse monoclonal anti‐BrdUrd antibody was obtained from Becton Dickinson (San Jose, CA, USA) and the peroxidase method was used to stain BrdUrd‐positive hepatocytes. Peroxidase‐conjugated goat antimouse immunoglobulin was obtained from Dako (Dako EnVisionTM Peroxidase Mouse, Dako Corporation, Carpinteria, CA, USA). Four micron sections were deparaffinized, treated with 2N HCl for 1 h, then with 0.1% trypsin type II (crude from porcine pancreas, Sigma, Milano, Italy) for 20 min and treated sequentially with normal goat serum 1 : 10 (Dako Corporation), mouse anti‐BrdUrd 1 : 100 and Dako EnVisionTM Peroxidase Mouse ready‐to‐use. Sites of peroxidase binding were detected by 3,3′‐diaminobenzidine.
Labelling index
The labelling index (LI) was expressed as number of BrdUrd‐positive nuclei/100 nuclei. Results are expressed as means ± SE of 5–10 rats per group. At least 4000 hepatocyte nuclei per liver were scored.
Northern blot analysis
Total RNA was prepared by using TRIzol isolation reagent (Invitrogen, San Giuliano Milanese, Italy); 30 µg of total RNA were fractionated on formaldehyde‐agarose gel and were transferred to Hybon‐XL‐membrane (Amersham Biosciences, Buckinghamshire, UK). The following 32P‐labelled probes were used for hybridization: probes for Cyclin D1 were prepared from total RNA, reverse‐transcribed with Thermo‐Script Real‐Time Polymerase Chain Reaction (RT‐PCR) System (Invitrogen) and PCR amplified with Platinum Taq DNA Polymerase (Invitrogen) using the following primers: forward primer: CCCAGACCCTCACACTCAGA and reverse primer: ATCCACTCAGGCATCGACAT; probe for 18S was prepared from total RNA using SuperScriptTM IIIOne‐Step RT‐PCR System with Platinum Taq DNA Polymerase (Invitrogen). PCR primers for 18S were forward: CGGCTACCACATCCAAGGAA and reverse: GCTGGAATTACCGCGGCT. DNA probes were labelled with [α32P]dCTP by random priming (ready to go DNA labelling beads or Megaprime DNA Labeling Systems; Amersham Bioscience). Unincorparated nucleotides were removed with Microspin G50 coloumns (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Membranes were exposed to autoradiographic film (Eastman Kodak, Sigma Chemical Co.).
Western blot analysis
Nuclear cell extracts were prepared from 100 mg of liver tissue according to the method of Timchenko et al. (1996), in the presence of 10 µg/mL leupeptin/aprotinin, 10 µg/mL pepstatin, 10 mm NaF, 1 mm phenylmethylsulphonylfluoride and 5 mm iodoacetic acid. Protein concentration of the resulting nuclear extracts was determined according to Bradford (1976) using bovine serum albumin as standard (DC Protein Assay, Bio‐Rad Laboratories, Hercules, CA, USA). For immunoblot analysis, equal amounts (from 100 to 150 g/lane) of protein were electrophoresed on 12% or 8% SDS polyacrylamide gels and electrotransfered onto nitrocellulose membranes (MSI). To ensure equivalent protein loading and transfer in all lanes, membranes and gels were stained with 0.5% (w/v) Ponceau S red (ICN Biochemicals, Irvine, CA, USA) in 1% acetic acid and with Coomassie blue (ICN) in 10% acetic acid, respectively. After blocking in Tris Buffered Saline (TBS) containing 0.05% Tween‐20 (Sigma Chemical Co.) and 5% non‐fat dry milk, membranes were incubated with primary antibodies diluted in blocking buffer. Depending on the origin of primary antibody, filters were incubated at room temperature with either antimouse, antirabbit or antigoat horseradish peroxidase‐conjugated IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immuno‐reactive bands were identified with chemiluminescence detection systems, as described by the manufacturer (Supersignal Substrate, Perbio Pierce, Erembodegen, Belgium). When necessary, antibodies were removed from filters by 30 min incubation at 60 °C in stripping buffer (100 mm 2‐mercaptoethanol, 2% SDS, 62.5 mm Tris‐HCl pH 7.6) and membranes reblotted as above.
Antibodies
For immunoblotting experiments, mouse monoclonal antibodies directed against p27 (anti‐Kip1/p27, Transduction Laboratories, Lexington, KY, USA), cyclin D1 (72‐13) and PCNA (PC‐10) (Santa Cruz) were used. Goat polyclonal antibody against p107 (C‐18), and rabbit polyclonal antibody against actin (C‐19) were also from Santa Cruz.
Statistical analysis
Comparison between the two groups was performed using Student's t‐test.
RESULTS
Experimental protocol 1
T3 administration causes immediate up‐regulation of cyclin D1 in young rats (Pibiri et al. 2001). Accordingly, we evaluated whether the response to T3 was still present in aged rats. As indicated in Fig. 1a and b, while cyclin D1 gene expression was very low before treatment, T3 strongly induced mRNA levels in 5 h after a single administration, suggesting that inducibility of cyclin D1 is age‐independent in rats. Induction of cyclin D1 was followed by a significant increase in the number of hepatocytes entering into S phase by 48 h, compared to untreated rats (LI was 7.8% versus 1.3% of controls; P < 0.02) (Fig. 1b,c). With this time interval, there was no significant difference in liver mass (control = 2.83 ± 0.33 g versus treated = 2.96 ± 0.16 g; P < 0.48). Normal hepatocytes of rat liver show a wide range of ploidy. By 48 h, many cells had finished S phase or completed the entire cell cycle, apparent by presence of BrdUrd‐labelled daughter cell pairs. These cell pairs were found for both diploid and tetraploid cells (Fig. 1c). Thus, even in these aged rats, T3 induced a full cell cycle including G2 and M phases, and stimulated hepatocytes of different ploidy.
Figure 1.
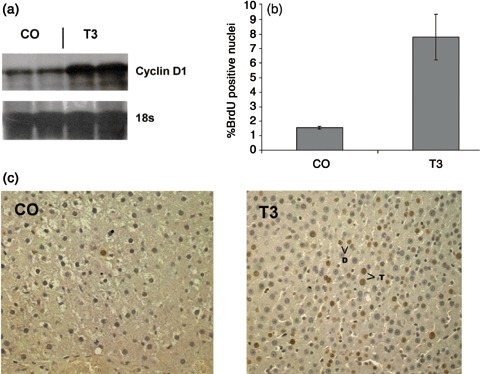
(a) Induction of cyclin D1 mRNA levels in old Wistar rats. Total RNA was prepared from rat liver 5 h after treatment with T3 (20 µg/100 g body weight) and was subjected to Northern blot analysis as described in the Materials and Methods section. Each lane represents an individual sample and 18 s was used as a loading control. CO, controls. (b) Labelling index of hepatocytes from old rats given a single dose of T3, and sacrificed 48 h later. Animals were given BrdUrd in drinking water (1 mg/mL) throughout the whole experiment. At least 4000 hepatocyte nuclei per liver were scored. LI was expressed as number of BrdUrd‐positive hepatocyte nuclei/100 nuclei. Results are expressed as means ± SE of five rats per group. (c) Representative photomicrographs that illustrate the effect of T3 on hepatocyte proliferation in 14‐month‐old rats. Two labeled daughter cell pairs are marked, one diploid (d) and the other tetraploid (T). A proportion of other labeled cells were binucleate. Sections were stained for BrdUrd and counterstained with haematoxylin (×200). Rats received a single dose of T3 and were sacrificed 48 h later.
Experimental protocol 2
It has previously been shown (Pibiri et al. 2001) that T3‐induced hepatocyte proliferation occurs in the absence of changes such as NF‐κB and STAT3 activation and increased expression of immediate early genes (c‐fos, c‐myc), considered to be essential for liver regeneration after partial hepatectomy, suggesting that nuclear receptor‐mediated pathways are different from those activated by membrane‐associated receptors. Therefore, we asked whether pre‐treatment with T3 could positively influence liver regeneration after 70% PH in old animals. Five hours after T3 administration, rats were subjected to 70% PH and were killed 24 h after surgery; a further group of rats was subjected to 70% PH, in the absence of treatment with T3. In agreement with the literature (Bucher et al. 1964; Stocker & Heine 1971), liver regeneration was severely impaired in old rats subjected to 70% PH; indeed, 24 h after surgery, very few hepatocyte nuclei were BrdUrd‐positive (Fig. 2a,b). On the other hand, pre‐treatment with 20 µg/100 g of T3, 5 h prior to PH, induced a much higher proliferative response (LI was 10.3% ± 0.32 in T3 + PH rats versus LI 2.28% ± 0.82 of rats subjected to PH alone; P < 0.001) (Fig. 2a,b). It thus appears that the two mitogenic stimuli are independent and additive. Moreover, induction of proliferation was quite rapid, indicated by presence of labelled hepatocytes in metaphase already apparent at 24 h. The higher proliferation rate, however, was not associated with increased growth at this early time point (liver mass was 1.23 ± 0.14 g in T3 + PH rats and 1.35 ± 0.20 g after PH alone, P < 0.04).
Figure 2.
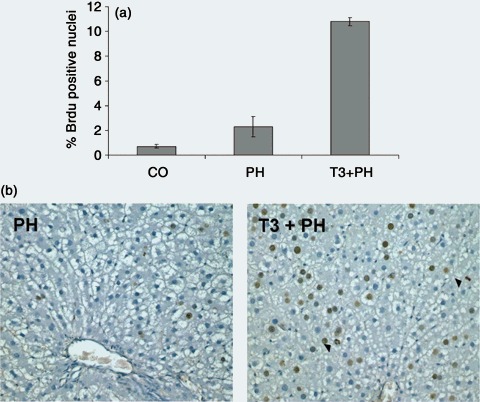
(a) Labelling index of hepatocytes from old rats subjected to 70% PH with or without T3 pre‐treatment. Rats were subjected to 70% PH with or without pre‐treatment with a single dose of T3 (20 µg/100 g body weight) and were sacrificed 24 h later. All rats were given BrdUrd (1 mg/mL) in drinking water until the time of sacrifice. At least 4000 hepatocyte nuclei per liver were scored. Labelling index (LI) was expressed as number of BrdUrd‐positive hepatocyte nuclei/100 nuclei. Results are expressed as means ± SE of five to six rats per group. (b) Representative photomicrographs that illustrate the effect of pre‐treatment with T3 on hepatocyte proliferation in old rats subjected to PH. Two BrdUrd cells in metaphase are indicated (arrowheads). Sections were stained for BrdUrd and counterstained with haematoxylin (×200).
Increased BrdUrd incorporation observed in rats receiving T3 prior to surgery was associated with higher levels of cyclin D1 and PCNA protein (Fig. 3). Because the CDK inhibitor p27 can block activation of cyclin/CDK complexes, inhibition of this protein might provide an alternative mechanism for increased entry of hepatocytes into S phase, observed in T3‐pre‐treated rats. As shown in Fig. 3, p27 protein was expressed at high levels in untreated aged animals and was further enhanced by PH. Pre‐treatment with T3 did not significantly modify PH‐induced enhancement of levels of this protein, suggesting that higher levels of expression of p27 did not inhibit entry into the cell cycle.
Figure 3.
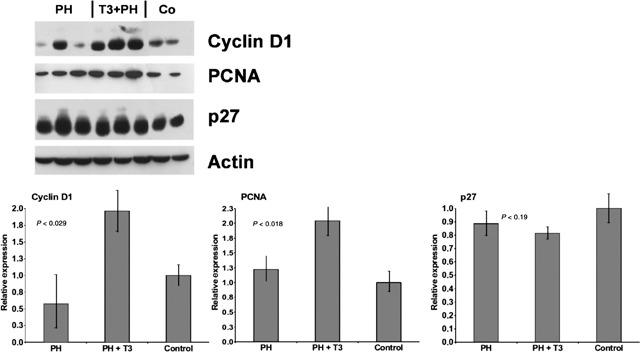
Nuclear extracts for cyclin D1, PCNA and p27 (100–150 µg/lane) were prepared from the livers and Western blot analysis was performed as described in the Materials and Methods section. Above, Western blots; below, densitometric quantification. Values are normalized to levels of untreated liver. Actin was used as a loading control. Each lane represents an individual sample. CO, controls.
Experimental protocol 3
After 90% PH, the proliferative response is significantly delayed, presumably because resources of the remnant liver are required for metabolic homeostasis (Gaub & Iversen 1984; Zieve et al. 1985). However, this form of massive hepatic injury is relevant to human states in which therapeutic intervention to augment regeneration would be valuable (e.g. surgical resection, transplantation and hepatic necrosis). We therefore evaluated the regenerative response of liver in rats treated with a single dose of T3, 5 h prior to performing 90% PH. At 24 h, pre‐treatment with T3 had greatly accelerated the proliferative response of the liver (Fig. 4a,b). While the LI of hepatocytes from rats subjected to PH alone was 14.22%, the LI of rats receiving a single dose of T3 was 27.88% (P < 0.02). Increased BrdUrd incorporation was paralleled by increased protein levels of cyclin D1, PCNA and p107 (Fig. 5). There was, however, no significant difference in liver growth by 24 h (liver mass was 0.64 ± 0.12 g following T3 + PH and 0.63 ± 0.06 g following PH alone; P < 0.38). Moreover, cyclin A expression was induced in T3‐treated, but not in control animals indicating earlier progression through the cell cycle. No mortality was observed following PH, with or without T3 administration.
Figure 4.
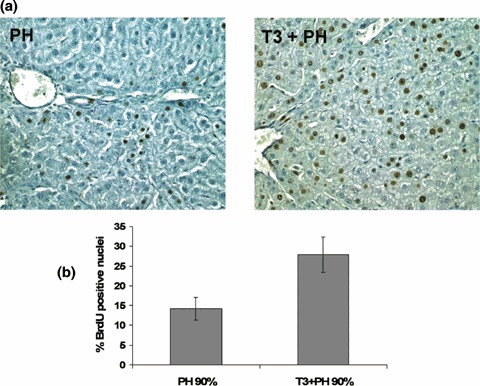
(a) Representative photomicrographs illustrating the effects of T3 on liver regeneration in 10‐week‐old rats subjected to 90% PH. Rats received a single dose of T3, 5 h prior to 90% PH and were sacrificed 24 h after surgery. Sections were stained for BrdUrd and counterstained with haematoxylin (×200). (b) Labelling index (LI) of hepatocytes from 10‐week‐old rats subjected to 90% PH with or without T3 pre‐treatment. Rats were subjected to 90% PH with or without pre‐treatment with a single dose of T3 (20 µg/100 g body weight) and were sacrificed 24 h later. All rats were given BrdUrd (1 mg/mL) in drinking water until time of sacrifice. At least 4000 hepatocyte nuclei per liver were scored. LI was expressed as number of BrdUrd‐positive hepatocyte nuclei/100 nuclei. Results are expressed as means ± SE of 10 rats per group.
Figure 5.
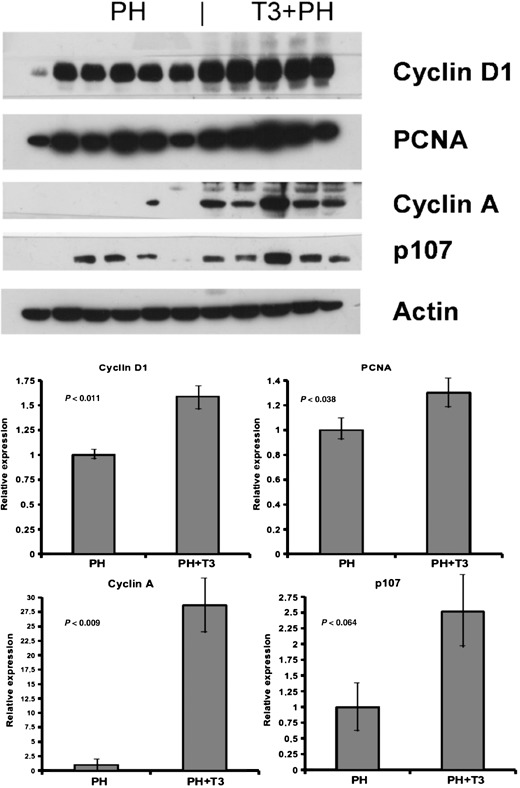
Western blot analysis of cell cycle‐associated proteins. Nuclear extracts for cyclin D1, PCNA, cyclin A and p107 (100–150 µg/lane) were prepared from the livers and Western blot analysis was performed as described in the Materials and Methods sections. Actin was used as a loading control. Each lane represents an individual sample. CO, controls. Above, Western blots; below, densitometric quantification. Values are normalized to the levels of animals treated only with PH.
DISCUSSION
Several studies have shown that regeneration following 70% PH is reduced and delayed in aged rodents (Bucher et al. 1964; Stocker & Heine 1971; Fry et al. 1984; Wang et al. 2001; Wang et al. 2002). However, our previous findings (Ledda‐Columbano et al. 2004) have shown that the primary mitogen TCPOBOP, a ligand for the nuclear receptor CAR, is able to induce hepatocyte DNA synthesis and mitosis in aged mice to the same levels found in young mice. The same study thus demonstrated that capacity of hepatocytes to enter the cell cycle is maintained during ageing, if an appropriate proliferative stimulus is provided. Moreover, our unpublished data have indicated that this response to TCPOBOP can augment liver regeneration in aged mice. However, TCPOBOP is a potentially toxic hydrocarbon, which is not a ligand for human CAR. We therefore turned to a model system more relevant to human therapy. As in the mouse, rat liver regeneration is attenuated with ageing. Ninety per cent hepatectomy in the rat is a well‐established model in which liver regeneration is delayed compared to 70% hepatectomy (Gaub & Iversen 1984; Zieve et al. 1985). T3, a drug that could readily be applied to human therapy, is a strong mitogen in rat liver with growth kinetics similar to TCPOBOP treatment in mouse. Because signal transduction pathways responsible for transition from G0 to G1 phase of hepatocytes in mitogen‐induced direct hyperplasia are different from those classically associated with liver regeneration (Ledda‐Columbano & Columbano 2003), results stemming from the above studies could have significant clinical relevance as they suggest a potential therapeutic approach to relieve the proliferative block after liver injury, that is observed in the elderly. Treatment with mitogens could also have clear applications in liver transplantation, gene therapy and hepatic failure.
Here, we have shown that T3 is able to induce a significant proliferative response in liver of aged rats; in addition, we have also demonstrated that while no regeneration of the liver occurred in old rats in the first 24 h after 70% PH, pre‐treatment with T3, a ligand of the nuclear receptor thyroid hormone receptor, was able to elicit conspicuous hepatocyte proliferation. Increased BrdUrd incorporation observed in rats receiving T3 prior to surgery was associated with higher levels of cell cycle‐related proteins. These results, together with our previous findings (Ledda‐Columbano et al. 2004) demonstrate that the capacity of hepatocytes to enter the cell cycle is maintained during ageing; the results also demonstrate that mitogens and liver injury activate proliferation by different signal transduction pathways. Hence, the use of mitogens like T3 may represent a valuable form of therapy to stimulate rapid liver growth in conditions where regeneration is impaired, such as, for example, when major liver resection is needed in aged patients.
Liver regeneration is also impaired in 90% PH, presumably because of metabolic demands on the remnant liver. Despite these metabolic requirements, forced induction of proliferation did not induce mortality in our experiments, and in human liver injury, metabolism can be therapeutically compensated. Previous studies (Malik et al. 2003; Malik et al. 2005) have focused on the potential utility of primary mitogens within the clinical context of donor conditioning, prior to liver transplantation. Those studies have shown that T3 was able to accelerate liver regeneration after 70% PH in young animals and that when the liver mass was increased by an injection of T3, given 10 days previously and 70% PH performed, there was a larger remnant liver mass and total hepatic DNA content, compared with rats subjected to PH in the absence of T3 treatment.
Although the normal liver has striking regenerative capacity, old age and massive loss of hepatocytes are two important clinical settings where regenerative capacity is impaired. Treatment of human liver injury with mitogens has considerable potential. The choice of a therapeutic mitogen, however, is complex because agents that activate CAR (e.g. TCPOBOP, phenobarbital) are strong promoters of carcinogenesis. In contrast, T3 mitogenesis is quite different, because this agent has an antipromotional effect on carcinogenesis (Ledda‐Columbano et al. 2000b). Whether interspecies differences (rat versus humans) will prevent application of this approach in man will require further experimentation. However, it is important to stress that techniques that could enhance hepatocyte proliferation, could extend the possibility of surgery to a greater proportion of patients (Redaelli et al. 2002), and the use of primary mitogens like T3 may form part of these strategies.
ACKNOWLEDGEMENTS
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC), Ministero Università e Ricerca Scientifica (PRIN ex 40%, and 60%), Fondazione Banco di Sardegna, Italy, and National Institute of Health Grant R01‐CA104292‐01, USA.
REFERENCES
- Akerman P, Cote P, Yang S‐Q, McClain C, Nelson S, Bagby GJ, Diehl AM (1992) Antibodies to tumor necrosis factor‐α inhibit liver regeneration after partial hepatectomy. Am. J. Physiol. 263, G579–G585. [DOI] [PubMed] [Google Scholar]
- Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein‐dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Bucher NLR, Swaffield MN, Di Troia JF (1964) The influence of age upon the incorporation of thymidine‐2‐C14 into the DNA of regenerating rat liver. Cancer Res. 24, 509–512. [PubMed] [Google Scholar]
- Columbano A, Shinozuka H (1996) Liver regeneration versus direct hyperplasia. FASEB J. 10, 1118–1128. [DOI] [PubMed] [Google Scholar]
- Cressmann DE, Greenbaum LE, Deangelis RA, Ciliberto G, Furth EE, Poli V, Taub R (1996) Liver failure and defective hepatocyte regeneration in interleukin‐6 deficient mice. Science 274, 1379–1383. [DOI] [PubMed] [Google Scholar]
- Fry MF, Silber J, Loeb LA, Martin GM (1984) Delayed and reduced cell replication and diminishing levels of DNA polymerases‐α in regenerating liver of aging mice. J. Cell Physiol. 118, 225–232. [DOI] [PubMed] [Google Scholar]
- Gaub J, Iversen J (1984) Rat liver regeneration after 90% partial hepatectomy. Hepatology 4, 902–904. [DOI] [PubMed] [Google Scholar]
- Higgins GM, Anderson RM (1931) Experimental pathology of the liver I. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. 12, 186–202. [Google Scholar]
- Ledda‐Columbano GM, Columbano A (2003) Mitogenesis by ligands of nuclear receptors: an attractive model for the study of the molecular mechanisms implicated in liver growth. Cell Death Differ. 10(Suppl. 1), S19–S21. [DOI] [PubMed] [Google Scholar]
- Ledda‐Columbano GM, Curto M, Piga R, Zedda AI, Menegazzi M, Sartori C, Shinozuka H, Bluethmann H, Poli V, Ciliberto G, Columbano A (1998) In vivo hepatocyte proliferation is inducible through a TNF and IL‐6‐independent pathway. Oncogene 17, 1039–1044. [DOI] [PubMed] [Google Scholar]
- Ledda‐Columbano GM, Perra A, Loi R, Shinozuka H, Columbano A (2000b) Cell proliferation induced by triiodothyronine in rat liver is associated with nodule regression and reduction of hepatocellular carcinoma. Cancer Res. 60, 603–609. [PubMed] [Google Scholar]
- Ledda‐Columbano GM, Pibiri M, Cossu C, Molotzu F, Locker J, Columbano A (2004) Aging does not reduce the hepatocyte proliferative response of mice to the primary mitogen TCPOBOP. Hepatology 40, 981–988. [DOI] [PubMed] [Google Scholar]
- Ledda‐Columbano GM, Pibiri M, Loi R, Perra A, Shinozuka H, Columbano A (2000a) Early increase in cyclin D1 expression and accelerated entry of mouse hepatocytes into S phase after administration of the mitogen 1,4‐bis[2‐(3,5‐dichloropyridyloxy) ]benzene. Am. J. Pathol. 56, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Habib M, Tootle R, Hodgson H (2005) Exogenous thyroid hormone induces liver enlargement whilst maintaining regenerative potential. A study relevant to donor preconditioning. Am. J. Transplant 5, 1801–1807. [DOI] [PubMed] [Google Scholar]
- Malik R, Mellor N, Selden C, Hodgson H (2003) Triiodothyronine enhances the regenerative capacity of the liver following partial hepatectomy. Hepatology 37, 79–86. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC (1997) Liver regeneration. Science 276, 60–66. [DOI] [PubMed] [Google Scholar]
- Pibiri M, Ledda‐Columbano GM, Cossu C, Simbula G, Menegazzi M, Shinozuka H, Columbano A (2001) Cyclin D1 is an early target in hepatocyte proliferation induced by thyroid hormone (T3). FASEB J. 15, 1006–1013. [DOI] [PubMed] [Google Scholar]
- Qatanani M, Moore DD (2005) CAR, the continuously advancing receptor, in drug metabolism and disease. Curr. Drug Metab. 6, 329–339. [DOI] [PubMed] [Google Scholar]
- Redaelli CA, Wagner M, Krahenbuhl L, Loor B, Schilling MK, Dufour JF, Buchler MW (2002) Liver surgery in the era of tissue‐preserving resections: early and late outcome in patients with primary and secondary hepatic tumors. World J. Surg. 26, 1126–1132. [DOI] [PubMed] [Google Scholar]
- Schultz JR, Tu H, Repa JJ, Medina JC, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B (2000) Role of LXRs in control of lipogenesis. Genes Dev. 14, 2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker E, Heine W‐D (1971) Regeneration of liver parenchyma under normal and pathological conditions. Mol. Cell. Biol. 144, 400–408. [PubMed] [Google Scholar]
- Timchenko NA, Wilde M, Nakanishi M, Smith JR, Darlington GJ (1996) CCATT/enhancer binding protein α (C/EBPα) inhibits cell proliferation through the p21 (WAF‐1/Cip‐1/SD‐1) protein. Genes Dev. 10, 804–815. [DOI] [PubMed] [Google Scholar]
- Wang X, Krupczak‐Hollis K, Tan Y, Dennewitz MB, Adami GR, Costa RH (2002) Increased hepatic forkhead box M1B (Fox M1B) levels in old‐aged mice stimulated liver regeneration through diminished p27kip1 protein levels and increased cdc25B expression. J. Biol. Chem. 277, 44310–44316. [DOI] [PubMed] [Google Scholar]
- Wang X, Quail E, Hung N‐J, Tan Y, Ye H, Costa RH (2001) Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age‐related proliferation defects in regenerating liver. Proc. Natl. Acad. Sci. USA 98, 11468–11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Kirillova I, Peschon JJ, Fausto N (1997) Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc. Natl. Acad. Sci. USA 94, 1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve L, Anderson WR, Lindblad S (1985) Course of hepatic regeneration after 80% to 90% resection of normal rat liver. Comparison with two‐lobe and one‐lobe hepatectomy. J. Lab. Clin. Med. 105, 331–336. [PubMed] [Google Scholar]


