Abstract
Abstract. A type II membrane protein similar to CD69 (TIIMPSC) has been isolated in human embryo fibroblasts treated with IFN‐α. Structural analysis and immunofluorescence detection has suggested that this protein is located on the surface of fibroblasts, generally considered, a receptor. Cell proliferation assay has revealed that activation of TIIMPSC elevates the level of fibroblast proliferation. Further, examination of signal transduction has indicated that expression of this protein is up‐regulated by IFN‐α stimulation, and that it is involved in the regulation of fibroblast growth through the JAK‐STAT signalling pathway.
INTRODUCTION
As a family of well‐characterized proteins secreted in the innate immune response, interferons (IFNs) possess the ability to defend against viral infection, regulate cell growth and activate immune cell proliferation (Goodbourn et al. 2000). The family is composed of several members, which can be classified into two types and are capable of functioning as signalling proteins to trigger a series of cellular responses (Kerr & Stark 1991; Malmgaard 2004). Some molecules induced in these responses regulate cell physiological activities and interfere with virus replication (Chesler et al. 2004). IFN‐α, a type I interferon, functions mainly in non‐immune cells and in establishment of an antiviral state in cells (Ivashkiv 2003). Studies performed to investigate the mechanism of this antiviral activity of IFN‐α have indicated that stimulation by IFN‐α of cells is capable of inducing the transcription of a significant number of genes and the synthesis of certain functional proteins in cells, including dsRNA‐dependent protein kinase R (PKR), those of the 2′–5′ oligoadenylate synthetase system (2–5OAS), and Mx protein, among others (Thyrell et al. 2004). Other proteins have functions related to regulation of cell biological activity, for example, nitric oxide synthase (NOS), which can improve the production of NO (Chesler et al. 2004).
Recent studies also suggest that IFN‐α is capable of inducing expression of many more potent genes in cells (Caraglia et al. 2005). With use of differential display‐PCR (DD‐PCR) and DNA chip technology, many novel cDNA genes specifically related to IFN‐α stimulation have been isolated from cells and examined for biological function (Jurecic & Belmont 2000; Kettunen et al. 2004). Undoubtedly, investigation of such novel genes induced in cellular responses to IFN‐α or other IFNs will be useful for detailed study of the molecular mechanisms of IFN‐α. In the present study, a novel gene has been isolated from human embryo fibroblasts treated with IFN‐α, and has been found to have the same encoding region as a cDNA gene isolated from a human full‐length cDNA bank (Yokoyama‐Kobayashi et al. 1999), which encodes a putative type II membrane protein. This protein, which includes a C‐type lectin domain, appears to belong to the C‐type lectin superfamily and probably functions as a receptor interacting with a still‐unknown ligand. A typical representative of this superfamily is CD69, an early activating marker in mature T cells. Although little is known concerning its ligand or mechanism of signal transduction, this protein in the T‐cell membrane is probably involved in controlling thymocyte export and the trafficking of T cells (Weis et al. 1998). In this case, the proteins of the C‐type lectin superfamily might be receptor proteins affecting cellular biological properties. Based on a structural comparison of this protein and CD69, it was named ‘type II membrane protein similar to CD69’ (TIIMPSC) (Yokoyama‐Kobayashi et al. 1999). The gene encoding it was cloned from human embryo fibroblasts treated with IFN‐α, and was examined for transcription related to IFN‐α stimulation. Further, investigation of the protein coded for by this gene was performed to determine its location, biological effects and signal transduction. The results of this study suggest that expression of this protein, which is up‐regulated by IFN‐α stimulation, is involved in the control of fibroblast proliferation through the JAK‐STAT signalling pathway.
MATERIALS AND METHODS
Cells
Human embryo fibroblast KMB‐17 strain cells were used, originated from a primary clone established by the Institute of Medical Biology in 1970; they were maintained in Dulbecco's modified Eagle's medium (DMEM)−5% fetal calf serum (FCS) with 1% penicillin–streptomycin and 2 mm glutamine at 37 °C in 5% CO2.
Differential display PCR
mRNA was harvested from 5 × 106 KMB‐17 cells treated with IFN‐α (Sigma, St Louis, MO, USA) at a concentration of 100 µ/ml for 6 h at 37 °C by the QuickPrep micro mRNA purification kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The mRNA of control cells was treated by the same procedure, but without IFN‐α treatment. Approximately 0.4 µg mRNA re‐suspended in 12 µl of sterile water was mixed with 2.5 mm oligo(dT) primer and incubated at 70 °C for 10 min. After snap cooling on ice, this reaction was continued at 37 °C with 10 mm dithiothreitol (Sigma), 20 µm dNTP (Sigma) and 200 units of superscript RNaseH−ve reverse transcriptase for 90 min. PCR was performed in a 25‐µl reaction system containing 1 µl cDNA, 1.2 mm MgCl2, 0.05% W‐1 detergent, 2 µm oligo(dT) primer and 0.5 µm random 10mer primers. Cycling parameters were 94 °C for 30 s, then 40 cycles of 94 °C for 30 s, 40 °C for 2 min, and 72 °C for 30 s followed by final extension at 72 °C for 5 min. The 5‐µl labelled PCR product was run on a 6% PAGE‐Urea gel. After drying, the gel was exposed to X‐film.
TIIMPCS gene cloning and expression
Based on the results of DD‐PCR and blasting of the TIIMPSC gene, two primers were synthesized: (1) AGTGGATCCATGATGACCAAACATAA and (2) AGTGGATCCTTAGTGTATTCTTTTCC, for cloning of the TIIMPSC encoding gene. cDNA was produced with primer 2 and mRNA, as described above, in a reverse transcription system. The complete gene fragment was amplified by PCR reaction including primer 1, primer 2, dNTP and Taq DNA polymerase, using a standard protocol. Cycling parameters were 94 °C for 50 s, then 35 cycles of 94 °C for 30 s, 54 °C for 40 s, and 72 °C for 50 s followed by extension of 72 °C for 5 min. The purified gene fragment was digested with BamHI and was then cloned into pcDNA3.0 (Novagen; EMD Bioscience, Inc., San Diego, CA, USA) and pET29a (Novagen) for further experiments. After confirmation by sequencing, the recombined pET‐TIIMPSC was induced with 1 mm IPTG to express TIIMPSC. After purification, as described in the standard protocol, the expressed protein was used to produce a specific antibody in rabbits.
Structure analysis of TIIMPSC molecule
By submitting the protein sequence of TIIMPSC to Swiss‐Model Server (http://swissmodel.expasy.org), the 3‐D structure was generated using modelling templates in the Protein Data Bank (PDB) database (http://www.pdb.org). The C‐lectin (accession no. cd0037.2) domain was modelled in the same server and was set as the structure control.
Northern blot analysis
The probe for the TIIMPSC gene for northern blotting was synthesized in a PCR reaction containing 50 nm primer 1, 50 nm primer 3 (5′‐AGTTTAACTATG‐3′), 2.5 mm dNTP, 0.2 µCi 32P‐α‐dATP, 2 units Taq DNA polymerase and 1 nm TIIMPSC gene template as described above. A 5‐µg aliquot of total RNA harvested from KMB‐17 cells treated with IFN‐α or control as described above, was subjected to electrophoresis in a denaturing 1.2% agarose, 6% formaldehyde gel and was transferred to a nylon membrane (NEN; New England Nuclear of PE Life Science, Inc., Wellesley, MA, USA). The filter was screened with the probe as described above, washed at 1 × SSC (150 mm NaCl and 15 mm sodium citrate) – 0.1% sodium dodecyl sulfate (SDS) at 60 °C for 1 h, and exposed to X‐film.
Transfection of KMB‐17 cells
The constructed pcDNA‐TIIMPSC was transfected into KMB‐17 cells not treated by IFN‐α, with lipofectin (Bio‐Rad Laboratories, Hercules, CA, USA) according to a standard protocol (Welsh et al. 1990). The cells were maintained in DMEM serum‐free medium for 2 h and were washed with the same medium twice before transfection. After transfection, the cells were incubated at 37 °C and 5% CO2 for 15 h, and maintained in DMEM−5% FCS replacement medium for further experiments.
Flow cytometry and cell proliferation assay
KMB‐17 cells treated with specific antiserum against TIIMPSC, or control serum, were collected with a rubber policeman at 12, 24 and 36 h post‐transfection. They were washed in phosphate‐buffered saline (PBS), fixed in 70% ethanol at 4 °C for 30 min, and analysed by flow cytometry. Each experiment was repeated three times. The same cells were grown in DMEM−5% FCS and collected at 24, 48 and 72 h by stimulation with the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) method according to the described protocol (Plumb 2004). The cells for each detection were collected from three wells, and results were statistically analysed.
Immunofluorescence examination
Transfected and control KMB‐17 cells were grown on glass cover‐slips for 24 h after transfection, then were fixed with 4% paraformaldehyde in PBS at 4 °C for 1 h, washed with PBS three times and continuously incubated in 1 : 300 dilution of fluorescent isothiocyanate‐conjugated goat anti‐rabbit antibody IgG (Sigma) for further analysis by fluorescence microscopy.
Immunoprecipitation
Cells were grown in methionine‐free minimal essential medium (MEM) or phosphate‐free MEM for 1 h and were then stimulated with specific antiserum to TIIMPSC, or control serum, for 30 min at 37 °C. Then, the same media with 35S‐methioine or 32P‐phosphate were used to replace the previous media. Cells in this labelled media were incubated at 37 °C for 1 h. The harvested cells were broken by detergent in buffer and were interacted with the antibody in RIPA‐containing buffer (150 mM NaCl, 1% Nonidet P‐40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris‐HCl, pH 8.0). Immune complexes were precipitated by protein A–sepharose buffer washing. Finally, the labelled protein, which was separated from the immune complexes by boiling in the presence of detergent, was subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and auto‐exposed on X‐film.
Western blot
The transfected cells were lysed at 4 °C in 1% Nonidet P‐40 (Sigma), 150 mm NaCl, 10 mm EDTA, 10 mm Tris‐HCl (pH 8.0), 1 mm phenylmethyl(sulphonyl) fluoride and 5 mm iodoacetamide. Lysates were cleared by centrifugation at 13 000 g for 10 min. Samples were boiled for 3 min in 2.5% SDS sample buffer and were separated on a 12% SDS–PAGE gel. They were then transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech), which was treated with blocking solution containing 2% bovine serum albumin, 0.5% triton 20, and 10 mm Tris‐HCl pH 7.4. After washing with TE (pH 7.4) containing 0.5% triton 20 twice, the membrane was incubated with specific antibody at room temperature for 1 h and then washed three times. Then, the membrane was interacted with coupled protein kinase B (AKT) mouse antibody to rabbit IgG for 1 h at room temperature. Finally, nitro‐blue tetrazolium (NBT) (Sigma) was used as a reactive substance to visualize target bands.
Statistical analysis
The confidence interval of mean of the data collected in each experiment was performed according to the standard protocol (Steinijans & Diletti 1983). Results were expressed as mean ± SD. Each experiment was repeated at least three times. Statistical significance between each group was analysed using a two‐way statistical analysis of variance (anova) and student t‐test using spss 11.0 statistical software (SAS Institute, Cary, NC, USA). Values of P ≤ 0.05 were considered to be significant.
RESULTS
Transcription of the TIIMPSC gene is specifically enhanced by IFN‐α stimulation
Studies of the mechanisms of IFNs have indicated that IFN‐α can induce transcription of some genes, which probably mediate the establishment of an antiviral state and interfere with proliferation of cells (Gil et al. 2001). Using various procedures, products of many genes have been found to have antiviral activity. In our mRNA differential display of gene responses induced by IFN‐α, TIIMPSC, a member of the C‐type lectin superfamily, was found to exhibit enhanced transcription in human fibroblasts stimulated with IFN‐α (Fig. 1a). In the work reported by Yokoyama‐Kobayashi et al. (1999), this cDNA gene was isolated from a human full‐length cDNA bank (Fig. 1b) (GenBank accession no. AB015628), but its function is unclear. This result thus provides clues to investigating the protein's molecular biological function. In northern blots of cells treated with IFN‐α and control cells, enhanced transcription was observed in fibroblasts stimulated by IFN‐α, compared with control cells (Fig. 1c), indicating that transcription of TIIMPSC in human fibroblasts is probably related to IFN‐α stimulation.
Figure 1.

Transcription of the TIIMPSC gene is specifically enhanced by IFN‐α stimulation. (a) mRNA differential display of gene responses induced by IFN‐α. The mRNA was harvested from 5×106 KMB‐17 cells untreated/treated with 100 µ/ml IFN‐α over time. Lane 1, negative control, untreated cells; lanes 2, 3 and 4, mRNA extracted from KMB‐17 cells treated with IFN‐α for 2, 4 and 6 h, separately. Arrowed differential band indicates the TIIMPSC transcript. (b) Nucleotide sequence and deduced amino acid sequence of TIIMPSC. (c) Northern blotting of TIIMPSC expression in human embryo fibroblasts stimulated with IFN‐α. KMB‐17 cells mock‐treated or treated with IFN‐α, and total mRNA isolated for northern blotting. The TIIMPSC transcript was detected using a specific probe (a fragment of 77 nt, nucleotides 1‐77 of TIIMPSC). β‐Actin mRNA was detected as a control with a 491‐nucleotide probe. Lane 1, KMB‐17 cells control; lane 2, KMB‐17 cells treated with IFN‐α for 6 hours.
Location of TIIMPSC in human fibroblasts
Findings have suggested that most members of the C‐type lectin superfamily are receptor proteins. In addition, analysis of TIIMPSC structure and its comparison with other members of the C‐type lectin superfamily have indicated that TIIMPSC has a C‐type lectin‐like domain and a membrane‐crossing region (Fig. 2) (Drickamer 1999). Results of our immunofluorescence detection with specific antibody to TIIMPSC has suggested that this protein is located on the surface of fibroblasts transfected with pcDNA‐TIIMPSC (Fig. 3). This finding strongly suggests that TIIMPSC is a receptor protein in human fibroblasts.
Figure 2.
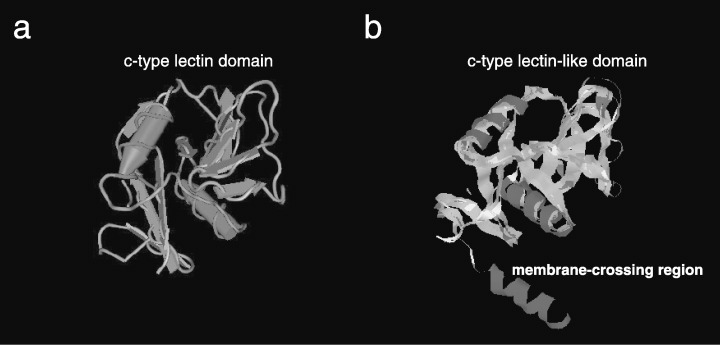
Modelling of the TIIMPSC structure and comparison with the conserved C‐type lectin domain. The amino acid sequence of TIIMPSC and classic C‐type lectin domain are submitted and generated to 3‐D structure in Swiss‐Model Server (http://swissmodel.expasy.org). (a) Modelling of a classic C‐type lectin domain (accession no. cd00037.2). (b) Modelling of TIIMPSC. A C‐type lectin‐like domain and 44 residues at the N‐terminus folding as a membrane‐crossing region.
Figure 3.
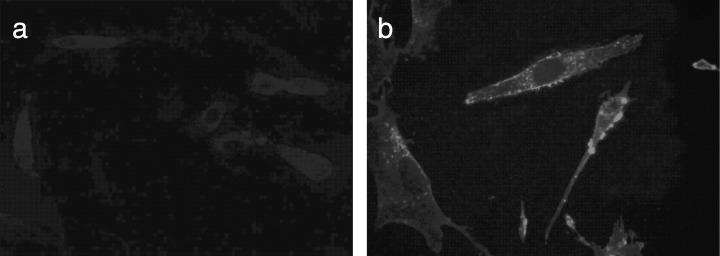
Subcellular localization of TIIMPSC in human embryo fibroblasts. Subcellular localization of TIIMPSC was examined in KMB‐17 cells transfected with pcDNA‐TIIMPSC, in the presence of control vector. Immunofluorescence detection was observed in KMB‐17 cells, with specific antibody to TIIMPSC, at 24 h after transfection (magnification, ×400). (a) KMB‐17 cells transfected with pcDNA3.0. No TIIMPSC expression was detected. (b) KMB‐17 cells transfected with pcDNA‐TIIMPSC. The TIIMPSC protein is located on the surface of fibroblasts.
Antibody binding to TIIMPSC elevates the level of cell proliferation
As a putative receptor located in the cell membrane, TIIMPSC should have a related physiological ligand, which is unknown at present. However, the specific interaction of TIIMPSC and its antibody is capable of mimicking, to some extent, the interaction of TIIMPSC and its ligand – activating TIIMPSC as a receptor. In this case, the antibody raised with TIIMPSC protein expressed in Escherichia coli was used to investigate TIIMPSC biological activity in cells. Results indicated that the presence of specific antibody to TIIMPSC caused growth in the cell population size of human embryo fibroblasts treated with IFN‐α compared with control cell populations (Fig. 4a). Cell cycle analysis of these fibroblasts has suggested that binding of antibody to TIIMPSC increases the ratio of S‐phase cells compared with control levels (Fig. 4b). These findings indicate that activation of TIIMPSC increases the level of fibroblast proliferation.
Figure 4.
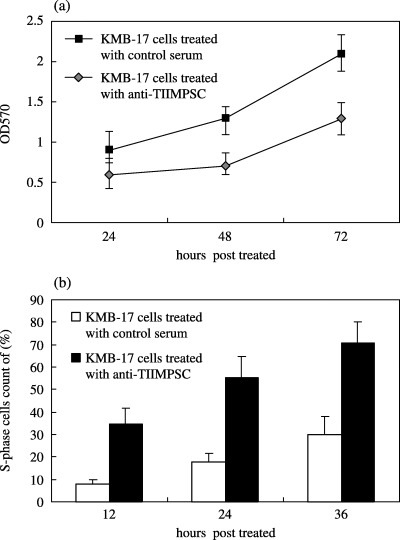
Cell proliferation elevated by activation of TIIMPSC in IFN‐α treated or TIIMPSC‐transfected human embryo fibroblasts. (a) Cell proliferation analysis with the MTT assay in IFN‐α‐treated KMB‐17 cells. KMB‐17 cells pre‐stimulated with IFN‐α (100 µ/ml) were treated with specific antiserum to TIIMPSC, or with control serum, and were collected at 24, 48 and 72 h. Each experiment was performed in triplicate and results were analysed statistically. (b) Flow cytometric analysis of increase in the proportion of S‐phase cells after TIIMPSC activation in pcDNA‐TIIMPSC‐transfected KMB‐17 cells. The KMB‐17 cells treated with IFN‐α (100 µ/ml) followed by specific antiserum to TIIMPSC or control serum were collected at 12, 24 and 36 h post‐transfection. S‐phase cell sorting analysis was performed, based on DNA content. The mean percentage and standard deviation of triplicate samples were determined.
Signal transduction by TIIMPSC is mediated via the JAK‐STAT pathway
To identify the signalling pathway activated by binding of TIIMPSC by a specific antibody, several signal molecules were screened for their phosphorylated forms with specific antibody after cells had been treated with IFN‐α and interacted with the specific antibody to TIIMPSC. Interestingly, the results suggested that the signal molecule phosphorylated as a result of antibody triggering of TIIMPSC is in fact STAT‐1α (Fig. 5a). Further co‐immunoprecipitation with the antibody against phosphorylated tyrosine and the antibody against STAT‐1α confirmed that the phosphorylated STAT‐1α was precipitated by the antibodies to tyrosine and to STAT‐1α. (Fig. 5b). Additionally, while STAT‐1α was activated after stimulation of TIIMPSC + its antibody, immunoprecipitation of Erk also suggested that this target molecule of STAT‐1α was phosphorylated in relation to cell proliferation (Fig. 5c).
Figure 5.
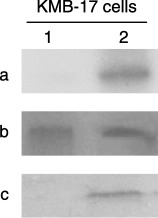
Immune precipitation of STAT‐1α involved in the JAK‐STAT pathway in KMB‐17 cells treated with IFN‐α and bound by specific antibody to TIIMPSC. (a) Immunoprecipitation of STAT‐1α in KMB‐17 cells stimulated by TIIMPSC antiserum. IFN‐α‐treated KMB‐17 cells were interacted with TIIMPSC antiserum in 32P‐phosphate labelling media, and were lysed in RIPA. The extract of cells was immunoprecipitated with STAT‐1α antibody. The conjugated complex was then absorbed to protein A–Sepharose 4B beads. After washing with RIPA, the samples were eluted in sample buffer and were run on a 12% SDS–PAGE gel. Lane 1, extract of negative cells control, which were not treated with TIIMPSC antiserum, immunoprecipitated with STAT‐1α antibody; lane 2, extract of cells treated with TIIMPSC antiserum, immunoprecipitated by STAT‐1α antibody. (b) Identification of the STAT‐1α phosphorylated form in cells stimulated with TIIMPSC antiserum. IFN‐α‐treated KMB‐17 cells interacted with TIIMPSC antiserum in 32P‐phosphate labelling media and lysed in RIPA. This extract of cells was immunoprecipitated with STAT‐1α antibody and phosporylated tyrosine antibody. The conjugated complex was then absorbed to protein A–Sepharose 4B beads. After washing with RIPA, the samples were eluted in sample buffer and run on a 12% SDS–PAGE gel. Lane 1, extract of cells treated by TIIMPSC antiserum immunoprecipitated with STAT‐1α antibody; lane 2, extract of cells treated with TIIMPSC antiserum immunoprecipitated with phosporylated tyrosine antibody. (c) Immunoprecipitation of Erk in KMB‐17 cells stimulated with TIIMPSC antiserum. IFN‐α‐treated KMB‐17 cells were interacted with TIIMPSC antiserum in 32P‐phosphate labelling media and were lysed in RIPA. The extract of cells was immunoprecipitated with Erk antibody. The conjugated complex was then absorbed to protein A–Sepharose 4B beads. After washing with RIPA, the samples were eluted in sample buffer and run on a 12% SDS–PAGE gel. Lane 1, extract of negative cells control, which were not treated with TIIMPSC antiserum, immunoprecipitated with Erk antibody; lane 2, extract of cells treated with TIIMPSC antiserum, immunoprecipitated with Erk antibody.
DISCUSSION
With DD‐PCR analysis, a novel cDNA clone was isolated from human fibroblasts treated by IFN‐α. The gene was up‐regulated when cells were stimulated with IFN‐α and it was identified as the cellular response to IFN‐α (Fig. 1). The protein encoded by the gene is a type II membrane protein, TIIMPSC, and is similar to CD69. Structural analysis indicated that TIIMPSC is a novel member of the C‐type lectin superfamily, which includes a group of proteins containing a C‐type carbohydrate recognition domain attached to further domains responsible for the physiological functions of molecules (Turville et al. 2003). As we know, the C‐type lectin family is comprised of two subtypes, one soluble C‐type lectin, the other a transmembrane C‐type lectin. The latter are usually cell membrane receptors, like CD69, which is an important functional receptor of T cells, involved in regulation of trafficking and export (Feng et al. 2002). Although its physiological ligand is yet unknown, TIIMPSC possesses a similar structure to members of the C‐type lectin superfamily, and has been observed on the surface of fibroblasts (2, 3), suggesting that it probably functions as a receptor. The observation of its transcription enhanced by IFN‐α stimulation suggests that TIIMPSC, as a trans‐membrane protein, has a specific biological role(s) related to alterations in cell function induced by IFN‐α. Intriguingly, this functional alteration of cells through TIIMPSC elevating levels of cell proliferation is in contrast to the inhibition of cell cycle progression caused by IFN‐α, which we have previously observed in the cellular response to IFN‐α. Moreover, that interaction of the antibody to TIIMPSC and fibroblasts treated with IFN‐α enhanced cell proliferation and increased the proportion of S‐phase cells, indicated that TIIMPSC is probably a receptor protein related to cell division. During stimulation of the TIIMPSC receptor, there was phosphorylation of STAT‐1α followed by activation of Erk. The findings here indicate that any extracellular message for cells to replicate, is transduced after TIIMPSC binding, via the STAT pathway. We cannot yet prove directly that the interaction between TIIMPSC and its specific antibody is comparable with binding of TIIMPSC with its physiological ligand. However, it is reasonable to predict that antibody‐bound TIIMPSC is able to induce activation of intracellular signalling and is capable of setting it in motion. Theory of the structure of such proteins and their functions finds a precedent in the anti‐insulin receptor antibody. Once bound, the antibody/receptor compound is capable of mimicking insulin's own activation of ribosomal protein S6 kinase. Also, the monoclonal antibody to the gp130 component of the interleukin‐6 type cytokine stimulates cell proliferation in hepatocellular carcinoma cells (Sung et al. 1989; Muller‐Newen et al. 2000). Results observed (4, 5) in this work confirmed the theory.
An important cytokine, IFN‐α is capable of inducing the expression of various genes to establish an antiviral state, in some types of cell (Jablonowski 2003). These genes (and thus, their proteins) have antiviral functions and play roles in the regulation of cell population states, for example, cell cycle arrest (Hilkens et al. 2003). Through alternative modes of function, IFN‐α is able to inhibit virus replication in cells. Findings have suggested that IFN‐α is capable of slowing the rate of proliferation of target cells or of making them more susceptible to apoptosis (Tissari et al. 2005). This indicates that the antiviral effect of IFN‐α also is manifested through altering the cellular environment to inhibit virus replication, and this also raises the question of whether inhibition of cell proliferation could bring about the death of cells during viral infection; this death could be a means by which IFN‐α defends cells against viral infection. If this should be the case, the antiviral effect of IFN‐α would injure many uninfected cells in vivo by causing apoptosis. However, this conflicts with our experimental and clinical treatment observations using IFN‐α. Thus, it appears likely that a mechanism exists in which uninfected cells are protected against the antiviral activity of IFN‐α. The TIIMPSC molecule described in this paper probably plays a role in such a mechanism. Expression of the TIIMPSC molecule on the surface of fibroblasts probably is a part of the cells’ response to IFN‐α function. This could antagonize the apoptotic effect incurred by IFN‐α in uninfected cells, by increasing cell proliferation in them until loss of cells infected by the virus has been repealed. In this case, any imbalance in the cell population after virus infection would be addressed by IFN‐α, then any excess in cell number would induce apoptosis. Previous data provided by Caraglia et al. (2003), found increased activity of Ras and Raf‐1 in IFN‐α‐treated cells; in addition, a 50% increase of phosphorylated isoforms and activity of Erk‐1/2 in IFN‐α‐treated cells was observed in their work. This is also supportive to the above points. Certainly, more evidence for this prediction are needed from future experiments. However, all results described above support in part our hypothesis that the antiviral effects of IFN‐α include a compensatory mechanism enabling uninfected cells to recover proliferate activity.
ACKNOWLEDGEMENTS
We would like to thank Dr Paul Kretchmer (kretchmer@sfedit.net) at San Francisco Edit for his assistance in editing this manuscript. This work was supported by a grant from the National Science Funds of China (grant no. 30370065).
REFERENCES
- Caraglia M, Tagliaferri P, Marra M, Giuberti G, Budillon A, Gennaro ED, Pepe S, Vitale G, Improta S, Tassone P, Venuta S, Bianco AR, Abbruzzese A (2003) EGF activates an inducible survival response via the RAS → Erk‐1/2 pathway to counteract interferon‐α‐mediated apoptosis in epidermoid cancer cells. Cell Death Differ. 10, 218. [DOI] [PubMed] [Google Scholar]
- Caraglia M, Marra M, Pelaia G, Maselli R, Caputi M, Marsico SA, Abbruzzese A (2005) α‐Interferon and its effects on signal transduction pathways. J. Cell Physiol. 202, 323. [DOI] [PubMed] [Google Scholar]
- Chesler DA, McCutcheon JA, Reiss CS (2004) Post‐transcriptional regulation of neuronal nitric oxide synthase expression by IFN‐γ. J. Interferon Cytokine Res. 24, 141. [DOI] [PubMed] [Google Scholar]
- Drickamer K (1999) C‐type lectin‐like domains. Curr. Opin. Struct. Biol. 9, 585. [DOI] [PubMed] [Google Scholar]
- Feng C, Woodside KJ, Vance BA, El Khoury D, Canelles M, Lee J, Gress R, Fowlkes BJ, Shores EW, Love PE (2002) A potential role for CD69 in thymocyte emigration. Int. Immunol. 14, 535. [DOI] [PubMed] [Google Scholar]
- Gil MP, Bohn E, O'Guin AK, Ramana CV, Levine B, Stark GR, Virgin HW, Schreiber RD (2001) Biologic consequences of Stat1‐independent IFN signaling. Proc. Natl Acad. Sci. USA 98, 6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S, Didcock L, Randall RE (2000) Interferons: cell signalling, immune modulation, anti‐viral response and virus countermeasures. J. Gen. Virol. 81, 2341. [DOI] [PubMed] [Google Scholar]
- Hilkens CM, Schlaak JF, Kerr IM (2003) Differential responses to IFN‐α subtypes in human T cells and dendritic cells. J. Immunol. 171, 5255. [DOI] [PubMed] [Google Scholar]
- Ivashkiv LB (2003) Type I interferon modulation of cellular responses to cytokines and infectious pathogens: potential role in SLE pathogenesis. Autoimmunity 36, 473. [DOI] [PubMed] [Google Scholar]
- Jablonowski H (2003) IFN‐α: therapeutic options in HIV and HIV/H|CV, HIV/HBV dual infection. Infection 31, 131. [PubMed] [Google Scholar]
- Jurecic R, Belmont JW (2000) Long‐distance DD‐PCR and cDNA microarrays. Curr. Opin. Microbiol. 3, 316. [DOI] [PubMed] [Google Scholar]
- Kerr IM, Stark GR (1991) The control of interferon‐inducible gene expression. FEBS Lett. 285, 194. [DOI] [PubMed] [Google Scholar]
- Kettunen E, Vivo C, Gattacceca F, Knuutila S, Jaurand MC (2004) Gene expression profiles in human mesothelioma cell lines in response to interferon‐γ treatment. Cancer Genet. Cytogenet. 152, 42. [DOI] [PubMed] [Google Scholar]
- Malmgaard L (2004) Induction and regulation of IFNs during viral infections. J. Interferon Cytokine Res. 24, 439. [DOI] [PubMed] [Google Scholar]
- Muller‐Newen G, Kuster A, Wijdenes J, Schaper F, Heinrich PC (2000) Studies on the interleukin‐6‐type cytokine signal transducer gp130 reveal a novel mechanism of receptor activation by monoclonal antibodies. J. Biol. Chem. 275, 4579. [DOI] [PubMed] [Google Scholar]
- Plumb JA (2004) Cell sensitivity assays: the MTT assay. Meth. Mol. Med. 88, 165. [DOI] [PubMed] [Google Scholar]
- Steinijans VW, Diletti E (1983) Statistical analysis of bioavailability studies: parametric and non‐parametric confidence intervals. Eur. J. Clin. Pharmacol. 24, 127. [DOI] [PubMed] [Google Scholar]
- Sung CK, Maddux BA, Hawley DM, Goldfine ID (1989) Monoclonal antibodies mimic insulin activation of ribosomal protein S6 kinase without activation of insulin receptor tyrosine kinase. Studies in cells transfected with normal and mutant human insulin receptors. J. Biol. Chem. 264, 18951. [PubMed] [Google Scholar]
- Thyrell L, Hjortsberg L, Arulampalam V, Panaretakis T, Uhles S, Dagnell M, Zhivotovsky B, Leibiger I, Grander D, Pokrovskaja K (2004) Interferon α‐induced apoptosis in tumor cells is mediated through the phosphoinositide 3‐kinase/mammalian target of rapamycin signaling pathway. J. Biol. Chem. 279, 24152. [DOI] [PubMed] [Google Scholar]
- Tissari J, Siren J, Meri S, Julkunen I, Matikainen S (2005) IFN‐α enhances TLR3‐mediated anti‐viral cytokine expression in human endothelial and epithelial cells by up‐regulating TLR3 expression. J. Immunol. 174, 4289. [DOI] [PubMed] [Google Scholar]
- Turville S, Wilkinson J, Cameron P, Dable J, Cunningham AL (2003) The role of dendritic cell C‐type lectin receptors in HIV pathogenesis. J. Leukoc. Biol. 74, 710. [DOI] [PubMed] [Google Scholar]
- Weis WI, Taylor ME, Drickamer K (1998) The C‐type lectin superfamily in the immune system. Immunol. Rev. 163, 19. [DOI] [PubMed] [Google Scholar]
- Welsh N, Oberg C, Hellerstrom C, Welsh M (1990) Liposome mediated in vitro transfection of pancreatic islet cells. Biomed. Biochim. Acta 49, 1157. [PubMed] [Google Scholar]
- Yokoyama‐Kobayashi M, Yamaguchi T, Sekine S, Kato S (1999) Selection of cDNAs encoding putative type II membrane proteins on the cell surface from a human full‐length cDNA bank. Gene 228, 161. [DOI] [PubMed] [Google Scholar]


