Abstract
Objectives: Preparations rich in growth factors (PRGF) release them plus bioactive proteins at localized sites, with the aim of triggering healing and regenerative processes. The prevailing paradigm suggests that their influence on proliferation, angiogenesis and the extracellular matrix synthesis is minimal. However, variations in their composition and impact on different cell phenotypes have not been examined.
Materials and methods: Sixteen fibroblast cultures obtained from three different anatomical sites (skin, synovium and tendon) of 16 donors were exposed to the molecular pool released from PRGF scaffolds, with increasing amounts of platelets. We evaluated cell proliferation, secretion of angiogenic growth factors (VEGF and HGF), synthesis of type I collagen and hyaluronic acid (HA), considering platelet dose and anatomical origin of the cells. Activity of transforming growth factor‐beta (TGF‐β) in type I procollagen and HA synthesis was examined by adding exogenous TGF‐β to plasma preparations.
Results: All plasma preparations induced a significant proliferative response compared to non‐stimulated cells (P < 0.05). Maximum proliferation rate was obtained with PRGF with 2‐fold or 4‐fold platelet concentration. Exposure to PRGF stimulated VEGF synthesis exclusively in tendon cells (P < 0.05), which also exhibited a different pattern of HGF production (P < 0.05). PRGF enhanced HA synthesis (P < 0.05), but did not alter collagen I production. Platelet‐secreted TGF‐β may be involved in HA, but not in type I procollagen synthesis.
Conclusions: Optimizing composition and use of platelet‐rich products is crucial to enhancing the therapeutic potential of this technology. Our data show that the biological effects of PRGF may depend on concentration of platelets and on the anatomical source of the cells.
Introduction
Tissue repair is a complex process, determined by the nature of the tissues themselves and the vast number of molecules involved therein (1, 2). Developing new biological technologies to improve healing not only involves delivering the correct combination of growth factors but also targeting the appropriate cells. Fibroblasts are common cells in connective tissues that contribute to the maintenance of structural integrity. Their dynamic roles in physiological and pathological processes are also extremely important, initiating the earliest molecular events leading to tissue repair (3).
It is now accepted that platelets have a major role in inflammatory and healing responses (4, 5, 6). During normal tissue repair in vivo, platelets release high concentrations of biologically active proteins, such as growth factors and other substances (7). In doing so they are able to influence a range of processes promoting recruitment, growth and morphogenesis of cells. Based on this knowledge, a novel technology that aims to replace the initial haematoma (containing a bulk of red blood cells and a small proportion of platelets and leucocytes) with a preparation rich in growth factors (PRGF) has emerged. This approach provides supra‐physiological concentrations of growth factors at the injury environment and can be used therapeutically to accelerate natural healing (8, 9).
Developing therapeutic autologous formulations that control the dose of growth factors and their local release into injured tissue is critical to achieving a successful outcome (10). By regulating the processing technique and centrifugation parameters (among other variables), it is possible to control the concentration of platelets and therefore, the dose of platelet‐derived growth factors. More importantly, controlling dose of platelet‐derived growth factors will also allow for regulation of the growth factor ratio, as platelet‐rich preparations contain a mixture of bioactive agents derived from both platelets and plasma. For example, insulin‐like growth factor 1 (IGF‐1) which enhances fibroblast proliferation, is principally found in plasma. Other factors, such as hepatocyte growth factor (HGF), fibronectin, tissue inhibitors of metalloproteinases, matrix metalloproteinases and hyaluronic acid (HA), are also present in plasma. Among the platelet‐released factors are platelet‐derived growth factors (PDGF AB or C), transforming growth factor (TGF‐β1), platelet factor‐4, vascular endothelial growth factor (VEGF), endostatins and thrombospondin‐1 (6, 9).
Fibroblasts express numerous surface receptors and can simultaneously sense multiple molecules that trigger behavioural responses (11). Because the function of fibroblasts is critical during repair, shaping their activities with the correct proportion of growth factors might positively influence the outcome of injured tissue (12). To address this challenge, it is necessary to understand how platelet density and therefore growth factor concentration, may influence cell activities. Additionally, we also hypothesized whether fibroblasts from different anatomical sources may exhibit different activities in response to plasma exposure. Hence, 16 primary fibroblast cultures obtained from three different anatomical sites (skin, synovium and tendon) in 16 donors have been propagated in vitro and exposed to the molecular pool released from fibrin matrices with increasing amounts of platelets. By regulating processing of peripheral blood, it is possible to control platelet number, thereby allowing various autologous formulations. To this end, we prepared various plasmas with differing levels of platelet‐secreted molecules in a fixed volume of platelet‐poor plasma. These mixtures contrast both in platelet secretome concentration (more than 300 proteins) and in molecular ratio between the secretome and bioactive agents derived from plasma. The purpose of this study is to examine the biological effect of these preparations and their clinical relevance in regulating tissue repair. Hence, we have evaluated several parameters relevant to tissue repair, including cell proliferation, secretion of angiogenic growth factors (VEGF and HGF), and synthesis of primary molecules of the extracellular matrix (type I collagen and HA).
Materials and methods
Cell isolation and characterization
Fibroblasts were isolated from biopsies of skin, synovium or tendon removed during joint surgery from consenting patients, and approval from our Institutional Review Board. Cells were isolated following our standard protocol with minor modification (13). Briefly, tissue fragments collected in phosphate‐buffered saline supplemented with antibiotics were minced and treated with 0.3% collagenase II (Gibco Life Technologies, Gaithersburg, MD, USA) at 37 °C for 90 min with gentle stirring. The resulting cell suspension was filtered and centrifuged at 460 g for 10 min. Cells were seeded into culture flasks and maintained with Dulbecco's modified Eagle's medium (DMEM)/F12 (1 : 1 volume) (Gibco) culture medium supplemented with 15% human serum (PAA Laboratories GmbH, Haidmannweg, Pasching, Austria), 2 mm glutamine (Sigma, St. Louis, MO, USA), 50 µg/ml gentamicin and 2.5 µg/ml amphotericin B (Sigma) in a humidified atmosphere at 37 °C with 5% CO2. When the cells reached confluence, they were detached with animal origin‐free trypsin‐like enzyme (TrypLE Select, Gibco). Cell viability was assessed by trypan blue dye exclusion. The fibroblast‐like morphology of cells isolated from the three anatomical localizations was checked by phase‐contrast microscopy. Cells were characterized by immunofluorescence using two monoclonal antibodies directed against prolyl 4‐hydroxylase (Dako Cytomation, Glostrup, Denmark) and CD90 antigen (BD Biosciences Pharmingen, San Diego, CA, USA). Briefly, 9500 cells/well were plated on 24‐well plates (Nunc) with poly‐l‐lysine‐coated glass coverslips (BD BioCoat). Cells were fixed in 4% buffered paraformaldehyde, permeabilized with Triton X‐100 (only for prolyl 4‐hydroxylase), blocked with foetal bovine serum (10% in phosphate‐buffered saline), and stained for 1 h with the primary antibodies. Next, cells were incubated with the secondary antibody, goat anti‐mouse immunoglobulin G conjugated with Alexa Fluor 488 fluorochrome (Molecular Probes, Eugene, OR, USA). Finally, preparations were nuclear stained with Hoechst 33342, mounted, and examined under a fluorescence microscope (Leica DM IRB, Leica Microsystems, Wetzlar, Germany).
Plasma preparations rich in growth factors
Blood from two healthy young male donors was collected into 9‐ml tubes with 3.8% (wt/v) sodium citrate. Samples were centrifuged at 4500 g for 12 min at 4 °C to separate platelet‐poor plasma. Samples were centrifuged at 460 g for 8 min at room temperature to separate PRGF2x, and care was taken to avoid the buffy coat. To further concentrate platelets and prepare PRGF4x, PRGF2x was re‐centrifuged at 4000 g for 12 min at room temperature, and platelets were resuspended in a sufficient volume of platelet‐poor plasma. Platelet counts were performed with a haematological analyser Micros 60 from Horiba ABX (Montpellier, France), not only with peripheral blood but also with platelet‐poor plasma, PRGF2x and PRGF4x before clotting. Platelet‐poor and PRGF fibrin matrices were formed by adding calcium chloride at a final concentration of 22.8 mm to samples in glass tubes and incubating at 37 °C. Matrices were allowed to retract for 1 h and the released supernatants were collected by aspiration. Three different preparations were used to supplement the culture medium: (i) supernatant released from a platelet‐poor fibrin matrix; (ii) supernatant released from PRGF fibrin containing a platelet concentration of 200% of the venous blood count (PRGF2x); or (iii) supernatant released from PRGF fibrin containing a platelet concentration of 400% of the venous blood count (PRGF4x). Growth factors (TGF‐β1, PDGF‐AB, VEGF, EGF, HGF and IGF‐1) were measured in the supernatants using commercially available Quantikine colorimetric sandwich enzyme‐linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA).
Subconfluent cultures of cells were detached and cell viability was assessed by trypan blue dye exclusion. Passage 3–4 cells were plated at a density of 20 000 cells per cm2 in 48‐well tissue‐culture plates and maintained with serum‐free medium for 24 h. Then, culture medium was replaced by serum‐free medium supplemented with either: (i) 20% (v/v) platelet‐poor supernatant, 16 ± 1 × 106 platelets/ml; (ii) 20% (v/v) PRGF2x supernatant, 404 ± 39 × 106 platelets/ml; or (iii) 20% (v/v) PRGF4x supernatant, 767 ± 95 × 106 platelets/ml. The study period was 72 h. Wells with these three supplements were maintained in the same conditions and used for background correction. Additional cultures were maintained for 72 h in serum‐free DMEM/F12 supplemented with 0.1% human serum to examine constitutive secretion (non‐stimulated cells). All experiments were run in parallel. Cell proliferation was evaluated using the WST‐1 (tetrazolium salt,(4‐[3,4‐iodophenyl)‐2‐(4‐nitrophenyl)‐2H‐5‐tetrazolio]‐1,3‐benzenedisulphonate) colourimetric assay (Roche, Basel, Switzerland). Absorbance at 450/620 nm was directly proportional to the number of living cells in the culture. As an index of cell number, calibration curves ranging from 10 000 to 90 000 cells per well were established for skin, tendon and synovial fibroblasts using the WST‐1 cell counting kit.
Secretion of extracellular matrix components
Culture medium was collected on day 3 of treatment, centrifuged for 5 min at 2000 g, and stored at –80 °C until assayed. HA concentration was determined by an enzyme‐linked binding protein assay (Corgenix Inc., Broomfield, CO, USA). Human procollagen type I C‐peptide was measured in the media conditioned by fibroblasts after 3 days of culture using an in vitro solid phase enzyme immunoassay kit, according to the manufacturer's instructions (TaKaRa, Shiga, Japan). A substudy was scheduled to assay activity of TGF‐β in the supernatants released from platelet‐poor and PRGF fibrin matrices. For this purpose, additional cultures (a total of six independent cultures) from different donors and appropriate anatomical sources (two from skin, two from synovium and two from tendon), were analysed for effects of TGF‐β1 in plasma supernatants; these cultures were identical to those described above except that platelet‐poor supernatants and PRGF2x were supplemented with TGF‐β (40 ng/ml; R&D Systems), and PRGF2x was incubated at 37 °C with TGF neutralizing antibody (4000× anti‐human TGF‐β1, R&D Systems) for 1 h. Therefore, when adding exogenous TGF‐β, we matched exactly the levels found in PRGF2x and PRGF4x, respectively. All samples were assayed in duplicate and results are expressed as ng or µg/106 cells.
Production of VEGF and HGF
Concentrations of VEGF and HGF were also measured in the culture media conditioned by tendon, synovial or skin fibroblasts using ELISA kits (R&D Systems). The results were normalized for cell number and expressed as ng/106 cells.
Statistical analysis
Results are expressed as mean ± standard deviation. The Levene test was applied to check homogeneity of variances, then, one‐way analysis of variance was used to assess the biological effects of plasma preparations, considering platelet dose and anatomical origin of cells as factors. In order to identify differences between various treatments and anatomical origin of fibroblasts, post‐hoc analysis was carried out using the Fisher least significant difference test. Statistical differences between groups were accepted for P‐values lower than 0.05 (Statgraphics Plus, Manugistic, MS, USA).
Results
Cultured fibroblasts from the diverse sites showed similar morphology, appearing as elongated, spindle‐shaped cells. Immunofluorescence microscopy confirmed that fibroblast cultures were uniformly positive for prolyl 4‐hydroxylase and CD90 (Fig. 1), but negative for markers of haematopoietic, endothelial, epithelial and smooth muscle cells (data not shown).
Figure 1.
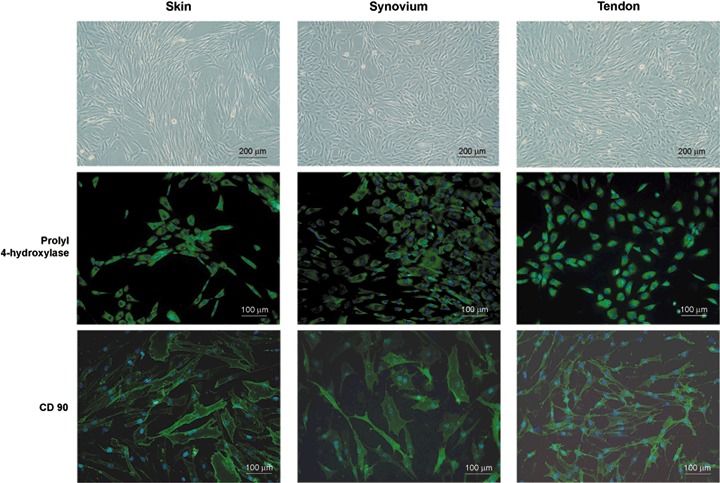
Fibroblasts isolated from diverse anatomical sites (skin, synovium and tendon). Representative phase contrast photomicrographs show the typical shape of diverse fibroblasts cultured on a plastic surface, and immunofluorescence microscopy confirmed that the fibroblastics were uniformly positive for prolyl 4‐hydroxylase and CD90, as revealed by immunostaining. Blue, Hoechst; green, prolyl 4‐hydroxylase and CD90.
Table 1 shows platelet count in each preparation and concentration of relevant growth factors.
Table 1.
Platelet and leucocyte count and concentrations of a range of growth factors in three different plasma preparations
| Plasma preparation | Leucocyte count (× 106/ml) | Platelet count (× 106/ml) | Growth factor concentration | ||||
|---|---|---|---|---|---|---|---|
| TGF‐β1 (ng/ml) | PDGF‐AB (ng/ml) | IGF‐1 (ng/ml) | VEGF (pg/ml) | HGF (pg/ml) | |||
| PPP | 0.0 | 16 ± 1 | 3.37 ± 0.45 | 1.31 ± 0.06 | 94 ± 22 | 13.9 ± 4.6 | 347 ± 64 |
| PRGF2x | < 0.2 | 404 ± 39 | 36.5 ± 3.3 | 17.6 ± 3.8 | 97 ± 32 | 142 ± 17 | 324 ± 44 |
| PRGF4x | < 0.2 | 767 ± 95 | 70.2 ± 13.9 | 37.2 ± 7.4 | 96 ± 29 | 214 ± 2 | 300 ± 24 |
PPP, platelet‐poor preparation; PRGF2x, preparation‐rich in growth factors (enriched in platelets 2‐fold over peripheral blood); PRGF4x, preparation rich in growth factors (enriched in platelets 4‐fold over peripheral blood). Peripheral blood contained (180 ± 5) × 103 platelets/µl. Concentrations are expressed as mean ± standard deviation (n = 2 donors).
Cell proliferation
Effects of different plasma preparations on fibroblasts are shown in Fig. 2. After 72 h, cells of every plasma preparation (including platelet‐poor plasma) showed statistically significant proliferative response compared to non‐stimulated cells (P < 0.05); maximum proliferation was obtained with plasma containing elevated platelet concentration (PRGF2x and PRGF4x). The increases in tendon cell proliferation induced by PRGF4x (767 ± 95 × 106 platelets/ml) and PRGF2x (404 ± 39 × 106 platelets/ml) were similar, while synovial cells showed a dose‐dependent response. On the other hand, dermal fibroblasts proliferated similarly with each plasma preparation (platelet‐poor, PRGF2x or PRGF4x).
Figure 2.
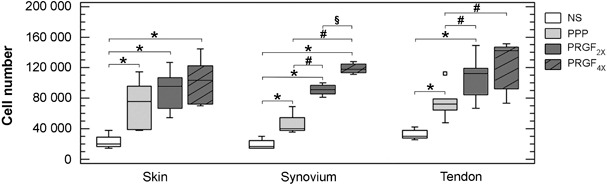
Effect of plasma preparations (platelet poor, PRGF2x and PRGF4x) on proliferation of fibroblasts from the skin, synovium and tendon. Cells were seeded at a density of 20 000 cells/cm2, and treated for 72 h with 20% of the supernatants released from platelet‐poor (PPP, light grey) and preparation rich in growth factors (PRGF2x, dark grey; PRGF4x, hatched bars) matrices. Box plot representation based on the median (line across the box) and 25th and 75th percentiles. Data summarize combined values obtained for different cell donors (skin, n = 6; synovium, n = 4; and tendon, n = 6). *P < 0.05 comparing with non‐stimulated cells; # P < 0.05 comparing with platelet‐poor preparation; §P < 0.05 comparing with PRGF2x.
Production of angiogenic factors
Angiogenic activity was assessed by measuring the production of two angiogenic factors (VEGF and HGF) that are important regulators of endothelial cell proliferation and migration. As shown in Fig. 3a, all fibroblasts showed constitutive secretion of VEGF. After 72 h of PRGF2x or PRGF4x treatment, VEGF production by tendon cells was significantly higher (P < 0.05). In contrast, VEGF levels were not affected by plasma preparations for either synovial or dermal fibroblasts. Of note, the angiogenic response to plasma preparations depended on the anatomical source of cells (P < 0.001). Avascular tendon fibroblasts responded with higher intensity than synovium or skin fibroblasts to pro‐angiogenic signals contained in plasma preparations (P < 0.05).
Figure 3.
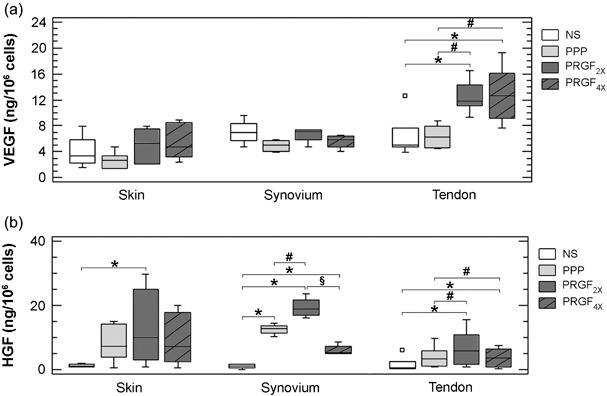
Effect of plasma preparations (platelet poor, PRGF2x and PRGF4x) on the secretion of angiogenic factors (VEGF and HGF) from skin, synovial and tendon fibroblast. Box plot representation based on the median (line across the box) and 25th and 75th percentiles. Grey boxes represent the group of experiments performed with the different plasma preparations: platelet‐poor (PPP, light grey) and preparation rich in growth factors (PRGF2x, dark grey; or PRGF4x, hatched bars). * P < 0.05 compared to non‐stimulated cells (NS); # P < 0.05 compared to platelet‐poor preparation; § P < 0.05 compared to PRGF2x.
As shown in Fig. 3b, HGF levels were up‐regulated following exposure to PRGF2x in every fibroblast phenotype (P < 0.05, compared to non‐stimulated cells); however, exposure to PRGF4x did not further increase HGF synthesis and there were regional differences in HGF synthesis after treatment. Once more, tendon cells responded differently from synovium or skin cells (P < 0.05). Of note, increase in HGF synthesis by tendon cells was observed following exposure to PRGF2x but not to PRGF4x.
Effect of plasma preparations on extracellular matrix
Type I procollagen levels were not greatly affected following exposure to different plasma preparations (Fig. 4a). This result was unexpected because TGF‐β1 is a potent inducer of collagen synthesis, and PRGF2x and PRGF4x contained high amounts of TGF‐β compared to platelet‐poor supernatants. To study TGF‐β activity, we added TGF‐β to platelet‐poor supernatants at concentrations that matched exactly the levels present in PRGF2x (40 ng/ml). This was designed as a strategy to examine the effect of TGF‐β1 in a similar milieu but without other proteins released from platelets. In addition, TGF‐β1 was added to PRGF2x at concentrations matching those in PRGF4x. As shown in Table 2 and confirming previous results, there was no difference between platelet‐poor‐, PRGF2x‐ and PRGF4x‐induced collagen synthesis. By contrast, an increase in collagen synthesis was observed in platelet‐poor supernatants and PRGF2x supplemented with exogenous TGF‐β1. Adding to the complexity is that blockade of platelet‐released TGF‐β1 induced only a slight decrease in procollagen (data not shown). All these data taken together point to the presence of modulatory molecules of platelet‐secreted TGF‐β1. Furthermore, all these data taken together confirm that TGF‐β1 is directly involved in collagen synthesis but at the same time is highly influenced by molecular complexity of the pool secreted by the growth factors and highlight the complexity of the molecular pool secreted from platelets.
Figure 4.
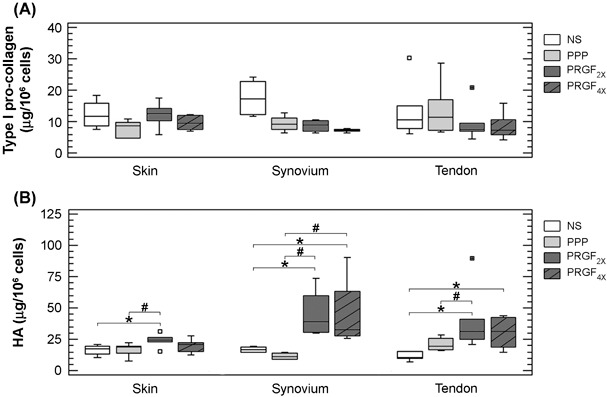
Effect of plasma preparations (platelet poor, PRGF2x and PRGF4x) on secretion of hyaluronic acid (HA) and type I procollagen by skin, synovial and tendon fibroblasts. Box plot representation based on the median (line across the box) and 25th and 75th percentiles. Grey boxes represent the group of experiments performed with the different plasma preparations: platelet‐poor (PPP, light grey) and preparation rich in growth factors (PRGF2x, dark grey; or PRGF4x, hatched bars) *P < 0.05 compared to non‐stimulated cells (NS); #P < 0.05 compared to platoelet‐poor preparation; § P < 0.05 compared to PRGF2x.
Table 2.
Effect of TGF‐β1 on collagen type I and hyaluronic acid secretion
| Baseline | PPP | PPP + TGF‐β1 | PRGF2x | PRGF2x + TGF‐β1 | PRGF4x | ||
|---|---|---|---|---|---|---|---|
| Skin | |||||||
| Procollagen I | µg/106 cells (SD) | 11.7 (4.0) | 24.1 (11.5) | 59.6 (0.3) | 16.1 (2.4) | 33.3 (15.3) | 11.2 (1.7) |
| Fold over baseline | 1.0× | 2.1× | 5.1× | 1.4× | 2.9× | 1.0× | |
| HA | µg/106 cells (SD) | 12.6 (3.9) | 10.3 (3.8) | 17.5 (3.8) | 18.8 (4.3) | 22.4 (13.6) | 17.2 (4.7) |
| Fold over baseline | 1.0× | 0.8× | 1.4× | 1.5× | 1.8× | 1.4× | |
| Synovium | |||||||
| Procollagen I | µg/106 cells (SD) | 7.5 (3.2) | 11.2 (0.9) | 19.3 (2.2) | 9.1 (3.3) | 15.9 (2.1) | 7.6 (1.5) |
| Fold over baseline | 1.0× | 1.5× | 2.6× | 1.2× | 2.1× | 1.0× | |
| HA | µg/106 cells (SD) | 16.8 (1.9) | 11.6 (2.4) | 38.1 (8.5) | 38.7 (10.7) | 51.4 (2.0) | 40.3 (2.4) |
| Fold over baseline | 1.0× | 0.7× | 2.3× | 2.3× | 3.1× | 2.4× | |
| Tendon | |||||||
| Procollagen I | µg/106 cells (SD) | 10.9 (6.1) | 16.4 (8.0) | 22.0 (1.7) | 20.4 (2.0) | 28.8 (7.1) | 13.2 (3.6) |
| Fold over baseline | 1.0× | 1.5× | 2.0× | 1.9× | 2.6× | 1.2× | |
| HA | µg/106 cells (SD) | 12.4 (2.3) | 9.5 (5.0) | 58.9 (12.1) | 37.3 (4.7) | 112.5 (10.1) | 42.6 (4.6) |
| Fold over baseline | 1.0× | 0.8× | 4.8× | 3.0× | 9.1× | 3.4× | |
Two primary cultures from each anatomical source were chosen randomly (n = 6). Cells were maintained simply in serum‐free media to examine constitutive secretion (baseline: non‐stimulated cells) or stimulated with 20% plasma preparations or 20% plasma preparations supplemented with TGF‐β1 (40 ng/ml). Concentrations are expressed as mean (SD) (n = 2 independent cultures). Relative secretion to non‐stimulated cells (basal) was expressed as fold over baseline. PPP, platelet‐poor preparation; PRGF2x, preparation‐rich in growth factors (enriched in platelets 2‐fold over peripheral blood); PRGF4x, preparation‐rich in growth factors (enriched in platelets 4‐fold over peripheral blood).
At 72 h, there were statistically significant increases in HA for PRGF2x and PRGF4x treatment in every type of fibroblast, independent of their anatomical location (P < 0.05) (Fig. 4b). Secretion of HA after exposure to plasma preparations depended on the anatomical source of the cells. Essentially, synovial and tendon cells secreted the highest concentration of HA in response to platelet‐rich treatment (P < 0.05 compared to skin cells).
Stimulation induced by PRGF but not platelet‐poor preparation, supports the participation of platelet‐secreted factors on HA synthesis. Interestingly, our results show that PRGF stimulatory action in HA synthesis may be attributed to platelet‐secreted TGF‐β1. Moreover, as shown in Table 2, the anatomical location may govern the magnitude of the cell response.
Discussion
Recent ideas and developments in regenerative medicine have led to the concept of PRGF technology, which is based on using autologous biomaterials in multiple configurations for regenerative purposes in different medical conditions (10, 14, 15). The term ‘PRGF’ identifies 100% autologous and biocompatible products obtained using centrifugation, and sodium citrate and calcium chloride as anticoagulant and activator, respectively. The latter is preferred over thrombin because it enables a more sustained and physiological release of platelet constituents; moreover since the preparation is 100% autologous, it can be easily translated in clinics. Additionally, leucocyte content has been eliminated from PRGF with the aim of avoiding pro‐inflammatory effects of proteases and acid hydrolases contained in white blood cells.
PRGF has a moderate platelet concentration which has been associated with optimal biological benefits. However, in‐depth knowledge is critical to establishing the number of platelets required for optimal healing responses for each tissue condition. Cell experiments may provide information on the biological effects of different platelet doses on specific cells. In the present study, we prepared plasma with varying densities of platelets in the absence of leucocytes; in this way, supernatants released after plasma coagulation differ only in concentration of platelet‐secreted products, while supernatants released from platelet‐poor plasma provide a subtractive molecular pool for analysing, in a simple manner, the influence of platelet‐secreted molecules on different biological effects.
What primarily emerges is the point of view that fibroblasts can initiate tissue repair by proliferating. The data reported herein show that all preparations, including the supernatants of platelet‐poor plasma, have mitogenic effects on each and all fibroblasts. This may be due to cooperative action of several molecules activated and/or released after plasma coagulation (13, 16, 17). For example, IGF‐1, which stimulates cell replication, is equally present in all plasma supernatants since it is not synthesized in the megakaryocyte but in the liver (9). Megakaryocytes endocytose IGF‐1 and the modulating protein IGFBP‐3 for storage in alpha granules (18). However, from a quantitative viewpoint, IGF‐1 released from platelets is trivial compared to plasma IGF‐1 concentration (picograms vs. nanograms). Thus, IGF‐1 is almost constant in all preparations (14). Still, when plasma contains platelets, IGF‐1 does not work alone but in cooperation with platelet‐secreted growth factors such as PDGF (19). The latter is a powerful stimulator of cell proliferation and its concentration correlates with number of platelets present in the preparation. Higher number of platelets (400–800 × 106 platelets/ml) further enhanced mitogenic potential of the preparations. These observations are likely to be of great interest for both clinicians and scientists; certainly the downstream applicability of the findings is evident since enhancing fibroblast expansion is relevant to preparation of tissue‐engineered products used for a variety of clinical applications in dermatology, ulcer care and/or reconstructive surgery (20, 21).
Presence of rapidly proliferating fibroblasts is thought to stimulate healing as they remain metabolically active and produce appreciable amounts of extracellular matrix molecules, such as type I collagen and HA. Unexpectedly, both platelet‐poor and platelet‐rich supernatants induced similar collagen synthesis despite differences in TGF‐β1 concentration. One may speculate that platelets release other proteins involved in signalling pathways that interfere with SMAD proteins. SMAD proteins serve as major conduits for transmission of TGF‐β signals from receptors to the nucleus, and are focal points for cross‐talk with a long and expanding list of other signal mediators and transcription factors (22). Molecular mechanisms behind these cellular responses form the next level of understanding and are the focus of our ongoing work.
The finding that HA secretion is increased after exposure of cells to PRGF suggests changes in tissue hydration, cell adhesion and motility (23, 24, 25). Moreover, that PRGF raised HA secretion by synovial cells might be important in formulating autologous treatment for joint pathology (25).
Our results did not show a platelet dose–response effect. Seemingly, in any plasma supernatant‐treated cells, the final effect might not depend only on concentration of platelet‐secreted molecules but on the ratio between platelet secretome and plasma proteins. Differences in secretome concentrations and secretome : plasma ratios could change the mechanism of action of the preparation, thereby precluding a linear response.
On the other hand, tissue repair is highly dependent on new vessel formation, which provide conduits for nutrients to rapidly proliferating fibroblasts. Thus, both events – cell proliferation and neovascularization – should be closely interconnected in normal physiology. One important feature of platelet‐rich preparations is its angiogenic capacity, controlled by an ambivalent partnership between pro‐angiogenic (TGF‐β1, VEGF, HGF, angiopoietin‐1 and CD40L) and anti‐angiogenic factors (thrombospondin‐1, β‐thromboglobulin, platelet factor‐4 and endostatins) (6). Our previous data have suggested that cellular conversations that take place between endothelia and underlying stromal fibroblasts are influenced by platelet‐released proteins and could be crucial to defining angiogenic status of the healing tissue (26, 27). Local fibroblasts may intensify or not, the angiogenic capacity of platelet‐secreted molecules by synthesizing further amounts of VEGF which exert trophic effects on endothelial cells through a cognate receptor kinase family expressed by endothelial cells to stimulate blood vessel formation. VEGF can also be pro‐inflammatory and stimulate adhesion of leucocytes to endothelial cells, a function suppressed by HGF acting through nuclear factor kappaB transcription factor (28). Once more, this shows interplay between newly synthesized factors.
Corroborating other findings (29), our data show that fibroblasts are diverse, exhibiting different patterns of biosynthetic activity in response to growth factors. Our results indicate that environmental milieu and anatomical location probably govern magnitude of the response. These findings may be also related to positional memory; tendon fibroblasts living in an avascular milieu might demand a stronger angiogenic response for successful repair. Tendon fibroblasts exhibited a distinct angiogenic response (in terms of VEGF and HGF synthesis) contrasting with synovial or skin fibroblasts. This topographic variation hints at richness and tissue specificity of fibroblasts’ response to platelet secretome. In tissue repair, higher requirements of avascular tissues for angiogenic growth factors may enhance the angiogenic response to injury and could be beneficial in overall tissue homeostasis. A major question in cell physiology is how these cells expanded in vitro‘know’ that they belong to an avascular tissue (30)? Another challenge is to identify major convergent points in the cell where interactions between growth factors induced signalling might occur (31). In doing so, it may be possible to ascertain transcriptional regulatory networks likely to control dynamics of receptors’ up‐ and down‐regulation (32, 33).
Accordingly, increased knowledge in molecular and cell biology has recognized fibroblasts as being diverse in their gene expression profiles (1). Furthermore, there is substantial diversity regarding their capacity for participating in disease processes after molecular activation. Such diversity is not unique to the fibroblast since vascular endothelial cells cultured from different anatomical sites also exhibit diverse transcriptional and proteomic profiles (34, 35).
HGF production did not show a secretome dose–response relationship and is almost inhibited at high platelet number. It is possible that by increasing platelet number, one may surpass threshold dose of some inhibitory molecules such as IL‐1β (25). Some authors have highlighted the ability of platelets to release IL‐1β into the extracellular milieu (36).
To obtain the most of platelet‐rich preparations, their formulations and use should be tailored to meet biological requirements of the specific tissue. Our study has shown the influence of defined platelet‐rich preparations on various biological effects evaluated in fibroblasts from diverse anatomical sites. In summary, these preparations enhanced proliferation and HA secretion in all studied fibroblasts, though angiogenic response depended on anatomical origin of the cells.
Acknowledgements
The authors wish to thank J. J. Aguirre for his statistical advice. The work of this group is partially funded by the Basque and Spanish governments.
References
- 1. Chang HY, Chi JT, Dudoit S, Bondre C, Van De Rijn M, Botstein D, Brown PO (2002) Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl Acad. Sci. USA 99, 12877–12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Werner S, Grose R. (2003) Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83, 853–870. [DOI] [PubMed] [Google Scholar]
- 3. Haniffa MA, Wang X‐N, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP (2007) Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J. Immunol. 179, 1595–1604. [DOI] [PubMed] [Google Scholar]
- 4. Von Hundelshausen P, Weber C (2007) Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ. Res. 100, 27–40. [DOI] [PubMed] [Google Scholar]
- 5. Weyrich AS, Prescott SM, Zimmerman GA (2002) Platelets, endothelial cells, inflammatory chemokines, and restenosis. Complex signalling in the vascular play book. Circulation 106, 1433–1435. [DOI] [PubMed] [Google Scholar]
- 6. Anitua E, Andía I, Ardanza B, Nurden P, Nudern AT (2004) Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 91, 4–15. [DOI] [PubMed] [Google Scholar]
- 7. Reed GL (2004) Platelet secretory mechanisms. Semin. Thromb. Hemost. 30, 441–450. [DOI] [PubMed] [Google Scholar]
- 8. Anitua E, Sánchez. M , Nurden AT, Nurden P, Gorka O, Andia I (2006) New insights into and novel applications for platelet‐rich fibrin therapies. Trends Biotechnol. 24, 227–234. [DOI] [PubMed] [Google Scholar]
- 9. Nurden AT, Nurden P, Sanchez. M , Andia I, Anitua E (2007) Platelets and wound healing. Front Biosci. 13, 3532–3548. [DOI] [PubMed] [Google Scholar]
- 10. Anitua E, Sanchez. M , Orive G, Andia I (2007) The potential impact of the preparations rich in growth factors (PRGF) in different medical fields. Biomaterials 28, 4551–4560. [DOI] [PubMed] [Google Scholar]
- 11. Gianchandani EP, Brautigan DL, Papin JA (2006) Systems analyses characterize integrated functions of biochemical networks. Trends Biochem. Sci. 31, 284–291. [DOI] [PubMed] [Google Scholar]
- 12. Wong T, McGrath JA, Navsaria H (2007) The role of fibroblasts in tissue engineering and regeneration. Br. J. Dermatol. 156, 1149–1155. [DOI] [PubMed] [Google Scholar]
- 13. Anitua E, Andia I, Sanchez. M , Azofra J, Del Mar. Zalduendo M, De La Fuente M, Nurden P, Nurden AT (2005) Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J. Orthop Res. 23, 281–286. [DOI] [PubMed] [Google Scholar]
- 14. Sanchez. M , Anitua E, Azofra J, Andia I, Padilla S, Mujika I (2007) Comparison of surgically repaired Achilles tendon tears using platelet‐rich fibrin matrices. Am. J. Sports Med. 35, 245–251. [DOI] [PubMed] [Google Scholar]
- 15. Anitua E, Sanchez. M , Orive G, Andia I (2008) Delivering growth factors for therapeutics. Trends Pharmacol. Sci. 29, 37–41. [DOI] [PubMed] [Google Scholar]
- 16. Han J, Meng HX, Tang JM, Chen ZB (2007) The effect of different platelet‐rich plasma concentrations on proliferation and differentiation of human periodontal ligament cells in vitro . Cell Prolif. 40, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M (2006) The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin. Oral Implants Res. 17, 212–219. [DOI] [PubMed] [Google Scholar]
- 18. Chan K, Spencer EM (1998) Megakaryocytes endocytose insulin‐like growth factor (IGF) I and IGF‐binding protein‐3: a novel mechanism directing them into α granules of platelets. Endocrinology 139, 559–565. [DOI] [PubMed] [Google Scholar]
- 19. Narine K, De Wever O, Van Vanckelborgh D, Francois K, Bracke M, Desmet S, Mareel M, Van Nooten G (2006) Growth factor modulation of fibroblast proliferation, differentiation, and invasion: implications for tissue valve engineering. Tissue Eng. 12, 2707–2715. [DOI] [PubMed] [Google Scholar]
- 20. Aiba‐Kojima E, Tsuno NH, Inoue K, Matsumoto D, Shigeura T, Sato T, Suga H, Kato H, Nagase T, Gonda K, Koshima I, Takahashi K, Yoshimura K (2007) Characterization of wound drainage fluids as a source of soluble factors associated with wound healing: comparison with platelet‐rich plasma and potential use in cell culture. Wound Repair Regen. 15, 511–520. [DOI] [PubMed] [Google Scholar]
- 21. Anitua E, Aguirre JJ, Algorta J, Ayerdi E, Cabezas AI, Orive G, Andia I (2008) Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J. Biomed. Mater. Res. B Appl. Biomater. 84, 415–421. [DOI] [PubMed] [Google Scholar]
- 22. Shi Y, Massague J (2003) Mechanisms of TGF‐β signalling from cell membrane to the nucleus. Cell 113, 685–700. [DOI] [PubMed] [Google Scholar]
- 23. Cheung WF, Cruz. T , Turley EA (1999) Receptor for hyaluronan‐mediated motility (RHAMM), a hyaladherin that regulates cell responses to growth factors. Biochem. Soc. Trans. 27, 135–142. [DOI] [PubMed] [Google Scholar]
- 24. Macri L, Silverstein D, Clark RA (2007) Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv. Drug Deliv. Rev. 59, 1366–1381. [DOI] [PubMed] [Google Scholar]
- 25. Anitua E, Sanchez. M , Nurden AT, Zalduendo MM, De La Fuente M, Azofra J, Andía I (2007) Platelet‐released growth factors enhance the secretion of hyaluronic acid and induce hepatocyte growth factor production by synovial fibroblasts from arthritic patients. Rheumatology (Oxford). 46, 1769–1772. [DOI] [PubMed] [Google Scholar]
- 26. Anitua E, Sanchez. M , Nurden AT, Zalduendo M, De La Fuente M, Orive G, Azofra J, Andía I (2006) Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J. Biomed. Mater. Res. A 77, 285–293. [DOI] [PubMed] [Google Scholar]
- 27. Anitua E, Sanchez. M , Nurden AT, Zalduendo M, De La Fuente M, Azofra J, Andia I (2007) Reciprocal actions of platelet‐secreted TGF‐β1 on the production of VEGF and HGF by human tendon cells. Plast. Reconstr. Surg. 119, 950–959. [DOI] [PubMed] [Google Scholar]
- 28. Min JK, Lee YM, Kim JH, Kim YM, Kim SW, Lee SY, Gho YS, Oh GT, Kwon YG (2005) Hepatocyte growth factor suppresses vascular endothelial growth factor‐induced expression of endothelial ICAM‐1 and VCAM‐1 by inhibiting the nuclear factor‐β B pathway. Circ. Res. 96, 300–307. [DOI] [PubMed] [Google Scholar]
- 29. Smith TJ (2005) Insights into the role of fibroblasts in human autoimmune diseases. Clin. Exp. Immunol. 141, 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY (2006) Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2, 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ball SG, Shuttleworth CA, Kietly CM (2007) Vascular endothelial growth factor can signal through platelet‐derived growth factors receptors. J. Cell Biol. 177, 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Danielpour D, Song K (2006) Cross‐talk between IGF‐I and TGF‐β signaling pathways. Cytokine Growth Factor Rev. 17, 59–74. [DOI] [PubMed] [Google Scholar]
- 33. Novosyadlyy R., Dudas J, Pannem R., Ramadori G, Scharf J‐G (2006) Crosstalk between PDGF and IGF‐I receptors in rat liver myofibroblasts: implication for liver fibrogenesis. Lab. Invest. 86, 710–723. [DOI] [PubMed] [Google Scholar]
- 34. Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, Van De Rijn M, Botstein T, Brown PQ (2003) Endothelial cell diversity revealed by global expression profiling. Proc. Natl. Acad. Sci. USA 100, 10623–10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Durr E, Yu J, Krasinska KM, Carver LA, Yates JR, Testa JE, Oh P, Schnitzer JE (2004) Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat. Biotechnol. 22, 985–992. [DOI] [PubMed] [Google Scholar]
- 36. Lindeman S, Tolley ND, Dixon DA, McIntire TM, Prescott SM, Zimmerman GA, Weyrich AS (2001) Activated platelets mediate inflammatory signaling by regulated interleukin 1b synthesis. J. Cell Biol. 154, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]


