Abstract
Oridonin, an active diterpenoid isolated from traditional Chinese herbal medicine, has drawn rising attention for its remarkable apoptosis‐ and autophagy‐inducing activity and relevant molecular mechanisms in cancer therapy. Apoptosis is a well known type of cell death, whereas autophagy can play either pro‐survival or pro‐death roles in cancer cells. Accumulating evidence has recently revealed relationships between apoptosis and autophagy induced by oridonin; however, molecular mechanisms behind them remain to be discovered. In this review, we focus on highlighting updated research on oridonin‐induced cell death signalling pathways implicated in apoptosis and autophagy, in many types of cancer. In addition, we further discuss cross‐talk between apoptosis and autophagy induced by oridonin, in cancer. Taken together, these findings open new perspectives for further exploring oridonin as a potential anti‐tumour agent targeting apoptosis and autophagy, in future anti‐cancer therapeutics.
Introduction
Oridonin, a diterpenoid isolated from Rabdosia rubescens, has been widely used in cancer therapy owing to its remarkable anti‐tumour activities in many types of human cancer 1, 2. In normal conditions, DNA can identify genetic defects during replication and induce cell‐cycle arrest to repair these errors, or if fail to do so, trigger programmed cell death to remove the damaged and deleterious cells. However, almost all tumour cells are able to evade signals that result in programmed cell death (PCD) 3, 4. PCD can be considered as referring to apoptosis, autophagy and programmed necrosis (death of a cell by any process mediated by an intracellular program). These three forms of PCD may decide the fate of cancer cells; apoptosis and programmed necrosis invariably end in cancer cell death, whereas autophagy plays a two‐faced role and can determine life or death of the cell 5. Apoptosis is featured by some morphological characteristics, including shrinkage of cytoplasm and nucleus, membrane blebbing and formation of discrete apoptotic bodies 6. Usually, but not exclusively, it is associated with activation of cysteine‐dependent specific aspartate proteases, termed caspases. Both extrinsic and intrinsic apoptotic pathways finally converge to a common process, the caspase cascade 7.
Autophagy is different from apoptosis. It is a lysosomal‐dependent pathway, a crucial self‐catabolic process for degradation and recycling of cell components 8. Hereafter, macroautophagy is referred to as autophagy – featured by presence of autophagosomes. These are double‐membraned vesicles that sequester cytoplasm or superfluous organelles; then they fuse with lysosomes followed by digestion of their cellular constituents 9, 10. Activation of autophagy is a multistep signalling cascade under strict control of evolutionarily conserved autophagy‐related genes. Autophagy is well known to be crucial for cell survival under extreme conditions, where degradation of intracellular macromolecules provides the energy required for minimal cell functioning when nutrients are lacking or scarce 11, 12. Autophagy‐mediated elimination of altered cytosolic constituents, such as aggregated proteins or damaged organelles, preserves cells from further damage. Thus, activation of autophagy can play a protective role for cells in early stages of cancer progression 13. On the other hand, autophagy can play a death‐promoting role as type II PCD, compared to apoptosis 14.
Numerous reports have recently shown that oridonin possesses remarkable anti‐tumour activity both in vitro and in vivo. Effective inhibition of various cancer cells’ survival in vitro has been clarified in breast, prostate, non‐small cell lung cancer, glioblastoma multiforme 15, human fibrosarcoma (HT1080 cells), human epidermoid carcinoma (A431 cells) and murine aneuploid fibrosarcoma (L929 cells). Oridonin also been found to suppress proliferation of human colon carcinoma HT29 cells both in vitro and in vivo, and to prolonged lifespan of mice suffering from P388 lymphocytic leukaemia and Ehrlich ascites 16, 17, 18, 19, 20. Furthermore, oridonin has been shown to trigger apoptosis in acute myeloid leukaemia cells in vitro, and has exhibited significant anti‐leukaemia efficacy, with few side‐effects, in vivo. Apart from autophagy‐ and apoptosis‐inducing activities, oridonin has been shown to induce G2/M phase cell cycle arrest in L929 cells 21. With accumulating insights into molecular mechanisms of apoptosis and autophagy, emerging anti‐tumour small molecules such as oridonin have been drawing rising attention and could possibly be considered as potential new anti‐cancer agents.
Oridonin‐induced apoptosis pathways in cancer
Classical apoptosis pathways induced by oridonin in cancer
The death receptor pathway
Death receptors (DRs) are type I transmembrane proteins, belonging to the tumour necrosis factor receptor (TNFR) superfamily, characterized by their death domain (DD) component. After ligand binding at the cell surface [for example, by tumour necrosis factor‐α (TNF‐α) or Fas ligand (FasL)], recruitment of cytosolic adaptor protein, Fas‐associated death domain (FADD) and caspase‐8 initiate and lead to formation of a death‐inducing signalling complex (DISC). As a result, the formed DISC transmits the death signal in a direct way by triggering effector caspases‐3, ‐6 and ‐7 or indirectly via cleavage of Bid, leading to the mitochondria‐mediated intrinsic apoptotic pathway, on translocation to mitochondria 22. Oridonin has been shown to inhibit cancer cell expansion by blocking TNF‐α and lipopolysaccharide‐stimulated NF‐κB activity, in Jurkat cells as well as in RAW264.7 murine macrophages. Moreover, oridonin has been shown to suppress proliferation of adult T‐cell leukaemia cells, and those of acute lymphoblastic leukaemia, chronic lymphocytic leukaemia and non‐Hodgkin's lymphoma, by inhibition of NF‐κB DNA‐binding activity 23. It is widely believed that murine L929 fibrosarcoma cells are extremely sensitive to TNF‐α, and previous researchers have pointed out that caspases can exert a pro‐survival role in TNF‐α‐treated L929 cells 24.
Apoptosis has been shown to be triggered by both oridonin and TNF‐α intervention, this observation being supported by evidence indicating that apoptotic characteristics were altered, whereas DNA fragmentation was merely found in TNF‐α‐treated L929 cells. One interesting phenomenon here was that only oridonin‐induced L929 cell death was required for degradation of poly ADP‐ribose polymerase (PARP) in caspase‐3‐independent manner. In general, PARP is the target of caspase‐3, its normal function being to repair DNA damage and facilitate cell survival, it is usually cleaved to an 85‐kDa fragment in response to apoptotic signals. However, caspase‐3 was not involved in this apoptotic process, suggesting that additional proteases other than caspase‐3 might be responsible for degradation of PARP 24. Investigation of effects of oridonin on intracellular TNF‐α expression have demonstrated that oridonin augmented inhibitor of κB (IκB) phosphorylation [through which nuclear factor‐κB (NF‐κB) is released and activated], thus upregulating expression of TNF‐α 24. Other reports have shown that initiation of NF‐κB participates in generation of cytokines such as IL‐1 and TNF‐α, in oridonin‐treated cancer cells 25.
Oridonin has been reported to induce Fas/FasL‐mediated apoptotic cell death by activating ERK in human histocytic lymphoma U937 cells. The outcome of oridonin treatment was that Fas/FasL, FADD and ERK levels were increased. However, ERK activation was FADD‐independent, thus this initiation might be via a further adaptor, receptor‐interacting protein (RIP) 26. Yet another study has described that inhibition of EGFR signalling by tyrphostin AG1478 can enhance oridonin‐induced apoptosis in human laryngeal HEp‐2 cancer cells (Fig. 1) characterized by EGFR gene amplification.
Figure 1.
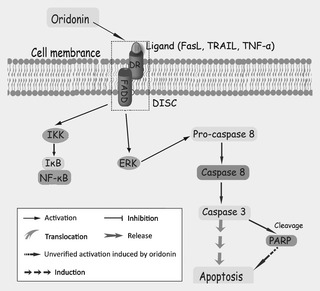
Mechanisms of oridonin‐induced death receptor‐mediated apoptosis.
The mitochondrial pathway
The mitochondrion, the organelle that chemically modifies adenosine triphosphate to supply a cells available energy, thus able to determine a cell's life or death, clearly plays a pivotal role in maintaining normal physiological function of eukaryotic cells 27, 28. Established evidence has revealed that mitochondria‐mediated apoptotic pathway, a most important form of this mode of cell death, is characterized by cytochrome c release from it into the cytosol; mitochondrial fragmentation as well as depolarization of mitochondrial membrane potential also occurs 29, 30, 31. This intrinsic pathway of apoptosis is under strict control of Bcl‐2 protein family members, which regulate irreversible mitochondrial outer membrane permeabilization of the pathway. Bcl‐2 family proteins are composed of anti‐apoptotic (for example, Bcl‐2 and Bcl‐XL) and pro‐apoptotic members (for example, Bax and Bid) and can activate a series of proteins such as death protease caspases, downstream 32, 33.
Oridonin has been reported to significantly inhibit cell expansion and to induce apoptosis in diverse cancer cell lines, through the mitochondria‐dependent apoptosis pathway in vitro, including in L929 cells, acute promyelocytic leukaemia NB4 cells and human hepatic carcinoma BEL‐7402 cells 34, 35, 36, 37, 38. Supportive of this notion, marked morphological changes including chromatin condensation, nuclear fragmentation, apoptotic body formation and DNA laddering indicative of apoptosis have been clearly observed following oridonin intervention 39, 40. Exposure to oridonin disrupted membrane potential of mitochondria, and altered the balance between pro‐ and anti‐apoptotic proteins in favour of apoptosis by up‐regulating Bax and Bid, as well as by downregulating Bcl‐2 and Bcl‐XL 36, 37, 38, 39. Pre‐treatment with oridonin promoted release of cytochrome c, AIF, and Smac which inhibited IAP (inhibitor of apoptosis protein) from mitochondrion, as well as facilitated cleavage of PARP in cancer cells 38, 39. Moreover, release of cytochrome c into the cytosol recruited Apaf–1 and pro‐caspase‐9 (composing the apoptosome), by activating the signalling cascade of caspases–9/3, leading to PARP cleavage, eventually triggering apoptosis 38, 39. In addition, oridonin selectively induced apoptosis of t(8;21) leukaemia cells, caused cleavage of AML1‐ETO oncoprotein, and interacted with glutathione and thioredoxin/thioredoxin reductase. This elevated intracellular reactive oxygen species, which in turn activated caspase‐3 in t(8;21) cells, suggesting oridonin to be a potential lead compound for molecular target‐based therapy of leukaemia 41 (Fig. 2).
Figure 2.
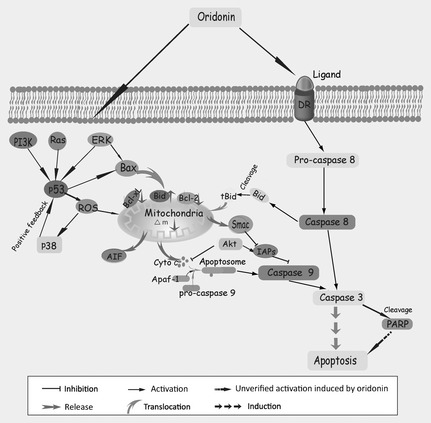
Mechanisms of oridonin‐induced mitochondria‐mediated apoptosis.
Other apoptosis pathways induced by oridonin in cancer
Mitogen‐activated protein kinase (MAPK) families are conserved enzymes in evolution, and play essential roles in transduction of extracellular stimuli 42, 43, 44. MAPKs are involved in diverse cell processes including cell proliferation, migration, and differentiation; deregulation of the MAPK family is indicated in aetiology of cancer 43, 44, 45. The MAPK pathway can be activated by triple cascade kinase reactions, and three distinct groups of MAPK have been identified in mammalian cells: ERK, JNK and p38 44, 45. ERK has dual regulatory functions, mediating apoptosis or preventing cells from initiating the apoptotic response, or inhibition of apoptosis performed by phosphorylation or promotion of expression of anti‐apoptotic Bcl‐2, depending on different conditions 45. JNK and p38 are preferentially initiated by stress stimuli, while the ERK pathway is apt to be induced by growth factor receptor binding such as by epidermal growth factor receptor (EGFR) and insulin‐like growth factor 1 receptor (IGFR) 44.
Oridonin has been shown to induce apoptosis by activation of pro‐apoptotic ERK1/2 signalling and inactivation of pro‐survival p38/JNK pathways, for example in human macrophage‐like U937 cells, murine fibrosarcoma L929 cells, and A375‐S2 cells 16, 22. Activation of ERK in A375‐S2 cells can serve as a bridge linking MAPK‐mediated pathways to the mitochondria‐related cascade, where ERK accounts for release of cytochrome c and balance of Bax/Bcl‐XL 16. Initiation of ERK also connects mitochondrial pathways with death receptor Fas/FasL‐mediated signalling pathways in U937 cells. And, ERK activation might be mediated by RIP, but not by FADD in response to Fas/FasL signalling 26. Elevation of pro‐apoptotic ERK and reduction in pro‐survival p38/JNK have been clarified in oridonin‐induced apoptosis of L929 cells also. Although this type of apoptotic cell death is quite different, DNA fragmentation (a main hallmark of apoptosis) was not found, and degradation of PARP was in a caspase‐independent manner 34. Recent reports have demonstrated that oridonin‐induced apoptosis of L929 cells was by increasing the ERK‐p53 apoptotic pathway and inhibiting the PTK‐mediated Ras‐Raf‐JNK survival pathway 22.
There have existed two discrete reports that verified oridonin treatment induced marked inactivation of ERK and activation of p38/JNK, in osteosarcoma cell lines and in HepG2 cells 46, 47. ROS generation was implicated in oridonin‐induced HepG2 cell apoptosis, of which, p53 regulated generation of ROS that was followed by triggering of p38. In addition, ROS reduced mitochondrial membrane potential and provoked cytochrome c release; this process associated the MAPK cascade with mitochondria‐mediated signalling pathways 48. Release of TNF‐α and interleukin‐1β (IL‐1β) under regulation of the MAPK cascade induced by oridonin, was also involved in the process of phagocytosis 49, 50, 51. After exposure of U937 cells to oridonin, levelsof ERK increased and led to phagocytosis. ERK positively regulated oridonin‐augmented phagocytosis, but p38 and JNK negatively regulated it 49. Additionally, further reports have demonstrated that the Ras/Raf/ERK pathway participates in mediation of phagocytosis and synthesis of IL‐1β, coupled with IκB‐α degradation induced by oridonin in U937 cells 50.
Besides regulation of phagocytosis, oridonin also fills a crucial role of regulating growth factor‐related apoptosis 52. Oridonin has been reported to induce apoptosis in A431 cells, possibly by inhibiting total tyrosine kinases and blocking phosphorylation of EGFR 53. Further reports have demonstrated that reduced expression of EGFR might be responsible for suppressing the Ras/Raf/ERK signalling cascade and finally triggered apoptosis in A431 cells 47. Insulin‐like growth factor 1 receptor (IGFR) signalling has been described as a potential survival pathway required for activation of Ras or p38, but not of PI3K or ERK, in oridonin‐treated A375‐S2 cells. When faced with higher concentrations of oridonin, A375‐S2 cells were not protected from apoptotic cell death by IGF‐1 and this was coupled with severe IGF‐1R damage. In this process, p53 was taken to be a pivotal transducer between pro‐apoptotic and pro‐survival signalling 54. A further growth factor, fibroblast growth factor‐2 (FGF‐2), also protected L929 cells from oridonin‐induced apoptosis, in which ERK was responsible for their rescue. Activated ERK decreases the ratio of Bcl‐2/Bax by mediating phosphorylation of Bcl‐2. Inhibition of Bcl‐2, presence of FGF‐2 and initiation of Ras/Raf/ERK signalling, were involved in protection against oridon‐induced apoptosis 55 (Fig. 3).
Figure 3.
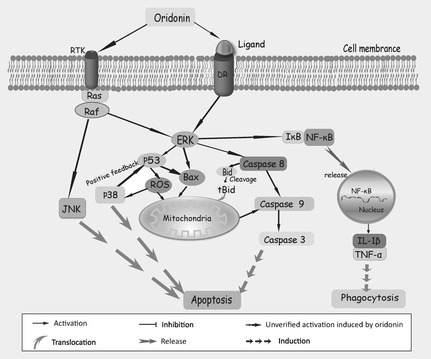
MAPK ‐modulated apoptosis in oridonin‐treated cancer cells.
Oridonin has been suggested to intensify phagocytosis of UV‐irradiated apoptotic U937 cells by regulating TNF‐α and IL‐1β secretion 56, 57. Further investigation demonstrated that this process relied on activating PI3K, PKC and ERK. PI3K promoted oridonin‐enhanced phaogocytosis by initiating parallel downstream targets, PKB/Akt and PLCγ2, the latter relocating to the intracellular membrane, thereby activating protein kinase C (PKC). Activation of PKC was independent of secretion of cytokines IL‐1β and TNF‐α, but ERK was under their control, thus regulating phagocytosis in oridonin‐treated U937cells 49. Remarkably, Akt, as a substrate of PI3K, exerts a potent anti‐apoptotic effect by upregulating pro‐survival factor IAP, as well as phosphorylating and inactivating glycogen synthase kinase 3 (GSK3), fork‐head transcription factors (FKHR), caspase‐9, Bad and several transcriptional factors such as NF‐κB 58, 59, 60, 61. Oridonin has been shown to inhibit proliferation and trigger caspase‐dependent apoptosis by downregulating the PI3K/Akt survival pathway, in the cervical carcinoma HeLa cell line and in osteosarcoma cells 46, 62. Consistent with previous reports, Akt, FOXO, GSK3 and IAP were constitutively reduced by oridonin treatment. Meanwhile, oridonin prompted release of cytochrome c accompanied by activation of caspase‐9, caspase‐3 and cleavage of PARP 49, 62. Inactivation of ERK and activation of p38 MAPK and JNK signalling pathways might contribute to oridonin‐triggered proliferation suppression and apoptosis in osteosarcoma cells 46. Additionally, oridonin can induce apoptosis and senescence in colorectal cancer cells by increasing histone hyperacetylation and regulation of p16, p21, p27 and c‐myc 63, and it can induce apoptosis by reducing c‐Myc protein levels in vitro and in vivo, this reduction being mediated by the ubiquitin‐proteasome system. Fbw7, a component of the ubiquitin‐proteasome system and an E3 ubiquitin ligase of c‐Myc, has been described to be upregulated rapidly in K562 cells and further leukaemia and lymphoma cells, resulting in rapid turnover of c‐Myc, indicating oridonin to be an Fbw7‐c‐Myc pathway targeting agent in cancer 64 (Fig. 4).
Figure 4.
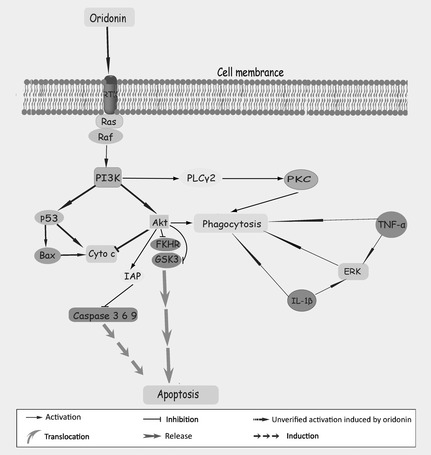
Further apoptosis‐related pathways in oridonin‐treated cancer cells.
Oridonin‐induced autophagy pathways in cancer
In addition to apoptosis‐inducing activities, more recent studies are emerging concentrating on autophagic cell death induced by oridonin. Oridonin has been reported to induce autophagy in A431 cells, in which autophagic activation is required for participation of Ras and class I PI3K. Ras negatively regulates this step by downregulation of Akt phosphorylation, which served as the target of PI3K 10. Interestingly, not autophagic hallmarks, but apoptotic features, such as collapse of mitochondrial membrane potential and upregulation of Bax/Bcl‐2 ratio, were observed that might be partially responsible for oridonin‐induced autophagy here 10. Oridonin has also been shown to trigger autophagy in human cervical carcinoma HeLa cells, supportive of the notion that inhibition of Ras, as well as promotion of p38 and JNK, is implicated in autophagic death initiated by oridonin. Although a different phenomenon has been detected compared to oridonin‐induced autophagy in A431cells, characteristics of autophagy, including conversion of LC3I to LC3II and Beclin1 increase, have been shown to correlate with activation of dose‐ and time‐dependent autophagic cell death in HeLa cells following oridonin 65. In addition to apoptosis and autophagy, programmed necrosis, type III PCD caused by oridonin, has been observed in several types of cancer cell, but predominant cell death mode in malignant cells was still apoptosis. Merely one piece of research has shown that oridonin could induce A431 cell death by affecting the balance between apoptosis and necrosis. After progressively increasing concentration of oridonin, number of apoptotic cells increased, while numbers of necrotic ones decreased 53.
Cross‐talk between oridonin‐induced apoptosis and autophagy
The relationship between apoptosis and autophagy is complex, suggesting that these two forms of PCD may be regulated by presence of a ‘molecular switch’ 66, 67. In some cases, autophagy can facilitate or regulate apoptotic cell death, but under certain conditions, autophagy can be initiated only when apoptosis is suppressed. Additionally, it has been described that these two forms of PCD share some common factors as well as overlap of identical biological functions 18. Apoptosis and autophagy caused by oridonin have been demonstrated in HeLa cells, in which autophagy might serve as a survival mechanism to protect cells from apoptotic death. Exploration of molecular mechanisms involved in this process found that PI3K/Akt signalling is augmented in autophagy, but is suppressed in apoptosis, whereas what accounted for the fate of the cell was the balance between Bax and Bcl‐2, determining whether the cells would proceed to apoptotic cell death or not 68. Recent research investigated the role of the PKC signalling pathway involved in oridonin‐induced cell death in HeLa cells, the results of which were consistent with previous results that autophagy protected cells against apoptosis. Involvement of PKC revealed the mechanistic features by which autophagy was facilitated, further giving rise to inhibition of apoptosis through regulation of Raf‐1 and JNK 69. In contrast though, autophagy induced by oridonin in MCF‐7 cells played a death‐prompting role, in which autophagy exerted a synergic effect on apoptosis, contemporarily contributing to cell death via the MAPK pathway 70. Exposure of L929 cells to oridonin induced both apoptosis and autophagy, in which reactive oxygen species (ROS) mediated apoptotic cell death by Bax translocation, cytochrome c release and ERK activation. This autophagic cell death represented by increasing of LC3‐II/LC3‐I ratio and Beclin 1 activation, may also function as a survival mechanism protecting cells from apoptosis through upregulation of the p38‐NF‐κB survival mechanism 71, 72. In contrast, a further study has demonstrated that the role of NF‐κB facilitated both apoptosis and autophagy induced by oridonin in human fibrosarcoma HT1080 cells by triggering activation of p53 18. Yet, previous reports have confirmed that there exists an interesting caspase‐independent apoptotic phenomenon induced by oridonin in L929 cells; the study proposed to trace the impact of calpain (calcium‐dependent cysteine protease) on apoptosis and autophagy in L929 cells pre‐treated with oridonin. Calpain suppressed apoptosis while autophagy was elevated in oridonin‐treated L929 cells; augmentation of apoptosis was thought to be ascribed to inhibition of autophagy 19. ROS after oridonin treatment can induce apoptosis and autophagy in HeLa cells 73 although autophagy enhanced phagocytosis of apoptotic human histocytic lymphoma U937 cells after oridonin treatment 74, 75. Briefly, this multiple cross‐talk between apoptosis and autophagy induced by oridonin is composed of intricate processes, and deciphering the molecular mechanisms may guide further study on the complexity of cancer treatment using oridonin (Fig. 5).
Figure 5.
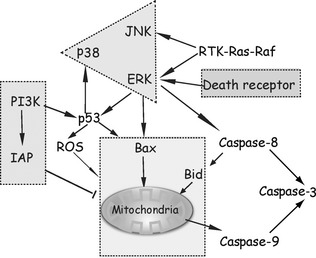
The intricate relationship in cells between oridonin‐caused apoptosis and autophagy.
Conclusions
Oridonin, an active diterpenoid isolated from traditional Chinese herbal medicine, has been drawing increased attention due to its versatile anti‐proliferation effects. Numerous studies have revealed that its significant apoptosis‐ and autophagy‐inducing activities are associated with anti‐tumour outcomes of oridonin treatment. Additionally, there is considerable cross‐talk between apoptosis and autophagy in oridonin‐treated cancer cells, and even some key signalling pathways implicated in them, overlap. Tremendous amounts of work have been carried out in deciphering the mechanistic features of oridonin in the anti‐cancer field, and exploration of these anti‐proliferative molecular mechanisms will advance their understanding for anti‐cancer therapy. Accumulating data concerning anti‐proliferative properties of oridonin may demonstrate that it will emerge as a valuable anti‐tumour agent.
Nevertheless, the challenge ahead of developing oridonin as an anti‐cancer drug is that some essential pathways and cell specificity remain ambiguous and many trials performed in vitro and in vivo are still preliminary. Further validation is urgently needed to introduce this compound into pre‐clinical and clinical applications. Oridonin bears remarkable anti‐neoplastic properties, which make it an ideal agent to be developed into a potent anti‐cancer drug. With the molecular mechanisms of oridonin‐induced anti‐proliferative activities in various types of cancer cells gradually being clarified, a new therapeutic strategy should be provided to utilize it as a new anti‐neoplastic drug, in the near future.
Acknowledgements
We thank Chun‐yang Li and Xu Zhao for reading this manuscript. This work was supported by the grants from the “Eleventh Five‐year Plan” military special fund (No. 08BJ01).
References
- 1. Zhou GB, Kang H, Wang L, Gao L, Liu P, Xie J et al (2007) Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1‐ETO fusion protein and shows potent antitumor activity with low adverse effects on t(8;21) leukemia in vitro and in vivo. Blood 109, 3441–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ikezoe T, Chen SS, Tong XJ, Heber D, Taguchi H, Koeffler HP (2003) Oridonin induces growth inhibition and apoptosis of a variety of human cancer cells. Int. J. Oncol. 23, 1187–1193. [PubMed] [Google Scholar]
- 3. Zhou GB, Chen SJ, Wang ZY, Chen Z (2007) Back to the future of oridonin: again, compound from medicinal herb shows potent antileukemia efficacies in vitro and in vivo. Cell Res. 17, 274–276. [DOI] [PubMed] [Google Scholar]
- 4. Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100, 57–70. [DOI] [PubMed] [Google Scholar]
- 5. Wen X, Lin ZQ, Liu B, Wei YQ (2012) Targeting caspase‐mediated programmed cell death pathways for cancer therapy. Cell Prolif. 45, 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu JJ, Lin M, Yu JY, Liu B, Bao JK (2011) Targeting apoptotic and autophagic pathways for cancer therapeutics. Cancer Lett. 300, 105–114. [DOI] [PubMed] [Google Scholar]
- 7. Chen M, Wang J (2002) Initiator caspases in apoptosis signaling pathways. Apoptosis 7, 313–319. [DOI] [PubMed] [Google Scholar]
- 8. Tsuchihara K, Fujii S, Esumi H (2009) Autophagy and cancer: dynamism of the metabolism of tumor cells and tissues. Cancer Lett. 278, 130–138. [DOI] [PubMed] [Google Scholar]
- 9. Li ZY, Yang Y, Ming M, Liu B (2011) Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem. Biophys. Res. Commun. 414, 5–8. [DOI] [PubMed] [Google Scholar]
- 10. Li CY, Wang EQ, Cheng Y, Bao JK (2011) Oridonin: an active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int. J. Biochem. Cell Biol. 43, 701–704. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B (2012) Targeting autophagic pathways by plant natural compounds in cancer. Cell Prolif. 45, 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fu LL, Wen X, Bao JK, Liu B (2012) MicroRNA‐modulated autophagic signaling networks in cancer. Int. J. Biochem. Cell Biol. 44, 733–736. [DOI] [PubMed] [Google Scholar]
- 13. Wang SY, Yu QJ, Zhang RD, Liu B (2011) Core signaling pathways of survival/death in autophagy‐related cancer networks. Int. J. Biochem. Cell Biol. 43, 1263–1266. [DOI] [PubMed] [Google Scholar]
- 14. Liu B, Cheng Y, Liu Q, Bao JK, Yang JM (2010) Autophagic pathways as new targets for cancer drug development. Acta Pharmacol. Sin. 31, 1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang CL, Wu LJ, Zuo HJ, Tashiro S, Onodera S, Ikejima T (2004) Cytochrome c release from oridonin‐treated apoptotic A375‐S2 cells is dependent on p53 and extracellular signal‐regulated kinase activation. J. Pharmacol. Sci. 96, 155–163. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Wu Y, Wu D, Tashiro S, Onodera S, Ikejima T (2009) NF‐kappab facilitates oridonin‐induced apoptosis and autophagy in HT1080 cells through a p53‐mediated pathway. Arch. Biochem. Biophys. 489, 25–33. [DOI] [PubMed] [Google Scholar]
- 17. Li D, Cui Q, Chen SG, Wu LJ, Tashiro S, Onodera S et al (2007) Inactivation of ras and changes of mitochondrial membrane potential contribute to oridonin‐induced autophagy in a431 cells. J. Pharmacol. Sci. 105, 22–33. [DOI] [PubMed] [Google Scholar]
- 18. Cheng Y, Qiu F, Huang J, Tashiro S, Onodera S, Ikejima T (2008) Apoptosis‐suppressing and autophagy‐promoting effects of calpain on oridonin‐induced L929 cell death. Arch. Biochem. Biophys. 475, 148–155. [DOI] [PubMed] [Google Scholar]
- 19. Zhu Y, Xie L, Chen G, Wang H, Zhang R (2007) Effects of oridonin on proliferation of HT29 human colon carcinoma cell lines both in vitro and in vivo in mice. Pharmazie 62, 439–444. [PubMed] [Google Scholar]
- 20. Node M, Sai M, Fuji K, Fujita E, Takeda S, Unemi N (1983) Antitumor activity of diterpenoids, trichorabdals A, B, and C, and the related compounds: synergism of two active sites. Chem. Pharm. Bull. (Tokyo) 31, 1433–1436. [DOI] [PubMed] [Google Scholar]
- 21. Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera S, Ikejima T (2009) Oridonin induces G2/M arrest and apoptosis via activating ERK‐p53 apoptotic pathway and inhibiting PTK‐Ras‐Raf‐JNK survival pathway in murine fibrosarcoma L929 cells. Arch. Biochem. Biophys. 490, 70–75. [DOI] [PubMed] [Google Scholar]
- 22. Kumar R, Herbert PE, Warrens AN (2005) An introduction to death receptors in apoptosis. Int. J. Surg. 3, 268–277. [DOI] [PubMed] [Google Scholar]
- 23. Ikezoe T, Yang Y, Bandobashi K, Saito T, Takemoto S, Machida H et al (2005) Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits the proliferation of cells from lymphoid malignancies in association with blockade of the NF‐kappa B signal pathways. Mol. Cancer Ther. 4, 578–586. [DOI] [PubMed] [Google Scholar]
- 24. Huang J, Wu L, Tashiro S, Onodera S, Ikejima T (2005) A comparison of the signal pathways between the TNF alpha‐ and oridonin‐induced murine L929 fibrosarcoma cell death. Acta Med. Okayama 59, 261–270. [DOI] [PubMed] [Google Scholar]
- 25. Xu Y, Xue Y, Wang Y, Feng D, Lin S, Xu L (2009) Multiple‐modulation effects of Oridonin on the production of proinflammatory cytokines and neurotrophic factors in LPS‐activated microglia. Int. Immunopharmacol. 9, 360–365. [DOI] [PubMed] [Google Scholar]
- 26. Liu YQ, Mu ZQ, You S, Tashiro S, Onodera S, Ikejima T (2006) Fas/FasL signaling allows extracellular‐signal regulated kinase to regulate cytochrome c release in oridonin‐induced apoptotic U937 cells. Biol. Pharm. Bull. 29, 1873–1879. [DOI] [PubMed] [Google Scholar]
- 27. Arismendi‐Morillo G (2009) Electron microscopy morphology of the mitochondrial network in human cancer. Int. J. Biochem. Cell Biol. 41, 2062–2068. [DOI] [PubMed] [Google Scholar]
- 28. Newmeyer DD, Ferguson‐Miller S (2003) Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112, 481–490. [DOI] [PubMed] [Google Scholar]
- 29. Van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P (2002) The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 9, 1031–1042. [DOI] [PubMed] [Google Scholar]
- 30. Oberst A, Bender C, Green DR (2008) Living with death: the evolution of the mitochondrial pathway of apoptosis in animals. Cell Death Differ. 15, 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Waterhouse NJ, Ricci JE, Green DR (2002) And all of a sudden it's over: mitochondrial outer‐membrane permeabilization in apoptosis. Biochimie 84, 113–121. [DOI] [PubMed] [Google Scholar]
- 32. Korsmeyer SJ (1999) BCL‐2 gene family and the regulation of programmed cell death. Cancer Res. 59, 1693s–1700s. [PubMed] [Google Scholar]
- 33. Tsujimoto Y, Shimizu S (2000) Bcl‐2 family: life‐or‐death switch. FEBS Lett. 466, 6–10. [DOI] [PubMed] [Google Scholar]
- 34. Zhang CL, Wu LJ, Tashiro S, Onodera S, Ikejima T (2004) Oridonin induces a caspase‐independent but mitochondria‐ and MAPK‐dependent cell death in the murine fibrosarcoma cell line L929. Biol. Pharm. Bull. 27, 1527–1531. [DOI] [PubMed] [Google Scholar]
- 35. Huang J, Wu L, Tashiro S, Onodera S, Ikejima T (2005) Bcl‐2 up‐regulation and P‐p53 down‐regulation account for the low sensitivity of murine L929 fibrosarcoma cells to oridonin‐induced apoptosis. Biol. Pharm. Bull. 28, 2068–2074. [DOI] [PubMed] [Google Scholar]
- 36. Liu J, Huang R, Lin D, Wu X, Peng J, Lin Q et al (2005) Apoptotic effect of oridonin on NB4 cells and its mechanism. Leuk. Lymphoma 46, 593–597. [DOI] [PubMed] [Google Scholar]
- 37. Zhang JF, Liu JJ, Liu PQ, Lin DJ, Li XD, Chen GH (2006) Oridonin inhibits cell growth by induction of apoptosis on human hepatocelluar carcinoma BEL‐7402 cells. Hepatol. Res. 35, 104–110. [DOI] [PubMed] [Google Scholar]
- 38. Liu JJ, Huang RW, Lin DJ, Wu XY, Lin Q, Peng J et al (2005) Antiproliferation effects of ponicidin on human myeloid leukemia cells in vitro. Oncol. Rep. 13, 653–657. [PubMed] [Google Scholar]
- 39. Zhang CL, Wu LJ, Tashiro S, Onodera S, Ikejima T (2004) Oridonin induced A375‐S2 cell apoptosis via bax‐regulated caspase pathway activation, dependent on the cytochrome c/caspase‐9 apoptosome. J. Asian Nat. Prod. Res. 6, 127–138. [DOI] [PubMed] [Google Scholar]
- 40. Liu JJ, Huang RW, Lin DJ, Wu XY, Peng J, Pan XL et al (2005) Oridonin‐induced apoptosis in leukemia K562 cells and its mechanism. Neoplasma 52, 225–230. [PubMed] [Google Scholar]
- 41. Zhen T, Wu CF, Liu P, Wu HY, Zhou GB, Lu Y et al (2012) Targeting of AML1‐ETO in t (8;21) leukemia by oridonin generates a tumor suppressor‐like protein. Sci. Transl. Med. 4, 127–138. [DOI] [PubMed] [Google Scholar]
- 42. Dan I, Watanabe NM, Kusumi A (2001) The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11, 220–230. [DOI] [PubMed] [Google Scholar]
- 43. Whitmarsh AJ, Davis RJ (1996) Transcription factor AP‐1 regulation by mitogen‐activated protein kinase signal transduction pathways. J. Mol. Med. (Berl) 74, 589–607. [DOI] [PubMed] [Google Scholar]
- 44. Krens SF, Spaink HP, Snaar‐Jagalska BE (2006) Functions of the MAPK family in vertebrate‐development. FEBS Lett. 580, 4984–4990. [DOI] [PubMed] [Google Scholar]
- 45. Roux PP, Blenis J (2004) ERK and p38 MAPK‐activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jin S, Shen JN, Wang J, Huang G, Zhou JG (2007) Oridonin induced apoptosis through Akt and MAPKs signaling pathways in human osteosarcoma cells. Cancer Biol. Ther. 6, 261–268. [DOI] [PubMed] [Google Scholar]
- 47. Li D, Wu LJ, Tashiro S, Onodera S, Ikejima T (2007) Oridonin‐induced A431 cell apoptosis partially through blockage of the Ras/Raf/ERK signal pathway. J. Pharmacol. Sci. 103, 56–66. [DOI] [PubMed] [Google Scholar]
- 48. Huang J, Wu L, Tashiro S, Onodera S, Ikejima T (2008) Reactive oxygen species mediate oridonin‐induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J. Pharmacol. Sci. 107, 370–379. [DOI] [PubMed] [Google Scholar]
- 49. Liu YQ, You S, Tashiro S, Onodera S, Ikejima T (2005) Activation of phosphoinositide 3‐kinase, protein kinase C, and extracellular signal‐regulated kinase is required for oridonin‐enhanced phagocytosis of apoptotic bodies in human macrophage‐like U937 cells. J. Pharmacol. Sci. 98, 361–371. [DOI] [PubMed] [Google Scholar]
- 50. Liu YQ, You S, Tashiro S, Onodera S, Ikejima T (2006) Roles of Ras and extracellular signal‐regulated kinase‐dependent IkappaBalpha degradation in oridonin‐enhanced phagocytosis of apoptotic cells by human macrophage‐like U937 cells. Int. Immunopharmacol. 6, 260–268. [DOI] [PubMed] [Google Scholar]
- 51. Foukas LC, Katsoulas HL, Paraskevopoulou N, Metheniti A, Lambropoulou M, Marmaras VJ (1998) Phagocytosis of Escherichia coli by insect hemocytes requires both activation of the Ras/mitogen‐activated protein kinase signal transduction pathway for attachment and beta3 integrin for internalization. J. Biol. Chem. 273, 14813–14818. [DOI] [PubMed] [Google Scholar]
- 52. Kyriakis JM, Avruch J (2001) Mammalian mitogen‐activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869. [DOI] [PubMed] [Google Scholar]
- 53. Li D, Wu LJ, Tashiro S, Onodera S, Ikejima T (2007) Oridonin inhibited the tyrosine kinase activity and induced apoptosis in human epidermoid carcinoma A431 cells. Biol. Pharm. Bull. 30, 254–260. [DOI] [PubMed] [Google Scholar]
- 54. Wang HJ, Li D, Yang FY, Tashiro S, Onodera S, Ikejima T (2008) Oridonin induces human melanoma A375‐S2 cell death partially through inhibiting insulin‐like growth factor 1 receptor signaling. J. Asian Nat. Prod. Res. 10, 787–798. [DOI] [PubMed] [Google Scholar]
- 55. Huang J, Wu L, Tashiro S, Onodera S, Ikejima T (2006) Fibroblast growth factor‐2 suppresses oridonin‐induced L929 apoptosis through extracellular signal‐regulated kinase‐dependent and phosphatidylinositol 3‐kinase‐independent pathway. J. Pharmacol. Sci. 102, 305–313. [DOI] [PubMed] [Google Scholar]
- 56. Engelman JA (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer 9, 550–562. [DOI] [PubMed] [Google Scholar]
- 57. Liu YQ, You S, Zhang CL, Tashiro S, Onodera S, Ikejima T (2005) Oridonin enhances phagocytosis of UV‐irradiated apoptotic U937 cells. Biol. Pharm. Bull. 28, 461–467. [DOI] [PubMed] [Google Scholar]
- 58. Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y et al (1997) Akt phosphorylation of BAD couples survival signals to the cell‐intrinsic death machinery. Cell 91, 231–241. [DOI] [PubMed] [Google Scholar]
- 59. Romashkova JA, Makarov SS (1999) NF‐kappaB is a target of AKT in anti‐apoptotic PDGF signalling. Nature 401, 86–90. [DOI] [PubMed] [Google Scholar]
- 60. Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E et al (1998) Regulation of cell death protease caspase‐9 by phosphorylation. Science 282, 1318–1321. [DOI] [PubMed] [Google Scholar]
- 61. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS et al (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868. [DOI] [PubMed] [Google Scholar]
- 62. Hu HZ, Yang YB, Xu XD, Shen HW, Shu YM, Ren Z et al (2007) Oridonin induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta Pharmacol. Sin. 28, 1819–1826. [DOI] [PubMed] [Google Scholar]
- 63. Gao FH, Hu XH, Li W, Liu H, Zhang YJ, Guo ZY et al (2010) Oridonin induces apoptosis and senescence in colorectal cancer cells by increasing histone hyperacetylation and regulation of p16, p21, p27 and c‐myc. BMC Cancer 10, 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang HL, Weng HY, Wang LQ, Yu CH, Huang QJ, Zhao PP et al (2012) Triggering Fbw7‐mediated proteasomal degradation of c‐Myc by Oridonin induces cell growth inhibition and apoptosis. Mol. Cancer Ther. 11, 1155–1165. [DOI] [PubMed] [Google Scholar]
- 65. Cui Q, Tashiro S, Onodera S, Minami M, Ikejima T (2007) Oridonin induced autophagy in human cervical carcinoma HeLa cells through Ras, JNK, and P38 regulation. J. Pharmacol. Sci. 105, 317–325. [DOI] [PubMed] [Google Scholar]
- 66. Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411, 342–348. [DOI] [PubMed] [Google Scholar]
- 67. Huang J, Klionsky DJ (2007) Autophagy and human disease. Cell Cycle 6, 1837–1849. [DOI] [PubMed] [Google Scholar]
- 68. Cui Q, Tashiro S, Onodera S, Ikejima T (2006) Augmentation of oridonin‐induced apoptosis observed with reduced autophagy. J. Pharmacol. Sci. 101, 230–239. [DOI] [PubMed] [Google Scholar]
- 69. Zhang Y, Wu Y, Tashiro S, Onodera S, Ikejima T (2009) Involvement of PKC signal pathways in oridonin‐induced autophagy in HeLa cells: a protective mechanism against apoptosis. Biochem. Biophys. Res. Commun. 378, 273–278. [DOI] [PubMed] [Google Scholar]
- 70. Cui Q, Tashiro S, Onodera S, Minami M, Ikejima T (2007) Autophagy preceded apoptosis in oridonin‐treated human breast cancer MCF‐7 cells. Biol. Pharm. Bull. 30, 859–864. [DOI] [PubMed] [Google Scholar]
- 71. Cheng Y, Qiu F, Ye YC, Guo ZM, Tashiro S, Onodera S et al (2009) Autophagy inhibits reactive oxygen species‐mediated apoptosis via activating p38‐nuclear factor‐kappa B survival pathways in oridonin‐treated murine fibrosarcoma L929 cells. FEBS J. 276, 1291–1306. [DOI] [PubMed] [Google Scholar]
- 72. Cheng Y, Qiu F, Ikejima T (2009) Molecular mechanisms of oridonin‐induced apoptosis and autophagy in murine fibrosarcoma L929 cells. Autophagy 5, 430–431. [DOI] [PubMed] [Google Scholar]
- 73. Zhang YH, Wu YL, Tashiro S, Onodera S, Ikejima T (2011) Reactive oxygen species contribute to oridonin‐induced apoptosis and autophagy in human cervical carcinoma HeLa cells. Acta Pharmacol. Sin. 32, 1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zeng R, Chen Y, Zhao S, Cui GH (2012) Autophagy counteracts apoptosis in human multiple myeloma cells exposed to oridonin in vitro via regulating intracellular ROS and SIRT1. Acta Pharmacol. Sin. 33, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zang L, Xu Q, Ye Y, Li X, Liu Y, Tashiro S et al (2012) Autophagy enhanced phagocytosis of apoptotic cells by oridonin‐treated human histocytic lymphoma U937 cells. Arch. Biochem. Biophys. 518, 31–41. [DOI] [PubMed] [Google Scholar]


