Abstract
Abstract. Low‐frequency electromagnetic fields are suspected of being involved in carcinogenesis, particularly in processes that could be related to cancer promotion. Because development of cancer is associated with deregulated cell growth and we previously observed a magnetic field‐induced decrease in DNA synthesis [Lange et al. (2002) Alterations in the cell cycle and in the protein level of cyclin D1p, 21CIP1, and p16INK4a after exposure to 50 HZ. MF in human cells. Radiat. Environ. Biophys. 41, 131], this study aims to document the influence of 50 Hz, 1 mT magnetic fields (MF), with or without initial γ‐ionizing radiation (IR), on the following cell proliferation‐relevant parameters in human amniotic fluid cells (AFC): cell cycle distribution, expression of the G1 phase‐regulating proteins Cdk4, cyclin D1, p21CIP1 and p16INK4a, and Cdk4 activity. While IR induced a G1 delay and a dose‐dependent G2 arrest, no discernible changes in cell cycle kinetics were observed due to MF exposure. However, a significant decrease in the protein expression of cyclin D1 and an increase in p21CIP1‐ and p16INK4a‐expression could be detected after exposure to MF alone. IR‐exposure caused an augmentation of p21CIP1‐ and p16INK4a‐ levels as well, but did not alter cyclin D1 expression. A slight diminution of Cdk4 activity was noticed after MF exposure only, indicating that Cdk4 appears not to act as a mediator of MF‐ or IR‐induced changes in the cell cycle of AFC cells. Co‐exposure to MF/IR affected neither cell cycle distribution nor protein expression or kinase activity additionally or synergistically, and therefore MF seems not to modify the mutagenic potency of IR.
INTRODUCTION
The question of possible health effects due to exposure to electromagnetic fields (EMF) has attracted a great deal of public attention during the last 25 years. A wealth of epidemiological studies has revealed that occupational or residential exposure to EMF might be correlated with increased cancer risk (Auvinen et al. 2000; Bethwaite et al. 2001; Pollan et al. 2001; Weiderpass et al. 2003). Various in‐vitro studies showed alterations in parameters also known to be involved in carcinogenesis, mainly modifications in cell proliferation and signal transduction pathways (Katsir & Parola 1998; Demattei et al. 2001; Ishido et al. 2001; Lange et al. 2002; McCreary et al. 2002; Richard et al. 2002) and, therefore, substantiate the assumption that exposure to EMF could play a role in the carcinogenic process. However, numerous investigations have also reported negative correlation between EMF and cancer risk (Linet et al. 1997; UK Childhood Cancer Study Investigators 1999) or have failed to identify biological effects of EMF on the cellular and molecular level (Loberg et al. 1999; Morehouse & Owen 2000; Shahidain et al. 2001; Supino et al. 2001; Griefahn et al. 2002; Cho & Chung 2003; Ikeda et al. 2003). The inconsistent results and the lack of an established chemical or molecular mechanism through which EMF exposure may contribute to carcinogenesis still generate significant controversy. However, as it is generally accepted that an EMF itself is not genotoxic (Repacholi & Greenebaum 1999; Simkóet al. 2001; Juutilainen et al. 2000), one potential mechanism for the action of EMF in cancer development is the modulation of cellular responses to known mutagenic agents, which generally result in changes in signal transduction pathways and cell cycle disturbances.
Although it appears that oncogenic defects may target any major cell cycle checkpoint, the steps most frequently deregulated are the control of G1 phase and G1/S transition, respectively. Cyclin‐dependent kinase 4 (Cdk4) plays a crucial role in the regulation of the progression through the G1‐phase and also has an impact on G1/S transition (Morgan 1995). The most important positive regulating subunits of Cdk4 are the D‐type cyclins (Matsushime et al. 1994). They qualify as proto‐oncogenes and are overabundant in a wide spectrum of human malignancies (Bartkova et al. 1997). The negative regulation of the cell cycle machinery is achieved through association of Cdk with Cdk inhibitory proteins (CKIs), which induce cell cycle arrest in response to both internal and external signals (Sherr & Roberts 1995; Harper & Elledge 1996). Given their function, all CKIs are considered to be potential tumour suppressor genes. The implication in carcinogenesis is demonstrated by p16INK4a and p21CIP1, as their genes are commonly mutated or lost in a variety of tumours (Bartkova et al. 1997).
In a previous study (Lange et al. 2002), we showed that horizontally applied 50 Hz, 1 mT magnetic fields (MF) induced a decrease in DNA synthesis as well as alterations in expression levels of G1 phase‐regulating proteins, whereas changes in cell cycle distribution could not be observed. In the present study, we investigated the protein expression of Cdk4, cyclin D1, p21CIP1 and p16INK4a after exposure to EMF and ionizing radiation, this latter a genotoxic agent known to cause a cell cycle arrest prior to S phase in many eukaryotic cells. Additionally, to obtain more detailed information concerning the influence of MF on G1 phase regulation, analysis of the Cdk4 complex and its kinase activity were also performed.
MATERIALS AND METHODS
Cell culture
Human diploid amniotic fluid cells (AFC) (Coriell Institute for Medical Research, Repository No. GM 00472, Camden, NJ, USA) were cultured in RPMI‐1640 medium (Gibco BRL, Grand Island, NY, USA) supplemented with 20% FCS (Biochrom KG, Berlin, Germany) at 37 °C in humidified 5% CO2, 95% air.
Exposure to EMF and ionizing radiation
Cell cultures were exposed to a 50 Hz, 1 mT root mean squared magnetic field applied horizontally to the cell monolayer and generated by a pair of Helmholtz coils (diameter 400 mm, distance between the coils 200 mm, resistance 2.1 Ω, 154 turns of copper wire; Phywe Systeme, Göttingen, Germany) which were located inside a CO2‐incubator (Heraeus/Kendro Instruments, Hanau, Germany) (Simkóet al. 1998). Magnetic flux density and temperature were monitored with an F. W. Bell Gauss/Tesla Meter model 6010 (Bell, Orlando, FL, USA) with integrated temperature sensor. Cells were placed in the region of spatially uniform magnetic flux, that is in the central area of the coils. Control cultures were maintained in an identical incubator without the Helmholtz system. The AC magnetic background field in the control incubator was measured to be 1 µT.
For exposure to ionizing radiation a 60Co‐exposure system (Theratron 780‐C, Philips Medizin Systeme, Hamburg) was used. Cells were irradiated with 0, 2, 4 and 8 Gy at a dose rate of 0.63 Gy/min at room temperature (20 °C).
Flow cytometry
Cells were harvested at regular intervals for a total of 30 h, centrifuged at 194 × g for 5 min at 4 °C, resuspended in 100 µl phosphate‐buffered saline (PBS) and fixed with 70%[v/v] ethanol at −20 °C. Fixed cells were centrifuged at 328 g for 12 min, re‐suspended in 0.5 ml of a pre‐warmed solution of 1 mg/ml RNase (100 Kunitz units/mg solid; Sigma, St Louis, MO, USA) in HEPES‐buffered saline (HBS: 150 mm NaCl, 14 mm HEPES, pH 7.4) and incubated for 30 min at 37 °C. Propidium iodide (500 µg/ml HBS) was added and cells were incubated for an additional 30 min at 37 °C. DNA content was analysed within 24 h in a flow cytometer (Epics Elite, Coulter Electronics, Hialeah, FL, USA).
Preparation of cell extracts
Cells were scraped off in ice‐cold PBS and collected by centrifugation at 194 g for 5 min at 4 °C. For immunoblotting, the pellets were re‐suspended in 100 µl lysis buffer I containing 50 mm Tris‐HCl (pH 7.5), 150 mm NaCl, 1 mm each of EDTA, EGTA, NaF, NaO4Va and phenylmethylsulphonyl fluoride (PMSF), 0.1% Tween‐20 and 1 µg/ml each of aprotinin, leupeptin and pepstatin A. In‐vitro kinase assays were performed after immunoprecipitation. For this, cells were lysed in 500 µl lysis buffer II containing 50 mm HEPES (pH 7.5), 150 mm NaCl, 1 mm EDTA, 2.5 mm EGTA, 10% glycerol, 0.1% Tween‐20, 10 mmβ‐glycerophosphate, 1 mm NaF, 0.1 mm Na3VO4, 1 mm PMSF, 1 mm 1,4‐dithiothreitol (DTT), and 5 µg/ml each of pepstatin A, aprotinin and leupeptin. Lysates were sonicated and clarified by microcentrifugation at 14 000 g for 10 min at 4 °C. The supernatants were boiled for 5 min with equal volumes of sodium dodecyl sulphate (SDS) sample buffer (0.76% Tris, 2% SDS, 10% glycerol, 20%β‐mercaptoethanol, 0.005% bromphenol blue) and subsequently size fractionated by SDS–polyacrylamide gel electrophoresis (PAGE) or immunoprecipitated for the kinase assay and the analysis of Cdk4‐associated proteins.
Antibodies
The following antibodies were used for immunoprecipitation and immunoblotting: monoclonal mouse antibodies to cyclin D1/2/3, p16INK4a and p21CIP1 were purchased from PharMingen, San Diego, CA, USA. Monoclonal mouse antibodies to Cdk4 and α‐tubulin were obtained from Sigma. The goat anti‐mouse IgG conjugated to alkaline phosphatase and the agarose‐conjugated polyclonal rabbit antibody to Cdk4 were provided by Santa Cruz Biotechnology, Santa Cruz, CA, USA.
Immunoblotting
Equal amounts of protein per lane were separated on a 12.5% SDS‐polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane. Protein quantities were determined as described elsewhere (Coppock et al. 1995). Briefly, western blots were blocked overnight at 4 °C with 5% non‐fat dry milk in TBS containing 0.05% Tween‐20 (TTBS). For analysis of proteins in the whole cell extract, membranes were probed for 2 h with a mix of the following antibodies: 0.5 µg/ml anti‐p16INK4a, 0.17 µg/ml anti‐p21CIP1, 0.6 µg/ml anti‐α‐tubulin, and either 0.5 µg/ml anti‐cyclin D1/2/3 or 10 µg/ml anti‐Cdk4 in 1% non‐fat dry milk in TTBS. For investigation of Cdk4‐associated proteins, an antibody mix containing 0.625 µg/ml anti‐p16INK4a, 0.17 µg/ml anti‐p21CIP1, and 0.5 µg/ml anti‐cyclin D1/2/3 was used for an incubation time of 3.5 h. Then blots were incubated with 0.13 µg/ml anti‐mouse IgG for 1 h and signals were developed using the Immun‐Star™ Chemiluminescent Protein Detection System according to the manufacturer's instructions (ECL, Bio‐Rad Laboratories, Hercules, CA, USA). Blots were scanned with the Fluor‐S MultiImager and relative amounts of the proteins were quantified using the ‘Quantity One’‐software (Bio‐Rad Laboratories).
Immunoprecipitation and Cdk4 kinase assay
For kinase assay and analysis of the Cdk4‐associated proteins, 500 and 1000 µg of cell extract was used for immunoprecipitation, respectively. Extracts were adjusted to a total volume of 500 µl with the appropriate lysis buffer mentioned above and incubated with 10 µg of polyclonal agarose‐conjugated anti‐Cdk4 antibody overnight at constant agitation and 4 °C. Agarose‐adsorbed immunocomplexes were precipitated by centrifugation at 960 g and 1 °C for 5 min. The supernatants were discarded and the pellets were washed four times with lysis buffer. For analysis of Cdk4‐associated proteins, agarose‐bound proteins were eluted by boiling in 40 µl SDS‐sample buffer for 5 min. Samples were analysed by 12.5% SDS–PAGE followed by immunoblotting. Immunoprecipitates for kinase assay were washed twice additionally with ice‐cold kinase buffer (50 mm HEPES, pH 7.5, 10 mm MgCl2, 2.5 mm EGTA, 10 mmβ‐glycerophosphate, 1 mm NaF, 0.1 mm Na3VO4, 1 mm DTT) and then re‐suspended in 30 µl of a freshly prepared glutathion‐S‐transferase‐(GST)‐Rb reaction buffer (20 µm ATP in kinase buffer, supplemented with 5 µCi γ‐[32P]ATP and 1 µg GST‐pRb per reaction). After incubation for 30 min at 30 °C, the reaction was stopped by the addition of an equal volume of SDS‐sample buffer and boiling for 5 min. The samples were centrifuged for 5 min at 13 000 r.p.m. (960 g) and the supernatants were separated in SDS‐polyacrylamide gels. Phosphorylated proteins were visualized by phosphorimaging (Bio‐Imaging Analyser BAS‐1000, Fuji, Düsseldorf, Germany) of dried gels, and phosphorylated Rb was quantified with the ‘Quantity One’‐software (Bio‐Rad Laboratories).
Statistics
Results of the flow cytometric measurements were assessed by the Student's t‐test. For statistical analysis of the immunobloting experiments, the Wilcoxon matched pairs signed rank test was carried out. The quantified raw data of band intensities of whole cell lysates were analysed after correction for α‐tubulin as an internal standard. As a result of the absence of α‐tubulin in the immunoprecipitates, the quantification of Cdk4‐associated proteins was performed without this correction. For each approach, three or more independent experiments were conducted and differences between exposed and control cells with an error probability of P < 0.01 were considered to be statistically significant.
RESULTS
Effect of exposure to MF and/or IR on cell cycle kinetics
We examined the influence of EMF alone and in combination with ionizing radiation on the cell cycle kinetics of AFCs using flow cytometry. The normal distribution pattern of exponentially growing AFC cells is about 55% in G0/G1 phase, 24% in S phase and 21% in G2/M phase (data not shown). Exposure to 1 mT EMF had no effect on the cell cycle of the cells (Table 1), whereas exposure to different doses of ionizing radiation led to an activation of G1‐ as well as G2‐checkpoints. As shown in Table 1, the IR‐irradiated cells progressed out of S phase and amassed in G2/M. The extent of the G2 arrest was directly related to the dose of IR. The maximum accumulation in G2 was reached at 6 h (2 Gy) and 12 h (4, 8 Gy) after irradiation, followed by a gradual decline through the following hours (2 and 4 Gy), although the percentage of cells in G2 was sustained above the initial level for more than 30 h in 4‐Gy‐irradiated cells. The transient G2 arrest lasted approximately 6 h and 6–12 h for cells irradiated with 2 and 4 Gy, respectively, as documented by the increase in G1 phase cells at these time points. The exposure to 8 Gy induced a prolonged delay in G2, as shown by the concomitant absence of cells entering G1. Because the IR‐induced G2 block causes modulations of the subsequent G1 phase, for analysis of the G1 arrest, the proportion of cells in S phase was evaluated. The uniform decrease of S phase cells to a minimum of 4% 12 h after exposure indicates that γ‐rays at 2–8 Gy eliminated almost completely the proliferating potential of AFC cells, and the retardation to exit G1 occurred independently of the radiation dose. During the subsequent period, the consistently low population in S phase confirmed the inability of cells to overcome G1 arrest within 30 h. The combined exposure to MF and IR did not result in alteration in cell cycle distribution compared with the treatment with IR alone. Hence, the flow cytometric analysis showed that neither exposure to MF alone nor co‐exposure to γ‐irradiation and MF could corroborate the previously seen reduction in DNA‐synthesis in AFC cells.
Table 1.
Cell cycle distribution of AFC cells after irradiation with 60Co γ‐rays and subsequent continuous exposure to 1 mT MF (control; 1 mT = MF exposure; 2, 4, 8 Gy = IR exposure; × Gy + 1 mT = exposure to MF and IR). The proportions of cells at different stages in the cell cycle were determined by single parameter flow cytometry. Mean of data from three independent exposures are shown. Asterisks mark statistically significant differences from control (P < 0.05, Student's t‐test)
| Exposure time (h) | ||||||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 30 | |
| G0/G1 phase | ||||||
| Control | 54.5 ± 2.9 | 50.7 ± 7.3 | 55.8 ± 5.9 | 59.8 ± 8.0 | 60.1 ± 5.1 | 63.7 ± 2.4 |
| 1 mT | 54.5 ± 2.9 | 50.3 ± 6.2 | 55.8 ± 5.6 | 58.6 ± 8.5 | 60.4 ± 5.3 | 63.4 ± 4.7 |
| 2 Gy | 56.0 ± 1.0 | 48.8 ± 7.2 | 63.5 ± 5.1 | 69.9 ± 6.5 | 68.8 ± 3.5* | 71.1 ± 0.5* |
| 2 Gy + 1 mT | 56.0 ± 1.0 | 47.1 ± 7.2 | 63.1 ± 5.0 | 69.4 ± 6.8 | 70.4 ± 3.0* | 70.8 ± 1.0* |
| 4 Gy | 56.3 ± 1.6 | 45.5 ± 6.4 | 50.1 ± 7.3 | 61.3 ± 8.4 | 64.1 ± 5.7 | 65.2 ± 3.6 |
| 4 Gy + 1 mT | 56.3 ± 1.6 | 44.8 ± 7.1 | 52.7 ± 7.5 | 62.2 ± 3.8 | 65.9 ± 1.6 | 65.0 ± 1.3 |
| 8 Gy | 54.9 ± 2.1 | 45.0 ± 3.7 | 48.0 ± 4.2 | 47.3 ± 4.1* | 52.7 ± 3.3* | 56.5 ± 1.5* |
| 8 Gy + 1 mT | 54.9 ± 2.1 | 45.5 ± 4.9 | 46.8 ± 4.6* | 47.1 ± 3.8* | 49.5 ± 6.0* | 52.1 ± 4.3* |
| S phase | ||||||
| Control | 24.4 ± 3.5 | 27.7 ± 7.8 | 23.1 ± 6.0 | 20.7 ± 5.4 | 20.1 ± 7.3 | 17.7 ± 4.7 |
| 1 mT | 24.4 ± 3.5 | 26.5 ± 6.8 | 21.2 ± 4.6 | 21.6 ± 8.2 | 20.1 ± 4.6 | 17.7 ± 3.5 |
| 2 Gy | 23.7 ± 4.3 | 14.1 ± 9.0* | 4.2 ± 2.8* | 7.2 ± 5.6* | 6.7 ± 3.7* | 6.5 ± 4.4* |
| 2 Gy + 1 mT | 23.7 ± 4.3 | 18.8 ± 15.5 | 3.6 ± 2.9* | 6.5 ± 3.8* | 6.1 ± 2.9* | 6.0 ± 3.3* |
| 4 Gy | 24.7 ± 4.0 | 12.6 ± 7.0* | 3.6 ± 2.8* | 3.8 ± 2.5* | 4.9 ± 4.4* | 4.1 ± 4.1* |
| 4 Gy + 1 mT | 24.7 ± 4.0 | 13.8 ± 8.8* | 2.9 ± 1.7* | 4.0 ± 2.8* | 3.6 ± 2.4* | 3.8 ± 3.2* |
| 8 Gy | 25.6 ± 6.0 | 14.2 ± 5.2* | 3.7 ± 1.7* | 4.6 ± 5.2* | 2.9 ± 2.1* | 3.7 ± 3.2* |
| 8 Gy + 1 mT | 25.6 ± 6.0 | 13.5 ± 4.4* | 2.4 ± 0.9* | 3.4 ± 3.2* | 6.3 ± 6.5* | 6.6 ± 5.6* |
| G2/M phase | ||||||
| Control | 21.1 ± 2.3 | 21.5 ± 0.6 | 21.1 ± 0.8 | 19.5 ± 3.7 | 19.8 ± 2.3 | 18.6 ± 3.1 |
| 1 mT | 21.1 ± 2.3 | 23.2 ± 3.0 | 23.0 ± 1.6 | 19.7 ± 2.0 | 19.5 ± 1.3 | 18.8 ± 3.3 |
| 2 Gy | 20.3 ± 3.7 | 37.1 ± 2.1* | 32.3 ± 2.5* | 22.9 ± 1.0* | 24.4 ± 0.5* | 22.4 ± 4.1* |
| 2 Gy + 1 mT | 20.3 ± 3.7 | 34.1 ± 8.9 | 33.2 ± 2.7* | 24.1 ± 2.9 | 23.5 ± 0.8* | 23.3 ± 3.1 |
| 4 Gy | 19.0 ± 2.4 | 41.9 ± 3.6* | 46.3 ± 5.0* | 34.9 ± 6.5* | 31.0 ± 2.6* | 30.8 ± 0.5* |
| 4 Gy + 1 mT | 19.0 ± 2.4 | 41.4 ± 1.7* | 44.5 ± 5.8* | 33.8 ± 1.1* | 30.5 ± 1.8* | 31.2 ± 63* |
| 8 Gy | 19.5 ± 4.0 | 40.8 ± 3.3* | 48.4 ± 2.6* | 48.1 ± 3.2* | 44.4 ± 3.2* | 39.9 ± 2.0* |
| 8 Gy + 1 mT | 19.5 ± 4.0 | 41.0 ± 1.7* | 50.8 ± 3.7* | 49.5 ± 3.2* | 44.3 ± 3.6* | 41.3 ± 1.3* |
Expression patterns of the G1 regulatory proteins Cdk4, cyclin D1, p21CIP1, and p16INK4a in whole cell lysates
In order to gain an insight into the molecular events of G1 regulation in AFC cells, expression patterns and kinetics of regulation of the G1 proteins Cdk4, cyclin D1, p21CIP1 and p16INK4a were investigated by immunoblotting and subsequent ECL detection. Cells were exposed to 1 mT and/or 8 Gy for various periods and abundance of proteins was determined. The immunobiochemical analysis of Cdk4 revealed that neither exposure of AFC cells to 1 mT MF nor the irradiation with 8‐Gy γ‐rays, nor the combined exposure to 1 mT/8 Gy led to changes of the Cdk4 level during the time course studied (Fig. 1a). Examination of cyclin D family expression was limited to cyclin D1, as previous experiments had shown that cyclin D1 was the most prominent D‐type cyclin in AFC cells, while cyclin D2 and D3 bands were only faintly detectable after using a monoclonal mouse antibody that recognizes all D‐type cyclins (data not shown). Within the first 18 h after exposure to MF, the protein content of cyclin D1 remained unaffected. However, a longer lasting exposure of 24 and 30 h caused a decrease of protein expression of 23 and 31%, respectively (Fig. 1b). Treatment with ionizing radiation at a dose of 8 Gy as well as the combined exposure to 1 mT/8 Gy did not exert an influence on the expression of cyclin D1 (Fig. 1b).
Figure 1.
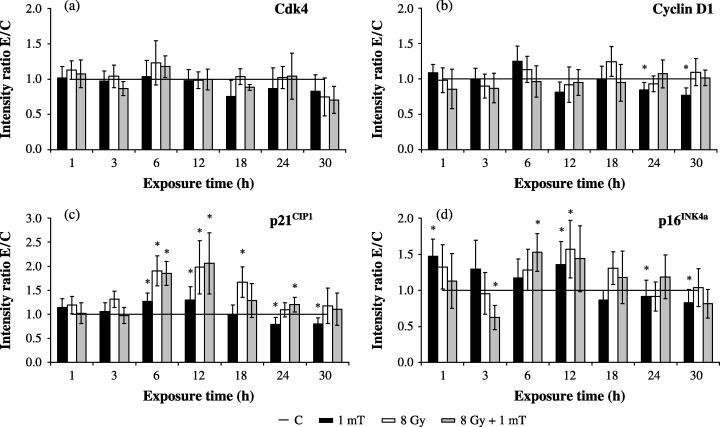
Expression of G1 phase‐regulating proteins in whole cell lysates after 8 Gy γ‐irradiation followed by MF exposure. The bands were quantified by ECL and subsequent optical scanning in a Fluor‐S‐MultiImager. Protein levels were normalized to α‐tubulin signals for lanes and represented as ratio of 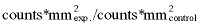 (C, control; 1 mT, MF exposure; 8 Gy, IR exposure; 8 Gy + 1 mT, exposure to MF and IR). Diagrams show the time‐dependent protein expression of (a) Cdk4 (n = 6–11), (b) cyclin D1 (n = 8–18), (c) p21CIP1 (n = 8–18) and (d) p16INK4a (n = 14–23). Columns show the mean values ± SEM; data points marked with an asterisk are statistically significant (*P < 0.01, Wilcoxon matched pairs signed rank test).
(C, control; 1 mT, MF exposure; 8 Gy, IR exposure; 8 Gy + 1 mT, exposure to MF and IR). Diagrams show the time‐dependent protein expression of (a) Cdk4 (n = 6–11), (b) cyclin D1 (n = 8–18), (c) p21CIP1 (n = 8–18) and (d) p16INK4a (n = 14–23). Columns show the mean values ± SEM; data points marked with an asterisk are statistically significant (*P < 0.01, Wilcoxon matched pairs signed rank test).
Proteins of the CIP/KIP and INK4 family act as effective inhibitors of Cdk4 activity. Examination of the expression of p16INK4a and p21CIP1 should clarify whether, after exposure to MF and/or IR, the regulation of Cdk4 kinase activity would be influenced by modulation in protein levels of these inhibitors. Exposure to extremely low frequency MF induced an augmentation of p21CIP1 expression by approximately 30% after 6–12 h of exposure, whereas 24 and 30 h of exposure resulted in a diminution of p21CIP1 level below control values (Fig. 1c). As expected, irradiation with γ‐rays led to a marked up‐regulation of p21CIP1 by 98%, which was detected within 6 h. The expression level remained elevated for 12 h and then decreased gradually and regained control level 30 h after irradiation (Fig. 1c). Additional exposure of 8 Gy‐irradiated cells to 1 mT MF caused only an additional decrease of 26% of p21CIP1 at 3 h. From immunoblot analysis of a specific Cdk4 inhibitor, it follows that MF induced an initial elevation of p16INK4a protein of 47% and 35% at 1 and 12 h, respectively. In concordance with the kinetics of cyclin D1 and p21CIP1 expression, a prolonged exposure of 24 and 30 h caused a reduction of p16INK4a to 86% and 73% (Fig. 1d). In response to IR as well as to MF/IR exposure, an increase of p16INK4a level by 50% could be demonstrated after 6 and 12 h. Although the combined treatment at 3 h led to a decrease of p16INK4a by 38%, a comparison of cells exposed to MF and IR with cells treated with IR alone showed no differences (data not shown).
Determination of Cdk4‐associated proteins in immunoprecipitates after exposure to MF and/or IR
Changes in expression of cyclin D1, p21CIP1 and p16INK4a could influence Cdk4 activity; therefore the proportions of these proteins bound in a Cdk4 complex were determined by western blot analysis in Cdk4 immunoprecipitates. Quantitative analysis of Cdk4/cyclin D1 complex revealed that the rate of cyclin D1 was enhanced by 48% after 3 h of MF exposure, whereas longer exposure times of 18 and 30 h caused a reduction of complex‐associated cyclin D1 by 19% and 29%, respectively (Fig. 2a). Irradiation alone resulted in an increase of Cdk4‐associated cyclin D1 by 65% at 3 and 12 h and 29% at 30 h exposure; however, these alterations were not evident after combined exposure to MF/IR (Fig. 2a).
Figure 2.
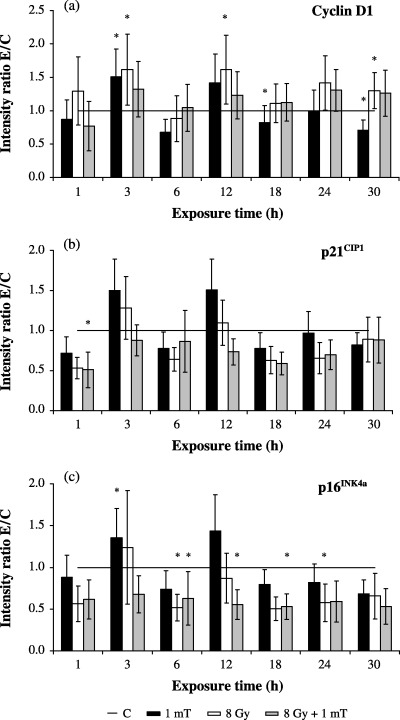
Expression of G1 phase‐regulating proteins in Cdk4 immunocomplexes after exposure to 1 mT and/or 8 Gy. The bands were quantified by ECL and subsequent optical scanning in a Fluor‐S‐MultiImager. Protein levels were represented as ratio of 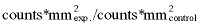 (C, control; 1 mT, MF exposure; 8 Gy, IR exposure; 8 Gy + 1 mT, exposure to MF and IR). Diagrams show the time‐dependent complex formation of Cdk4 with (a) cyclin D1 (n = 9–16), (b) p21CIP1 (n = 9–16) and (c) p16INK4a (n = 6–12). Columns show the mean values ± SEM, data points marked with an asterisk are statistically significant (*P < 0.01, Wilcoxon matched pairs signed rank test).
(C, control; 1 mT, MF exposure; 8 Gy, IR exposure; 8 Gy + 1 mT, exposure to MF and IR). Diagrams show the time‐dependent complex formation of Cdk4 with (a) cyclin D1 (n = 9–16), (b) p21CIP1 (n = 9–16) and (c) p16INK4a (n = 6–12). Columns show the mean values ± SEM, data points marked with an asterisk are statistically significant (*P < 0.01, Wilcoxon matched pairs signed rank test).
As shown in Fig. 3(b), the overall expression of p21CIP1 co‐immunoprecipated with a Cdk4‐specific antibody remained unchanged regardless of treatment. Thus, the observed augmentation in total p21CIP1 was not connected with alterations in Cdk4/p21CIP1 complex formation and had therefore no direct influence on the Cdk4 kinase activity. Extremely low‐frequency MF led to a short‐term enhancement of Cdk4/p16INK4a association by 41% after 3 h compared with corresponding control cells (Fig. 2c). Cells exposed to ionizing radiation were characterized by a loss of Cdk4/p16INK4a complexes. Following exposure of γ‐irradiated cells to 1 mT, no further influence on complex formation could be observed (data not shown).
Figure 3.
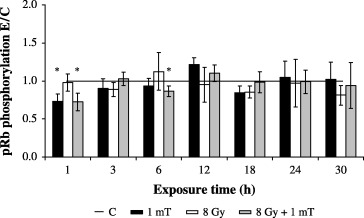
Time course of relative kinase activities associated with anti‐Cdk4 immunocomplexes in MF‐exposed (1 mT), γ‐irradiated (8 Gy) or co‐exposed (8 Gy + 1 mT) AFC cells as assayed with a 32P‐labelled GST‐pRb fusion protein. Columns show the mean ± SEM (*P < 0.01, Wilcoxon matched pairs signed rank test).
Regulation of Cdk4 kinase activity in AFC cells following exposure to MF and/or IR
To test whether the altered expression patterns of cyclin D1, p21CIP1 and p16INK4a were associated with functional modifications of Cdk4, we examined the effect of 1 mT and/or 8 Gy on the ability of immunoprecipitated Cdk4 to phosphorylate a GST‐pRb fusion protein as pRb is a well‐established critical substrate for Cdk4 kinase (Weinberg 1995; Sherr 1996). The data in Fig. 3 show that exposure to 1 mT slightly diminished the kinase activity at 1 h by 26%, while during the remaining period no modification of Cdk4 activation could be determined. Irradiation of cells to 8 Gy did not influence the phosphorylating activity of Cdk4 during the 30 h. Although combined exposure to 1 mT/8 Gy weakly reduced kinase activity after 1 and 6 h by 28% and 14%, respectively, the statistical analysis of the co‐exposure experiments (1 mT/8 Gy) versus 8 Gy alone revealed no changes in Cdk4 activity (data not shown).
DISCUSSION
In this study, we characterized the effect of MF and IR on cell cycle kinetics and relevant proteins of the G1 phase in AFC cells. In response to γ‐irradiation with doses of 2–8 Gy AFC cells were arrested in both the G1 and G2 phases, as already demonstrated for several other human cell types in a multitude of studies (Gadbois & Lehnert 1997; Olivier et al. 1998; Chazotte‐Aubert et al. 2001; Savell et al. 2001; Desimone et al. 2003). In contrast, extremely low‐frequency electromagnetic fields failed to induce any discernible effects and exposure to 50 Hz MF following γ‐irradiation caused no additional modulations of radiation‐induced cell cycle delays. These results are in agreement with recent studies: Ding et al. (2001) reported that exposure of MCF‐7 cells to ionizing radiation resulted in G1 and G2 phase arrest, which was not affected by additional exposure to MF. Tian et al. (2002) revealed that 1 Gy‐irradiation slightly altered the cell cycle in CHO‐K1 cells, but there was no significant difference in the cell cycle distribution between exposure to 60 Hz, 5 mT MF together with 1 Gy and IR‐exposure alone. In contrast, under the same experimental conditions, xrs5 cells decreased the G1 arrest induced by X‐irradiation when exposed to MF, although a magnetic field alone had no effect. Thus, exposure to MF following irradiation can affect cell cycle phase distribution, but it has to be taken into account that different cell lines may show different responses to ELF magnetic fields.
Because a previous study (Lange et al. 2002) showed influence of MF‐exposure on cell cycle distribution in AFC cells, we investigated the expression patterns of various G1 phase‐regulating proteins to clarify the molecular mechanism of this specific cell cycle delay. For this, the use of γ‐radiation was limited to a dose of 8 Gy, because it produced the most consistent delay in entering into the G1 phase and, hence, ensured the highest percentage of cells arrested in G1. Moreover, Wharton (1995) described a γ‐radiation dose of 8 Gy as the minimum dose required, inducing a uniform permanent G1 arrest.
Several lines of evidence have revealed that the availability of cyclin‐dependent kinase Cdk4 is essential for progression of cells through the G1 phase and for the G1/S transition, and the variation of expression of Cdk4 results in modifications in the G1 phase (Reed et al. 1998; Jinno et al. 1999). However, we could not identify any alterations in Cdk4 level during 30‐h exposure of AFC cells to MF and/or IR. Savell et al. (2001) have also reported the consistency of Cdk4 level after exposure to 8 Gy γ‐radiation of normal human fibroblasts.
The expression patterns of cyclin D1, that also play a crucial role during passage through the G1 phase in the mammalian cell cycle, were not altered due to exposure to ionizing radiation. Similar results were published by Dulic et al. (1994) and Savell et al. (2001). In both cases, irradiation with γ‐rays at doses of 6 and 8 Gy did not change the accumulation of cyclin D1 in human fibroblasts. Exposure to 1 mT MF was found to diminish the cyclin D1 expression by 23–31% at 24–30 h, but additional treatment of IR‐irradiated AFC cells with MF caused no variation in cyclin D1 level. Moreover, modifications in the complex formation of cyclin D1 with Cdk4 could be observed in both MF‐ and IR‐exposed cell cultures. After MF exposure, a short‐term enrichment of Cdk4‐bound cyclin D1 (+48%) could be detected at 3 h, however, this was independent of the availability of either protein. At the same time point (3 h after MF‐exposure) an increase (+41%) of the inhibitor p16INK4a could be detected. Although the members of the INK4 family often prevent interaction between Cdk4 and cyclin D by forming binary complexes with the kinase, ternary association of INK4 proteins with cyclin D/Cdk‐complexes have been proven by Ruas & Peters (1998). Furthermore, Gabrielli et al. (1999) showed that the half‐life of Cdk4 in association with p16INK4a increases 2–3‐fold. Thus, the inhibitor ensures a higher stability of these complexes and could therefore cause the enhancement of Cdk4/cyclin D1 level at 3‐h exposure. Because of the inhibitory properties of p16INK4a, this ternary complex contains the kinase in its inactive form which can explain the previously detected decrease in DNA synthesis (Lange et al. 2002). The reduction of Cdk4/cyclin D1 complexes after 18–30‐h MF exposure was consistent with the concomitantly depleted total cyclin D1 content in AFC cells. Because no variations in the protein levels of p16INK4a and p21CIP1 bound to Cdk4 were detected at these time points, it can be concluded that modifications in the Cdk4/cyclin D1 complexes at later times are caused by other factors. After application of IR alone, an accumulation of Cdk4/cyclin D1 (+29–65%) was detectable at 3, 12 and 30 h after exposure. This effect was counteracted by MF exposure together with IR at these time points.
Analyses of cell cycle inhibitory proteins show that 1 mT fields influenced the expression of p21CIP1, but no changes in the association of p21CIP1 with Cdk4 were detected. In contrast, decreased p21CIP1 levels were noted when an electromagnetic signal pulsed at 72 Hz was employed (Phillips et al. 1993). No EMF effects on transcription level of p21CIP1 gene were seen in two human breast epithelial cell systems after exposure to a 60‐Hz EMF with flux densities of 10, 100 and 1000 µT (Loberg et al. 1999). Therefore, the nature of the EMF‐induced effect on p21CIP1 expression may be signal‐dependent. In the present study, an increase of p21CIP1 due to ionizing radiation alone was presented. Hence, an arrest of AFC cells in the G1 phase seems not to be caused by direct inhibition of Cdk4 activity by an elevated complex formation of p21CIP1 with the kinase. Although both MF and IR exposure resulted in an increase of p21CIP1 level after 6–12 h in whole cell lysates, the co‐exposure to MF and IR did not show any additive or synergistic effects. Similarly, Ding et al. (2001) found that 60‐Hz MF failed to affect p21CIP1 expression induced by 4 Gy X‐ray irradiation. Tian et al. (2002) could not demonstrate any changes in the level of this protein in CHO‐K1 cells exposed to X‐rays alone or co‐exposed to X‐rays and MF.
p16INK4a is well established as an antagonist for formation and activation of Cdk4/cyclin D and Cdk6/cyclin D complexes. As several studies have shown that fibroblasts express no or only very low levels of p16INK4a (Meyerson & Harlow 1994; Tam et al. 1994), we assumed that MF‐ and IR‐induced alterations in p16INK4a mainly would be connected with the regulation of Cdk4 in the fibroblast‐like AFC cells. The amount of Cdk4‐associated p16INK4a in MF‐exposed cells exhibited an elevated level (+41%) at 3 h. Thus, the MF‐induced changes in the expression of this inhibitor appear to temporarily stabilize the association of Cdk4 with cyclin D1 in an inactive kinase complex, as mentioned above. The immunoprecipitates of 8‐Gy irradiated cells and the co‐exposed cells indicated a reduced integration of p16INK4a into the Cdk4 complex, despite the detected increase in protein expression after 6 and 12 h in whole cell lysates. Because the cell cycle inhibitors affect complex formation of Cdks with cyclins and/or other inhibitors (Jiang et al. 1998; Lukas et al. 1999; Grimison et al. 2000), the diminished p16INK4a/Cdk4 association could be a consequence of antagonistic effects of other inhibitors and their mutually influenced interaction with Cdk/cyclin‐complexes. The demonstrated modulations of p16INK4a level in whole cell lysates and immunoprecipitates suggest the involvement of p16INK4a in radiation‐induced G1‐retardation in AFC cells.
Kinase activity of Cdk4 plays a critical role in the G1 phase; therefore, phosphorylation activity was determined using the pRb protein. pRb is the transcriptional negative regulator of G1 phase progression which acts as a substrate for the cyclin D/Cdk4 complex. Exposure to 1 mT MF alone caused a reduction of kinase activity by 26% after 1 h. Because this decrease was not accompanied by modulations of complex formation of Cdk4 with cyclin D1, p21CIP1 or p16INK4a, an influence on Cdk4 as a result of the investigated proteins seems not to be likely. However, the complexity of cell cycle regulation allows other proteins to be involved in the control of kinases. The lack of variation in Cdk4 kinase activity of irradiated cells was consistent with the results of Cdk4/cyclin D1 complex formation. Thus, an influence of Cdk4 on the radiation‐induced G1 arrest can be ruled out in AFC cells, although co‐exposure to MF and IR faintly decreased Cdk4 activity at 1 and 6 h exposure compared with control. Because exposure to IR alone and IR/MF caused similar effects, a biological relevance of combined exposure to 1 mT/8 Gy on Cdk4 kinase activity seems to be unlikely.
In conclusion, our data suggest that exposure to 50 Hz, 1 mT MF and/or IR induce a significant increase in total protein expression of p16INK4a and p21CIP1 This, however, does not result in Cdk4/p16INK4a and/or Cdk4/p21CIP1 complex formation. Furthermore, slight modification of Cdk4 kinase activity was detected, although this effect could not be connected to the former observed alterations in the G1 phase, indicating that Cdk4 seems not to be the mediator of MF‐ or IR‐induced changes in cell cycle distribution in AFC cells. Increase in the total amount of p16INK4a and p21CIP1 after MF or IR exposure could be induced by other factors leading to a delay in the G1 phase, such as the impairment of Cdk2, which also plays a pivotal role in regulation of the G1 phase and is of eminent importance for the G1/S transition. To confirm this further studies are required.
ACKNOWLEDGEMENTS
The authors would like to thank Volkmar Henschel for help with the statistical analyses. This work was supported by the Federal Office for Radiation Protection, Salzgitter, Germany (StSch 4129).
REFERENCES
- Auvinen A, Linet MS, Hatch EE, Kleinerman RA, Robison LL, Kaune WT, Misakian M, Niwa S, Wacholder S, Tarone RE (2000) Extremely low‐frequency magnetic fields and childhood acute lymphoblastic leukemia: an exploratory analysis of alternative exposure metrics. Am. J. Epidemiol. 152, 20. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Lukas J, Bartek J (1997) Aberrations of the G1‐ and G1/S‐regulating genes in human cancer. Prog. Cell Cycle Res. 3, 211. [DOI] [PubMed] [Google Scholar]
- Bethwaite P, Cook A, Kennedy J, Pearce N (2001) Acute leukemia in electrical workers: a New Zealand case‐control study. Cancer Causes Control 12, 683. [DOI] [PubMed] [Google Scholar]
- Chazotte‐Aubert L, Pluquet O, Hainaut P, Ohshima H (2001) Nitric oxide prevents gamma‐radiation‐induced cell cycle arrest by impairing p53 function in MCF‐7 cells. Biochem. Biophys. Res. Commun. 281, 766. [DOI] [PubMed] [Google Scholar]
- Cho YH, Chung HW (2003) The effect of extremely low frequency electromagnetic fields (ELF‐EMF) on the frequency of micronuclei and sister chromatid exchange in human lymphocytes induced by benzo(a)pyrene. Toxicol. Lett. 143, 37. [DOI] [PubMed] [Google Scholar]
- Coppock DL, Buffolino P, Kopman C, Nathanson L (1995) Inhibition of the melanoma cell cycle and regulation at the G1/S transition by 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA) by modulation of CDK2 activity. Exp. Cell Res. 221, 92. [DOI] [PubMed] [Google Scholar]
- Demattei M, Caruso A, Pezzetti F, Pellati A, Stabellini G, Sollazzo V, Traina GC (2001) Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect. Tissue Res. 42, 269. [DOI] [PubMed] [Google Scholar]
- Desimone JN, Bengtsson U, Wang X, Lao XY, Redpath JL, Stanbridge EJ (2003) Complexity of the mechanisms of initiation and maintenance of DNA damage‐induced G2‐phase arrest and subsequent G1‐phase arrest: TP53‐dependent and TP53‐independent roles. Radiat. Res. 159, 72. [DOI] [PubMed] [Google Scholar]
- Ding GR, Nakahara T, Tian FR, Guo Y, Miyakoshi J (2001) Transient suppression of X‐ray‐induced apoptosis by exposure to power frequency magnetic fields in MCF‐7 cells. Biochem. Biophys. Res. Commun. 286, 953. [DOI] [PubMed] [Google Scholar]
- Dulic V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, Elledge SJ, Reed SI (1994) p53‐dependent inhibition of cyclin‐dependent kinase activities in human fibroblasts during radiation‐induced G1 arrest. Cell 76, 1013. [DOI] [PubMed] [Google Scholar]
- Gabrielli BG, Sarcevic B, Sinnamon J, Walker G, Castellano M, Wang XQ, Ellem KA (1999) A cyclin D‐Cdk4 activity required for G2 phase cell cycle progression is inhibited in ultraviolet radiation‐induced G2 phase delay. J. Biol. Chem. 274, 13961. [DOI] [PubMed] [Google Scholar]
- Gadbois DM, Lehnert BE (1997) Temporal position of G1 arrest in normal human fibroblasts after exposure to gamma‐rays. Exp. Cell Res. 232, 161. [DOI] [PubMed] [Google Scholar]
- Griefahn B, Kunemund C, Blaszkewicz M, Lerchl A, Degen GH (2002) Effects of electromagnetic radiation (bright light, extremely low‐frequency magnetic fields, infrared radiation) on the circadian rhythm of melatonin synthesis, rectal temperature, and heart rate. Ind. Health 40, 320. [DOI] [PubMed] [Google Scholar]
- Grimison B, Langan TA, Sclafani RA (2000) P16Ink4a tumor suppressor function in lung cancer cells involves cyclin‐dependent kinase 2 inhibition by Cip/Kip protein redistribution. Cell Growth Differ. 11, 507. [PubMed] [Google Scholar]
- Harper JW, Elledge SJ (1996) Cdk inhibitors in development and cancer. Curr. Opin. Genet. Dev. 6, 56. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Shinmura Y, Mizoe H, Yoshizawa H, Yoshida A, Kanao S, Sumitani H, Hasebe S, Motomura T, Yamakawa T, Mizuno F, Otaka Y, Hirose H (2003) No effects of extremely low frequency magnetic fields found on cytotoxic activities and cytokine production of human peripheral blood mononuclear cells in vitro . Bioelectromagnetics 24, 21. [DOI] [PubMed] [Google Scholar]
- Ishido M, Kurokawa Y, Nitta H, Kabuto M (2001) Magnetic fields (MF) of 50 Hz at 1.2 microT as well as 100 microT cause uncoupling of inhibitory pathways of adenylyl cyclase mediated by melatonin 1a receptor in MF‐sensitive MCF‐7 cells. Carcinogenesis 22, 1043. [DOI] [PubMed] [Google Scholar]
- Jiang H, Chou HS, Zhu L (1998) Requirement of cyclin E‐Cdk2 inhibition in p16INK4A‐mediated growth suppression. Mol. Cell Biol. 18, 5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Hung SC, Yamamoto H, Lin J, Nagata A, Okayama H (1999) Oncogenic stimulation recruits cyclin‐dependent kinase in the cell cycle start in rat fibroblast. Proc. Natl Acad. Sci. USA 96, 13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juutilainen J, Lang S, Rytomaa T (2000) Possible cocarcinogenic effects of ELF electromagnetic fields may require repeated long‐term interaction with known carcinogenic factors. Bioelectromagnetics 21, 122. [DOI] [PubMed] [Google Scholar]
- Katsir G, Parola AH (1998) Enhanced proliferation caused by a low frequency weak magnetic field in chick embryo fibroblasts is suppressed by radical scavengers. Biochem. Biophys. Res. Commu. 252, 753. [DOI] [PubMed] [Google Scholar]
- Lange S, Richard D, Viergutz T, Kriehuber R, Weiss DG, Simkó M (2002) Alterations in the cell cycle and in the protein level of cyclin D1p, 21CIP1, and p16INK4a after exposure to 50 Hz MF in human cells. Radiat. Environ. Biophys. 41, 131. [DOI] [PubMed] [Google Scholar]
- Linet MS, Hatch EE, Kleinerman RA, Robison LL, Kaune WT, Friedman DR, Severson RK, Haines CM, Hartsock CT, Niwa S, Wacholder S, Tarone RE (1997) Residential exposure to magnetic fields and acute lymphoblastic leukemia in children. N. Engl. J. Med. 337, 1. [DOI] [PubMed] [Google Scholar]
- Loberg LI, Gauger JR, Buthod JL, Engdahl WR, McCormick DL (1999) Gene expression in human breast epithelial cells exposed to 60 Hz magnetic fields. Carcinogenesis 20, 1633. [DOI] [PubMed] [Google Scholar]
- Lukas J, Sorensen CS, Lukas C, Santoni‐Rugiu E, Bartek J (1999) p16INK4a, but not constitutively active pRb, can impose a sustained G1 arrest: molecular mechanisms and implications for oncogenesis. Oncogene 18, 3930. [DOI] [PubMed] [Google Scholar]
- Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato JY (1994) D‐type cyclin‐dependent kinase activity in mammalian cells. Mol. Cell Biol. 14, 2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreary CR, Thomas AW, Prato FS (2002) Factors confounding cytosolic calcium measurements in Jurkat E6.1 cells during exposure to ELF magnetic fields. Bioelectromagnetics 23, 315. [DOI] [PubMed] [Google Scholar]
- Meyerson M, Harlow E (1994) Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell Biol. 14, 2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehouse CA, Owen RD (2000) Exposure of Daudi cells to low‐frequency magnetic fields does not elevate MYC steady‐state mRNA levels. Radiat. Res. 153, 663. [DOI] [PubMed] [Google Scholar]
- Morgan DO (1995) Principles of CDK regulation. Nature 374, 131. [DOI] [PubMed] [Google Scholar]
- Olivier M, Bautista S, Valles H, Theillet C (1998) Relaxed cell‐cycle arrests and propagation of unrepaired chromosomal damage in cancer cell lines with wild‐type p53. Mol. Carcinog. 23, 1. [PubMed] [Google Scholar]
- Phillips JL, Haggren W, Thomas WJ, Ishida‐Jones T, Adey WR (1993) Effect of 72 Hz pulsed magnetic field exposure on ras p21 expression in CCRF‐CEM cells. Cancer Biochem. Biophys. 13, 187. [PubMed] [Google Scholar]
- Pollan M, Gustavsson P, Floderus B (2001) Breast cancer, occupation, and exposure to electromagnetic fields among Swedish men. Am. J. Ind. Med. 39, 276. [DOI] [PubMed] [Google Scholar]
- Reed MF, Liu VF, Ladha MH, Ando K, Griffin JD, Weaver DT, Ewen ME (1998) Enforced CDK4 expression in a hematopoietic cell line confers resistance to the G1 arrest induced by ionizing radiation. Oncogene 17, 2961. [DOI] [PubMed] [Google Scholar]
- Repacholi MH, Greenebaum B (1999) Interaction of static and extremely low frequency electric and magnetic fields with living systems: health effects and research needs. Bioelectromagnetics 20, 133. [DOI] [PubMed] [Google Scholar]
- Richard D, Lange S, Viergutz T, Kriehuber R, Weiss DG, Simkó M (2002) Influence of 50 Hz magnetic fields in combination with tumour promoting phorbol ester on protein kinase C and cell cycle in human cells. Mol. Cell Biochem. 232, 133. [DOI] [PubMed] [Google Scholar]
- Ruas M, Peters G (1998) The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta 1378, F115. [DOI] [PubMed] [Google Scholar]
- Savell J, Rao S, Pledger WJ, Wharton W (2001) Permanent growth arrest in irradiated human fibroblasts. Radiat. Res. 155, 554. [DOI] [PubMed] [Google Scholar]
- Shahidain R, Mullins RD, Sisken JE (2001) Calcium spiking activity and baseline calcium levels in ROS 17/2.8 cells exposed to extremely low frequency electromagnetic fields (ELF EMF). Int. J. Radiat. Biol. 77, 241. [DOI] [PubMed] [Google Scholar]
- Sherr CJ (1996) Cancer cell cyclins. Science 274, 1672. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM (1995) Inhibitors of mammalian G1 cyclin‐dependent kinases. Genes Dev. 9, 1149. [DOI] [PubMed] [Google Scholar]
- Simkó M, Kriehuber R, Lange S (1998) Micronucleus formation in human amnion cells after exposure to 50 Hz MF applied horizontally and vertically. Mutat. Res. 418, 101. [DOI] [PubMed] [Google Scholar]
- Simkó M, Richard D, Kriehuber R, Weiss DG (2001) Micronucleus induction in syrian hamster embryo cells following exposure to 50 Hz magnetic fields, benzo(a)pyrene, and TPA in vitro . Mutat. Res. 495, 43. [DOI] [PubMed] [Google Scholar]
- Supino R, Bottone MG, Pellicciari C, Caserini C, Bottiroli G, Belleri M, Veicsteinas A (2001) Sinusoidal 50 Hz magnetic fields do not affect structural morphology and proliferation of human cells in vitro . Histol. Histopathol 16, 719. [DOI] [PubMed] [Google Scholar]
- Tam SW, Theodoras AM, Shay JW, Draetta GF, Pagano M (1994) Differential expression and regulation of cyclin D1 protein in normal and tumor human cells: association with Cdk4 is required for cyclin D1 function in G1 progression. Oncogene 9, 2663. [PubMed] [Google Scholar]
- Tian F, Nakahara T, Yoshida M, Honda N, Hirose H, Miyakoshi J (2002) Exposure to power frequency magnetic fields suppresses X‐ray‐induced apoptosis transiently in Ku80‐deficient xrs5 cells. Biochem. Biophys. Res. Commun. 292, 355. [DOI] [PubMed] [Google Scholar]
- Uk Childhood Cancer Study Investigators. (1999) Exposure to power‐frequency magnetic fields and the risk of childhood cancer. Lancet 354, 1925. [PubMed] [Google Scholar]
- Weiderpass E, Vainio H, Kauppinen T, Vasama‐Neuvonen K, Partanen T, Pukkala E (2003) Occupational exposures and gastrointestinal cancers among Finnish women. J. Occup. Environ. Med. 45, 305. [DOI] [PubMed] [Google Scholar]
- Weinberg RA (1995) The retinoblastoma protein and cell cycle control. Cell 81, 323. [DOI] [PubMed] [Google Scholar]
- Wharton W (1995) Cell cycle constraints on peroxide‐ and radiation‐induced inhibitory checkpoints. Cancer Res. 55, 5069. [PubMed] [Google Scholar]


