Abstract
Objectives
This study aimed to investigate the influence of hypoxia on angiogenesis in a 3D gel, with co‐culturing adipose‐derived stromal cells (ASCs) and endothelial cells (ECs).
Materials and methods
ASCs from green fluorescent protein‐labeled mice and ECs from red fluorescent protein‐labeled mice were co‐cultured in 3D collagen gels at 1:1 ratio, in normal and hypoxic oxygen conditions, and morphology of angiogenesis was observed using confocal laser scanning microscopy. To discover changes in growth factors between monoculture ASCs and ECs, transwell co‐cultures of ASCs and ECs were applied. Semi‐quantitative PCR was performed to explore mRNA expression of growth factors.
Results
Enhanced angiogenesis was observed in 3D gels implanted with 1:1 mixture of ASCs and ECs after 7 days hypoxia. Genes including VEGFA/B,EGF‐1,HIF‐1a,IGF‐1,PDGF,TGF‐β1 and BMP‐2/4 in ECs, both monoculture and co‐culture, were significantly enhanced after being cultured under hypoxia. In comparison, genes VEGFA/B,EGF‐1,HIF‐1a, TGF‐β1 and BMP‐2 in ASCs increased. In all, factors VEGFA/B, EGF‐1, HIF‐1a, TGF‐β1 and BMP‐2 increased in both ASCs and ECs after being cultured in hypoxia no matter whether as monoculture or co‐culture.
Conclusions
Co‐culture of ASCs and ECs at 1:1 ratio in a 3D gel under hypoxia promoted angiogenesis. Those growth factors which were increased in both ASCs and ECs, indicate that VEGFA/B,EGF‐1, HIF‐1a, TGF‐β1 and BMP‐2 might be responsible for enhancement in angiogenesis triggered by hypoxia.
Introduction
The application of adult's own stem cells can not only solve the problem of cell source, but also avoid the immune rejection concerns, moral and ethical controversy and other irreducible aspects 1. ASCs which are isolated from subcutaneous fat have successfully caught researchers’ attention since 2001, Zuk 2. ASCs like other stem cells can obtain stable and high proliferation ability in vitro under certain conditions 3. Many researches on ASCs indicate that ASCs could be differentiated into adipose 4, bone 5, cartilage 5, muscle 6, epithelial cells 7, myocardial cells 8, nerve cells 9 and hepatocytes 10 direction when induced in vitro under specific conditions. It is also acknowledged that ASCs still maintain the ability of differentiation after long time being cultured 3. Therefore, ASCs have been successfully applied into tissue repair and regeneration. Our research group had tested the stem cell properties of ASC that obtained from adipose tissue. The method of obtaining ASC is mature in our group.
Moreover, it is well known that the aim of tissue engineering is to create functional tissues and organs, vascularization becomes one of the key challenges in tissue engineering 11. In current strategies to create vascularized tissues, one is based on the endothelial cells for their ability to form new vessels known as neoangiogenesis. There are many approaches in vascularization techniques by using various biomolecules, such as growth factors, cytokines, peptides and proteins as well as cells 12. We also have acknowledged that co‐implantation of ASCs with ECs can stimulate the formation of vascular network 8, 13, 14. Therefore, in this study we apply the co‐culturing ASCs and ECs into 3D gel to observe the change of vessel network.
Most cell cultures are maintained in vitro at air like condition which is almost 20% oxygen. However, cells in natural microenvironment are maintained much lower oxygen tensions: the mean oxygen concentration of arterial blood almost 12%, and that of tissue is approximates 3%, with variation depended on different locations 15. It is illustrated that oxygen status is critical determinant of tumor angiogenesis and also influenced cellular functions, including metabolism, survival rate, proliferation, migration and angiogenesis 16. It is also known that the cells in hypoxia (2% oxygen) shows improved proliferation, migration, and secretion of growth factors, moreover, it has been found that hypoxia is a strong stimulus to promote angiogenesis in vitro 17. And all of these cellular functions are directly or indirectly controlled by a master transcription factor called hypoxia‐inducible factor‐1a (HIF‐1a) 18. As culturing ASCs in hypoxia is rarely reported especially co‐culture with ECs, in this study we first establish an angiogenesis model by 3D collagen gel with co‐culturing ASCs and ECs at 1:1 ratio at normal and hypoxia conditions. We then detect the gene expressions of vascular endothelial growth factor A and B (VEGFA and B), and investigate the gene profile of relevant growth factors (epidermal growth factor‐1 (EGF‐1), fibroblast growth factor‐1/‐2 (FGF‐1/‐2), hypoxia inducible factor‐1a (HIF‐1a), insulin‐like growth factor‐1 (IGF‐1), platelet‐derived growth factor (PDGF), transforming growth factor‐beta1 (TGF‐β1), VE‐cadherin (VE‐ca), Vinculin (Vcl), and bone morphogenetic proteins‐2/‐4/‐5/‐6/‐7BMPs (BMP‐2, ‐4, ‐5, ‐6, ‐7)) in both ASCs and ECs. By confirming the key growth factors induced by hypoxia participated in promoting angiogenesis, we hope to detect those relevant growth factor changes under hypoxia in the formation of the vascular system.
Methods and materials
Cell culture
The animal materials used in this study were obeyed according to ethical principles and the protocol was reviewed and permitted by our Institutional Review Board (IRB). The methods to obtain ASCs and ECs were mature and formed as a standard way in our study group.
The ASCs that we used were obtained from subcutaneous adipose tissue of 4‐week female mouses. Then, the collected subcutaneous adipose tissue was cut into small pieces and trypsinised (0.25%) for 30 min at 37 °C. The trypsin‐contained supernatant was then removed and replaced with 0.5% collagenase type I for 3 h at 37 °C. The collagenase type I‐treated solution (ASCs suspension) was collected and mixed at 1:1 (v/v) with fresh 10% heat‐activated foetal bovine serum (FBS) a‐MEM (0.1 mM nonessential amino acids, 4 mm L‐glutamine, 1% penicillin‐streptomycin solution). The mixed suspension was centrifuged at 1,000 rpm for 5 min. After removing the supernatant, the 10% FBS a‐MEM was added into centrifuge tube to resuspend the ASCs. Then, the suspended ASCs were seeded into flasks at 37 °C in a humidified atmosphere of 5% CO2 till usage no matter used under normal and hypoxia condition or mono‐culture and co‐culture. The purified ASCs could be obtained the same nature after two passages as previously described 19.
In order to obtain purified ECs in a high density, brain microvascular tissue was collected from neonatal mouses. The microvascular tissue was cut into small pieces and trypsinised (0.25%) for 30 min at 37 °C. The trypsin‐contained supernatant was then removed and replaced with 0.5% collagenase type II for 3 h at 37 °C. The collagenase type II‐treated solution (ASCs suspension) was collected and mixed 1:1 (v/v) with fresh 10% heat‐activated foetal bovine serum (FBS) DMEM (high‐glucose DMEM, 0.1 mm nonessential amino acids, 4 mm L‐glutamine, 1% penicillin‐streptomycin solution). The mixed suspension was centrifuged at 1000 rpm for 5 min. After removing the supernatant, the 10% FBS DMEM was added into centrifuge tube to resuspend the ECs. The suspended ECs were seeded into plates at 37 °C in a humidified atmosphere of 5% CO2 till usage no matter used under normal and hypoxia condition or mono‐culture and co‐culture.
To acquire green fluorescent protein (GFP)‐positive ASCs and DsRed‐Express‐positive ECs, the subcutaneous adipose tissue and brain microvascular tissue were collected from enhanced GFP transgenic mice (The Centre of Genetically Engineered Mice, West China Hospital, Sichuan University, Chengdu, China) and the DsRed‐Express transgenic mice (The Genetic Centre of Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences and Centre of Comparative Medicine, Peking Union Medical College, Beijing, China), separately. The cell isolation and culture were the same as described above.
Mono‐culture and Co‐culture at normal oxygen and hypoxia
The cell‐cell co‐culture between GFP‐ASCs and RFP‐ECs was carried out in a 3D collagen gel model. ASCs and ECs were mixed at a 1:1 ratio and suspended in DMEM‐HG (Hyclone, Logan, UT, USA) and rat tail tendon collagen type I (Shengyou Biotechnology, Hangzhou, China). The seeded cells were divided into two groups, one is placed under normal oxygen condition while the other is placed under hypoxia condition (the oxygen concentrations is 2%). The two groups seeded cells were transferred into 96‐well plates to form 3D gel samples in 37 °C and cultured for 1 week.
To detect the gene profile of growth factors in ASCs and ECs both under normal and hypoxia condition, so the transwell co‐culture system is applied into this study. Firstly, ECs were allowed to equilibrate for 24 h. The culture media were then replaced with 2% FBS DMEM for a 12 h starvation. The ECs which were seeded onto six‐well plates were about 1–5 × 106 cells per well (85–95% confluence). The seeded ECs were split into four groups namely, the normal mono‐culture group, the normal co‐culture group, the hypoxia mono‐culture group, the hypoxia co‐culture group. The media of the mono‐culture ECs were replaced with fresh 1% FBS DMEM both in the normal condition and hypoxia condition; meanwhile, the transwell chamber with no ASCs was filled with 1% FBS a‐MEM for culture media control. As counterpart, the media of the co‐culture ECs were replaced with fresh 1% FBS DMEM and the transwell chamber was also filled with 1% FBS a‐MEM but ASCs were synchronously seeded on to the chamber at the beginning of ECs seeding. As for ASCs, there still contain two mono‐culture groups which is mono‐culture under normal condition and hypoxia condition. Similarly, ASCs were allowed to equilibrate for 24 h. The culture media were then replaced with 2% FBS a‐MEM for a 12 h starvation. The ASCs seeded onto six‐well plates were also about 1–5 × 106 cells per well (85–95% confluence). The media of the two groups of mono‐culture ASCs were replaced with fresh 1% FBS a‐MEM and the transwell chamber was also filled with 1% FBS DMEM. The cell lysate samples (1000 μl) of ASCs and ECs were collected at 7 days and repeated 4 times after mono‐culture and co‐culture under normal and hypoxia condition.
Confocal laser scanning microscopy (CLSM) for images of co‐cultured 3D model
The morphologies of normal and fluorescent ASCs and ECs were observed and captured by using IX71 inverted microscope (Olympus, Tokyo, Japan).
The morphologies of 3D vascular like structures in collagen gel were inspected and scanned by a Leica DMIRE2 confocal laser scanning microscope (TCS SP2, Leica Microsystems, Wetzlar, Germany, parameter: 20×, Leica Microsystems original image: 1024 × 1024, 100 μm) equipped with a 60× oil immersion objective lens. The 3D images were analysed by software‐Imaris 7.0.0 (Bitplane, Zurich, Switzerland). The main data was got by three‐dimensional reconstruction.
Semi‐quantitative polymerase chain reaction (PCR)
The RNA samples of ASCs and ECs from the four groups were isolated using the RNeasy Plus Mini Kit (Qiagen, Shanghai, China) with a genomic DNA eliminator. The isolated RNA was then dissolved in RNase‐free water and quantified according the absorbance at 260 nm with a spectrophotometer. The RNA samples from the four groups were then disposed with DNase I (Mbi, Glen Burnie, MD, USA) and cDNA was set up from each sample, using 0.5 μg of total RNA and cDNA synthesis kit (Mbi) in a final volume of 20 μl.
To estimate the expression levels of growth factors and BMPs family in different treated groups as normalised to the glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) and beta‐actin (β‐actin), semi‐quantitative PCR was performed with a PCR kit (Mbi) by a thermo‐cycler (Bio‐Rad, Hercules, CA, USA). The selected sets of primers are exhibited in table 1. BLAST was used to search for all primer sequences to ensure gene specificity. Semi‐quantitative PCR reactions were carried out in a 25 μl volume comprising a 1 μl cDNA sample. The PCR program including a 30 s denaturisation cycle at 94 °C, a 30 s annealing cycle at 55–65 °C and 72 °C, a 30 s elongation cycle, 28–32 amplification cycles. The final products were then resolved by 2% agarose gel electrophoresis in trisborate/ethylenediaminetetraacetic acid (EDTA) buffer and visualised by staining with ethidium bromide.
Table 1.
Primers of housekeeping genes (GAPDH and β‐actin) and related growth factor genes designed for semi‐quantitative PCR
| mRNA | Primer pairs |
|---|---|
| GAPDH (233 bp) |
Forward GGTGAAGGTCGGTGTGAACG Reverse CTCGCTCCTGGAAGATGGTG |
| β‐ACTIN (266 bp) |
Forward GTCCCTCACCCTCCCAAAAG Reverse GCTGCCTCAACACCTCAACCC |
| VEGFA (106 bp) |
Forward CTGCTGTGGACTTGTGTTGG Reverse AAAGGACTTCGGCCTCTCTC |
| VEGFB (128 bp) |
Forward GCAACACCAAGTCCGAATG Reverse CTGGCTTCACAGCACTCTCC |
| TGF‐β1(113 bp) |
Forward TGGAGCCTGGACACACAGTA Reverse TAGTAGACGATGGGCAGTGG |
| IGF‐1(171 bp) |
Forward CTGCTTGCTCACCTTCACC Reverse TCATCCACAATGCCTGTCTG |
| HIF‐1α (129 bp) |
Forward TGAACATCAAGTCAGCAACG Reverse CACAAATCAGCACCAAGCAC |
| EGF‐1(112 bp) |
Forward GCTCTTCTGGGTTCAGGACA Reverse AGACAAACTGTGCCGTGCTT |
| VE‐ca (102 bp) |
Forward CATCGCAGAGTCCCTCAGTT Reverse TCAGCCAGCATCTTGAACCT |
| Vcl(122 bp) |
Forward GTCTGTGAGCGAATCCCAAC Reverse ATGAACCAGCATCTCTGTGG |
| PGF(107 bp) |
Forward CCGATAAAGACAGCCAACATC Reverse CATTCACAGAGCACATCCTGA |
| FGF‐1(124 bp) |
Forward CCACAGCCCAGCAGTTATC Reverse CTCCTACGCCCACTCTTCAG |
| FGF‐2(138 bp) |
Forward AGGAAGATGGACGGCTGCT Reverse GCCCAGTTCGTTTCAGTGC |
| BMP‐2(106 bp) |
Forward TGAGGATTAGCAGGTCTTTGC Reverse CGTTTGTGGAGCGGATGT |
| BMP‐4(103 bp) |
Forward TCTTCAACCTCAGCAGCATC Reverse AAGCCCTGTTCCCAGTCAG |
| BMP‐5(100 bp) |
Forward CACCAGGGAAACAAGCATCT Reverse CTCACCAAATACTCCGACTCCT |
| BMP‐6(124 bp) |
Forward AGGTTCCATTCCCAGCAAG Reverse TCACACCACCGAGAGTCAAC |
| BMP‐7(101 bp) |
Forward TCCTGCATCCACACAAAGAA Reverse TTCCAGGGACACAGACATGA |
Statistical analysis
All experiments were executed at 4 separate times. Statistical analysis of data was performed with SPSS 16.0 (IBM, Silicon Valley, CA, USA) using one‐way ANOVA to compare the means of all groups, and Student–Newman–Keuls (SNK‐q) test to compare the means of each two groups. If the two‐tailed P value was <0.05, it can be considered that data were significantly different.
Results
Hypoxia promotes the formation of vessel‐like structure in 3D collagen gel
We first established a 3D collagen gel model with co‐culturing green fluorescent protein‐labeled ASCs and red fluorescent protein‐labeled ECs in both normal and hypoxia condition. We then found hypoxia could significantly induced the formation of vessel‐like structures more than that in normal condition in 3D gel (Fig. 1a) by 3D construction post 20‐layer CLSM scaning. After quantification analysis, we found hypoxia could induced longer (up to 4.68‐fold) and wider (up to 2.13‐fold) (Fig. 1b) vessel‐like structure compared to that of normal conditions, respectively. Further, after 7 days co‐culture, we found more network connection formation in the hypoxia conditioned group (Fig. 1a, right lane).
Figure 1.
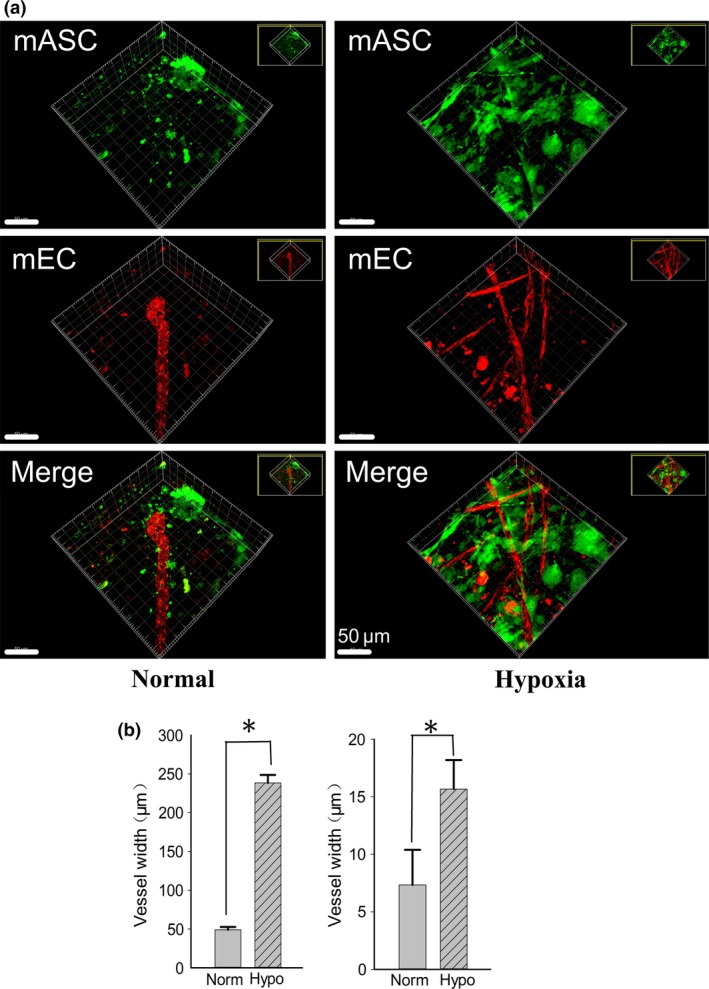
Hypoxia promotes angiogenesis in ASC ‐ EC co‐cultured 3D gel. (a) Morphologies of vessel‐like structure in co‐cultured fluorescent adipose stromal cells (ASCs) and endothelial cells (ECs) in 3D gels under normal and hypoxia condition. The experiments by modified CLSM were repeated at least three times (n = 3), the 3D images were constructed by Imaris 7.0.0. (b) Histograms showing comparsion of the vessel length and vessel width formed in 3D gel between normal and hypoxia condition.
Hypoxia up‐regulated the expressions of VEGF genes in both ASCs and ECs in co‐culture system
In order to explore the variation of angiogenesis‐related genes in each 3D gel, we compared VEGFA and B between the normal and hypoxia condition. The transwell chambers were used to achieve non‐contact co‐culture between ASCs and ECs. After 7 days’ co‐culture by using semi‐quantitative PCR, we found that VEGFA and B in ASCs and ECs were all up‐regulated in hypoxia condition both in the mono‐culture and co‐culture groups (Fig. 2a). After quantification by OD method with Quantity One 4.6.3 software, we found that in the hypoxia mono‐cultured ECs group VEGFA and B were up to 1.60‐fold and 1.88‐fold relative to the normal mono‐cultured group, respectively. Meanwhile, in the co‐cultured group, VEGFA and B were up to 2.32‐fold and 2.33‐fold in hypoxia condition and up to 2.01‐fold and 1.48‐fold in normal condition relative to the normal mono‐cultured group. As for the ASCs groups, we found that in the hypoxia mono‐cultured ASCs group VEGFA and B were up to 1.63‐fold and 1.34‐fold relative to the normal mono‐cultured group, respectively. Moreover, in the co‐cultured group, VEGFA and B were up to 2.30‐fold and 1.77‐fold in hypoxia condition and up to 1.37‐fold and 1.36‐fold in normal condition relative to the normal mono‐cultured group (Fig. 2b).
Figure 2.
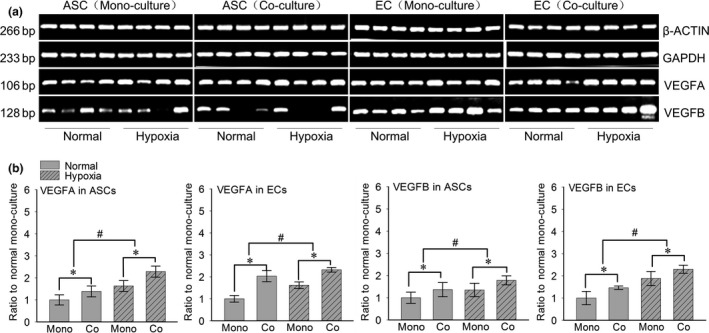
Hypoxia increases the gene expressions of VEGFA/B in both ASC s and EC s. (a) Semi‐quantitative PCR showed increased gene expressions of VEGFA/B in both ASCs and ECs. Housekeeping genes (GAPDH and β‐ACTIN) were set as the inner controls. The samples were taken at 7 days. Four lanes in each group represent the four repeats in an individual experiment. The gels shown were representative of three experiments (n = 3). (b) The fold changes were calculated by OD method with Quantity One 4.6.3 software (bio‐Rad) based on the semi‐quantitative PCR (Fold changes represented the mean average ratio of the other three groups to the normal mono‐culture group). #Significant difference with respect to the normal mono‐culture group control (P < 0.05).
Hypoxia induced gene expression of relevant growth factors in both ASCs and ECs in co‐culture system
In order to screen growth factors which might function as angiogenesis‐related genes, we detected the expression of EGF, FGF‐1/‐2, HIF‐1a, IGF‐1, PDGF, TGF‐β1, VE‐ca and Vcl between the normal and hypoxia condition. After 7 days’ co‐culture by using semi‐quantitative PCR, we found that EGF and HIF‐1a in ASCs and ECs were all up‐regulated in hypoxia condition in both the mono‐culture and co‐culture groups but we did not detect the expression of FGF‐1 in ECs. (Fig. 3a). After quantification by OD method, we found that in the hypoxia mono‐cultured ECs group EGF‐1, TGF‐β1 showed a slightly increase (EGF‐1 was as high as 1.43‐fold in the hypoxia mono‐cultured group relative to the normal mono‐cultured group, TGF‐β1 was up to 1.37‐fold in the hypoxia mono‐cultured group relative to the normal mono‐cultured group) (Fig. 3b). FGF‐2, Vcl and VE‐ca showed no significant variation; HIF‐1a, IGF‐1 and PDGF all showed significant increases (HIF‐1a was up to 1.72‐fold in the hypoxia mono‐culture group relative to the normal mono‐culture group, IGF‐1 was up to 2.34‐fold, PDGF was up to 3.82‐fold total quantification), Vcl showed a slightly increase (Vcl was up to 1.21‐fold in the hypoxia mono‐culture group relative to the normal mono‐culture group). Meanwhile, in the co‐cultured ECs group, EGF‐1, FGF‐2, HIF‐1a, IGF‐1, TGF‐β1, PDGF and VE‐ca all showed significant increases (EGF‐1 was as high as 1.93‐fold in the hypoxia co‐culture group relative to the normal mono‐culture group, FGF‐2 was up to 2.34‐fold, HIF‐1a was up to 1.56‐fold, IGF‐1 was up to 2.20‐fold, PDGF was up to 3.99‐fold, TGF‐β1 was up to 1.66‐fold total quantification and VE‐ca was up to 2.28‐fold).
Figure 3.
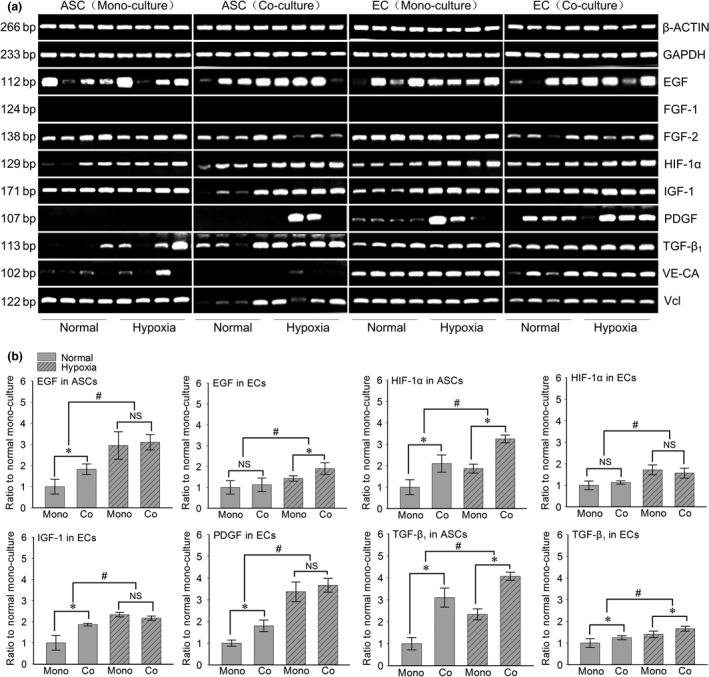
Hypoxia induced the different gene expressions of growth factors in both ASC s and EC s in co‐culture system. (a) Semi‐quantitative PCR showed the increased gene expressions of EGF‐1, HIF‐1α and TGF‐β1 in both ASCs and ECs, while IGF‐1 and PDGF in ECs. Housekeeping genes (GAPDH and β‐ACTIN) were set as the inner controls. The samples were taken at 7 days. Four lanes in each group represent the four repeats in an individual experiment. The gels shown were representative of three experiments (n = 3). (b) The fold changes were calculated by OD method with Quantity One 4.6.3 software (bio‐Rad) based on the semi‐quantitative PCR (Fold changes represented the mean average ratio of the other three groups to the normal mono‐culture group). #Significant difference with respect to the normal mono‐culture group control (P < 0.05).
As for ASCs groups, FGF‐1 and PDGF were not detected. In the hypoxia mono‐culture ASCs group, we found that EGF‐1, HIF‐1a and TGF‐β1 showed significant increases (EGF‐1 was as high as 2.95‐fold in the hypoxia mono‐culture group relative to the normal mono‐culture group, HIF‐1a was up to 1.86‐fold in the hypoxia mono‐culture group, TGF‐β1 was up to 4.63‐fold in the hypoxia mono‐culture group), but IGF‐1 and VE‐ca showed no significant variation. Meanwhile FGF‐2 and Vcl were decreased. In the hypoxia co‐culture ASCs group, we found that EGF‐1, HIF‐1a, TGF‐β1, Vcl and VE‐ca showed significant increases (EGF‐1 was up to 3.20‐fold in the hypoxia co‐culture group, HIF‐1a was up to 3.27‐fold, TGF‐β1 was up to 5.36‐fold, Vcl was up to 1.53‐fold and VE‐ca was up to 2.26‐fold), but FGF‐2 showed decreasing, EGF‐1 did not expressed. As for other growth factors, i.e. IGF‐1, PDGF and FGF‐1 were all increased at different level.
Hypoxia also induced the changes of BMP genes in both ASCs and ECs in co‐culture system
We also detected the gene variations of BMPs family (Fig. 4a). In our study we did not detect BMP‐5 and BMP‐7 in ASCs and ECs, moreover BMP‐4 was also not detected in ASCs. In ECs the hypoxia mono‐culture group, BMP‐2 and BMP‐4 showed significant changes (BMP‐2 was as high as 2.49‐fold in the hypoxia mono‐culture group relative to the normal mono‐culture group, meanwhile BMP‐4 was up to 1.32‐fold in the hypoxia mono‐culture group) (Fig. 4b), but BMP‐6 was not increased. In ECs the hypoxia co‐cultured group, we still found that BMP‐2 and BMP‐4 showed significant changes (BMP‐2 was as high as 4.51‐fold in the hypoxia co‐culture group relative to the normal mono‐culture group, meanwhile BMP‐2 was up to 3.05‐fold in the normal co‐culture group relative to the normal mono‐culture group, BMP‐4 was up to 1.93‐fold in the hypoxia co‐culture group relative to the normal mono‐culture group and in the normal co‐culture group BMP‐4 was up to 1.53‐fold), but BMP‐6 was even decreasing after under hypoxia condition. In ASCs the mono‐culture and co‐culture groups, BMP‐2 showed significant changes (BMP‐2 was as high as 1.76‐fold in the hypoxia mono‐culture group relative to the normal mono‐culture group, while in the hypoxia co‐culture group BMP‐2 was up to 2.25‐fold relative to the normal mono‐culture group), but BMP‐6 was only significantly enhanced in the ASCs co‐cultured group, there was no change in the mono‐culture group.
Figure 4.
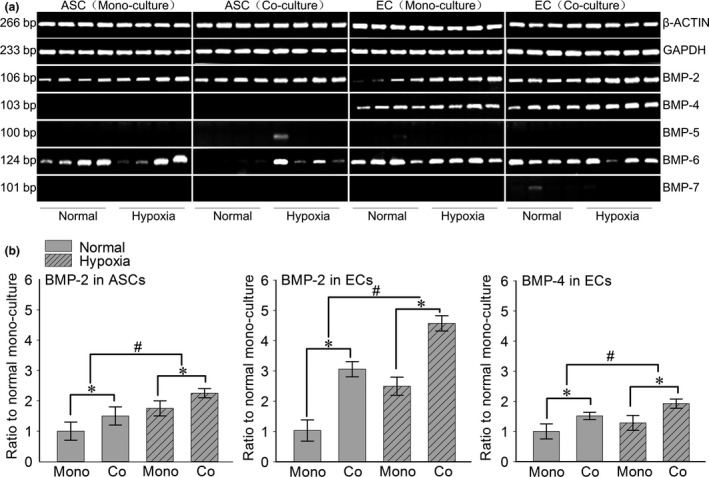
Hypoxia induced the different gene expressions of BMPs in both ASC s and EC s in co‐culture system. (a) Semi‐quantitative PCR showed increased gene expressions of BMP‐2 in both ASCs and ECs, while BMP‐4 was increased only in ECs. Housekeeping genes (GAPDH and β‐ACTIN) were set as the inner controls. The samples were taken at 7 days. Four lanes in each group represent the four repeats in an individual experiment. The gels shown were representative of three experiments (n = 3). (b) The fold changes were calculated by OD method with Quantity One 4.6.3 software (bio‐Rad) based on the semi‐quantitative PCR (Fold changes represented the mean average ratio of the other three groups to the normal mono‐culture group). #Significant difference with respect to the normal mono‐culture group control (P < 0.05).
Discussion
In modern medicine, tissue engineering and regenerative medicine are too important to emphasize. Obviously, the application of stem cells is very common since iPS (induced pluripotent stem cell) appeared 20. ASCs, as adult stem cells, are one of the most promising stem cells because they obtain stable and high proliferation ability under certain conditions 21, 22, furthermore human adipose tissue is ubiquitous and easily obtained with little donor site morbidity and patient discomfort 21. Therefore, ASCs as research tools are popular and have been shown to be both safe and effective in preclinical and clinical researches. Angiogenesis plays an important role in many physiological processes, including tissue growth, proliferation, and progression 23, 24, 25.
In our previous reports 13, 14, the mECs and mASCs were acquired from normal and fluorescent protein‐labeled mice. The isolated ECs were then identified by factor III (FIII) immunofluorescence and the ASCs were identified by stromal cell marker CD34, CD146, Sca‐1 and CD44, moreover they showed the abilities of adipogenic and osteogenic differentiation. Our research group had confirmed that at normal condition the co‐culture between mECs and mASCs could enhance the formation of vessel‐like structures. The phenomenon that the formation of vascular‐like structures increased after co‐culturing ECs with ASCs comparing with monolayer ECs was observed. The conclusion was in accordance with other researches’ that ASCs promoted angiogenesis by interacting with ECs. Knowing that angiogenesis could be triggered by various stresses for instance hypoxia, moreover hypoxia was considered to be the strongest inducer both in vitro and in vivo 26, 27. Even a number of papers 28, 29 have described ASCs are able to enhance angiogenesis when injected into ischemia tissue. Then in this study, we placed the co‐culturing system in hypoxia condition (2% oxygen), and finally we found that the formation of vascular like structures was more than that in normal condition. The observed phenomenon was in accord with other researchers’ results that hypoxia is a stimulus to promote angiogenesis; moreover, we had detected the change of the relevant growth factors and BMPs.
VEGF which serves as a major angiogenic factor in normal cardiac development is recognized as the most important and notable growth factor involved in angiogenesis 30. VEGF has been mainly associated with initiating the process of angiogenesis through the recruitment and proliferation of endothelial cells 31. In our study, the detected VEGFA and B were both increased in ASCs and ECs. The results agree with the phenomenon that observing enhanced vessel networks and promoted angiogenesis under hypoxia condition and also illustrate that VEGF expression can be regulated by hypoxia.
In response to hypoxia, the HIF‐1a which is a heterodimeric transcription factor and can be induced by hypoxia is increased as a symbol 32. In our study, it was also found that HIF‐1a increased in the hypoxia groups. Some researches illustrated that exposure to hypoxia significantly accelerated the rate of tubular morphogenesis by HIF‐1a regulating VEGF expression at the transcriptional level. Moreover some researchers supposed the reason might because there was also a sufficient level of angiogenic factors due to inflammation stimulated by hypoxia 3, 33. In our study, we had screened some other relevant growth factors and confirmed the enhanced ones in both ASCs and ECs after being cultured under hypoxia condition in angiogenesis. After analysing the results, we founded that in ECs the related factors such as VEGFA and B, HIF‐1a, EGF‐1, IGF‐1, PDGF and TGF‐β1 were significantly increasing under hypoxia condition. While in ASCs the related factors such as VEGFA and B, HIF‐1a, EGF‐1 and TGF‐β1 were significantly increasing under hypoxia condition. It was inferred that in the progress of angiogenesis stimulated by hypoxia, the factors including VEGFA and B, HIF‐1a, EGF‐1 and TGF‐β1 all played important role in ASCs and ECs about vascular formation, besides IGF‐1 and PDGF also promoted angiogenesis in ECs under hypoxia condition. Therefore, the main factors in up‐regulating angiogenesis processes stimulated by hypoxia were VEGFA and B, HIF‐1a, EGF‐1 and TGF‐β1 in this study.
As for the growth factors such as EGF‐1 and TGF‐β1, they were thought to influence angiogenesis by regulating VEGF 34. Other scholars verified that such growth factors achieved their effects by binding to their specific cell‐surface receptor, such as EGF receptor (EGFR), for initiating intracellular signaling cascades 35. In a study, the researchers found that ascofuranone inhibited angiogenesis by suppressing EGF‐1‐induced HIF‐1a and VEGF expression, thus they implied that the expression of HIF‐1a and VEGF could be regulated by EGF‐1 36. As for TGF‐β1, researchers considered it as a kind of indirect angiogenic factor giving that the synthesis of VEGF could be stimulated by TGF‐β1 37. The increased expression of EGF‐1 and TGF‐β1 in our study agreed with the other previous studies.
BMPs as osteoinductive growth factors also play an important role in angiogenesis 38. It has been reported that application of BMPs in vivo not only increases bone formation, but also enhances angiogenesis 14. It was reported that BMP‐2 could induce new vessel formation through stimulation of VEGF, besides VEGF expression could also be induced by BMP‐2/4 (BMP‐2 and BMP‐4 are highly homologous) 39. In our investigation, ASC co‐culture promoted the gene expressions of BMP‐2 and BMP‐4 in ECs.
In all, the factors including growth factors and BMPs family, means that VEGFA and B, EGF‐1, HIF‐1a, TGF‐β1 and BMP‐2 were increased in both ASCs and ECs after being cultured in hypoxia condition no matter mono‐culture or co‐culture.
It is necessary to mention that there are still some limitations in this study. Firstly, the hypoxia condition that the fraction of oxygen was set as 2%, but which fraction of oxygen is best for promoting angiogenesis is unknown. This test content will be conducted in our next in‐depth exploration about hypoxia. Secondly, the screened growth factor profile was based on the common gene‐bank, the other unscreened growth factors may also play a vital role in angiogenesis under hypoxia condition. Our study group has studied the research about IGF‐1 and got the results that adding IGF‐1 enhanced the angiogenesis compared with the control group. In our next work we will explore the increased growth factors and get more detail about hypoxia promoting angiogenesis. Moreover the relationship among the increased factors will be tested in the further study. Thirdly, the 3D gel angiogenesis system we made is a kind of cell–cell contact cross‐talk. As for detecting the gene expressions changes between ASCs and ECs, transwell co‐culture system is non‐contact. There will some difference between contact and non‐contact co‐cultures system. Fourthly, as we have known that the aim of tissue engineering is to create functional tissues and organs, vascularization is the key challenges in tissue engineering. The novelty of this study that enhancing angiogenesis may own its potential application in promote tissue regeneration. However, there will be many explorations need to be done. Based on this study we only detected the hypoxia‐induced angiogenesis on ECs, the non‐vasculature cells types will be considered in our further study.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
This work was funded by National Natural Science Foundation of China (81201211, 81471803).
Qiang Xie and Jing Xie contributed equally to this work.
References
- 1. Daar AS, Bhatt A, Court E, Singer PA (2004) Stem cell research and transplantation: Science leading ethics. Transpl. Proc. 36, 2504–2506. [DOI] [PubMed] [Google Scholar]
- 2. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ et al (2001) Multilineage cells from human adipose tissue: implications for cell‐based therapies. Tissue Eng. 7, 211–228. [DOI] [PubMed] [Google Scholar]
- 3. Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A (2004) Improvement of postnatal neovascularization by human adipose tissue‐derived stem cells. Circulation 110, 349–355. [DOI] [PubMed] [Google Scholar]
- 4. Cignarelli A, Perrini S, Ficarella R, Peschechera A, Nigro P, Giorgino F (2012) Human adipose tissue stem cells: relevance in the pathophysiology of obesity and metabolic diseases and therapeutic applications. Expert Rev. Mol. Med. 14, e19. [DOI] [PubMed] [Google Scholar]
- 5. Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A et al (2007) Cytokine profile of human adipose‐derived stem cells: expression of angiogenic, hematopoietic, and pro‐inflammatory factors. J. Cell. Physiol. 212, 702–709. [DOI] [PubMed] [Google Scholar]
- 6. Merfeld‐Clauss S, Lupov IP, Lu H, Feng D, Compton‐Craig P, March KL et al (2014) Adipose stromal cells differentiate along a smooth muscle lineage pathway upon endothelial cell contact via induction of activin A. Circ. Res. 115, 800–809. [DOI] [PubMed] [Google Scholar]
- 7. Drexler SK, Bonsignore L, Masin M, Tardivel A, Jackstadt R, Hermeking H et al (2012) Tissue‐specific opposing functions of the inflammasome adaptor ASC in the regulation of epithelial skin carcinogenesis. Proc. Natl Acad. Sci. USA 109, 18384–18389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao Y, Sun Z, Liao LM, Meng Y, Han Q, Zhao RCH (2005) Human adipose tissue‐derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 332, 370–379. [DOI] [PubMed] [Google Scholar]
- 9. Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M (2014) Stimulating the neurotrophic and angiogenic properties of human adipose‐derived stem cells enhances nerve repair. Stem Cells Dev. 23, 741–754. [DOI] [PubMed] [Google Scholar]
- 10. Xu D, Nishimura T, Zheng M, Wu M, Su H, Sato N et al (2014) Enabling autologous human liver regeneration with differentiated adipocyte stem cells. Cell Transplant. 23, 1573–1584. [DOI] [PubMed] [Google Scholar]
- 11. Takebe T, Koike N, Sekine K, Enomura M, Chiba Y, Ueno Y et al (2012) Generation of functional human vascular network. Transpl. Proc. 44, 1130–1133. [DOI] [PubMed] [Google Scholar]
- 12. Wang T, Ji X, Jin L, Feng Z, Wu J, Zheng J et al (2013) Fabrication and characterization of heparin‐grafted poly‐L‐lactic acid‐chitosan core‐shell nanofibers scaffold for vascular gasket. ACS Appl. Mater. Interfaces 5, 3757–3763. [DOI] [PubMed] [Google Scholar]
- 13. Cun X, Xie J, Lin S, Fu N, Deng S, Xie Q et al (2015) Gene profile of soluble growth factors involved in angiogenesis, in an adipose‐derived stromal cell/endothelial cell co‐culture, 3D gel model. Cell Prolif. 48, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou C, Cai X, Grottkau BE, Lin Y (2013) BMP4 promotes vascularization of human adipose stromal cells and endothelial cells in vitro and in vivo. Cell Prolif. 46, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdollahi H, Harris LJ, Zhang P, McIlhenny S, Srinivas V, Tulenko T et al (2011) The role of hypoxia in stem cell differentiation and therapeutics. J. Surg. Res. 165, 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ni X, Zhao Y, Ma J, Xia T, Liu X, Ding Q et al (2013) Hypoxia‐induced factor‐1 alpha upregulates vascular endothelial growth factor C to promote lymphangiogenesis and angiogenesis in breast cancer patients. J. Biomed. Res. 27, 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zimna A, Kurpisz M (2015) Hypoxia‐inducible factor‐1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int 2015, 549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi KS, Bae MK, Jeong JW, Moon HE, Kim KW (2003) Hypoxia‐induced angiogenesis during carcinogenesis. J. Biochem. Mol. Biol. 36, 120–127. [DOI] [PubMed] [Google Scholar]
- 19. Zhou C, Cai X, Fu Y, Wei X, Fu N, Xie J et al (2015) Tetraploid complementation proves pluripotency of induced pluripotent stem cells derived from adipose tissue. Cell Prolif. 48, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirschi KK, Li S, Roy K (2014) Induced pluripotent stem cells for regenerative medicine. Annu. Rev. Biomed. Eng. 16(16), 277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mizuno H, Tobita M, Uysal AC (2012) Concise review: adipose‐derived stem cells as a novel tool for future regenerative medicine. Stem Cells 30, 804–810. [DOI] [PubMed] [Google Scholar]
- 22. Frey JL, Stonko DP, Faugere MC, Riddle RC (2014) Hypoxia‐inducible factor‐1 alpha restricts the anabolic actions of parathyroid hormone. Bone Res. 2, 14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ji X, Yang W, Wang T, Mao C, Guo L, Xiao J et al (2013) Coaxially electrospun core/shell structured poly(L‐lactide) acid/chitosan nanofibers for potential drug carrier in tissue engineering. J. Biomed. Nanotechnol. 9, 1672–1678. [DOI] [PubMed] [Google Scholar]
- 24. Tanaka S, Sugimachi K, Yamashita Y, Shirabe K, Shimada M, Wands JR et al (2003) Angiogenic switch as a molecular target of malignant tumors. J. Gastroenterol. 38, 93–97. [PubMed] [Google Scholar]
- 25. Gong T, Xie J, Liao J, Zhang T, Lin S, Lin Y (2015) Nanomaterials and regenrative medicine. Bone Res. 10, 15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhong J, Guo B, Xie J, Deng S, Fu N, Lin S et al (2015) Crosstalk between adipose‐derived stem cells and chondrocytes: when growth factors matter. Bone Res. 3, 15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsipis CP, Sun X, Xu K, Lamanna JC (2014) Hypoxia‐induced angiogenesis and capillary density determination. Methods Mol. Biol. 1135, 69–80. [DOI] [PubMed] [Google Scholar]
- 28. Park IS, Chung PS, Ahn JC (2015) Adipose‐derived stromal cell cluster with light therapy enhance angiogenesis and skin wound healing in mice. Biochem. Biophys. Res. Commun. 462, 171–177. [DOI] [PubMed] [Google Scholar]
- 29. Toyserkani NM, Christensen ML, Sheikh SP, Sorensen JA (2015) Adipose‐derived stem cells: new treatment for wound healing? Ann. Plast. Surg. 75, 117–123. [DOI] [PubMed] [Google Scholar]
- 30. Ahluwalia A, Tarnawski AS (2012) Critical role of hypoxia sensor–HIF‐1alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr. Med. Chem. 19, 90–97. [DOI] [PubMed] [Google Scholar]
- 31. Korpisalo P, Karvinen H, Rissanen TT, Kilpijoki J, Marjomaki V, Baluk P et al (2008) Vascular endothelial growth factor‐A and platelet‐derived growth factor‐B Combination gene therapy prolongs angiogenic effects via recruitment of interstitial mononuclear cells and paracrine effects rather than improved pericyte coverage of angiogenic vessels. Circ. Res. 103, 1092–U1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu L, Cai X, Dong H, Jing W, Huang Y, Yang X et al (2010) Serum regulates adipogenesis of mesenchymal stem cells via MEK/ERK dependent PPARgamma expression and phosphorylation. J. Cell Mol. Med. 14, 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang W, Fu J, Wang D, Wang T, Wang H, Jin S et al (2010) Study on chitosan/polycaprolactone blending vascular scaffolds by electrospinning. J. Biomed. Nanotechnol. 6, 254–259. [DOI] [PubMed] [Google Scholar]
- 34. Burnouf PA, Juan PK, Su CY, Kuo YP, Chou ML, Su CH et al (2010) A novel virally inactivated human platelet lysate preparation rich in TGF‐beta, EGF and IGF, and depleted of PDGF and VEGF. Biotechnol. Appl. Biochem. 56, 151–160. [DOI] [PubMed] [Google Scholar]
- 35. Takeuchi K, Yanai R, Kumase F, Morizane Y, Suzuki J, Kayama M et al (2014) EGF‐like‐domain‐7 is required for VEGF‐induced Akt/ERK activation and vascular tube formation in an ex vivo angiogenesis assay. PLoS ONE 9, e91849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou C, Lin Y (2014) Osteogenic differentiation of adipose‐derived stem cells promoted by quercetin. Cell Prolif. 47, 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang XJ, Dong Z, Zhong XH, Shi RZ, Huang SH, Lou Y et al (2008) Transforming growth factor‐beta 1 enhanced vascular endothelial growth factor synthesis in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 365, 548–554. [DOI] [PubMed] [Google Scholar]
- 38. Cui QJ, Dighe AS, Irvine JN (2013) Combined angiogenic and osteogenic factor delivery for bone regenerative engineering. Curr. Pharm. Des. 19, 3374–3383. [DOI] [PubMed] [Google Scholar]
- 39. David L, Feige JJ, Bailly S (2009) Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 20, 203–212. [DOI] [PubMed] [Google Scholar]


