Abstract
Abstract. Objectives: Mesenchymal stromal cells (MSCs) have attracted considerable interest in both the scientific and clinical fields. In order to obtain a sufficient cell number for application, their in vitro expansion is necessary, but during this process their characteristics may be altered and cells may acquire oncogenic properties. We have investigated properties of MSC that may be related to oncogenesis, a critical parameter that has to be evaluated prior to MSC clinical use. Materials and methods: We studied the expression of p53, p16, RB, H‐RAS and human telomerase reverse transcriptase (hTERT) in MSCs from bone marrow of children diagnosed with idiopathic thrombocytopenic purpura (ITP) and autoimmune neutropenia. The same cells were seeded in soft agar to confirm their anchorage dependence and were karyotypically analysed. Finally, MSCs were subcutaneously transplanted into SCID mice and their ectopic osteogenic as well as tumorigenic potential was evaluated. Results: We have shown that MSCs derived from bone marrow of children with ITP and autoimmune neutropenia do not undergo transformation, the cells expressed normal levels of p53, p16, RB and H‐RAS. Expression of hTERT was undetectable, chromosome content remained stable, and their anchorage dependence was confirmed. In an in vivo model, when MSCs were subcutaneously transplanted into SCID mice, no tumorigenesis was observed. Conclusions: These findings suggest that MSCs from bone marrow of children do not have oncogenic properties and, therefore, represent validate candidates for applications in regenerative medicine.
INTRODUCTION
Mesenchymal stromal cells (MSCs) have attained much scientific interest as potential source of stem cells for cell and gene therapy, primarily due to their ability to self‐renew and contribute to the regeneration of mesenchymal tissues, such as bone, cartilage, muscle, ligament, tendon, adipose and stroma. MSCs are also essential in providing support for growth and differentiation of primitive haemopoietic cells within the bone marrow microenvironment (Dexter & Spooncer 1987; Pittenger et al. 1989; Devine & Hoffman 2000).
The potential role of MSCs in regenerative medicine has recently become a focus of interest and promising results exist from the first clinical applications. In more detail, MSCs have been used in orthopedic surgery for bone restoration (Barry 2003; , Kassem 2004) for therapeutic intervention in peripheral ischaemia of the lower limbs, some cases of severe idiopathic aplastic anaemia (Kassem 2004) and for recovery of patients with ischaemic stroke (Bang et al. 2005). In laboratory practice, MSCs can easily be isolated and expanded as they adhere to plastic surfaces, and through media containing appropriate growth factors and differentiation substances, may be stimulated to differentiate towards a multitude of cell lineages, such as cartilage, bone, adipose and muscle cells. Although laboratory isolation of MSCs is well established, the lack of an MSC‐specific antigenic marker (Kemp et al. 2005) and their low frequency in bone marrow necessitates their expansion in vitro prior to their use, in order to obtain sufficient cell numbers for application.
Recent studies on animal models support the notion that spontaneous transformation of adult stem cells can take place after long‐term culture in vitro (Rubio et al. 2005; Miura et al. 2006). Additionally, malignant cells with the ability to grow indefinitely may be generated in long‐term culture (Morshead et al. 2002; Sanai et al. 2005). Such a malignant cell phenotype is usually associated with genetic changes that involve many genes controlling the cell cycle, signal transduction and immortality. Thus, we hypothesized that MSCs expanded in long‐term culture may acquire oncogenic characteristics and eventually cause malignancy when used clinically.
The aim of this study was to investigate any effects of long‐term culture that may be related to acquired malignant transformation, in MSCs derived from the bone marrow of children with benign haematological diseases. We report that these MSCs isolated from bone marrow of children, and expanded in long‐term culture did not acquire oncogenic properties. They maintained a stable profile in respect of mRNA expression of tumour suppressor genes (p53, p16 and RB) and oncogenes (H‐RAS), anchorage dependence, chromosome content and structure, and absence of human telomerase reverse transcriptase (hTERT) mRNA expression. Most importantly, administration of in vitro cultured MSCs did not result in formation of tumours in an in vivo mouse model, suggesting that under suitable culture conditions MSCs derived from children satisfy the requirements of, and may be good candidates for, use in regenerative medicine.
MATERIALS AND METHODS
Patients
Bone marrow samples were obtained from children, aged 3 months to 12 years, diagnosed with idiopathic thrombocytopenic purpura (ITP; n = 10) and autoimmune neutropenia (AIN; n = 6) hospitalized at the Department of Pediatric Hematology‐Oncology. Bone marrow aspiration was performed as departmental policy, when necessary, to rule out other diseases. Parents were informed that cells from the bone marrow would be used for in vitro research. The procedure had the University Hospital of Heraklion Ethics Committee approval.
Mononuclear cell isolation and MSC expansion
Mononuclear cell (MNC) samples were isolated by Ficoll‐Hypaque separation (Lymphoprep‐Nycomed, Oslo, Norway; d = 1077 g/mL). Then, they were cultured in α‐modified minimum essential medium (α‐MEM; Invitrogen, Paisley, UK) with 10% selected foetal bovine serum (FBS; HyClone Perbio, Erembodegem, Belgium) and 1 ng/mL fibroblast growth factor‐2 (FGF‐2) (AbCys, Paris, France) at a concentration of 5 × 104 cells/cm2. When cultures reached confluence of ≥80%, cells were detached using 0.25% trypsin‐ethylenediaminetetraacetic acid (EDTA) (Gibco, Paisley, UK) and were replated at 1000 cells/cm2. Cell cultures were continued up to P6. Growth medium was changed every 72 h.
Immunophenotype analysis
For immunophenotype analysis, monoclonal antibodies CD105‐PE (Caltag Laboratories, Burlingame, CA, USA), CD146‐PE (BD Biosciences, San Jose, CA, USA) CD29 fluoroisothyocyanate (FITC), CD44 FITC, CD90 FITC, CD14 FITC (all from Immunotech, Marseille, France), CD45 FITC (Cytognos SL, Salamanca, Spain), CD34 PE (BD Biosciences) and CD95 FITC (BD Biosciences) were used. Cells were incubated at 1 × 105 cells/antibody for 20 min at room temperature. They were washed with phosphate‐buffered saline, and fixed in 2% paraformaldehyde solution. Expression of surface antigens was evaluated using an Epics Coulter flow cytometer, after acquisition of 30 000 events.
Colony‐forming unit‐fibroblastic assay
At day zero of culture (day of initial MNC plating) and in every passage thereafter, a colony‐forming unit‐fibroblastic (CFU‐F) assay was performed and cell doubling time was calculated. For the CFU‐F assay, at day zero, 1 × 105 bone marrow MNC were seeded in the culture medium described above without FGF‐2, in each well of a 24‐well plate (in triplicate). At subsequent passages, MSCs were plated, in duplicate, in 20 cm2 petri plates at a concentration of 10 cells/cm2. CFU‐Fs were quantified after 14 day culture at 37 °C and 5% CO2, with Giemsa staining.
RNA isolation
RNA was isolated using the SV Total RNA Isolation kit (Promega, Madison, WI, USA) from P2 and P6 MSCs. Quantity of RNA was determined by spectrophotometry at 260 nm and quality by 1% agarose gel electrophoresis. From each sample, 1 µg of RNA was used for cDNA synthesis using the Reverse Transcription System (Promega).
Reverse transcription–polymerase chain reaction and real‐time polymerase chain reaction
mRNA levels of p53, p16, RB and H‐RAS were measured using reverse transcription–polymerase chain reaction (RT‐PCR). One microlitre of cDNA, recombinant Taq DNA polymerase (Invitrogen), 200 mm deoxyribonucleotide triphosphate (Invitrogen), and 400 mm from each primer were used. Primers were designed according to gene sequences (BLAST) and Primer3 software (http://frodo.wi.mit.edu/cgi‐bin/primer3/primer3_www.cgi) (Table 1). All cDNAs used as templates were normalized throughout glyceraldehyde‐3‐phosphate‐dehydrogenase expression (GAPDH). As normal control for adherent cells, human umbilical vein endothelial cells (HUVECs) were used. Reactions included initial denaturation at 94 °C for 5 min, and 32–35 cycles 94 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min and were set in triplicate for each sample. PCR products were electrophoresed on 1% agarose gel electrophoresis. Density of bands corresponding to PCR products was quantified using Alpha Ease FC Stand Alone software (Alpha Innotech Corp., San Leandro, CA, USA). Relative expression of genes was defined by the ratio: density of gene of interest/density of GAPDH. Expression of hTERT mRNA was quantified with real‐time PCR (set up in triplicate), with primer sequences previously published (Ohyashiki et al. 2005). Amplification took place in an Mx3000P QPCR system (Stratagene, La Jolla, CA, USA) using QuantiTect SYBR Green PCR Kit (Qiagen, Hilden, Germany), and analysis of products was performed with the Mx3000P software (Stratagene). Due to absence of signal in test samples, products were also electrophoresed in 2% agarose gel electrophoresis. Expression of ABL gene was used as internal control, and as positive control for hTERT expression the M5 (human melanoma) cell line was used.
Table 1.
Primer sequences, PCR cycle number and expected product sizes
| Gene | Primer forward 5′–3′ | Primer reverse 5′–3′ | Cycles | Product size (base pair) |
|---|---|---|---|---|
| GAPDH | GGGCTGCTTTTAACTCTGGT | TGGCAGGTTTTTCTAGACGG | 32–35 | 710 |
| p53 | CCCCTCTGAGTCAGGAAACA | TCATCTGGACCTGGGTCTTC | 35 | 151 |
| p16 | TGCCTTTTCACTGTGTTGGA | AGCTTTGGTTCTGCCATTTG | 35 | 196 |
| RB | ACACAACCCAGCAGTTCGAT | CTTTGAGCAACATGGGAGGT | 35 | 187 |
| H‐RAS | GTTGGACATCCTGGATACCG | CTTCACCCGTTTGATCTGCT | 32 | 252 |
Soft agar colony formation assay
Four 35‐mm dishes were coated with 1.5 mL 0.5% base agar mix consisting of 2×α‐MEM (Invitrogen) supplemented with 20% selected FBS (HyClone Perbio) and 1% agar. The top layer was composed of 1.5 mL 0.4% agarose gel electrophoresis consisting of 2×α‐MEM (Invitrogen) supplemented with FBS (HyClone Perbio) and 0.8% agarose gel electrophoresis. Five thousand P2 or P6 MSCs (n = 9) were seeded in each plate. Incubation in 5% CO2 and at 37 °C for 14 days followed. HL‐60 cells (promyelocytic leukaemia) were used as positive control. Cell size was estimated with the Nikon Eclipse camera E400.
Karyotype analysis
Four in situ karyotype analyses were performed in P2 and P6 MSCs isolated from one patient diagnosed with ITP and in one with AIN. Chromosome samples were prepared and analysed with G‐banding techniques. For each individual sample, at least 30 metaphases of P2 MSCs and 15 for P6 MSCs were analysed.
Differentiation assay
The ability of MSCs to differentiate into adipocytes, osteocytes and chondrocytes was tested prior to transplantation. Differentiation was assessed by histochemical staining (oil red O staining for adipocytes, von Kossa staining for osteocytes and alcian blue staining for chondrocytes) and RT‐PCR analysis (lipoprotein lipase for adipocytes, alkaline phosphatase, osteocalcine and osteoprotegerin expression for osteocytes and finally aggrecan and Col II expression for chondrocytes). To induce adipocyte differentiation P2 MSCs were plated in tissue culture flasks at a cell density of 4 × 104 cells/cm2 and were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Paisley, UK) supplemented with 20% foetal calf serum (Gibco BRL), 100 nm roziglitazone (Cayman Chemicals, Ann Arbor, MI, USA), 1 µm dexamethasone, 60 µm indomethacin and 0.5 mm isobutylmethylxanthine (all from Sigma, Steinheim, Germany). For osteocyte lineage, P2 MSCs were plated in tissue culture flasks at a cell density of 1.5 × 104 cells/cm2 and were cultured in DMEM supplemented with 10% foetal calf serum, 0.1 µm dexamethasone, 3 mm NaH2PO4 and 0.15 mm ascorbate‐2‐phosphate (Sigma). For the formation of chondrocyte pellets, 2.5 × 105 P2 MSCs were cultured in a 15‐mL polypropylene in DMEM supplemented with 0.1 µm dexamethasone, 1 mm sodium pyruvate, 0.17 mm ascorbate‐2‐phosphate, 0.35 mm proline (Sigma), 1X ITS premix (Cambrex Bio Science, Berkshire, UK), 5.35 mg/mL linoleic acid (Sigma), 1.24 mg/mL bovine serum albumin and 10 ng/mL transforming growth factor‐β1 (R&D Systems, Abingdon, UK). Medium was replaced every 3–4 days for a total of 21 days.
Transplantation of MSCs in SCID mice and histological analysis of transplants
Osteogenic and tumourigenic ability of MSCs was assessed using an in vivo transplantation assay (Bianco et al. 1998). All animal procedures were approved by the relevant institutional committee and were conducted in compliance with the guiding principles of Care and Use of Animals (http://www.nap.edu/books/0309053773/html/). For each transplant, 2 × 106 cells, released by trypsin at P3, were incubated with 40 mg of hydroxyapatite/tricalcium phosphate (Zimmer, Warsaw, IN, USA), previously sterilized by heating at 220 °C overnight, in 1 mL of culture medium. Cells were loaded on carrier particles by mixing, with slow rotation (25 r.p.m.) for 70–100 min at 37 °C. After incubation, samples were briefly centrifuged and the supernatant was removed. Fibrinogen (40–50 µL; Sigma) and human thrombin (40–50 µL; Sigma) were then added to each vial in order to obtain a clot. The percentage of cell adhesion for each cell‐carrier construct was estimated by counting cells left in the incubation medium. Immunodeficient 8‐ to 15‐week‐old male SCID beige mice (Charles River Laboratories, Wilmington, MA, USA) were used as subcutaneous transplant recipients. Prior to transplantation, MSCs were grown with or without FGF‐2 (Martin et al. 1997). MSCs at P3 grown without FGF‐2 were transplanted into five mice, whereas the same cells grown with FGF‐2 were transplanted into eight mice. Operations were performed under anaesthesia, of intramuscular injection of Zoletil 20 (Virbac, Carros, France) + 2% xylazina, 4 µL/g of body weight. Midlongitudinal skin incisions of approximately 1 cm in length were made on the dorsal surface of each mouse, and subcutaneous pockets were formed by blunt dissection. A single transplant was placed into each pocket with up to four transplants per animal; incisions were closed with surgical staples. Samples were harvested 8 weeks after transplantation, washed with phosphate‐buffered saline and fixed with 4% phosphate‐buffered formaldehyde overnight at 4 °C. After decalcification with 10% EDTA (pH = 7), all transplants were embedded in paraffin wax. Five micron thick sections were cut from each block, stained with haematoxylin and eosin and then were analysed using light microscopy.
Statistical analysis
Statistical analysis was performed with the use of SPSS software (SPSS Inc., Chicago, IL, USA). Differences in gene expression among cell types were evaluated using the non‐parametric Mann–Whitney test and were considered statistically significant when P < 0.05. Data are presented as mean ± SD.
RESULTS
Descriptive characteristics of MSCs
From P1 to P6, MSCs had characteristic spindle‐shaped morphology. The total number of CFU‐F count was 37.8 ± 3.8 at P2 and 17 ± 3.8 at P6. At day zero, MNCs expressed CD34, CD45, CD44 and CD29; there was low expression of CD90 and the mesenchymal‐related markers, CD105 and CD146. From P1 to P6, MSCs constantly expressed >85% of CD90, CD29, CD44, CD105 and CD146.
MSCs had normal levels of tumour suppressor genes and oncogenes
By RT‐PCR, mRNA corresponding to p53, p16, RB and H‐RAS genes was measured in 16 samples of isolated MSCs, at P2 and P6 (Fig. 1). Relative mRNA levels of p53, p16, RB and H‐RAS, normalized by GAPDH expression, were similar between P2 and P6 MSCs (Fig. 2, Table 2). Comparisons between expression profiles of P2 and P6 MSCs did not exhibit substantial differences when compared to HUVECs (Table 2).
Figure 1.

Representative electrophoresis results of RT‐PCR products for the genes p53, p16, RB and H‐RAS. Analysis was performed at different mesenchymal stromal cell (MSC) passages of ITP and AIN samples. On the first row, the bands correspond to the gene of interest and on the second row to GAPDH.
Figure 2.
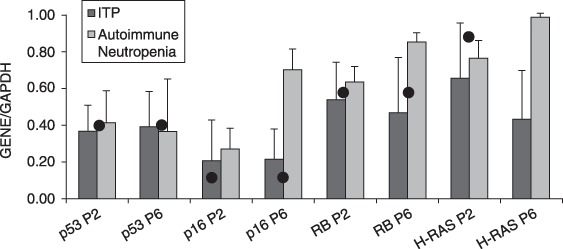
mRNA expression of p53, p16, RB, H‐RAS relative to GAPDH in mesenchymal stromal cells (MSC) according to disease group. Bar graphs represent mean expression levels of three independent experiments with SD (error bars). The expression levels of the same genes in HUVEC cells are denoted with a dot.
Table 2.
Mean expression levels of genes analysed at different mesenchymal stromal cell (MSC) passages (P2, P6) and human umbilical vein endothelial cells (HUVECs). The non‐statistically significant probability values P result from the Mann–Whitney non‐parametric test, conducted for comparisons among groups
| Disease | Relative expression | P2 MSC | P6 MSC | HUVEC | P P2 versus P6 | P P2 versus HUVEC | P P6 versus HUVEC |
|---|---|---|---|---|---|---|---|
| Idiopathic thrombocytopenic purpura | p53/GAPDH | 0.37 | 0.39 | 0.5 | 0.63 | 0.54 | 0.8 |
| p16/GAPDH | 0.2 | 0.21 | 0.23 | 0.63 | 0.9 | 0.8 | |
| RB/GAPDH | 0.53 | 0.48 | 0.73 | 0.82 | 0.6 | 0.8 | |
| H‐RAS/GAPDH | 0.64 | 0.42 | 0.93 | 0.33 | 0.8 | 0.4 | |
| Autoimmune neutropenia | p53/GAPDH | 0.41 | 0.36 | 0.5 | 0.85 | 0.85 | 1.0 |
| p16/GAPDH | 0.27 | 0.69 | 0.23 | 0.07 | 0.85 | 0.66 | |
| RB/GAPDH | 0.62 | 0.84 | 0.73 | 0.143 | 0.57 | 0.66 | |
| H‐RAS/GAPDH | 0.75 | 0.97 | 0.93 | 0.07 | 0.57 | 0.66 |
MSCs did not display anchorage‐independent growth
Development of ‘adherent’ cells without anchorage dependence is usually correlated with tumorigenicity and is a characteristic of cell transformation. Consistent with our gene expression profiles, none of the nine MSC samples seeded in soft agar showed anchorage‐independent growth either at P2 or at P6, after 14 days of culture. In contrast, HL‐60 cells, which were used as positive control, grew fast and foci were visible from day 7 of culture (Fig. 3).
Figure 3.
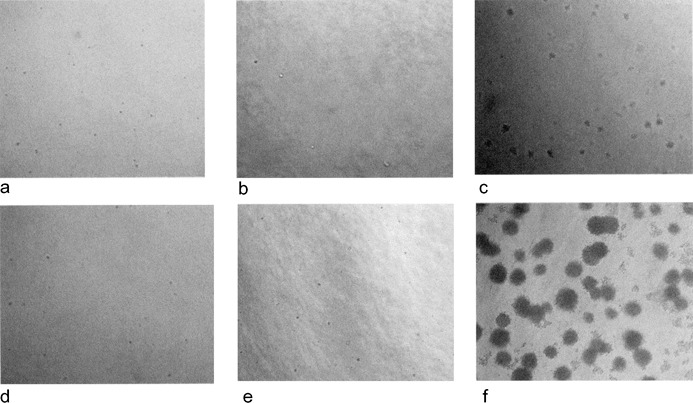
Culture of mesenchymal stromal cells (MSCs) on soft agar for the assay of anchorage dependence. Pictures were taken on day 7 (a, b, c) and 14 (d, e, f) and correspond to ΗL‐60 (c, f), P2 MSCs (a, d) and P6 MSCs (b, e). No colonies were observed in MSC samples in contrast with HL‐60 cells where foci were visible from day 7.
MSCs did not express hTERT and retained a normal karyotype
Expression of hTERT mRNA was quantified employing real‐time PCR. Levels of hTERT were undetectable both at P2 MSCs and P6 MSCs (Fig. 4). MSCs at the same passages had a normal karyotype and chromosome abnormalities, such as deletion, inversion, translocation or ring chromosomes were not seen (Fig. 5).
Figure 4.
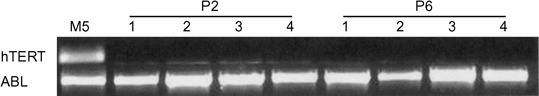
Electrophoresis of human telomerase reverse transcriptase (hTERT) real‐time PCR products. Four mesenchymal stromal cell (MSC) samples are shown at P2 and P6. hTERT is expressed in the cell line M5 but not in the MSC samples.
Figure 5.

Representative G‐banding karyotype results. P2 MSCs (a) and P6 MSCs (b) from two different male patients. All karyotypes were normal. MSC, mesenchymal stromal cell.
Transplanted MSCs showed no signs of tumorigenesis
The cardinal trait of a malignant cell is the capacity to generate tumours in vivo. To exclude the possibility that ex vivo expansion of MSCs might result in acquisition of a malignant phenotype, the MSCs were implanted in SCID mice. Prior to transplantation, MSCs at P2 were tested for their ability for tri‐lineage differentiation. Cells differentiated successfully into adipocytes, osteocytes and chondrocytes; this was confirmed by histochemical staining (Fig. 6) and molecular analysis of expression of lineage‐specific markers (data not shown). Cells originating from the same samples were cultured with and without FGF‐2 and their immunophenotype was analysed just before transplantation into the SCID mice (Fig. 7). MSCs grown without FGF‐2 were transplanted into five mice and those grown with FGF‐2 were transplanted into eight mice. Upon in vivo transplantation, MSCs grown in the presence of FGF‐2 (n = 8) were able to differentiate into osteogenic cells. In these samples, sparse bone trabeculae could be observed on carrier surfaces and vascularized fibrous tissue filled intertrabecular spaces. No bone was observed in transplants of untreated cells, in which carrier particles were found embedded in fibrous tissue. Of note, in none of the 13 animals receiving MSCs subcutaneously was any tumorigenesis found (Fig. 8).
Figure 6.
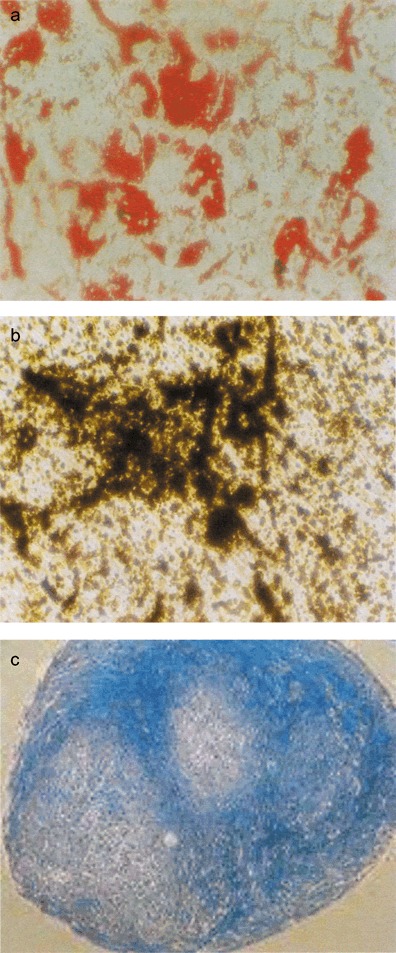
Histochemically stained differentiated mesenchymal stromal cells. (a) adipocytes stained with oil red O, (b) osteocytes stained with von Kossa and (c) chondrocytes stained with Alcian Blue.
Figure 7.

Mesenchymal stromal cell cultured with (a) and without fibroblast growth factor‐2 (b). Immunophenotype analysis results before subcutaneous transplantation into SCID mice are presented.
Figure 8.
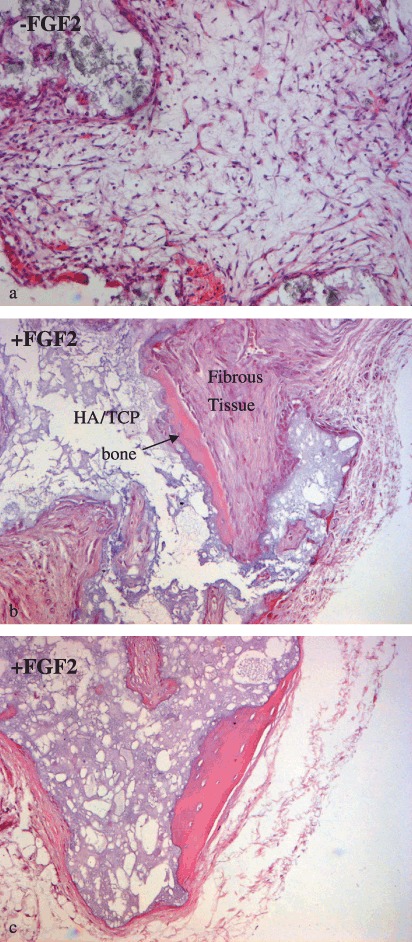
Histological analysis. Deposition of bone matrix onto the carrier in transplants of mesenchymal stromal cell (MSC) grown with fibroblast growth factor (FGF)‐2 (b, c) is shown. Only fibrous tissue and hydroxyapatite/tricalcium phosphate (HA/TCP) particles were detected in transplants of untreated cells (a). None of the samples showed evidence of tumour growth.
DISCUSSION
MSCs have been described as stem cells due to their ability to differentiate into multiple lineages and their extensive proliferative potential. Because of their possible role in regenerative medicine and tissue engineering, MSCs have attracted researchers’ interest and both preclinical and clinical studies have shown that they can be of therapeutic value (Campagnolli et al. 2002; Fouillard et al. 2003; Falanga et al. 2007).
Despite promise emerging from experimental and clinical use of MSCs, few studies have been conducted up‐to‐date assessing MSCs in relation to oncogenesis, and some of these describe MSCs mutating into malignant cells under certain circumstances leading to tumorigenesis. Recent published data show that MSCs isolated from adipose tissue spontaneously transform after long‐term culture over 4–5 months (Rubio et al. 2005). More experiments on MSCs derived from bone marrow of mice revealed that they favour oncogenesis only when transplanted in combination with cancer cell lines; however, these findings were not confirmed for human MSCs (Zhu et al. 2006). Additionally, hTERT transduced MSCs after long‐term culture showing loss of contact inhibition and anchorage dependence and the cells formed tumours in 10/10 mice transplanted (Serakinci et al. 2004). Djouad et al. reported that MSCs could cause side effects related to systemic immunosuppression and can lead to oncogenesis in vivo (Djouad et al. 2003). In in vitro models, MSCs transiently arrest the cell cycle of cancer cells in G1 phase. However, when tumour cells were injected into non‐obese diabetic‐SCID mice concomitantly with MSCs, tumour growth was much faster compared to the group receiving tumour cells only. It has been hypothesized that MSCs have the ability to form a cancer stem cell niche in which tumour cells can retain their proliferative potential and sustain the malignant process (Ramasamy et al. 2006). In the most recent study available and conducted on MSCs from 10 adult donors, it was shown that MSCs in long‐term culture reach cell senescence and do not transform (Bernardo et al. 2007).
In the present study, we investigated the effects of long‐term culture on MSCs (P2, P6), derived from bone marrow of children, related to oncogenic transformation and tumorigenesis. No remarkable changes of MSCs morphology were observed between P2 and P6 and during the same period MSCs antigen expression remained unaltered. For further characterization of MSCs with respect to transformation, we examined mRNA expression of the tumour suppressor genes p53, p16, RB and the oncogene H‐RAS. The expression profile of these genes, known to regulate cell cycle progression and signal transduction, did not show any significant alteration with the progression of passages in comparison with HUVECs, used as normal control.
Interestingly, culture of MSCs on soft agar confirmed their anchorage dependence for growth and proliferation. Loss of anchorage dependence allows transformed cells only to grow in suspension, and is considered to be a hallmark of cancer cells; therefore, according to this assay, our MSCs from children can be considered non‐transformed. To the same extent, karyotypic analysis of P2 and P6 MSCs showed normal diploid karyotypes (46XY) without any aneuploidy or polyploidy. Normal chromosome structure was verified by G‐banding analysis and did not reveal aberrations as has been reported in previous studies concerning in vitro spontaneous transformation (Rubio et al. 2005; Miura et al. 2006).
Telomerase activity found in 85% of human cancers is not observed in most normal tissues (Kim et al. 1994). Therefore, presence of telomerase is considered to be closely associated with attainment of cell immortality, while its absence is involved in cell senescence. At early and late passages of MSCs, expression of hTERT mRNA was undetectable, a finding that supports the absence of telomerase activity, as its activity correlates with hTERT expression (Takakura et al. 1998). Previous studies on MSCs have also shown that cells cultured in vitro lack telomerase activity (Zimmermann et al. 2003) and hTERT expression which results in telomere shortening with serial passageing (Simonsen et al. 2002). It is important to note that telomerase is related to malignancy but is not an oncogene, as it does not promote growth deregulation in cells. Telomerase only functions to maintain telomere length, thus permitting normal cell traits. Indeed, the vast majority of studies show that cell cycle checkpoint controls, such as contact growth inhibition, are maintained in the setting of hTERT overexpression.
Finally, a model of ectopic ossification was used to analyse behaviour of paediatric MSCs in vivo. In agreement with well‐known nature of MSCs as skeletal stem cells, this model showed that MSC populations used in this study were able to differentiate into osteogenic cells and to produce normal lamellar bone tissue. At the same time, it confirmed that paediatric MSCs do not undergo spontaneous transformation on in vitro expansion and are not tumorigenic in vivo.
The process of malignant transformation requires an initial step of immortalization and subsequent acquisition of the full malignant phenotype; different events are necessary to drive different cell types to malignancy (number of mutational events, oncogene expression levels, epigenetic changes, such as DNA methylation and more). It may be possible that cells from older donors or individuals with inherited defects affecting MSCs, or different intrinsic biological factors, are more prone to malignant transformation.
Moreover, MSCs can be isolated by using several different protocols, and expanded in different culture media. Hence, the resulting MSC population may be affected by the protocol applied. Such factors may play a role in the selection of cell types and they may favour expansion of cell populations with different properties. However, to date, there is no report where different isolation and culture conditions are compared with regard to transformation.
Spontaneous transformation of MSC and tumorigenesis seem to be affected by the source of origin and existing differences between species, ex vivo culture conditions and immune status of the recipient. The issues raised by these observations emphasize the need for careful analysis of human bone marrow cells expanded ex vivo for therapeutic administration, and the importance of karyotype analysis and other quality control measures to guard against inadvertent administration of transformed cells to patients.
The ex vivo expansion of MSCs derived from children under conditions studied here did not render them tumorigenic, and such a source of MSCs and culture conditions provide a supply of candidate cells for clinical application.
ACKNOWLEDGEMENT
This work was supported by the European 6th Framework Program GENOSTEM (contract no. 503161) and the University of Crete Secreteriat Research Committee.
REFERENCES
- Bang OY, Lee JS, Lee PH, Lee G (2005) Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 57, 874–882. [DOI] [PubMed] [Google Scholar]
- Barry FP (2003) Mesenchymal stem cell therapy in joint disease. Novartis Found. Symp. 249, 86–96. [PubMed] [Google Scholar]
- Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, Zuffardi O, Locatelli F (2007) Human bone marrow derived mesenchymal stem cells do not undergo transformation after long‐term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 67, 9142–9149. [DOI] [PubMed] [Google Scholar]
- Bianco P, Kuznetsov SA, Riminucci M, Fisher LW, Spiegel AM, Robey PG (1998) Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsalpha‐mutated skeletal progenitor cells. J. Clin. Invest. 101, 1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnolli C, Bellantuonno I, Kumar S, Fairnairn LJ, Roberts I, Fisk NM (2002) High transduction efficiency of circulating first trimester fetal mesenchymal cells: potential targets for in utero ex vivo gene therapy. BJOG 109, 952–954. [DOI] [PubMed] [Google Scholar]
- Devine SM, Hoffman R. (2000) Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr. Opin. Hematol. 7, 358–363. [DOI] [PubMed] [Google Scholar]
- Dexter TM, Spooncer E (1987) Growth and differentiation in the hemopoietic system. Annu. Rev. Cell Dev. Biol. 3, 423–421. [DOI] [PubMed] [Google Scholar]
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C (2003) Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 102, 3837–3844. [DOI] [PubMed] [Google Scholar]
- Kassem M (2004) Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning Stem Cells 6, 369–374. [DOI] [PubMed] [Google Scholar]
- Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P (2007) Autologous bone marrow‐derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 13, 1299–1312. [DOI] [PubMed] [Google Scholar]
- Fouillard L, Bensidhoum M, Bories D, Bonte H, Lopez M, Moseley AM, Smith A, Lesage S, Beaujean F, Thierry D, Gourmelon P, Najman A, Gorin NC (2003) Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia 17, 474–476. [DOI] [PubMed] [Google Scholar]
- Kemp KC, Hows H, Donaldson G (2005) Bone marrow derived mesenchymal cells. Leuk. Lymphoma 46, 1531–1544. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015. [DOI] [PubMed] [Google Scholar]
- Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R (1997) Fibroblast growth factor‐2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology 138, 4456–4462. [DOI] [PubMed] [Google Scholar]
- Miura M, Miura Y, Padilla‐Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S (2006) Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells 4, 1095–1103. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Benveniste P, Iscove NN, Van Der Kooy D (2002) Hematopoietic competence is a rare property of neural stem cell that may depend on genetic and epigenetic alterations. Nat. Med. 8, 268–273. [DOI] [PubMed] [Google Scholar]
- Ohyashiki JH, Hisatomi H, Nagao K, Honda S, Takaku T, Zhang Y, Sashida G, Ohyashiki K (2005) Quantitative relationship between functionally active telomerase and major telomerase components (hTERT and hTR) in acute leukaemia cells. Br. J. Cancer 92, 1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD (1989) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F (2006) Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia 21, 304–310. [DOI] [PubMed] [Google Scholar]
- Rubio D, Garcia‐Castro J, Martín MC, De La Fuente R, Cigudosa JC, Lloyd AC, Bernad A (2005) Spontaneous human adult stem cell transformation. Cancer Res. 65, 3035–3039. [DOI] [PubMed] [Google Scholar]
- Sanai N, Alvarez‐Buylla A, Berger MS (2005) Neural stem cells and the origin of gliomas. N. Engl. J. Med. 353, 811–822. [DOI] [PubMed] [Google Scholar]
- Serakinci N, Guldberg P, Burns JS, Abdallah B, Schrodder H, Jensen T, Kassem M (2004) Adult human mesenchymal stem cell as a target for neoplastic transformation. Oncogene 23, 5095–5098. [DOI] [PubMed] [Google Scholar]
- Simonsen JL, Rosada C, Serakinci N, Justesen J, Stenderup K, Rattan SI, Jensen TG, Kassem M (2002) Telomerase expression extends the proliferative lifespan and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 20, 592–596. [DOI] [PubMed] [Google Scholar]
- Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M (1998) Expression of human telomerase subunits and correlation with cervical cancer. Cancer Res. 58, 1558–1561. [PubMed] [Google Scholar]
- Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y (2006) Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo . Oncol. Rep. 16, 1021–1028. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Voss M, Kaiser S, Kapp U, Waller CF, Martens UM (2003) Lack of telomerase activity in human mesenchymal stem cells. Leukemia 17, 1146–1149. [DOI] [PubMed] [Google Scholar]


