Abstract
Abstract. Objectives: Iron is essential for DNA synthesis; its deficiency may lead to impaired DNA synthesis and subsequent alterations in levels of apoptosis. Here, we have aimed to investigate effects of iron deficiency anaemia (IDA) on apoptotic response of phagocytic cells and to understand whether the effect is reversible after iron supplementation. Materials and methods: Forty‐nine IDA patients and 26 healthy controls, aged between 6 months and 12 years with similar demographic status, were considered. Neutrophil‐ and monocyte‐apoptotic responses of IDA patients and the control group were compared by flow cytometry. Then, IDA patients were provided with oral iron supplementation. On day 15 of iron therapy, neutrophil‐ and monocyte‐apoptotic responses of IDA patients were rechecked and were compared to those of control group. Results: Neutrophil‐ and monocyte‐apoptotic responses in terms of early and late percentages of apoptosis, and percentages of necrotic cells, were significantly less in IDA patients compared to the control group. The significantly low apoptotic responses of IDA patients rose to levels of the control group by day 15 of iron therapy. Besides, the effect of IDA on apoptotic responses was found to be more enhanced in severe IDA patients that those of mild IDA patients. Conclusion: Correction of differences after iron supplementation therapy implies that IDA might be a cause for changes in neutophil‐ and monocyte‐apoptotic responses. The impact of this diminution of apoptotic cellular function in IDA should be further investigated, with longitudinal studies, in order to document the impact of any severe and/or long‐lasting IDA on autoimmunity and malignancy.
INTRODUCTION
Traces of elements such as iron, zinc, copper and manganese make up a small percentage of body mass but are vital for the body's proper functioning (Bridges 1992). These metals must be present in appropriate amounts, and they must be available to react with other elements to form critical molecules and participate in important chemical reactions. Iron is indispensable for cell growth and survival (Maclean et al. 2001). It participates in oxidation–reduction processes and its deficiency affects haem synthesis, electron transport and DNA synthesis (Reichard & Ehrenberg 1983; Bridges 1992). Impaired DNA synthesis as a result of iron deficiency may lead to subsequent alteration in levels of programmed cell death. Apoptosis can be initiated by activation of both intrinsic and extrinsic caspase pathways (Binet et al. 1996; Thiele & Kastan 2002). The intrinsic pathway begins with release of cytochrome c from mitochondrial membranes (Thiele & Kastan 2002). Iron plays a central role in the production of mature cytochrome c (Reed 1997). It is also well known that differentiation of monocytes and macrophages is also iron‐dependant (Kramer et al. 2002). Because apoptosis could be viewed as the last step in cell differentiation, it is possible that apoptosis of monocytes may also be affected by iron deficiency in vivo. Although there are some cell culture studies that demonstrate increased apoptotic response in the presence of hypoxia and iron chelation (Haq et al. 1995; Kovar et al. 1997; Simonart et al. 2000), further in vitro investigations have found that numbers of apoptotic neutrophils and mononuclear cells do not differ from those of control groups (Bergman et al. 2005). In the light of such conflicting reports, detailed clinical studies are needed to determine how the functions of neutrophils and monocytes are affected in iron deficiency, and how the reported in vitro effects can translate into clinical conditions.
We have aimed to investigate the impact of iron deficiency anaemia (IDA) on apoptotic responses of phagocytic cells and to understand whether any effect is reversible after iron supplementation.
MATERIALS AND METHODS
Study design and patients
The study to be conducted in Marmara University Medical Center and Goztepe Educational Hospital was approved prospectively by the local ethics committee of Marmara University Medical Center in Istanbul, Turkey.
Patients aged between 6 months and 12 years, with a prior haemogram and peripheral smear at their first visit to Marmara University Medical Center or to Goztepe Educational Hospital ‘Healthy Child Clinic’ or ‘Sick Child Clinic’ or ‘Paediatric Haematology Clinic’, were selected in order to determine possible IDA patients or to provide healthy children for the control group. Those with possible IDA or those who seemed to be among the healthy control group, were ordered haemograms, peripheral smears and ferritin level analysis. Heparinized blood for apoptosis tests and occult blood in faeces × 3 were collected.
Both IDA patients and the healthy control group or their parents were questioned regarding their eating habits (amount of milk, green vegetables and red meat consumed/day), pica; pattern of defecation, history of bleeding/operation, accident or chronic illness of any kind. Eating green vegetables and red meat (at least 250 g/week) and consuming less than 500 mL/day milk was considered as a program of good eating habits.
All patients had a physical examination including recording height and weight percentiles.
Patients with non‐anaemic iron deficiency, any chronic illness, any haematologic disease other than IDA, history of gastrointestinal bleeding or having had surgery, or who had any parasitic or other infection in the last month, or who had ingested any iron containing drug, were excluded from the study. Forty‐nine IDA patients and 26 healthy children as the control group, all aged between 6 months and 12 years with similar demographic status, were included in the study (Table 1). Apoptosis tests were performed on both IDA patients and control children on their inclusion to the study.
Table 1.
Characteristics of 49 iron deficiency anaemia (IDA) patients and 26 healthy children
| IDA (n = 49) | Controls (n = 26) | P | |
|---|---|---|---|
| Gender (n) | 28F/21M | 12F/14M | 0.467* |
| Age (years) (mean ± SD) | 3.8 ± 3.3 | 3.8 ± 3.1 | 0.996** |
| Weight (kg) (mean±SD) | 17.3 ± 9 | 17.4 ± 8.7 | 0.956** |
| Height (cm) (mean±SD) | 97.7 ± 22.4 | 98.4 ± 22.6 | 0.996** |
| ‘Good eating habits’ (n) | 15 | 21 | 0.00* |
| No ‘good eating habits’ (n) | 34 | 5 | |
| Hemoglobin (g/dL) (mean±SD) | 8.3 ± 1.5 | 12.3 ± 0.5 | 0.00** |
| Red blood cell count (1012/L) (mean±SD) | 4.2 ± 0.5 | 4.6 ± 0.3 | 0.00** |
| Mean corpuscular volume (fl) (mean±SD) | 63.9 ± 6.4 | 78.9 ± 4.1 | 0.00** |
| Red cell distribution width (mean±SD) | 17.5 ± 2.7 | 13.6 ± 0.8 | 0.00** |
| Ferritin (µg/L) | 5.9 ± 4.7 | 50.3 ± 18.1 | 0.00** |
F, female; M, male.
Fisher's exact chi‐squared test.
Mann–Whitney U‐test.
Oral ferrous sulfate supplementation (3 mg/kg/day in bid‐ferrosanol) was provided for the IDA patients. On day 15 of iron supplementation therapy, apoptosis tests were rechecked on 26 of the IDA patients. Tests could not be performed on 23 of the IDA patients as their parents refused extra blood withdrawal on day 15 (Table 2).
Table 2.
Characteristics of 26 iron deficiency anaemia (IDA) patients having apoptosis tests on day 15 of iron supplementation therapy and 23 IDA patients not having apoptosis tests on day 15 of iron supplementation therapy
| IDA with apoptosis tests performed on day 15 (n = 26) | IDA with apoptosis tests not performed on day (n = 23) | P | |
|---|---|---|---|
| Gender (n) | 16F/10M | 12F/11M | 0.572* |
| Age (years) (mean±SD) | 3.7 ± 3.5 | 3.8 ± 3.1 | 0.508** |
| Weight (kg) (mean±SD) | 17.7 ± 10.6 | 16.8 ± 7.1 | 0.673** |
| Height (cm) (mean±SD) | 96.7 ± 23.9 | 98.9 ± 20.9 | 0.514** |
| ‘Good eating habits’ (n) | 8 | 7 | 1.00* |
| No ‘good eating habits’ (n) | 18 | 16 | |
| Hemoglobin (g/dL) (mean±SD) | 8.3 ± 1.6 | 8.4 ± 1.5 | 0.865** |
| Red blood cell count (1012/L) (mean±SD) | 4.2 ± 0.4 | 4.2 ± 0.6 | 0.849** |
| Mean corpuscular volume (fl) (mean±SD) | 63.3 ± 7.4 | 64.7 ± 6.4 | 0.589** |
| Red cell distribution width (mean±SD) | 17.8 ± 3.3 | 17.1 ± 1.9 | 0.464** |
| Ferritin (µg/L) (mean±SD) | 4.9 ± 3.6 | 7.2 ± 5.6 | 0.203** |
F, female; M, male.
Fisher's exact chi‐squared test.
Mann–Whitney U‐test.
Isolation of white blood cells
White blood cells from venous blood of patients were isolated by an ammonium chloride‐based method. Briefly, 3 ml of blood was mixed with 30 ml of erythrocyte lysing solution (0.1 mm EDTA; 155 mm NH4Cl; 10 mm KHCO3) and was incubated at room temperature for 15 min. Following centrifugation at 600 g for 10 min, supernatant was discarded and the cell pellet was washed with phosphate‐buffered saline gel (PBS‐gel; PBS containing 0.1% gelatin) and cell count was adjusted to 3–4 × 106.
Evaluation of apoptosis and cell necrosis
Apoptosis was induced in cells by using phorbol myristate acetate (PMA, Sigma‐Aldrich, Taufkirchen, Germany) as previously described but with some modifications (Takei et al. 1996). In optimization studies, we used peripheral blood samples from normal children (n = 5). Cell death was induced by PMA in half of the cells, while the rest were incubated under the same conditions without PMA, for evaluating spontaneous cell death of neutrophils. Samples were taken at specific time points (0, 1st, 2nd, 3rd and 6th hour for spontaneous apoptosis, and 0, 3rd and 6th hour for PMA‐induced samples); differences were clearly seen (Fig. 1). Apoptosis was evaluated by the method defined below and the 3rd hour of evaluation was chosen as time point for the experiments. Briefly, two tubes were prepared for each apoptosis experiment and 1 × 106 cells/mL were dispensed into the tubes. One of these was induced for apoptosis using 100 ng/mL of PMA, at 37 °C for 3 h, while other was incubated at the same temperature without stimulation, as a control. Fluorogenic caspase‐3 substrate, Phi‐Philux (OncoImmune Inc., College Park, MD, USA) was used to stain then evaluate apoptotic events in monocytes and neutrophils. Following washing with PBS‐gel, cells were labelled with 50 µL Phi‐Philux (OncoImmunin Inc.) at 37 °C in the dark for 1 h. Cells were then washed again with PBS‐gel and, while evaluation by flow cytometry (FACScan, Becton Dickinson, Mountain View, CA, USA) 20 µg/mL of propidium iodide was added to the tubes, to reveal late stages of apoptotic cell death.
Figure 1.
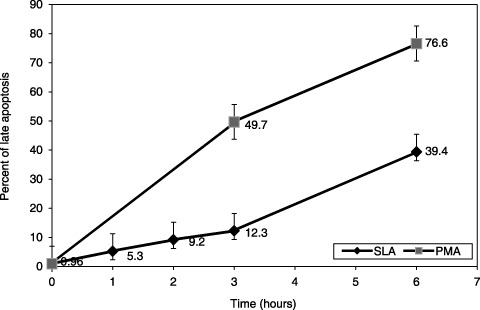
Optimization of phorbol myristate acetate (PMA)‐induced apoptosis in healthy subjects (n = 5) in comparison to spontaneous apoptosis of neutrophils. Half the samples treated with PMA while the other half was left without PMA. Late apoptosis of neutrophils positive for both Phi‐Philux and propidium iodide staining was checked in various time points and PMA induced cell death has been shown (P = 0.034, Mann–Whitney U‐test). SLA, spontaneous late apoptosis.
For analysis, monocytes and neutrophils were separately gated according to their granularity and size, on forward scatter versus side scatter plots. Early apoptosis, late apoptosis and cell necrosis were evaluated on fluorescence 1 (FL1 for Phi‐Philux) versus fluorescence 3 (FL3 for propidium iodide) plots. Percentage of cells stained with Phi‐Philux only was evaluated as early apoptosis; percentage of cells stained with both Phi‐Philux and propidium iodide was evaluated as late apoptosis and percentage of cells stained with propidium iodide only was evaluated as necrotic cells.
Statistical analysis
For comparisons of continuous variables, the Mann–Whitney U‐test was used; frequency of distribution of different parameters were compared among groups of patients by means of Fisher's exact chi‐squared test; for comparisons of pre‐ and post‐treatment variables the Wilcoxon signed‐rank test was used. Significance level was accepted with P < 0.05.
RESULTS
Haemoglobin, haematocrit and red blood cell counts at inclusion in the study in IDA patients were shown to have undergone a statistically significant increase on day 15 of iron supplementation treatment (Table 3).
Table 3.
Haemoglobin, red blood cell count, mean corpuscular volume and red cell distribution width values at admission to the study and on day 15 of iron treatment
| At admission to the study (n = 49) | At day 15 of iron treatment (n = 26) | P * | |
|---|---|---|---|
| Hemoglobin (g/dL) (mean±SD) | 8.3 ± 1.5 | 9.7 ± 1.6 | 0.000 |
| Red blood cell count (1012/L) | 4.2 ± 0.5 | 4.7 ± 0.5 | 0.000 |
| Mean corpuscular volume (fl) (mean±SD) | 63.9 ± 6.4 | 67.5 ± 7.3 | 0.000 |
| Red cell distribution width (mean±SD) | 17.5 ± 2.7 | 18.5 ± 4.1 | 0.322 |
Wilcoxon signed‐rank test.
Effects of IDA on parameters of apoptosis
In IDA patients, neutrophil apoptosis (in terms of percentages of early and late neutrophil apoptosis), and percentages of necrotic neutrophils were all found to be significantly lower than in the control group (Table 4; 2, 3, 4). In IDA patients, monocyte apoptosis (in terms of percentages of early and late monocyte apoptosis), and percentages of necrotic monocytes were all found to be significantly lower than the control group (Table 4; 5, 6, 7).
Table 4.
Neutrophil and monocyte apoptosis in iron deficiency anaemia (IDA) and control groups
| IDA at admission to the study (n = 49) (mean±SD) | P (IDA at admission versus control) | IDA at day 15 of iron treatment (n = 26) (mean±SD) | P (IDA at admission versus IDA at day 15 of iron treatment) | Controls (n = 26) (mean±SD) | P (IDA at day 15 of iron treatment versus control) | |
|---|---|---|---|---|---|---|
| Early neutrophil apoptosis a (%) | 26.2 ± 22.6 | 0.017* | 37.9 ± 24.1 | 0.058** | 41.4 ± 23.7 | 0.596* |
| Late neutrophil apoptosis b (%) | 12.5 ± 13.6 | 0.01* | 24.0 ± 23.9 | 0.049** | 24.7 ± 21.7 | 0.876* |
| Neutrophil necrosis c (%) | 9.2 ± 8.7 | 0.041* | 11.5 ± 14.8 | 0.269** | 14.2 ± 11.1 | 0.138 |
| Early monocyte apoptosis a (%) | 15.8 ± 21.1 | 0.007* | 29.3 ± 26.5 | 0.078** | 31.7 ± 32.5 | 0.583* |
| Late monocyte apoptosis b (%) | 0.9 ± 1.6 | 0.021* | 4.6 ± 12.8 | 0.017** | 3.2 ± 5.0 | 0.51* |
| Monocyte necrosis c (%) | 1.7 ± 2.4 | 0.009* | 6.9 ± 17.3 | 0.026** | 3.9 ± 4.8 | 0.84* |
Mann–Whitney U‐test.
Wilcoxon signed‐rank test.
Measured by percent fluoresence of Phi‐Philux that is cleaved by active caspase‐3.
Measured by percent fluoresence of Phi‐Philux that is cleaved by active caspase‐3 and propidium iodide.
Percentage of cells that are stained by propidium iodide only.
Figure 2.
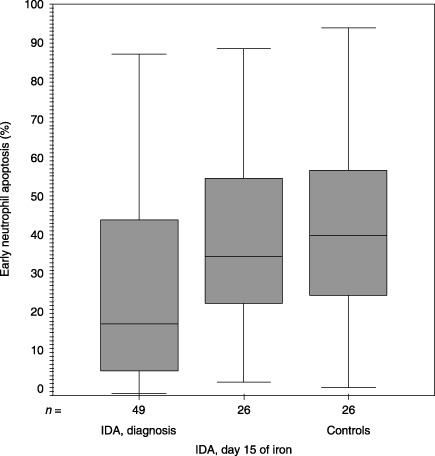
Median (inter‐quartile range) values for early neutrophil apoptosis (%) upon inclusion into the study and at day 15 of iron therapy and the controls were 18.33 (5.97–44.90), 35.55 (22.30–56.36) and 40.93 (24.84–58.09), respectively (P = 0.017, Mann–Whitney U‐test). IDA, iron deficiency anaemia.
Figure 3.
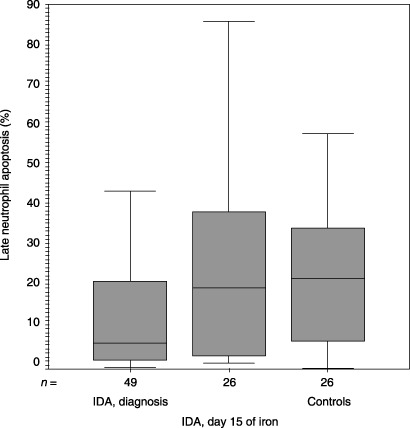
Median (inter‐quartile range) values for late neutrophil apoptosis (%) upon inclusion to the study and at day 15 of iron therapy and the controls were 6.42 (1.87–23.06), 19.97 (3.25–41.57) and 22.38 (5.96–35.32), respectively (P = 0.01, Mann–Whitney U‐test). IDA, iron deficiency anaemia.
Figure 4.
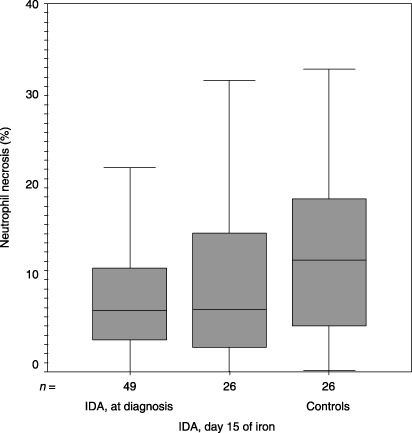
Median (inter‐quartile range) values for neutrophil necrosis (%) upon inclusion into the study and at day 15 of iron therapy and the controls were 6.62 (3.49–11.53), 6.77 (2.62–15.13) and 12.13 (4.83–20.28), respectively (P = 0.041, Mann–Whitney U‐test). IDA, iron deficiency anaemia.
Figure 5.
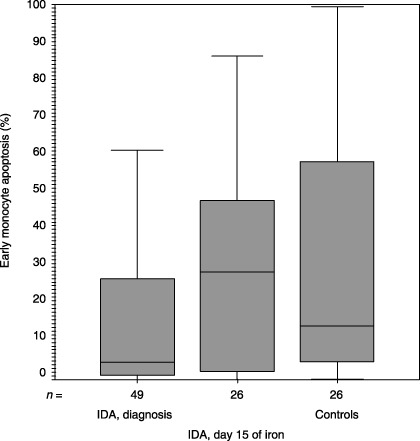
Median (inter‐quartile range) values for early monocyte apoptosis (%) upon inclusion to the study and at day 15 of iron therapy and the controls were 4.58 (1.12–28.54), 28.8 (1.99–48.56) and 14.31 (4.92–58.01), respectively (P = 0.007, Mann–Whitney U‐test). IDA, iron deficiency anaemia.
Figure 6.
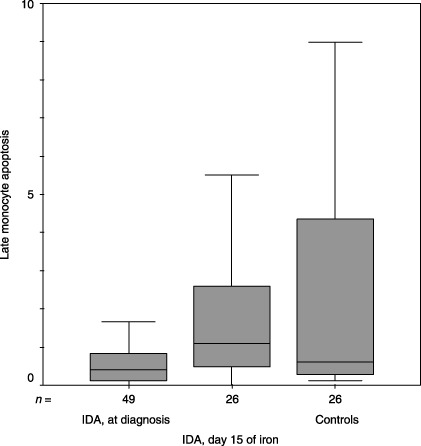
Median (inter‐quartile range) values for late monocyte apoptosis (%) upon inclusion to the study and at day 15 of iron therapy and the controls were 0.4 (0.12–0.84), 1.08 (0.45–2.66) and 0.61 (0.29–4.72), respectively (P = 0.021, Mann–Whitney U‐test). IDA, iron deficiency anaemia.
Figure 7.
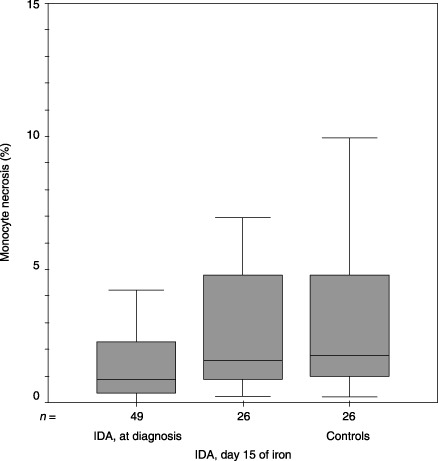
Median (inter‐quartile range) values for monocyte necrosis (%) upon inclusion to the study and at day 15 of iron therapy and the controls were 0.86 (0.36–2.38), 1.6 (0.83–4.85) and 1.77 (0.97–5.14), respectively (P = 0.009, Mann–Whitney U‐test). IDA, iron deficiency anaemia.
Effects of iron supplementation on parameters of apoptosis
In IDA patients, parameters of both neutrophil and monocyte apoptosis at inclusion into the study, were shown to increase to the values of those of control patients, on day 15 of iron supplementation treatment (Table 4). This increase was found to be statistically significant for all neutrophil and monocyte apoptosis responses, but not for percentages of necrotic neutrophils (P = 0.269), while percentages of neutrophil and monocyte apoptosis (P = 0.058 and P = 0.078, respectively) were found to have a borderline statistical significance (Table 4).
Effects of severe IDA on parameters of apoptosis
Iron deficiency anaemia patients were divided in two groups, severe IDA patients (those with haemoglobin levels of ≤ 8.0 g/dL) and mild IDA patients (those with haemoglobin levels of ≥ 8.01 g/dL), in order to demonstrate effects of severe IDA on parameters of apoptosis. In severe IDA patients, monocyte apoptosis (in terms of percentages early and late apoptosis), were found to be significantly lower than those of mild IDA patients, while neutrophil apoptosis (in terms of percentages of early and late apoptosis, P = 0.077 and P = 0.059, respectively) were found to have a borderline statistical significance (Table 5).
Table 5.
Neutrophil and monocyte apoptosis in severe and mild IDA patients
| Severe IDA at admission to the study (n = 20) (mean±SD) | Mild IDA at admission to the study (n = 29) (mean±SD) | P * | |
|---|---|---|---|
| Early neutrophil apoptosis a (%) | 18.9 ± 18.3 | 31.1 ± 24.2 | 0.077 |
| Late neutrophil apoptosis b (%) | 8.9 ± 12.8 | 15.0 ± 13.8 | 0.059 |
| Neutrophil necrosis c (%) | 7.8 ± 7.1 | 10.1 ± 9.7 | 0.433 |
| Early monocyte apoptosis a (%) | 7.1 ± 14.0 | 21.9 ± 23.2 | 0.002 |
| Late monocyte apoptosis b (%) | 0.7 ± 1.8 | 1.1 ± 1.5 | 0.02 |
| Monocyte necrosis c (%) | 1.6 ± 1.3 | 1.8 ± 3.0 | 0.464 |
Mann–Whitney U‐test.
Measured by percent fluoresence of Phi‐Philux that is cleaved by active caspase‐3.
Measured by percent fluoresence of Phi‐Philux that is cleaved by active caspase‐3 and propidium iodide.
Percentage of cells that are stained by propidium iodide only.
DISCUSSION
Iron deficiency is probably the most prevalent and most common micronutrient deficiency in the developing world today (Lukes 1995; Tatala et al. 1998; Asobayire et al. 2001; Abalkhail & Shawky 2002; Hashizume et al. 2003). According to the World Health Organization, frequency of paediatric IDA in developing countries is between 46% and 51% (World Health Organization 1998). Results from limited studies conducted in different provinces in Turkey have shown that iron deficiency is the most important cause of anaemia in children, with prevalence of between 3.2% and 18.3% (Gurel et al. 1981; Calvo & Gnazzo 1990; Kocak et al. 1995; Aydinok et al. 1998; Koc et al. 2000; Kilinc et al. 2002; Keskin et al. 2005). According to these results, Turkey has a comparatively moderate‐to‐high pre‐valance of IDA. Poor diet and low dietary iron bioavailability are the principal factors that contribute to this high incidence of iron deficiency (Bridges 1992; Tatala et al. 1998; Simonart et al. 2000; Sherry et al. 2001). Likewise, numbers of patients with poor eating habits in our study were found to be significantly higher in IDA group than controls.
In addition to its role in haemoglobin production, iron also contributes to changing levels of apoptosis (Bridges 1992; Thiele & Kastan 2002). There are both intrinsic and extrinsic pathways and mechanisms that result in apoptotic cell death (Binet et al. 1996). Both cascades converge at one point of activation, that of caspase‐3. Thus, measurement of caspase‐3 activation is a direct and relevant method for demonstrating cells that undergo apoptosis rather than some others (e.g. methods such as Annexin V staining), which also shows monocyte and neutrophil activation (Salvesen & Dixit 1997). The intrinsic pathway occurs with release of mitochondrial cytochrome c (Smith 2000); iron is an important component of mature cytochrome c (Reed 1997). Thus, impaired DNA synthesis as a result of iron deficiency may lead to subsequent alteration in levels of programmed cell death. Accordingly, several in vitro experiments have been performed to determine the status of neutrophil/monocyte apoptosis with iron chelation (Fukuchi et al. 1994; Hannah et al. 1995; Haq et al. 1995; Hileti et al. 1995; Binet et al. 1996; Kovar et al. 1997; Juckett et al. 1998; Kyriakou et al. 1998; Yuan 1999; Rakba et al. 2000; Simonart et al. 2000; Maclean et al. 2001; Kramer et al. 2002; Mecklenburgh et al. 2002; Bergman et al. 2005). Although iron deficiency and induction of apoptosis have well documented association, from several in vitro experiments (Fukuchi et al. 1994; Hileti et al. 1995; Kovar et al. 1997; Kyriakou et al. 1998; Yuan 1999; Rakba et al. 2000), hypoxia and/or iron chelation of neutrophils from healthy individuals have also been shown to cause a profound but reversible inhibition of apoptosis (Hannah et al. 1995; Mecklenburgh et al. 2002). Haq et al. (1995), induced iron deprivation in human leukaemic cells by incubation with either galium or desferioxamine and observed an increase in the number of apoptotic cells, which could be prevented by iron supplementation to the medium. Similar findings were reported by Maclean et al. (2001), Juckett et al. (1998), Simonart et al. (2000) and Kovar et al. (1997) on myeloid cells of normal mouse, on human umbilical vein endothelial cells, on Kaposi's sarcoma cells and on mouse B‐cell lymphoma cells, respectively. Yet, on the contrary, the in vitro study of Bergman et al. (2005) revealed that the number of apoptotic neutrophils and mononuclear cells from IDA patients do not differ from that of control groups. In our investigation, neutrophil and monocyte apoptosis responses, in terms of early and late apoptosis percentages, and necrotic cell percentages were all found to be significantly lower than those of control groups. In IDA patients, both neutrophil and monocyte apoptosis parameters upon their inclusion to the study were demonstrated to have increased to the values of controls on day 15 of iron supplementation treatment. Correction of differences after iron supplementation therapy and demonstration of the enhanced effect of IDA on apoptotic responses in severe IDA patients, when compared to mild IDA patients, implies that IDA might be a reason for changes in neutophil and monocyte apoptoses. Discrepancies between previous investigations and our results might be because those had been carried out in vitro, whereas this investigation was performed on clinical bases with healthy individuals. The discordance might emphasize differences from each other between in vivo and in vitro conditions.
Delayed neutrophil apoptosis has been associated with inflammatory states such as sepsis or pneumonia (Dibbert et al. 1999). In the resolution of inflammation, accumulated neutrophils need to be safely removed. Apoptosis of neutrophils controls the duration and the intensity of an inflammatory response and therefore the extent of neutrophil‐mediated tissue damage. Under normal conditions, cell death and cell proliferation have a critical balance that regulates the size of cell populations and apoptosis plays a key role in this balance. It is imperative for cells to undergo spontaneous apoptosis as a mechanism to facilitate normal cell turnover and immune system homeostasis. Sustained immune reactions may lead to disruption of the critical balance and contribute to a number of human diseases, ranging from neurodegenerative or autoimmune disorders to malignancy (Thompson 1995; Hanahan & Weinberg 2000; Okada & Mak 2004; Navratil et al. 2006). The notion that apoptosis might influence the malignant phenotype goes back to the early 1970s (Lowe & Lin 2000). Kinetic studies of tumour growth implied that cell loss from tumours could be massive and hence changes in this ‘cell loss factor’ could have a major impact on tumour growth or regression (Kerr et al. 1972; Wyllie et al. 1980). Besides, there are studies highlighting disruption of apoptosis can promote tumour initiation, progression and treatment resistance (Clarke et al. 1993; Lowe et al. 1993; Merritt et al. 1994; Symonds et al. 1994; Pritchard et al. 1998; Schmitt et al. 1999), and it seems likely that one or more apoptotic programs must be lost to reach the malignant state (Lowe & Lin 2000).
As the source of energy production, mitochondria have a critical importance in apoptosis. Mature cytochrome c from mitochondria that encompass iron requisition (Reed 1997) is essential for the flow of the apoptotic cascade. Thus, it is logical to observe inhibition of apoptosis in an iron deficiency setting in otherwise healthy human beings, as we have demonstrated in this clinical study.
In conclusion, inhibition of neutrophil and monocyte apoptosis as shown here might lead to disruption of the immune balance in IDA patients. Inhibition of apoptosis could initiate a pro‐inflammatory state for the immune system that could lead to autoimmunity or to malignancy in the long term. Hence, results of our research shown here – diminution of apoptotic function with iron deficiency anaemia – indicate the necessity of longitudinal studies in order to document the impact of severe and long‐lasting IDA on autoimmunity and malignancy.
REFERENCES
- Abalkhail B, Shawky S (2002) Prevalence of daily breakfast intake, iron deficiency anaemia and awareness of being anaemic among Saudi school students. Int. J. Food Sci. Nutr. 53, 519. [DOI] [PubMed] [Google Scholar]
- Asobayire FS, Adou P, Davidsson L, Cook JD, Hurrell RF (2001) Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: a study in Cote d’Ivoire. Am. J. Clin. Nutr. 74, 776. [DOI] [PubMed] [Google Scholar]
- Aydinok Y, Oztop S, Nisli G, Kavakli K, Cetingil N (1998) Percentile norms and curves for haematological values in Turkish adolescents. Turk. J. Haematol. 15, 169. [Google Scholar]
- Bergman M, Salman H, Pinchasi R, Straussberg R, Djaldetti M, Bessler H (2005) Phagocytic capacity and apoptosis of peripheral blood cells from patients with iron deficiency anemia. Biomed. Pharmacother. 59, 307. [DOI] [PubMed] [Google Scholar]
- Binet JL, Mentz F, Merle‐Beral H (1996) Apoptosis in blood diseases. Review new data. Hematol. Cell Ther. 38, 253. [DOI] [PubMed] [Google Scholar]
- Bridges R (1992) Iron metabolism and sideroblastic anemia In: Nathan DG, Oski FA, eds. Hematology of Infancy and Childhood, p. 391 Philadelphia, PA: W.B. Saunders Company. [Google Scholar]
- Calvo EB, Gnazzo N (1990) Prevalence of iron deficiency in children aged 9–24 mo from a large urban area of Argentina. Am. J. Clin. Nutr. 52, 534. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Purdie C, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH (1993) Thymocyte apoptosis induced by p53‐dependent and independent pathways. Nature 362, 849. [DOI] [PubMed] [Google Scholar]
- Dibbert B, Weber M, Nikolaizik WH, Vogt P, Schoni MH, Blaser K, Simon HU (1999) Cytokine‐mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc. Natl Acad. Sci. USA 96, 13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K, Tomoyasu S, Tsuruoka N, Gomi K (1994) Iron deprivation‐induced apoptosis in HL‐60 cells. FEBS Lett. 350, 139. [DOI] [PubMed] [Google Scholar]
- Gurel G, Calik A, Kurkcuoglu M, Tanyeri G, Yilmaz A (1981) Erzurum ili ilkokul çocuklarında demir eksikliği ile ilgili bir çalışma In: Tumay SB, Bilger M, Bedir O, Hatemi N, Cenani A, eds. Türk Çocuğunun Sağlık Sorunları XVIII Türk Pediatri Kongresi, p. 27 Istanbul, Turkey: Özlem Kardeşler Matbaası. [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100, 57. [DOI] [PubMed] [Google Scholar]
- Hannah S, Mecklenburgh K, Rahman I, Bellingan GJ, Greening A, Haslett C, Chilvers ER (1995) Hypoxia prolongs neutrophil survival in vitro . FEBS Lett. 372, 233. [DOI] [PubMed] [Google Scholar]
- Haq RU, Wereley J, Chitambar CR (1995) Induction of apoptosis by iron deprivation in human leukemic CCRF‐CEM cells. Exp. Hematol. 23, 428. [PubMed] [Google Scholar]
- Hashizume M, Kunii O, Sasaki S, Shimoda T, Wakai S, Mazhitova Z, Dauletbaev D, Caypil W, Aldiyarova M, Farmer A, Yamashiro Y, Chiba M (2003) Anemia and iron deficiency among schoolchildren in the Aral Sea region, Kazakhistan. J. Trop. Pediatr. 49, 172. [DOI] [PubMed] [Google Scholar]
- Hileti D, Panayiotidis P, Hoffbrand AV (1995) Iron chelators induce apoptosis in proliferating cells. Br. J. Haematol. 89, 181. [DOI] [PubMed] [Google Scholar]
- Juckett MB, Shadley J, Zheng Y, Klein JP (1998) Desferrioxamine enhances the effects of gamma radiation on clonogenic survival and the formation of chromosomal aberrations in endothelial cells. Radiat. Res. 149, 330. [PubMed] [Google Scholar]
- Kerr JF, Wyllie A, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide‐ranging implications in tissue kinetics. Br. J. Cancer 26, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin Y, Moschonis G, Dimitriou M, Sur H, Kocaoglu B, Hayran O, Manios Y (2005) Prevalence of iron deficiency among school children of different socio‐economic status in urban Turkey. Eur J. Clin. Nutr. 59, 64. [DOI] [PubMed] [Google Scholar]
- Kilinc M, Yuregir G, Ekerbicer H (2002) Anaemia and iron‐deficiency anaemia in south‐east Anatolia. Eur. J. Haematol. 69, 280. [DOI] [PubMed] [Google Scholar]
- Koc A, Kosecik M, Vural H, Erel O, Atas A, Tatli MM (2000) The frequency and etiology of anemia among children 6–16 years of age in the southeast region of Turkey. Turk. J. Pediatr. 42, 91. [PubMed] [Google Scholar]
- Kocak R, Alparslan Z, Agridag G, Baslamisli F, Aksungur PD, Koltas S (1995) The frequency of anaemia, iron deficiency, hemoglobin S and beta thalassemia in the south of Turkey. Eur. J. Epidemiol. 11, 181. [DOI] [PubMed] [Google Scholar]
- Kovar J, Stunz L, Stewart BC, Kriegerbeckova K, Ashman RF, Kemp JD (1997) Direct evidence that iron deprivation induces apoptosis in murine lymphoma 38C13. Pathobiology 65, 61. [DOI] [PubMed] [Google Scholar]
- Kramer JL, Baltathakis I, Alcantara OS, Boldt DH (2002) Differentiation of functional dendritic cells and macrophages from human peripheral blood monocyte precursors is dependent on expression of p21 (WAF1/CIP1) and requires iron. Br. J. Haematol. 117, 727. [DOI] [PubMed] [Google Scholar]
- Kyriakou D, Eliopoulos A, Papadakis A, Alexandrakis M, Eliopoulos GD (1998) Decreased expression of c‐myc oncoprotein by peripheral blood mononuclear cells in thalassaemia patients receiving desferrioxamine. Eur. J. Haematol. 60, 21. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Lin A (2000) Apoptosis in cancer. Carcinogenesis 21, 485–495. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Schmitt E, Smith SW, Osborne BA, Jacks T (1993) p53 is required for radiation‐induced apoptosis in mouse thymocytes. Nature 362, 847. [DOI] [PubMed] [Google Scholar]
- Lukes J (1995) Iron metabolism and iron deficiency In: Miller DR, ed. Blood Diseases in Infancy and Childhood, p. 193 St. Louis, MO: CV Mosby. [Google Scholar]
- Maclean K, Yang H, Cleveland JL (2001) Serum suppresses myeloid progenitor apoptosis by regulating iron homeost. J. Cell Biochem. 82, 171. [DOI] [PubMed] [Google Scholar]
- Mecklenburgh KI, Walmsley S, Cowburn AS, Wiesener M, Reed BJ, Upton PD, Deighton J, Greening AP, Chilvers ER (2002) Involvement of a ferroprotein sensor in hypoxia‐mediated inhibition of neutrophil apoptosis. Blood 100, 3008. [DOI] [PubMed] [Google Scholar]
- Merritt AJ, Potten C, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA (1994) The role of p53 in spontaneous and radiation‐induced apoptosis in the gastrointestinal tract of normal and p53‐deficient mice. Cancer Res. 54, 614. [PubMed] [Google Scholar]
- Navratil JS, Liu CC, Ahearn JM (2006) Apoptosis and autoimmunity. Immunol. Res. 36, 3. [DOI] [PubMed] [Google Scholar]
- Okada H, Mak TW (2004) Pathways of apoptotic and non‐apoptotic death in tumour cells. Nat. Rev. Cancer 4, 592. [DOI] [PubMed] [Google Scholar]
- Pritchard DM, Potten C, Hickman JA (1998) The relationships between p53‐dependent apoptosis, inhibition of proliferation, and 5‐fluorouracil‐induced histopathology in murine intestinal epithelia. Cancer Res. 58, 5453. [PubMed] [Google Scholar]
- Rakba N, Loyer P, Gilot D, Delcros JG, Glaise D, Baret P, Pierre JL, Brissot P, Lescoat G (2000) Antiproliferative and apoptotic effects of O‐Trensox, a new synthetic iron chelator, on differentiated human hepatoma cell lines. Carcinogenesis 21, 943. [DOI] [PubMed] [Google Scholar]
- Reed J (1997) Cytochrome c: can't live with it‐can't live without it. Cell 91, 559. [DOI] [PubMed] [Google Scholar]
- Reichard P, Ehrenberg A (1983) Ribonucleotide reductase‐a radical enzyme. Science 221, 514. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM (1997) Caspases: intracellular signaling by proteolysis. Cell 91, 443. [DOI] [PubMed] [Google Scholar]
- Schmitt CA, McCurrach M, De Stanchina E, Wallace‐Brodeur RR, Lowe SW (1999) INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 13, 2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B, Mei Z, Yip R (2001) Continuation of the decline in prevalence of anemia in low‐income infants and children in five states. Pediatrics 107, 677. [DOI] [PubMed] [Google Scholar]
- Simonart T, Degraef C, Andrei G, Mosselmans R, Hermans P, Van Vooren JP, Noel JC, Boelaert JR, Snoeck R, Heenen M (2000) Iron chelators inhibit the growth and induce the apoptosis of Kaposi's sarcoma cells and of their putative endothelial precursors. J. Invest. Dermatol. 115, 893. [DOI] [PubMed] [Google Scholar]
- Smith CW (2000) Clinical immunology, neutrophils In: Abbas AK, Lichtman A, Pober JS, eds. Cellular and Molecular Immunology, p. 15 Philadelphia, PA: W.B. Saunders Company. [Google Scholar]
- Symonds H, Krall L, Remington L, Saenz‐Robles M, Lowe S, Jacks T, Van Dyke T (1994) p53‐dependent apoptosis suppresses tumor growth and progression in vivo . Cell 78, 703. [DOI] [PubMed] [Google Scholar]
- Takei H, Araki A, Watanabe H, Ichinose A, Sendo F (1996) Rapid killing of human neutrophils by the potent activator phorbol 12‐myristate 13‐acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 59, 229. [DOI] [PubMed] [Google Scholar]
- Tatala S, Svanberg U, Mduma B (1998) Low dietary iron availability is a major cause of anemia: a nutrition survey in the Lindi District of Tanzania. Am. J. Clin. Nutr. 68, 171. [DOI] [PubMed] [Google Scholar]
- Thiele CJ, Kastan M (2002) Biology of childhood cancer In: Pizzo PA, Poplack DG, eds. Principles and Practice of Pediatric Oncology, p. 89 Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Thompson C (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1998) World Health Organization, UNICEF & UNU Iron Deficiency: Indicators for Assessment and Strategies for Prevention. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Wyllie AH, Kerr J, Currie AR (1980) Cell death: the significance of apoptosis. Int. Rev. Cytol. 68, 251. [DOI] [PubMed] [Google Scholar]
- Yuan X (1999) Apoptotic macrophage‐derived foam cells of human atheromas are rich in iron and ferritin, suggesting iron‐catalysed reactions to be involved in apoptosis. Free Radic. Res. 30, 221. [DOI] [PubMed] [Google Scholar]


