Abstract
Objectives
Gastric cancer is an important cause of cancer‐related mortality worldwide (1). There is increasing evidence that the existence of cancer stem cells (CSC) is responsible for tumour formation and maintenance.
Materials and methods
The present study was designed to recognise circulating CSCs from blood samples of patients with gastric cancer, using CD133 and ABCG2 as potential markers. CD133−, CD133+ ABCG2− and CD133+ ABCG2+ cells lines were analysed by flow cytometry, immunofluorescence staining, western blotting and real‐time PCR. Furthermore, functional assays (clonogenic assay in vitro and tumourigenic assay in vivo) were also performed using these cell lines.
Results
Higher percentages of CD133+ cells were identified in blood samples from gastric cancer patients compared to normal controls. In addition, we found by using Kaplan–Meier analysis, that numbers of CD133+ cells correlated with poor prognosis gastric cancer patients. Finally, tumourigenic properties of CD133+ ABCG2+ cells were determined in vitro and in vivo.
Conclusions
Our in vitro and in vivo experiments demonstrated that CD133+ ABCG2+ cells exhibited well‐known CSC characteristics; thus when circulating they could be used as a prognostic marker for gastric cancer.
Introduction
Gastric cancer is an important cause of cancer‐related mortality worldwide, with 988 000 new cases and 736 000 deaths per year 1. Of the world total, nearly 42% male and 19% female gastric cancer patients are found in China 2.
The cancer stem cell (CSC) hypothesis proposed that no more than a proportion of cells of a cancer self‐regenerate, proliferate and have multiple differentiation potential 3. It has been suggested that current therapies have failed to prevent gastric cancer relapse and metastasis due to the existence and activity of CSC 4. Previous studies have revealed that so‐called side population (SP) cells have stem cell‐like characteristics, which suggests that they are enriched with CSCs 5, 6, 7. SP cells exclude fluorescent DNA‐binding dye Hoechst 33342, due to the action of the ABCG2 (BCRP1) transporter molecule 8. Although most previous studies on CSC focussed on identification and characterization of CSCs from the primary tumour mass, more and more recent investigations have reported circulating CSCs isolated from patients’ blood 9, 10, 11. Fan et al. 12 highlighted the importance of circulating CSCs as a prognostic tool to illuminate early recurrence of hepatocellular carcinoma after hepatectomy. In the study of Li et al. 9, circulating CD44+ CSC provided more specific prognostic information regarding recurrence risk, than merely detecting circulating CSC in gastric cancer patients. A number of markers has been proposed to identify circulating CSCs, including CD133, a glycoprotein expressed on haematopoietic progenitors 13. Pilati et al. 14 confirmed CD133 to be a marker for circulating CSCs in patients with colorectal cancer.
However, up to now, no studies investigating prognostic and biological relevance of circulating CD133+ cells in gastric cancer, have been reported. Here, we identified CD133+ cells in patients’ blood and evaluated clinical characteristics of these patients.
Materials and methods
Gastric cancer patient specimens
The clinical investigation was conducted according to the principles expressed in the Helsinki Declaration of 1975. Blood samples were obtained from 36 patients who had had no chemotherapy nor radiotherapy prior to resection, at the Department of General Surgery, Center Hospital of Handan, between March 2008 and September 2012. Blood samples from 16 consenting healthy individuals were used as control.
Sorting of circulating CD133+ cells
Cellgro Lymphocyte Separation Medium (Mediatech, Herndon, VA, USA) was used to isolate mononuclear cells from blood samples, according to the manufacturer's instructions. A cocktail phycoerythrin‐conjugated mouse anti‐human CD133 antibody was made according to the manufacturer's protocol (StemCell Technologies, Miami, FL, USA). Briefly, cells from blood samples were suspended in PBS with 2% foetal bovine serum and 0.5 μm EDTA, labelled with anti‐CD133 antibody cocktail and mixed using appropriate magnetic microbeads; CD133+ and CD133− cells were then separated magnetically. Sorted cells were seeded on six‐well plates and allowed to adhere, floating ones being discarded. Purity of sorted cells was evaluated using flow cytometry (Becton Dickinson, San Jose, CA, USA).
Side population cell analysis
Adherent CD133+ and CD133− cells isolated from blood samples were suspended at 1 × 106 cells/ml then incubated at 37 °C for 60 min with 5 μg/ml Hoechst 33342 (Sigma Chemicals, St Louis, MO, USA). Control cells were cultured in the presence of 500 μm verapamil (Sigma). Analysis and sorting was performed using a FACS Vantage SE cytometer (Becton Dickinson). Hoechst 33342 was excited by UV laser at 350 nm and fluorescence emission was measured at both 402–446 nm (Hoechst blue) and 640 nm (Hoechst red).
Immunofluorescence
CD133+ ABCG2− and CD133+ ABCG2+ cells were stained with fluorescent nucleic acid dye 4,2‐diamidino‐2‐phenylindole dihydrochloride (DAPI; KeyGEN BioTECH, Nanjing, China) to identify nucleate cells. They were subsequently stained with fluorescently labelled monoclonal antibodies against CD45 (APC) (eBioscience, San Diego, CA, USA) and cytokeratin 8 (CK8) (PE) (eBioscience), to identify tumour cells.
Western blotting
Cells were lysed for protein extraction using the Total Protein Extraction Kit (KeyGEN) according to the manufacturer's instructions. Protein concentration was measured using BCA Protein Assay Kit (KeyGEN) as directed. Thirty micrograms of each protein were denatured at 95 °C then resolved on 10% SDS‐PAGE and transferred to PVDF membranes (Millipore Corporation, Bedford, MA, USA) using the wet transfer blotting system (Bio‐Rad, Hercules, CA, USA). Membranes were blocked with 2.5% non‐fat milk, and probed with the appropriate antibodies; primaries against CD133 (1:200; Millipore), ABCG2 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β‐actin (1:1000; Santa Cruz), with alkaline phosphatase‐conjugated IgG of the appropriate species (1:1000; KeyGEN) as secondary. Protein was detected using ECL Western Blotting Analysis System (Amersham Biosciences, Piscataway, NJ, USA).
Real‐time PCR
Total RNA was isolated from cells using RNeasy Mini Kit (Biomed, Beijing, China). cDNA was reverse transcribed with 1 μg total RNA using a TaKaRa Reverse Transcription Kit (TaKaRa, Dalian, China) and was amplified with the following primers. CD133 primers were 5′‐ACCGACTGAGACCCAACATC‐3′ (sense) and 5′‐GGTGCTGTTCATGTTCTCCA‐3′ (antisense). ABCG2 primers were 5′‐AGCTGCAAGGAAAGATCCAA‐3′ (sense) and 5′‐TCCAGACACACCACGGATAA‐3′ (antisense). GAPDH primers were 5′‐AGAAGGCTGGGGCTCATTTG‐3′ (sense) and 5′‐AGGGGCCATCCACAGTCTTC‐3′ (antisense) and used as an internal control. Amplification of CD133, ABCG2 and GAPDH was performed with 1 cycle at 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Calculation of relative expression of each transcript was performed using the 2−ΔΔCt method.
Cell proliferation
Cell Counting Kit‐8 (CCK‐8; Dojindo Molecular Technologies, Rockville, MD, USA) was employed to determine cell viability. In brief, 1000 cells/well were seeded on 96‐well plates and allowed to adhere. After 48 h, 10 μl of CCK‐8 solution was added to each well, and plates were incubated for 4 h; absorbance was measured at 450 nm using a microplate reader (Bio‐Rad).
Sphere assay
Cells were plated at 6 × 104 cells/well in six‐well, ultra‐low attachment plates, under serum‐free, sphere‐specific conditions, described by Gibbs and colleagues 15. Fresh aliquots of epidermal growth factor and basic fibroblast growth factor were added daily. After 5 days culture, spheres were visible by light microscopy (Olympus CX31; Olympus, Tokyo, Japan).
In vivo tumour study
All experiments with animals were performed according to the guidelines of the Liaoning Medical University Ethical Committee. Unsorted, CD133−, CD133+, CD133+ ABCG2− or CD133+ ABCG2+ cells (2 × 107 in 200 μl) were subcutaneously injected into 5‐ to 7‐week‐old, male BALB/c mice weighing 17–20 g (Charles River, Wilmington, MA, USA). Animals were kept with 12 h light/dark cycle (7 a.m. to 7 p.m.) at 22 °C with free access to food and water. Every 5 days until the end of the experiment, one mouse from each group was randomly selected to be anaesthetized, photographed and sacrificed. For each tumour, measurements were made using callipers, and tumour volumes were calculated as follows:
16.
Immunohistochemistry
Immunohistochemistry was performed on deparaffinized 5 μm sections according to previous reports 17. Briefly, deparaffinized sections were stained with the first antibody against CD133, ABCG2 or Ki‐67, as described in the western blotting assay, by incubating overnight at 4 °C. Secondary staining with biotinylated secondary antibodies and tertiary staining with streptavidin–horseradish peroxidase complex (Beyotime, Beijing, China) were performed for 60 min at room temperature. Then, sections were counterstained with haematoxylin (Beyotime) and mounted.
Statistical analysis
Data were analysed using GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA) and performed using one‐tailed Student's t‐test (unilateral and unpaired). Discrete variables were compared using chi‐squared testing. Kaplan–Meier survival plots were generated and comparisons between survival curves were made using log‐rank statistical analysis. P values <0.05 were considered to indicate statistically significant differences.
Results
Circulating CD133+ ABCG2+ cells from the patient's blood
CD133− and CD133+ cells in blood samples were isolated using magnetic microbeads and sorted cells were evaluated using flow cytometry (Fig. 1a). We found that the CD133+ fraction comprised 0.4–4.1% (mean = 1.6%) total peripheral blood mononuclear cells in these gastric cancer patients. Higher percentages of CD133+ cells were identified in 36 blood samples from gastric cancer patients compared to healthy controls (0.0–2.4%, mean = 0.6%) (P < 0.05, Fig. 1c). CD133− and CD133+ cells, after culture, were labelled with Hoechst 33342 and isolated using flow cytometry. We found that the SP fraction was comprised of 0.03% CD133− cells and 0.34% of CD133+ cells, and that this population disappeared following treatment with the selective ABCG2 transporter inhibitor, verapamil (Fig. 1a). Immunofluorescence assays were performed on CD133+ ABCG2− and CD133+ ABCG2+ cells. Both CD133+ ABCG2− and CD133+ ABCG2+ cells showed cytokeratin (CK) expression but not CD45 expression (Fig. 1b). Follow‐up information was available for the 36 patients for periods ranging from 1 month to 5 years (median = 32 months). In addition, we found that of these patients with gastric cancer, presence of CD133 cells correlated with poor prognosis, using Kaplan–Meier analysis (P < 0.05, Fig. 1d). Results of association of CD133+ cells with patients clinicopathological characteristics are summarized in Table 1. CD133+ cells related to tumour differentiation (P = 0.047), lymphatic invasion (P = 0.024) and venous invasion (P = 0.031).
Figure 1.
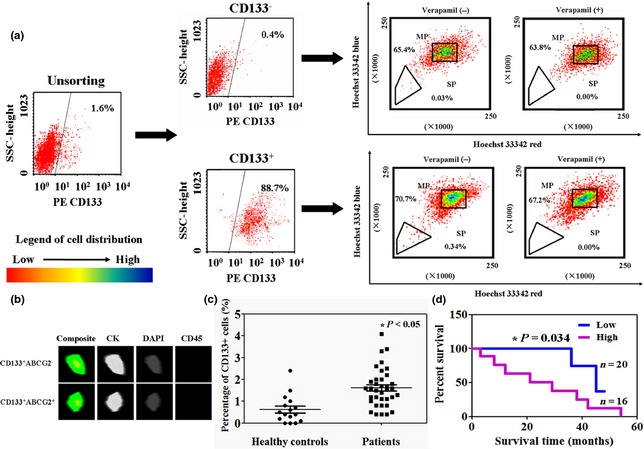
Circulating CD133 + cells in gastric cancer specimens. (a) Distribution of CD133+ and CD133− cells in blood samples from patients, detected by flow cytometry. Identification of side population (SP) and main population (MP) CD133+ and CD133− cells, using Hoechst 33342 exclusion assay. Control cells were cultured in the presence of 500 μm verapamil. (b) Representative images of staining of adherent CD133+ and CD133− cells from blood of gastric cancer patients, using immunofluorescence assay. Cells were identified following immunostaining with CK, DAPI and CD45. (c) Percentages of circulating CD133+ cells were determined using fluorescence‐activated cell sorting, of patients and healthy controls. (d) Kaplan–Meier curves of cumulative survival rate of patients with gastric cancer based on their percentage of circulating CD133+ cells.
Table 1.
Relationship between CD133 cells and clinicopathological parameters of patients with gastric cancer
| Clinicopathological features | CD133 cells | ||||
|---|---|---|---|---|---|
| n | Low | High | χ2 | P | |
| Sex | |||||
| Female | 13 | 7 | 6 | 0.038 | 0.846 |
| Male | 23 | 13 | 10 | ||
| Age (years) | |||||
| <65 | 11 | 4 | 7 | 1.376 | 0.241 |
| ≥65 | 25 | 16 | 9 | ||
| Differentiation | |||||
| Differentiated | 17 | 6 | 11 | 3.913 | 0.047 |
| Undifferentiated | 19 | 14 | 5 | ||
| Lymphatic invasion | |||||
| − | 12 | 3 | 9 | 5.077 | 0.024 |
| + | 24 | 17 | 7 | ||
| Venous invasion | |||||
| − | 21 | 8 | 13 | 4.641 | 0.031 |
| + | 15 | 12 | 3 | ||
| Lymph node metastasis | |||||
| − | 14 | 7 | 7 | 0.037 | 0.848 |
| + | 22 | 13 | 9 | ||
| Tumour size | |||||
| <4 cm | 18 | 8 | 10 | 1.012 | 0.314 |
| ≥4 cm | 18 | 12 | 6 | ||
| pN category | |||||
| pN0 | 10 | 5 | 5 | 0.944 | 0.815 |
| pN1 | 8 | 4 | 4 | ||
| pN2 | 7 | 5 | 2 | ||
| pN3 | 11 | 6 | 5 | ||
χ2 value, chi‐squared distribution.
Bold values mean the values have statistical significance (>0.05).
CD133+ ABCG2+ cells displayed tumourigenic properties in vitro and in vivo
Protein and mRNA levels of ABCG2 and CD133 were detected using western blotting and real‐time PCR respectively. Expressions of ABCG2 and CD133 protein and mRNA in CD133+ ABCG2+ cells were significantly higher than in other cells (Fig. 2a). MTT assays had been performed to detect proliferation of unsorted, CD133−, CD133+, CD133+ ABCG2− and CD133+ ABCG2+ cells. Growth curves demonstrated that CD133+ ABCG2+ cells had highest proliferation ratio compared to the other lines (P < 0.05, Fig. 2b). CD133− and CD133+ ABCG2− lines almost lost proliferation ability (P < 0.05, Fig. 2b). After 5 days culture, sphere clusters were clearly observed in CD133+ ABCG2+ cultures, while the other lines did not form spheres (Fig. 2c).
Figure 2.
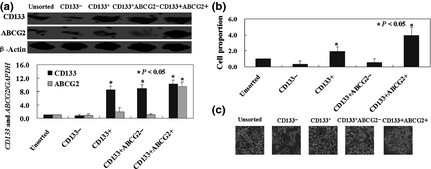
CD133 + ABCG2 + cells displayed tumourigenic properties in vitro. (a) Protein and mRNA levels of ABCG2 and CD133 were detected by using western blotting and real‐time PCR respectively. (b) Proliferation ratio of each cell line as described in the Materials and methods section, was determined by MTT assay. (c) Representative pictures of sphere cluster formed by CD133+ ABCG2+ cells, and no sphere cluster formed by other cells.
Furthermore, we found that unsorted, CD133−, CD133+, CD133+ ABCG2− and CD133+ ABCG2+ cells all had their ability to transfer growing tumour cells to immunocompromised mice (Fig. 3a). As shown in Fig. 3b, tumour volume of CD133+ ABCG2+‐injected mice was 1.8‐ to 4.3‐fold more than the other cell injected animals (P < 0.05). However, tumour volume of CD133− or CD133+ ABCG2−‐injected mice showed no significant changes from beginning to end of the experiment. Accordingly, survival rate of CD133+ ABCG2+‐injected mice was significantly lower than mice bearing tumours formed from the other lines (P < 0.05, Fig. 3c). Results of immunohistochemistry showed that both CD133+ cells and ABCG2+ cells regenerated positive and negative populations (Fig. 3d). Ki‐67 immunohistochemical staining showed higher Ki‐67 positivity in tissues of CD133+ ABCG2+‐injected mice than in others (Fig. 3d).
Figure 3.
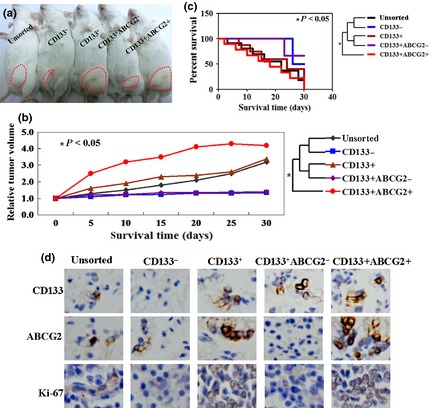
CD133 + ABCG2 + cells displayed tumourigenic properties in vivo. (a) Macroscopic appearance of subcutaneous tumours in each group, as described in the Materials and methods section. (b) Tumour volume of each group. (c) Kaplan–Meier survival curves of the groups. (d) Immunohistochemical staining of resected tumour tissues from each group using CD133, ABCG2 and Ki‐67 antibodies. Bound antibody detected with DAB appears brown.
Discussion
In this study, we identified CD133+ ABCG2+ cells in peripheral blood of patients with gastric cancer, by immunomagnetic selection or flow cytometry, on the basis of CD133 and ABCG2 expression. We demonstrated that presence of circulating CD133+ cells correlated with survival of these patients. In addition, sorted CD133+ ABCG2+ cells displayed tumourigenic properties in vivo and in vitro.
The CD133 molecule is expressed by numerous types of stem cell, including haematopoietic stem cells, endothelial progenitor cells and CSCs 17, 18, 19. Haematopoietic stem cells (identified as CD133+ CD34+ CD45+) have been found in peripheral blood of patients with lung cancer 19. Discoveries of Nadal et al. 20 also suggested CD133 expression in circulating tumour cells to be a promising marker of chemoresistance in breast cancer patients. Based on results of previous studies and those of our own, circulating CD133+ cells may be used as a potential biomarker in patients with cancer. However, CD133 is not a specific biomarker for circulating cancer cells. Thus, we used a further marker, ATP‐binding cassette super family G member 2 (ABCG2). ABCG2 has the capacity to export many cytotoxic drugs from cells and it is up‐regulated in SP cells 21. Previous studies have shown that the SP is enriched in primitive and CSCs 22, 23, 24. To the best of our knowledge, primitive progenitor cells expressing CD133 and ABCG2 are rare. Furthermore, we found that circulating CD133+ cells were related to tumour differentiation, lymphatic invasion and venous invasion. Nakamura et al. 25 found that CD133+ cells have enhanced invasive capacity as they elevate MMP expression and Ding et al. 26 found that CD133 facilitates epithelial–mesenchymal transition leading to invasion and metastasis of pancreatic cancer cells. Combined with previous studies, we infer that CD133+ ABCG2+ cells sorted in our study, were mainly cancer cells.
In conclusion, this study confirmed for the first time, existence of CD133+ ABCG2+ cells in peripheral blood of patients with gastric cancer. Additionally, we demonstrated that circulating CD133+ ABCG2+ cells could potentially be used as biomarker of tumour spread. Our in vitro and in vivo experiments demonstrated that CD133+ ABCG2+ cells have well‐known CSC characteristics; however, our study has some limitations. First, we could not entirely confirm CD133+ ABCG2+ cells are cancer cells and second, larger patient samples would be needed to confirm percentages of CD133+ ABCG2+ cells in future study.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We thank Miss Wang Jing‐Jing for her valuable comments and excellent technical assistance.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA. Cancer J. Clin. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2. An JY, Kim KM, Kim YM, Cheong JH, Hyung WJ, Noh SH (2012) Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: their risk factors and clinical relevance. Ann. Surg. Oncol. 19, 2452–2458. [DOI] [PubMed] [Google Scholar]
- 3. Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414, 105–111. [DOI] [PubMed] [Google Scholar]
- 4. Li K, Dan Z, Nie YQ (2014) Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J. Gastroenterol. 20, 5420–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirschmann‐Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U et al (2004) A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. USA 101, 14228–14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bussolati B, Bruno S, Grange C, Ferrando U, Camussi G (2008) Identification of a tumor‐initiating stem cell population in human renal carcinomas. FASEB J. 22, 3696–3705. [DOI] [PubMed] [Google Scholar]
- 7. Xia P, Gou WF, Wang JJ, Niu ZF, Chen S, Takano Y et al (2013) Distinct radiosensitivity of lung carcinoma stem‐like side population and main population cells. Cancer Biother. Radiopharm. 28, 471–478. [DOI] [PubMed] [Google Scholar]
- 8. Kondo T, Setoguchi T, Taga T (2004) Persistence of a small subpopulation of cancer stem‐like cells in the C6 glioma cell line. Proc. Natl. Acad. Sci. USA 101, 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li M, Zhang B, Zhang Z, Liu X, Qi X, Zhao J et al (2014) Stem cell‐like circulating tumor cells indicate poor prognosis in gastric cancer. Biomed. Res. Int. 2014, 981261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tinhofer I, Saki M, Niehr F, Keilholz U, Budach V (2014) Cancer stem cell characteristics of circulating tumor cells. Int. J. Radiat. Biol. 90, 622–627. [DOI] [PubMed] [Google Scholar]
- 11. Cheng SW, Tsai HW, Lin YJ, Cheng PN, Chang YC, Yen CJ et al (2013) Lin28B is an oncofetal circulating cancer stem cell‐like marker associated with recurrence of hepatocellular carcinoma. PLoS One 8, e80053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC, Wong J (2011) Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann. Surg. 254, 569–576. [DOI] [PubMed] [Google Scholar]
- 13. Faltas B (2012) Cornering metastases: therapeutic targeting of circulating tumor cells and stem cells. Front Oncol. 2, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pilati P, Mocellin S, Bertazza L, Galdi F, Briarava M, Mammano E et al (2012) Prognostic value of putative circulating cancer stem cells in patients undergoing hepatic resection for colorectal liver metastasis. Ann. Surg. Oncol. 19, 402–408. [DOI] [PubMed] [Google Scholar]
- 15. Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW et al (2005) Stem‐like cells in bone sarcomas: implications for tumorigenesis. Neoplasia 7, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ivković‐Kapicl T, Knelević‐Usaj S, Panjković M, Mastilović K (2006) Immunohistochemical analysis of angiogenesis in invasive ductal breast carcinoma with correlations to clinicopathological factor. Vojnosanit. Pregl. 63, 635–642. [DOI] [PubMed] [Google Scholar]
- 17. Xia P, Gou WF, Zhao S, Zheng HC (2013) Crizotinib may be used in Lewis lung carcinoma: a novel use for crizotinib. Oncol. Rep. 30, 139–148. [DOI] [PubMed] [Google Scholar]
- 18. Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S et al (2006) Identification and clinical significance of circulating endothelial progenitor cells in human non‐small cell lung cancer. Cancer Res. 66, 7341–7347. [DOI] [PubMed] [Google Scholar]
- 19. Vroling L, Lind JS, de Haas RR, Verheul HM, van Hinsbergh VW, Broxterman HJ et al (2010) CD133? Circulating haematopoietic progenitor cells predict for response to sorafenib plus erlotinib in non‐small cell lung cancer patients. Br. J. Cancer 102, 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nadal R, Ortega FG, Salido M, Lorente JA, Rodríguez‐Rivera M, Delgado‐Rodríguez M et al (2013) CD133 expression in circulating tumor cells from breast cancer patients: potential role in resistance to chemotherapy. Int. J. Cancer 133, 2398–2407. [DOI] [PubMed] [Google Scholar]
- 21. Szaka'cs G, Paterson JK, Ludwig JA, Booth‐Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5, 219–234. [DOI] [PubMed] [Google Scholar]
- 22. Larderet G, Fortunel NO, Vaigot P, Cegalerba M, Maltère P, Zobiri O et al (2006) Human side population keratinocytes exhibit long‐term proliferative potential and a specific gene expression profile and can form a pluristratified epidermis. Stem Cells 24, 965–974. [DOI] [PubMed] [Google Scholar]
- 23. Pacak CA, Cowan DB (2014) Growth of bone marrow and skeletal muscle side population stem cells in suspension culture. Methods Mol. Biol. 1210, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu HB, Meng QH, Du DW, Sun JF, Wang JB, Han H (2014) The effects of ABCG2 on the viability, proliferation and paracrine actions of kidney side population cells under oxygen‐glucose deprivation. Int. J. Med. Sci. 11, 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura M, Zhang X, Mizumoto Y, Maida Y, Bono Y, Takakura M et al (2014) Molecular characterization of CD133+ cancer stem‐like cells in endometrial cancer. Int. J. Oncol. 44, 669–677. [DOI] [PubMed] [Google Scholar]
- 26. Ding Q, Miyazaki Y, Tsukasa K, Matsubara S, Yoshimitsu M, Takao S (2014) CD133 facilitates epithelial‐mesenchymal transition through interaction with the ERK pathway in pancreatic cancer metastasis. Mol. Cancer. 13, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]


