Abstract
Abstract. Objectives: Recent data show that Imatinib mesylate (IM) also affects haematopoietic stem cells (HSC), T lymphocytes and dendritic cells that do not harbour constitutively active tyrosine kinases. Materials and methods: We evaluated possible effects of IM on human bone marrow‐derived mesenchymal stem cells (MSC) in vitro. Results: Screening the activity of 42 receptor tyrosine kinases revealed an exclusive inhibition of platelet‐derived growth factor receptorβ (PDGFRβ). Analysis of downstream targets of PDGFRβ demonstrated IM‐mediated reduction of Akt and Erk1/2 phosphorylation. Culture of MSC with IM led to the reversible development of perinuclear multi‐vesicular bodies. The proliferation and clonogenicity of MSC were significantly reduced compared to control cultures. IM favoured adipogenic differentiation of MSC whereas osteogenesis was suppressed. The functional deficits described led to a 50% reduction in the support of clonogenic haematopoietic stem cells, cultured for 1 month on a monolayer of MSC with IM. Conclusion: In summary, inhibition of PDGFRβ and downstream Akt and Erk signalling by IM has a significant impact on proliferation and differentiation of human MSC in vitro.
INTRODUCTION
Mesenchymal stem cells (MSC) have been identified in the bone marrow (BM) as multi‐potent non‐haematopoietic progenitor cells that differentiate into osteoblasts, adipocytes, chondrocytes, tenocytes, skeletal myocytes and cells of visceral mesoderm (Pittenger et al. 1999; Jiang et al. 2002; Smith et al. 2004). MSC do not express haematopoietic markers, but a specific pattern of molecules, such as SH2 (CD105), SH3 and SH4 (CD73), VCAM‐1 (CD106), THY‐1 (CD90) and STRO‐1 (Pittenger et al. 1999; Oswald et al. 2004). MSC interact with haematopoietic stem cells (HSC), influencing their homing and differentiation through cell–cell contact and the production of factors including chemokines (Majumdar et al. 2000). MSC can be cotransplanted with HSC to improve their engraftment in BM (Koc et al. 2000), and they may be used to repair or regenerate damaged or mutated bone or cartilage (Pereira et al. 1995; Horwitz et al. 1999). Finally, MSC have recently been shown to possess immunoregulatory functions (Le Blanc et al. 2004; Aggarwal & Pittenger 2005).
Imatinib mesylate (IM) is routinely used for the treatment of patients with Philadelphia chromosome positive chronic myeloid leukaemia (Druker et al. 2001; Kantarjian et al. 2002; O’Brien et al. 2003). The specific blockade of the tyrosine kinase activity of c‐kit and platelet‐derived growth factor receptorβ (PDGFRβ) by IM has been well described for leukaemic and other malignant cells (Apperley et al. 2002; Demetri et al. 2002). Recent data support the hypothesis that IM exerts non‐specific inhibitory effects on CD34+ normal haematopoietic progenitor cells and dendritic cells (Appel et al. 2004; Bartolovic et al. 2004). In addition, IM has been shown to inhibit antigen‐specific T cell proliferation, thereby potentially mediating immunosuppressive effects (Seggewiss et al. 2005).
Surprisingly, little is known about the effects of IM on benign non‐haematopoietic stem cells. Because the interaction of haematopoietic stem cells (HSC) with mesenchymal stem cells (MSC) in the bone marrow environment is of special importance for the maintenance of the quiescent HSC pool and MSC might be of relevance for the regeneration of mesenchymal tissue, we studied the effects of IM on primary cultures of human MSC.
MATERIALS AND METHODS
MSC isolation and culture
Bone marrow samples were obtained from healthy donors who underwent a standard bone marrow harvest under general anaesthesia. The volunteer donor's age ranged between 25 and 48 years and all gave informed consent to the research protocol, which had been approved by the institutional review board of the University Hospital of Dresden. MSC were isolated by density gradient (mononuclear fraction) and adherence capacity, based on previously described methods (Majumdar et al. 1998; Oswald et al. 2004) and cultured in low‐glucose Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Grand Island, NY, USA; http://www.invitrogen.com) supplemented with 10% foetal calf serum (FCS) (Biochrom, Cambridge, UK; http://www.biochrom.de). The immunophenotype (characteristically CD73+, CD90+, CD105+, CD34−, CD45−) and ability to form fibroblastoid colony‐forming units (CFU‐F) were checked in all samples. All experiments were performed using Imatinib mesylate (IM) at the indicated concentrations dissolved in dimethyl sulfoxide (DMSO) or with 0.5 µl/ml DMSO as control.
Receptor tyrosine kinases (RTK)‐phospho array and Western Blots
Nearly (70–80%) confluent MSC cultures were incubated for 24 h in DMEM with 10% FCS. Subsequently, reagents were added in concentrations and for times as indicated in the legend of Fig. 1. After incubation, cells were trypsinized, washed and lysed with extraction buffer (Illmer et al. 2004). Protein concentration was measured using the Bio‐Rad protein assay kit (Richmond, CA, USA; http://www.bio‐rad.com). The Proteome Profiler™ array – human phospho‐RTK array kit was used according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA; http://www.rndsystems.com) loading 250 µg protein/membrane. For immunoblotting, 30 µg total proteins were loaded in each lane. Electrophoresis was performed in 10% acrylamide gels. Nitrocellulose membranes (http://www.amershambiosciences.com) were incubated with primary antibodies at 4 °C overnight. Antibodies against phosphorylated and total p44/42 (Erk) and Akt were purchased from Cell Signalling (http://www.cellsignal.com;Danvers,MA,USA) and anti‐actin antibody from Sigma (Sigma, St. Louis, MO, USA; http://www.sigmaaldrich.com). The secondary antibodies used were horseradish peroxidase goat antimouse or horseradish peroxidase goat antirabbit (Dako, Carpinteria, CA, USA; http://www.dakocytomation.de). Blots were developed with the chemiluminescence detection kit ECL‐Plus (Amersham biosciences, Arlington Heights, IL, USA; http://www.amershambiosciences.com).
Figure 1.
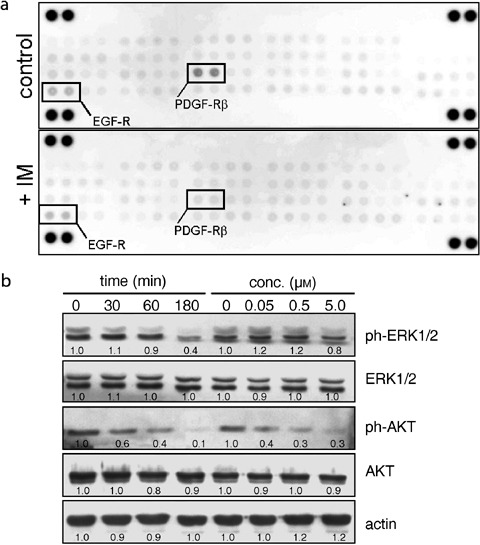
Imatinib mesylate blocks phosphorylation of PDGFRβ, Akt and Erk. (a) Phospho‐RTK array: MSC were cultured for 24 h in order to induce autophosphorylation of RTKs. Then, cells were incubated for 1 h with 0.1 µl/ml DMSO (control) or 1 µm IM. Phosphorylation of EGFR and PDGFRβ can be noticed, but only PDGFRβ is inhibited by IM (n = 2). (b) Phosphorylation of Akt and Erk1/2 were evaluated in MSC (n = 5). In order to evaluate the time‐dependent effect, samples were treated with 1 µm IM for 0, 30, 60 and 180 min, respectively. To test the concentration dependency, samples were incubated for 1 h with the respective concentrations of IM before cell lysis and protein extraction. Quantitative densitometric analyses relative to control (time 0 = 1.0; concentration 0 = 1.0) are provided.
Phase contrast and electron microscopy
For phase contrast imaging, 10 000 MSC/cm2 were seeded in 12‐well plates and cultured for 24 h to reach maximal attachment. The medium was changed and cells were incubated for 24 h then photographed using an Axiovert 25 phase contrast microscope (Carl Zeiss, Oberkochen, Germany). For transmission electron microscopy, MSC were cultured for 6 days with 10 µm IM or 1 µl/ml DMSO (control), fixed with 2.5% glutaraldehyde buffer for 1 h, washed and post‐fixed with 1% OsO4 for 1 additional hour. Samples were then dehydrated with increasing concentrations of ethanol and embedded in Epon™ (Vantico GmbH, Bad Sackingen, Germany). Finally, ultra‐thin slides (65 nm) were set in copper grids and stained with uranyl acetate and lead citrate. Samples were examined with a Zeiss EM 906 microscope (Zeiss, Oberkochen, Germany).
Proliferation and fibroblastoid colony‐forming units (CFU‐F) assays
For all proliferation curves, 6000 MSC/cm2 were seeded in 12‐well plates and allowed to attach for 24 h. Medium was subsequently replaced with DMEM/10% FCS containing 0.5 µl/ml DMSO (control), variable concentrations of IM, 10 ng/ml PDGFβ (human recombinant, R&D Systems; http://www.rndsystems.com) or 20 µm UO126 (http://www.cellsignal.com). Living cells were counted with trypan‐blue exclusion dye in a Neubauer chamber. Medium containing the respective supplements was changed at day 3. CFU‐F were determined by seeding 2.5 × 106 bone marrow‐derived mononuclear cells in one 25‐cm2 flask and cultured for 14 days with stem cell medium (NH‐Medium, Miltenyi Biotec, Auburn, CA, USA; http://www.miltenyibiotec.com), without any medium change. At day 14, samples were fixed with 4%P‐formaldehyde and stained with Azur‐eosin‐methylene blue solution (Giemsa, Merck, Darmstadt, Germany). Visible colonies (more than 50 cells) were counted and grouped according to size.
Osteogenic and adipogenic MSC differentiation
Osteogenic differentiation of MSC was induced by incubating 70–80% confluent cultures in standard medium containing 0.2 mm ascorbic acid (http://www.fagron.de; Fagron GmbH & Co., Barsbüttel, Germany), 0.1 µm dexamethasone and 10 mmβ‐glycerophosphate (Sigma; both http://www.sigmaaldrich.com). Medium was replaced every 3–4 days. Alkaline phosphatase (ALP) activity was measured at day 11. Briefly, cells were trypsinized and lysed with 1.5 m Tris‐HCl solution containing 1.0 mm ZnCl2, 1.0 mm MgCl2 and 1% Triton X‐100 for 10 min. Lysates were centrifuged at 20 800 g for 30 min and incubated with 3.7 mm P‐nitrophenylphosphate solution containing 0.1% Triton X‐100 for 30 min. Released P‐nitrophenolate was determined spectrophotometrically at 405 nm. Protein concentration was measured using the Bio‐Rad protein assay kit (http://www.bio‐rad.com). At day 14, calcium phosphate precipitates were stained for Kossa. Briefly, cells were fixed with a fresh solution of 10% formalin for 30 min, stained with 2% silver nitrate solution and developed with 1% pyrogallol. In order to induce MSC adipogenesis, cells were seeded in 6‐well plates and grown to 70–80% confluency. Then, medium was changed to MesenCult™ containing adipogenic stimulatory supplements (http://www.stemcell.com), changed every 3–4 days. At day 14, adipocytes were counted under phase contrast on a defined central area. Adipocytes were arbitrarily defined as cells containing five or more visible lipid vacuoles. The total number of living cells per well was determined as described above. The number of adipocytes was extrapolated to total well area (9.62 cm2) allowing the determination of MSC differentiated into adipocytes.
Four‐week culture‐initiating assays
Haematopoietic stem cells (HSC) were isolated from G‐CSF‐mobilized leukapheresis products and selected for CD34+ using a magnetic cell‐sorting system according to manufacturer's recommendations (http://www.miltenyibiotec.com). 3000 CD34+ HSC/cm2 were co‐cultured over a confluent MSC monolayer with long‐term culture medium (MyeloCult H 5100, http://www.stemcell.com) supplemented with 1 µm hydrocortisone for 4 weeks and with or without 5 µm IM. Afterwards cells were trypsinized, washed and counted. Approximately 50 000 recovered cells were plated in triplicate in 24‐well plates in CFU‐GM medium (Methocult GF H4435, http://www.stemcell.com) and assessed at day 14 for the presence of colony forming units. The number of CFU‐GM was used to calculate the frequency of colony‐forming cells per number of HSC originally seeded. Differential progenitor growth was detected by scoring granulocyte‐macrophage (CFU‐GM), macrophage (CFU‐M), mixed (CFU‐GEMM) and burst‐forming erythroid colonies (BFU‐E).
Statistical analysis
The number of MSC‐donors used for each experiment is indicated in the legend of the respective figure. Averages are shown with standard error of the mean as error bars. Statistical comparison was performed using a paired student t‐test. Observed differences were assumed as significant when the calculated two‐sided P‐value was < 0.05.
RESULTS
IM blocks the MAPK/ERK‐ and Akt/PI3K‐signalling pathways by specific inhibition of PDGFRβ in MSC
In order to determine the molecular target(s) of IM in MSC, 42 different receptor tyrosine kinases (RTK) were screened for auto‐phosphorylation and subsequent inhibition by 1 h of treatment with1 µm IM. As seen in Fig. 1(a), epidermal growth‐factor receptor (EGFR) and PDGFRβ were activated by phosphorylation after 24 h of culture, but only PDGFRβ phosphorylation was inhibited by IM. RTKs are the initial step for activation of several signalling pathways, including MAPK/Erk, Akt/PI3K and JNK/MAPK. We evaluated critical components of these pathways, finding inhibition of Erk1/2 and Akt in a concentration and time‐dependent manner (Fig. 1b), while JNK1 phosphorylation was not affected (not shown).
IM changes MSC morphology
Characteristic morphological changes could be observed in MSC incubated with IM: spindle‐shaped cells flattened, lost confluence and developed perinuclear granules (Fig. 3a). After 24 h of incubation, perinuclear granules were evident only at concentrations of 5 µm or higher. In addition, time‐lapse analyses showed the appearance of granula gradually in the cytosol within the first 12 h with a pronounced migration to the perinuclear region after 18–20 h. These granules resembled multi‐vesicular bodies as suggested by transmission electron microscopy (Fig. 3b). After removal of IM, the granules disappeared completely within 2 days.
Figure 3.
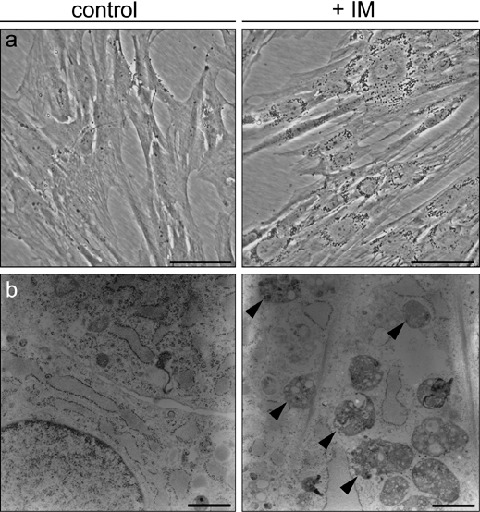
Imatinib mesylate alters the morphology of MSC. (a) Phase contrast microscopy of MSC cultured for 24 h with or without IM (n = 3). (b) Perinuclear granula resembled multi‐vesicular bodies (highlighted with arrowheads) as observed in MSC after 6 days with or without IM using transmission electron microscopy (n = 2).
IM decreased proliferation and clonogenicity of MSC
Addition of IM inhibited growth of MSCs in a concentration‐dependent manner. As shown in (Fig. 2a), the negative effect of IM on MSC proliferation was detectable after short times of culture, but seemed not to be due to toxicity because the number of living cells did not decrease below the original number seeded. Annexin V/PI staining after 3 days of culture with 5 µm IM showed no increase in apoptotic cells compared to controls (FACS measurements, data not shown). Because the lower proliferation rate was expected to be due to the inactivation of PDGFRβ in MSC leading to inhibition of the MAPK/Erk signalling pathway, we evaluated the effect of human recombinant PDGFβ and the MAPK kinase (MEK1/2)‐specific inhibitor UO126 on MSC proliferation. As expected, addition of 10 ng/ml PDGFβ‐favoured cell proliferation enormously while 20 µm UO126 inhibited the cell growth to a similar degree as did IM at 5 µm. To investigate effects of prolonged IM exposition, we kept MSC in medium containing 5 µm IM for up to 21 days with medium changes twice a week. Cell proliferation was blocked leading to constant cell numbers up to day 21. No signs of cell death or apoptosis could be detected.
Figure 2.
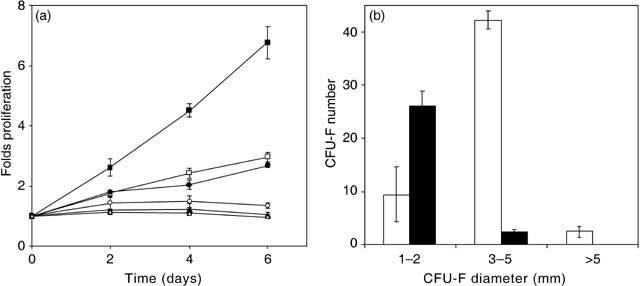
Proliferation (a) and clonogenicity (b) of MSC are negatively affected by Imatinib mesylate. (a) PDGFβ (10 ng/ml; ▪, n = 3) has a positive effect on cell proliferation as compared to controls (□, n = 14), while 20 µm UO126 (▵, n = 3) completely inhibits cell growth. IM inhibits cell proliferation in a concentration‐dependent manner [0.05 µm (•, n = 3), 0.5 µm (○, n = 6) and 5.0 µm (▴, n = 4)]. (b) Colony‐forming units assays denote a decrease of total CFU‐F in presence of 5 µm IM (black bars) as compared to controls (open bars). Specifically, IM favoured the presence of small colonies, while intermediate and large size CFU‐F were strongly inhibited as compared to controls (n = 4).
In accordance with these observations, IM also affected the clonogenicity of MSC, evaluated by the number and size of CFU‐F after 14 days of culture. The absolute number was drastically lower in IM‐treated samples compared to controls (Fig. 2b). Specifically, colonies of intermediate and large size (3 mm to 5 mm, and bigger than 5 mm diameter, respectively) were significantly reduced in IM‐treated samples (P = 0.0005), favouring the growth of small colonies (1–2 mm diameter). Therefore, the effect of IM on CFU‐F seemed to be predominantly inhibition of cell proliferation, rather than the induction of apoptosis.
IM affected osteogenic and adipogenic differentiation of MSCs
The negative effect of IM on MSC osteogenesis was apparent from reduced calcium phosphate precipitates compared to controls, after 14 days of culture under osteogenic stimuli. Although a typical disruption of the cell layer and loss of confluence were observed, differentiation was not fully inhibited (Fig. 4, upper left panels). In order to quantify the effect of IM on osteogenesis, we evaluated the induction of ALP activity on day 11 (Fig. 4, upper right panel). IM decreased ALP activity significantly from 4.4 fold induction (control) to 3.4 fold, while the specific MEK inhibitor UO126 led to only a 2.0 fold induction of ALP (data not shown). On the other hand, differentiation of MSC into adipocytes was favoured in the presence of IM (Fig. 4, lower panels). Adipogenic differentiation was induced to a similar degree by the specific MEK inhibitor UO126 supporting the role of Erk1/2 signalling for osteogenic differentiation (data not shown).
Figure 4.
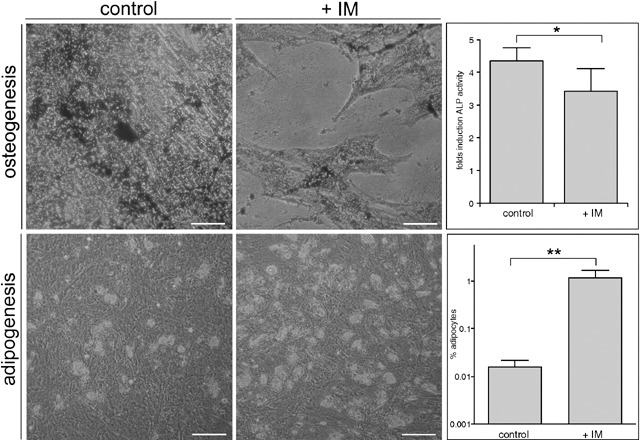
Imatinib mesylate affects osteogenic and adipogenic differentiation of MSC. Upper panels: Calcium phosphate precipitates stained with von Kossa at day 12 were diminished in presence of 5 µm IM as compared to control cultures. In addition, disruption of the cell layer and loss of confluence were detected. Upper right panel, effect of 5 µm IM on ALP activity of MSC after 11 days of osteogenic differentiation (n = 4). Lower panels: MSC cultured for 14 days with adipogenic medium containing 0.5 µl/ml DMSO (control) or 5 µm IM, representative regions were directly photographed. This increase of adipocytes was quantified as described in materials and methods (lower left panel, n = 4). Scale bars = 100 µm *P = 0.02, **P = 0.12.
IM affects MSC‐mediated support of HSC growth
A characteristic function attributed to MSCs is their ability to support long‐term growth of haematopoietic stem cells (HSC). In order to evaluate this feature, we performed a 4‐week co‐culture of CD34+ selected HSC over a monolayer of MSCs, followed by a CFU‐GM assay. Incubation of HSC over MSC in the presence of IM halved the number of haematopoietic cells with clonogenic potential, compared to control co‐cultures (P < 0.01) (Fig. 5). Nevertheless, this effect was mainly due to a reduced recovery of HSCs after 4 weeks of co‐culture, rather than to a direct negative effect on the clonogenic capacity of the recovered HSC. In fact, the number of CFU‐GM per dish was higher when HSCs harvested after 4 weeks of culture with IM were seeded at the same concentration as the controls (205.9% higher, P < 0.01) (data not shown), suggesting that early stem and progenitor cells are resistant to IM. Limiting the exposure of MSC to a 72‐h preincubation with 5 µm IM did not reduce the yield of CFU‐GM after 4 weeks arguing for a reversible effect of IM. In addition, we saw no significant difference in the proportion of CFU‐GM, CFU‐M, CFU‐GEMM and BFU‐E derived from HSC after 4 weeks between IM‐treated co‐cultures and controls. Therefore, we propose that the major effect of IM was the impairment of the MSC feeder‐layer's quality by inducing adipogenic differentiation thereby reducing the support of HSC, rather than by directly suppressing HSC proliferation.
Figure 5.
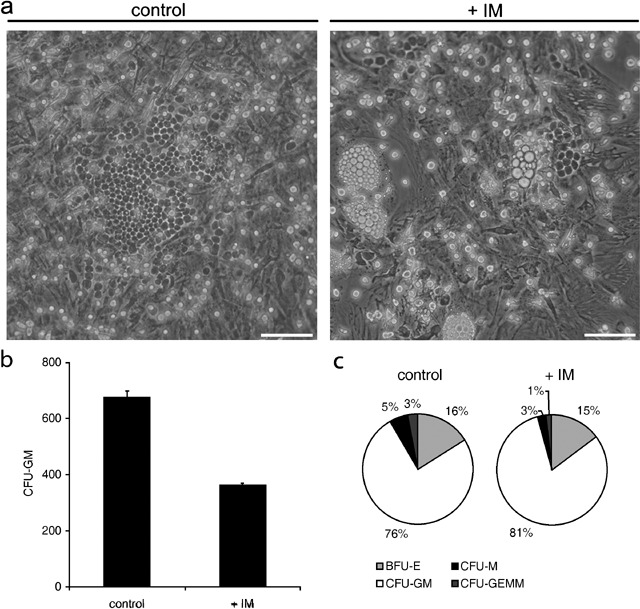
Imatinib mesylate affects MSC‐mediated support of HSC growth. (a) Control (left panel) shows CD34+ selected haematopoietic stem cells (HSC) aggregating in large cobblestone forming areas (CFA) over a confluent monolayer of MSC after 4 weeks of co‐culture. In contrast, co‐cultures of HSC and MSC in the presence of 5 µm IM (right panel) contained smaller and fewer CFA. In addition, a disrupted monolayer of MSC with spontaneous adipogenesis can be observed (n = 3). (b) Frequency of CFU‐GM, after 4‐week coculture (n = 3). Scale bar on (a) = 100 µm (c) Proportion of granulocyte‐macrophage (CFU‐GM), macrophage (CFU‐M), mixed (CFU‐GEMM) and burst‐forming eythroid (BFU‐E) colonies derived from HSC cultured for 4 weeks on MSC.
DISCUSSION
This is the first detailed report describing the effects of IM on bone marrow‐derived MSC. We evaluated the effect of IM on morphology, clonogenicity, differentiation potential, support of HSC growth and identified molecular targets that are related to the inhibitory effect of IM on MSC.
The data presented here support in different ways the importance of PDGFRβ and EGFR signalling for MSC proliferation and differentiation. First, the phospho‐RTK array revealed exclusive phosphorylation of EGFR and PDGFRβ in ex vivo‐expanded MSC. Second, IM blocked constitutive PDGFRβ signalling with subsequent inhibition of the PI3K/Akt and Erk pathway. Activation of the MAPK/Erk‐signalling pathway influences proliferation and differentiation in various types of mammalian cells (Zhang & Liu 2002), including MSC. Additionally, the MAPK/Erk pathway has been previously linked to osteogeneic differentiation, and inhibition of this pathway induces adipogenesis in MSC (Jaiswal et al. 2000). Specifically, the MAPK pathway can activate RUNX‐2 and blocks PPAR‐gamma, transcription factors that are critical for osteogenic and adipogenic differentiation (Xiao et al. 2000; Kim et al. 2001).
A recent publication provided evidence for differential signalling in MSC via EGFR or PDGFRβ (Kratchmarova et al. 2005). The authors showed that regulatory and catalytic members of the PI3Kinase family were critically affected by PDGFRβ stimulation, whereas other MAPKinases like Erk1/2 could be stimulated by either PDGFRβ or EGFR. As shown by us, the MAPKinase inhibitor UO126 had a negative effect on proliferation and osteogenic differentiation of MSC in their report. The set‐up of our experiments differed in that we did not add exogenous growth factors but specifically blocked PDGFRβ signalling. In accordance with the findings of Kratchmarova et al., we observed significant inhibition of the PI3/Akt pathway after the inhibition of PDGFRβ. Because EGFR was not blocked to any extent, the less pronounced reduction of Erk1/2 phosphorylation may be attributed to the residual activity of EGFR within our system.
Because multi‐vesicular bodies could only be observed at the highest concentration of IM, corresponding to maximum plasma concentrations achievable only for short‐time periods in patients, the relevance of these findings has to be discussed (Le Coutre et al. 2004). Nevertheless, the effects on proliferation and differentiation could be observed at IM concentrations comparable to trough plasma levels.
The inhibitory effects of IM in the co‐culture experiments may in part be explained by the direct inhibition of kinase activity in clonogenic haematopoietic progenitors (Bartolovic et al. 2004; Dewar et al. 2005). But, as mentioned above, the observed reduction in overall CFU‐GM was mainly due to a reduced recovery of HSCs after 4 weeks of co‐culture. As shown in Fig. 5(a), this was mainly due to the functional loss of the MSC layer incubated with IM leading to a reduced recovery of HSC after 4 weeks. Therefore, we propose that the major effect of IM is the impairment of the MSC feeder‐layer's quality by inducing adipogenic differentiation thereby reducing the support of HSC, rather than by directly suppressing HSC proliferation. Given the limitations of our in vitro model, we are not able to speculate on the chronic effects of IM on MSC and HSC in vivo.
No case of irreversible aplasia has been described after the use of IM, because the most primitive stem cells seem to be spared from the effects of IM. Available data from syngeneic animal transplant models and most clinical studies do not suggest that IM has a negative impact on the speed and durability of engraftment in vivo (Hoepfl et al. 2002; Bornhauser et al. 2006). Several effects observed in our short‐term in vitro culture model might therefore be compensated during long‐term use of IM in patients.
In summary, our experiments show that IM inhibits the capacity of human MSC to proliferate and to differentiate into the osteogenic lineage, favouring adipogenesis. This effect is mainly mediated by an inhibition of PDGFRβ autophosphorylation leading to a more pronounced inhibition of PI3K/Akt compared to Erk1/2 signalling. The selective effects of IM and other small molecule kinase inhibitors might be helpful to further delineate the signalling cascades involved in distinct differentiation pathways of adult mesenchymal stromal cells. Whether the observed in vitro effects can become relevant in specific clinical and therapeutic settings remains to be studied (Berman et al. 2006).
ACKNOWLEDGEMENTS
We thank the physicians of the department of haematology for performing the bone marrow harvests. We are indebted to Michael Kasper for performing electron microscopy. Special thanks to Michele Solimena for the critical discussion of the morphological findings. This work was supported in part by the German Ministery of Education and Science (BMBF grants 03 N4028 to MB and SB) and by the Deutsche Forschungsgemeinschaft (Graduiertenkolleg 864 ‘Molecular Cell Biology and Bioengineering’ to FF and SFB 655 to GE and MB).
REFERENCES
- Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822. [DOI] [PubMed] [Google Scholar]
- Appel S, Boehmler AM, Grunebach F, Muller MR, Rupf A, Weck MM, Hartmann U, Reichardt VL, Kanz L, Brummendorf TH, Brossart P (2004) Imatinib mesylate affects the development and function of dendritic cells generated from CD34+ peripheral blood progenitor cells. Blood 103, 538–544. [DOI] [PubMed] [Google Scholar]
- Apperley JF, Gardembas M, Melo JV, Russell‐Jones R, Bain BJ, Baxter EJ, Chase A, Chessells JM, Colombat M, Dearden CE, Dimitrijevic S, Mahon FX, Marin D, Nikolova Z, Olavarria E, Silberman S, Schultheis B, Cross NC, Goldman JM (2002) Response to Imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet‐derived growth factor receptor beta. N. Engl. J. Med. 347, 481–487. [DOI] [PubMed] [Google Scholar]
- Bartolovic K, Balabanov S, Hartmann U, Komor M, Boehmler AM, Buhring HJ, Mohle R, Hoelzer D, Kanz L, Hofmann WK, Brummendorf TH (2004) Inhibitory effect of imatinib on normal progenitor cells in vitro . Blood 103, 523–529. [DOI] [PubMed] [Google Scholar]
- Berman E, Nicolaides M, Maki RG, Fleisher R, Chanel S, Scheu K, Wilson BA, Heller G, Sauter NP (2006) Altered bone and mineral metabolism in patients receiving Imatinib mesylate. N. Engl. J. Med. 354, 2006–2013. [DOI] [PubMed] [Google Scholar]
- Bornhauser M, Kroger N, Schwerdtfeger R, Schafer‐Eckart K, Sayer HG, Scheid C, Stelljes M, Kienast J, Mundhenk P, Fruehauf S, Kiehl MG, Wandt H, Theuser C, Ehninger G, Zander AR (2006) Allogeneic haematopoietic cell transplantation for chronic myelogenous leukaemia in the era of imatinib: a retrospective multicentre study. Eur. J. Haematol. 76, 9–17. [DOI] [PubMed] [Google Scholar]
- Demetri GD, Von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H (2002) Efficacy and safety of Imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480. [DOI] [PubMed] [Google Scholar]
- Dewar AL, Cambareri AC, Zannettini AC, Miller BL, Doherty KV, Hughes T, Lyons PJ (2005) Macrophage colony‐stimulating factor receptor c‐fms is a novel target of imatinib. Blood 105, 3127–3132. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno‐Jones S, Sawyers CL (2001) Efficacy and safety of a specific inhibitor of the BCR‐ABL tyrosine kinase in chronic myeloid leukaemia. N. Engl. J. Med. 344, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Hoepfl J, Miething C, Grundler R, Gotze KS, Peschel C, Duyster J (2002) Effects of imatinib on bone marrow engraftment in syngeneic mice. Leukaemia 16, 1584–1588. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK (1999) Transplantability and therapeutic effects of bone marrow‐derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 5, 309–313. [DOI] [PubMed] [Google Scholar]
- Illmer T, Schaich M, Platzbecker U, Freiberg‐Richter J, Oelschlagel U, Von Bonin M, Pursche S, Bergemann T, Ehninger G, Schleyer E (2004) P‐glycoprotein‐mediated drug efflux is a resistance mechanism of chronic myelogenous leukaemia cells to treatment with Imatinib mesylate. Leukaemia 18, 401–408. [DOI] [PubMed] [Google Scholar]
- Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF (2000) Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen‐activated protein kinase. J. Biol. Chem. 275, 9645–9652. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz‐Gonzalez XR, Reyes M, Lenvik T, Lund T, Du Blackstad MJ, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti‐Passerini C, Niederwieser D, Resta D, Capdeville R, Zoellner U, Talpaz M, Druker B, Goldman J, O’Brien SG, Russell N, Fischer T, Ottmann O, Cony‐Makhoul P, Facon T, Stone R, Miller C, Tallman M, Brown R, Schuster M, Loughran T, Gratwohl A, Mandelli F, Saglio G, Lazzarino M, Russo D, Baccarani M, Morra E (2002) Hematologic and cytogenetic responses to Imatinib mesylate in chronic myelogenous leukaemia. N. Engl. J. Med. 346, 645–652. [DOI] [PubMed] [Google Scholar]
- Kim SW, Muise AM, Lyons PJ, Ro HS (2001) Regulation of adipogenesis by a transcriptional repressor that modulates MAPK activation. J. Biol. Chem. 276, 10199–10206. [DOI] [PubMed] [Google Scholar]
- Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM (2000) Rapid haematopoietic recovery after coinfusion of autologous‐blood stem cells and culture‐expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high‐dose chemotherapy. J. Clin. Oncol. 18, 307–316. [DOI] [PubMed] [Google Scholar]
- Kratchmarova I, Blagoev B, Haack‐Sorensen M, Kassem M, Mann M (2005) Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science 308, 1472–1477. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O (2004) Treatment of severe acute graft‐versus‐host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441. [DOI] [PubMed] [Google Scholar]
- Le Coutre P, Kreuzer KA, Pursche S, Bonin M, Leopold T, Baskaynak G, Dorken B, Ehninger G, Ottmann O, Jenke A, Bornhauser M, Schleyer E (2004) Pharmacokinetics and cellular uptake of imatinib and its main metabolite CGP74588. Cancer Chemother. Pharmacol. 53, 313–323. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL (2000) Human marrow‐derived mesenchymal stem cells (MSCs) express haematopoietic cytokines and support long‐term haematopoiesis when differentiated toward stromal and osteogenic lineages. J. Hematother. Stem Cell Res. 9, 841–848. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL (1998) Phenotypic and functional comparison of cultures of marrow‐derived mesenchymal stem cells (MSCs) and stromal cells. J. Cell Physiol. 176, 57–66. [DOI] [PubMed] [Google Scholar]
- O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ (2003) Imatinib compared with interferon and low‐dose cytarabine for newly diagnosed chronic‐phase chronic myeloid leukaemia. N. Engl. J. Med. 348, 994–1004. [DOI] [PubMed] [Google Scholar]
- Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C (2004) Mesenchymal stem cells can be differentiated into endothelial cells in vitro . Stem Cells 22, 377–384. [DOI] [PubMed] [Google Scholar]
- Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ (1995) Cultured adherent cells from marrow can serve as long‐lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc. Natl. Acad. Sci. USA 92, 4857–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147. [DOI] [PubMed] [Google Scholar]
- Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC, Dunbar CE, Wiestner A (2005) Imatinib inhibits T‐cell receptor‐mediated T‐cell proliferation and activation in a dose‐dependent manner. Blood 105, 2473–2479. [DOI] [PubMed] [Google Scholar]
- Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ (2004) Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells 22, 823–831. [DOI] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT (2000) MAPK pathways activate and phosphorylate the osteoblast‐specific transcription factor, Cbfa1. J. Biol. Chem. 275, 4453–4459. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu HT (2002) MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 12, 9–18. [DOI] [PubMed] [Google Scholar]


