Abstract
Cholinergic efferent neurons originating in the brainstem innervate the acoustico-lateralis organs (inner ear, lateral line) of vertebrates. These release acetylcholine (ACh) to inhibit hair cells through activation of calcium-dependent potassium channels. In the mammalian cochlea, ACh shunts and suppresses outer hair cell (OHC) electromotility, reducing the essential amplification of basilar membrane motion. Consequently, medial olivocochlear neurons that inhibit OHCs reduce the sensitivity and frequency selectivity of afferent neurons driven by cochlear vibration of inner hair cells (IHCs). The cholinergic synapse on hair cells involves an unusual ionotropic ACh receptor, and a near-membrane postsynaptic cistern. Lateral olivocochlear (LOC) neurons modulate type I afferents by still-to-be-defined synaptic mechanisms. Olivocochlear neurons can be activated by a reflex arc that includes the auditory nerve and projections from the cochlear nucleus. They are also subject to modulation by higher-order central auditory interneurons. Through its actions on cochlear hair cells, afferent neurons, and higher centers, the olivocochlear system protects against age-related and noise-induced hearing loss, improves signal coding in noise under certain conditions, modulates selective attention to sensory stimuli, and influences sound localization.
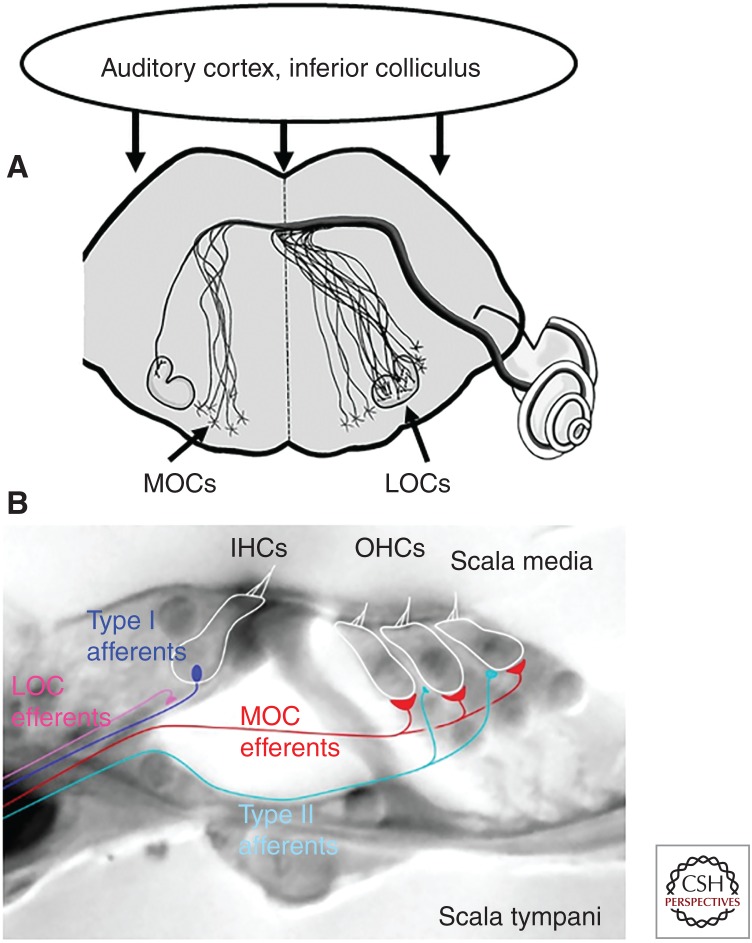
Efferent neurons originating in the brainstem project to the acoustic-lateralis organs of vertebrates, ranging from the fish lateral line to the mammalian cochlea (Klinke and Galley 1974). These efferent neurons release acetylcholine (ACh) to inhibit sensory hair cells. Although the general vertebrate pattern has both afferent and efferent synapses on individual hair cells, these connections are largely segregated to different types of hair cells in the mammalian cochlea (Warr and Guinan 1979). Cochlear outer hair cells (OHCs) are the sole targets of medial olivocochlear (MOC) efferent neurons in the adult. Cochlear OHCs also have sparse, weak connections with unmyelinated type II spiral ganglion neurons (SGNs). In contrast, cochlear inner hair cells (IHCs) have no direct efferent inputs at maturity but instead are the sole presynaptic source to the great majority (95%) of primary afferents, the myelinated type I SGNs. Immediately after birth, efferent neurons do make inhibitory synaptic contacts with cochlear IHCs (Glowatzki and Fuchs 2000; Simmons 2002) but these disappear by the onset of hearing, by postnatal day 12–14 in rodents (Katz et al. 2004; Roux et al. 2011). Smaller diameter LOC efferents remain connected with the dendrites of type I SGNs below the IHCs in the mature cochlea. It has proved more difficult to determine the synaptic effects of LOC efferents. Lesion experiments and pharmacological studies in vivo have produced mixed outcomes, possibly reflecting the more complex neurochemistry of LOC efferents that includes dopamine and GABA as well as ACh (Ruel et al. 2007). Efferent inputs can excite vestibular afferent neurons by activation of nicotinic cholinergic receptors (Jordan et al. 2013) and such an effect may occur in the cochlea (Fig. 1) (Felix and Ehrenberger 1992).
Figure 1.
Efferent neurons to the cochlea. (A) Schematized brainstem section showing location of medial olivocochlear (MOC) and lateral olivocochlear (LOC) somata in or near the olivary complex. MOCs project both contra- and ipsilaterally. LOCs are predominantly ipsilateral. (B) LOCs form synaptic contacts on the dendrites of type I cochlear afferents below the inner hair cells (IHCs). MOCs form synaptic contacts on outer hair cells (OHCs). Type II afferents also contact OHCs. (From Lauer et al. 2012; adapted, with permission, from Elsevier © 2012.)
INNERVATION AND ULTRASTRUCTURE
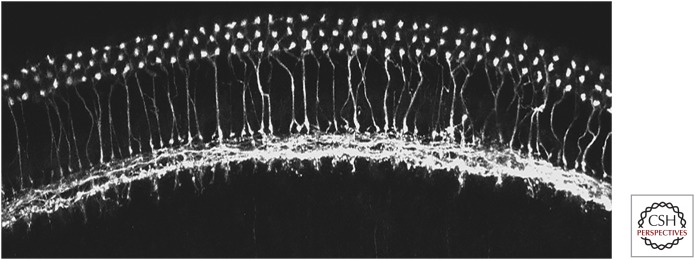
Labeling by silver stains for axonal degeneration after lesioning and cholinesterase histochemistry established the number, origins, and cholinergic identity of efferent neurons to both the cochlear and vestibular end organs (Klinke and Galley 1974). Retrograde tracing by horseradish peroxidase injection into the inner ear revealed 1700–1800 cochlear, and 400–500 vestibular efferents (Warr 1975). Since that time, various cholinergic and presynaptic markers have been used to describe efferent innervation of the inner ear. MOC and LOC efferent neurons are both positive for acetylcholinesterase histochemistry (Warr 1975; Emmerling et al. 1990) and can be immunolabeled for cholinergic markers such as choline acetyltransferase (ChAT) (Sobkowicz and Emmerling 1989; Huang et al. 2007; Roux et al. 2016) and vesicular ACh transporter (VAT) (Maison et al. 2003). In addition to cholinergic markers, MOC and LOC efferents also can be selectively immunolabeled for proteins involved in transmitter release that are absent from hair cells, among them synapsin (Bergeron et al. 2005), synaptophysin (Bartolome et al. 2009; Murthy et al. 2009), and synaptic vesicle protein 2 (SV2) (Kong et al. 2008). Immunolabeling for Na/K ATPase distinguishes MOC from LOC efferents (McLean et al. 2009). Antibodies to the neuropeptide calcitonin gene-related peptide (CGRP) label MOCs and LOCs (Cabanillas and Luebke 2002; Maison et al. 2003), although this peptide can be expressed by type II afferents as well (Wu et al. 2018). Antibody labeling has shown that MOC efferents reach OHCs of rodents in the first postnatal weeks, followed several days later by LOC efferents to the region beneath the IHCs (Simmons 2002). Prior to the arrival of LOC efferents, it is thought that MOC efferents form temporary synaptic contacts on IHCs like the mature inhibitory synapses on OHCs (Figs. 2 and 3) (Glowatzki and Fuchs 2000; Oliver et al. 2000; Lioudyno et al. 2004; Goutman et al. 2005; Ballestero et al. 2011).
Figure 2.
Efferent innervation of the cochlea. Surface view of a cochlear turn from a mouse in which choline acetyltransferase (ChAT)-Cre is driving the expression of the fluorescent reporter tdTomato. Medial olivocochlear (MOC) efferent neuron terminals are seen beneath three rows of outer hair cells, with labeled axons crossing the tunnel of Corti. Lateral olivocochlear (LOC) efferent axons run along the inner spiral bundle and give off terminals to the dendrites of type I afferents beneath the inner hair cells. (Image provided by K. Schrode.)
Figure 3.
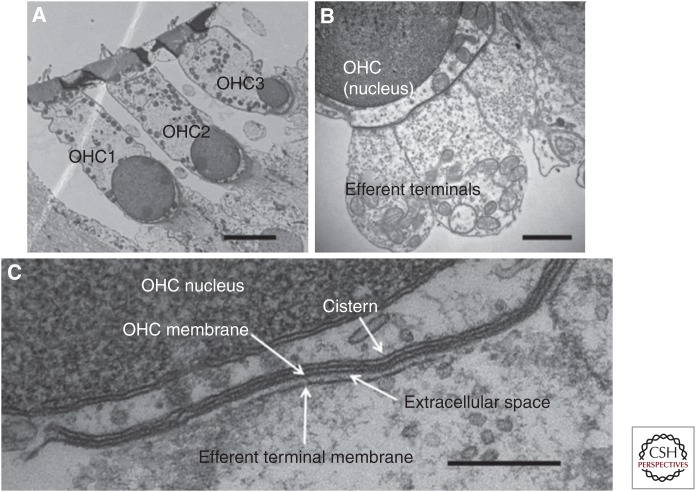
Synaptic ultrastructure in outer hair cells (OHCs) of mice. (A) Transverse section showing an OHC of each row. (B) Higher magnification showing three efferent terminals on one OHC. (C) Postsynaptic cistern of OHC, aligned with efferent terminal. Scale bars, 4 µm (A); 1 µm (B); 250 nm (C). (From Fuchs et al. 2014; adapted, courtesy of HHS Public Access.)
Efferent synapses on hair cells range from 0.5 to several microns in maximum extent of contact with the hair cell and are richly vesiculated (Smith and Sjostrand 1961; Sato et al. 1997, 1999; Fuchs et al. 2014). A near-membrane endoplasmic reticulum (subsynaptic cistern) is always found in the hair cell aligned with the efferent terminal where it forms a flattened sac closely secured to the plasma membrane. In cochlear OHCs, these cisterns are coextensive with one or more efferent terminals and so can be several microns in maximal extent. The hair cell’s subsynaptic cistern is reminiscent of the sarcoplasmic reticulum of muscle and may serve as a synaptic calcium store (Lioudyno et al. 2004; Im et al. 2014). Neurotransmitter receptors and ion channels that mediate the postsynaptic response cluster in the postsynaptic membrane beneath the efferent contact (Wersinger et al. 2010; Roux et al. 2011; Rohmann et al. 2015).
CELLULAR PHYSIOLOGY
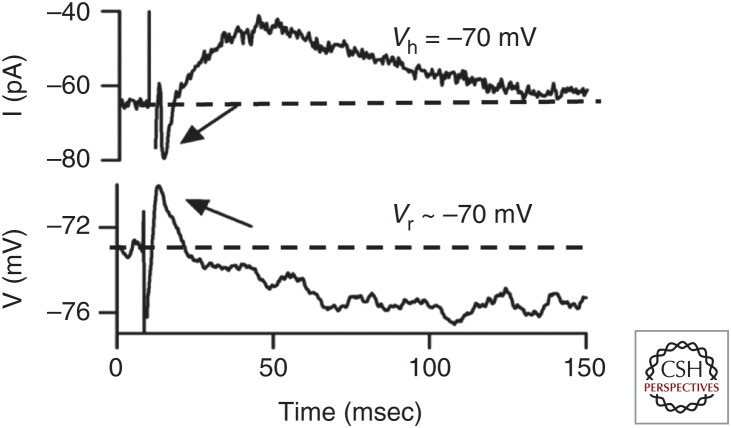
Intracellular recording of efferent synaptic effects was first accomplished on hair cells of fish and frogs (Flock and Russell 1973; Ashmore and Russell 1982) followed by a series of studies in the basilar papilla of turtle (Art et al. 1982, 1984, 1985). Recordings from hair cells isolated from the chicken basilar papilla (Fuchs and Murrow 1992a,b; McNiven et al. 1996; Yuhas and Fuchs 1999) and mammalian cochlea (Blanchet et al. 1996; Dallos et al. 1997; Evans et al. 2000) revealed that ACh activated a calcium-dependent potassium current to hyperpolarize and inhibit hair cells. Molecular cloning discovered that the hair cell’s acetylcholine receptor (AChR) is uniquely composed of two atypical members of the nicotinic receptor gene family, α9 and α10 (Elgoyhen et al. 1994, 2001). These are distinguished by their narrow range of effective agonists (not including nicotine) and blockade by strychnine and atropine as well as some nicotinic antagonists such as curare and α-bungarotoxin. α9α10-containing receptors form a ligand-gated ion channel with relatively high permeability to calcium, the resulting influx of which activates nearby calcium-dependent potassium channels. In most hair cells, these are small conductance SK channels (Fuchs and Murrow 1992a; Yuhas and Fuchs 1999; Glowatzki and Fuchs 2000; Oliver et al. 2000; Dawkins et al. 2005; Parks et al. 2017; Poppi et al. 2017), but also can include BK channels, particularly in OHCs of the basal turns of the mammalian cochlea (Wersinger et al. 2010; Rohmann et al. 2015). Oppositely directed flux through the AChR and potassium channels near the resting membrane potential gives rise to biphasic changes in membrane voltage. The potassium conductance increase is predominant and long lasting, hyperpolarizing the OHC for 100 msec or longer (Fig. 4).
Figure 4.
Evoked release of acetylcholine (ACh) from medial olivocochlear (MOC) efferent neuron terminals produces biphasic change in membrane current and voltage of an outer hair cell. Arrows point to inward current (upper record) or initial depolarization (lower record). Dotted line indicates resting current or membrane potential. (From Ballestero et al. 2011; adapted, with permission, from Journal of Neuroscience © 2011.)
Several features of this synapse are noteworthy. First is the unusual coupling of a ligand-gated cation channel (the AChR) with calcium-dependent potassium channels. The resulting inhibition and unexpected pharmacology (block by atropine) of the α9-containing AChR led to early supposition that this might be a muscarinic, G-protein-coupled mechanism akin to that in heart muscle. However, the cloning of α9 and α10 enabled genetic knockout and point mutation experiments that proved this was the necessary and sufficient receptor (Vetter et al. 1999; Taranda et al. 2009). The hair cell subunits are members of a gene family that includes the signature nicotinic receptor of muscle. In contrast to other members of this family, the hair cell subunits have been subject to positive selection in mammals, giving rise to enhanced calcium permeability in cochlear hair cells (Lipovsek et al. 2012, 2014). A second aspect is the long time course of the potassium conductance change itself. The time course of unitary synaptic currents in rat OHCs is explained by the gating kinetics of SK channels exposed to a brief pulse of calcium, decaying with a time constant, time course of 30 msec (Oliver et al. 2000). Thus, longer-lasting signals may reflect more slowly decaying calcium signals. The near-membrane postsynaptic cistern is likely to shape synaptic calcium signals (Lioudyno et al. 2004), either as a constraint on calcium diffusion, or by calcium-induced calcium release. A third feature involves the low quantum content (the average number of vesicles released for each action potential) of efferent terminals, about 0.3 during 1 Hz stimulation of MOCs onto OHCs (Ballestero et al. 2011) and about 1.0 for efferent synapses on immature IHCs (Goutman et al. 2005; Zorrilla de San Martin et al. 2010). Consequently, repetitive activation of efferents is required to facilitate transmitter release for effective inhibition, as observed for electrical stimulation of efferents to suppress afferent activity in vivo (Galambos 1956; Wiederhold and Kiang 1970).
Modulation of Efferent Transmission
Efferent terminals are richly endowed with synaptic vesicles (Lenoir et al. 1980; Nadol 1988; Simmons et al. 1996; Bruce et al. 2000; Fuchs et al. 2014) and release is enhanced with elevated extracellular calcium (Ballestero et al. 2011). Thus, short-term facilitation of efferent transmitter release is most likely a result of calcium accumulation in the presynaptic terminal as occurs generally (Zucker and Regehr 2002). In addition, there are several other modes of plasticity that affect the operation of efferent synapses, particularly those temporary ones found on IHCs before the onset of hearing. Efferent terminals on IHCs possess L-type voltage-gated calcium channels and calcium-dependent BK channels whose combined action limits the impact of presynaptic action potentials (Zorrilla de San Martin et al. 2010). Glutamate release from the hair cell negatively modulates efferent transmitter release through the activation of presynaptic metabotropic receptors (Ye et al. 2017). Activation of presynaptic metabotropic GABA receptors likewise suppresses efferent transmitter release onto IHCs and OHCs (Wedemeyer et al. 2013). Efferent transmission is enhanced when cytoplasmic stores release calcium in the postsynaptic IHC (Kong et al. 2013). This retrograde facilitation is communicated by calcium-dependent production of nitric oxide in the IHC. With the exception of GABAergic modulation, it is not known whether a similar wealth of modulatory mechanisms exists also at the MOC synapses on OHCs. It is possible that the transient efferent contacts on IHCs are particularly subject to modulation.
DEVELOPMENTAL PLASTICITY OF EFFERENT INNERVATION
Development and Maturation
Efferent innervation of the inner ear begins in mid-embryogenesis of rodents (Fritzsch 1996) with functional synaptic contacts on IHCs first detected at postnatal 2–3 and on OHCs several days later (Katz et al. 2004; Roux et al. 2011). The earliest responses to applied ACh include only cationic current through the hair cell’s nicotinic acetylcholine receptor (nAChR) (Dulon and Lenoir 1996; He and Dallos 1999; Roux et al. 2011), with outward current through SK channels and synaptic activity appearing 1 to 2 days later, in conjunction with numerous labeled clusters of AChRs (average of 16) and SK channels (average of 25) (Roux et al. 2011). Are SK channels necessary for the assembly of the postsynaptic complex? In SK-null mice efferent synapses on hair cells either fail to form or do so temporarily then retract (Kong et al. 2008; Murthy et al. 2009). SK channels may secure the synaptic complex through their interaction with the actin-binding protein α-actinin as suggested by coimmunoprecipitation (Lu et al. 2009; Scholl et al. 2014).
Efferent synapses disappear from IHCs just prior to the onset of hearing (about P12 in rodents) (Katz et al. 2004) at the same time that afferent synaptic contacts attain their adult functionality (Johnson et al. 2005, 2009). These changes may be reciprocal not only in time course, but perhaps also in causality. Null-mice lacking either the hair cell’s α9-AChR subunit, or with disabled efferent transmitter release, fail to undergo the normal maturation of afferent ribbon function that involves clustering of calcium channels for more efficient coupling to vesicular fusion (Johnson et al. 2013). Conversely, mice lacking the Cav1.3 voltage-gated calcium channels that normally cluster at the afferent ribbons maintain efferent synaptic contacts on IHCs at least 2 weeks longer than normal (Brandt et al. 2003).
Aging
Reciprocal changes of innervation are seen at the end of IHC life, as well as at the beginning. IHCs of aged, deaf mice lose many of their afferent synaptic contacts (Stamataki et al. 2006; Fernandez et al. 2015; Kujawa and Liberman 2015) but regain inhibitory efferent synapses (Lauer et al. 2012) that function like those present before hearing onset (Zachary and Fuchs 2015). The implication of these phenomena is that IHC synaptogenesis involves a degree of interaction between efferent and afferent synapses. Calcium influx at these opposing synapses may provide the underlying cross talk. Strong depolarization of the IHC can prolong the activation of SK current by ACh (Zachary et al. 2018), whereas sustained application of ACh can cause transmitter release from nearby ribbon synapses (Moglie et al. 2018). During IHC maturation, spontaneous calcium action potentials (Kros et al. 1998; Tritsch et al. 2007; Tritsch and Bergles 2010; Johnson et al. 2011) will greatly influence cytoplasmic calcium homeostasis and so the degree to which synaptic cross talk might occur. Whether aged IHCs revert to the immature pattern of excitability is not known. Finally, there is limited information about how efferent synapses on OHCs might vary throughout the lifetime, or as a consequence of trauma.
EFFECTS ON AUDITORY NERVE RESPONSES AND OTOACOUSTIC EMISSIONS
Responses in Quiet
Galambos (1956) first showed that electrical stimulation of olivocochlear axons in the floor of the IVth ventricle reduced the amplitude of the compound action potential (CAP) produced by an acoustic click. Suppression increased with shock number and frequency and was eliminated when olivocochlear axons were severed. The magnitude of efferent effects increases with increasing shock rate up to about 200 shocks/sec (Guinan and Gifford 1988a; Rajan and Johnstone 1988). Single-unit afferent recordings show that the rate-level function (acoustic sensitivity) is shifted to higher sound levels during efferent activation (Guinan and Gifford 1988a; Winslow and Sachs 1987), resetting the dynamic range. Efferent effects could be blocked by strychnine, now known to be a potent antagonist of the hair cell AChR. In further support, efferent effects are absent in mice lacking the α9 subunit of the hair cell’s AChR (Fig. 5) (Vetter et al. 1999).
Figure 5.
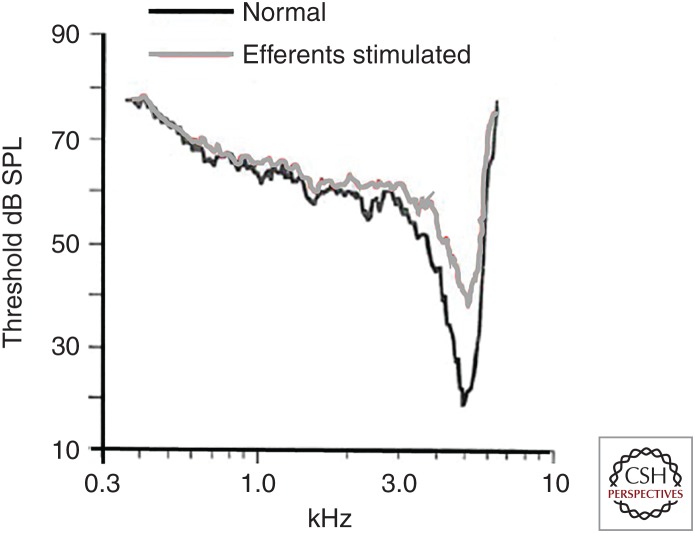
Efferent stimulation causes a frequency specific loss of sensitivity in type I cochlear afferents. Frequency sweep stimulation was used to determine acoustic threshold in a single afferent of the cat cochlea. When paired with electrical stimulation of medial olivocochlear (MOC) efferent neuron efferents (gray curve) the cochlear afferent was “detuned.” SPL, Sound pressure level. (From Guinan and Gifford 1998a; adapted, with permission, from Elsevier © 1988.)
When examined as a function of acoustic frequency, efferent suppression was greatest at the best frequency and had lesser effects on the flanks of the tuning curve (Guinan and Gifford 1988b), thus decreasing the frequency selectivity of cochlear tuning. The afferent’s acoustic response reflects the essential role of OHCs in cochlear sensitivity and tuning. Efferent hyperpolarization and shunting suppresses electromotility driven by acoustic receptor potentials in OHCs. When OHCs are inhibited, basilar membrane motion is reduced and detuned (Russell and Murugasu 1997). Consequently, the cochlear vibration that drives IHC receptor potentials is reduced and transmitter release driving afferent activity is reduced.
Lateral Olivocochlear Efferents
LOC efferents have proven more difficult to analyze. These small unmyelinated axons cannot be stimulated electrically without also activating larger diameter, lower threshold MOCs. Although uniformly positive for cholinergic markers, LOC efferents also label for GABAergic, dopaminergic, and peptidergic transmission. Two general strategies have been employed to determine LOC function, cochlear perfusion with candidate neurotransmitter agonists and antagonists, and selective lesions of central somata or axon tracts. Antibodies for the enzyme ChAT label all LOCs (Altschuler et al. 1985). Although microiontophoresis at the base of IHCs was reported to excite type I afferent activity (Felix and Ehrenberger 1992), little else has emerged on the effects of ACh. The most progress has been made for dopamine whose receptors are expressed in the cochlea (Maison et al. 2012). LOC efferents express tyrosine hydroxylase, an enzyme leading to production of dopamine (Eybalin et al. 1993; Darrow et al. 2006). Cochlear perfusion with dopamine suppressed spontaneous and driven activity of type I cochlear afferents (Ruel et al. 2001) via G-protein-coupled phosphorylation that reduced voltage-gated sodium current (Valdes-Baizabal et al. 2015). Central LOC axons have been lesioned surgically and by injection of neurotoxins with mixed effects on afferent activity. In addition to these small molecule neurotransmitters, a number of peptide transmitters also are implicated in LOC function. Given this still emerging story, the reader is referred to recent reviews (Ruel et al. 2007; Reijntjes and Pyott 2016).
Responses in Noise
In background noise, auditory nerve fibers show reduced firing rates as a result of adaptation to the noise. Electrical stimulation of the olivocochlear bundle reduces auditory nerve fiber firing rate to low-level tones, enabling an increase in the firing rates to higher-level tones. Efferent “unmasking” enhances the neural representation of acoustic transients in the presence of low-level noise (Wiederhold and Kiang 1970; Winslow and Sachs 1987, 1988; Guinan and Gifford 1988a; Kawase and Liberman 1993). Under these conditions, there is little change in threshold, but there is improved discrimination of small changes in tone intensity (Fig. 6).
Figure 6.
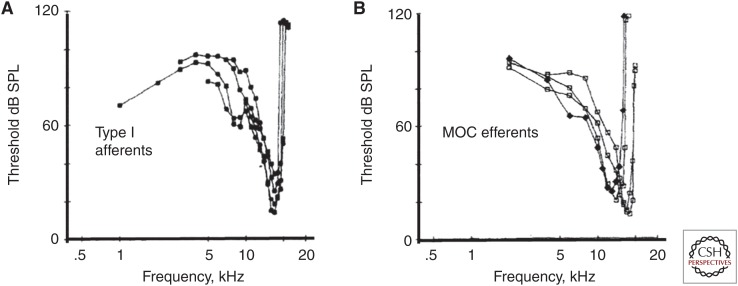
Medial olivocochlear (MOC) efferents are driven by sound. (A) Tuning curves of type I cochlear afferents from single-unit recording in guinea pig. (B) Single-unit recording from MOCs in the brainstem show similar sensitivity and tuning to that of type I afferents. That is, MOCs are driven by input from type I afferents via second-order cells in the cochlear nucleus. SPL, Sound pressure level. (From Robertson and Gummer 1985; adapted, with permission, from Elsevier © 1985.)
The effects on auditory nerve activity in noise may be elicited directly by sound-driven activation of the olivocochlear neurons, presumably by cochlear nucleus principal neurons, or by activation of higher-order central auditory structures that project to the olivocochlear neurons. Single-unit recordings from MOC neurons in vivo showed that these could be almost as sensitive and sharply tuned to acoustic stimuli as type I cochlear afferents in the same preparations (Robertson and Gummer 1985; Liberman and Brown 1986). Thus, there is a negative feedback loop from cochlear afferents to olivocochlear efferents that can regulate sensitivity in response to the acoustic environment (Fig. 7).
Figure 7.
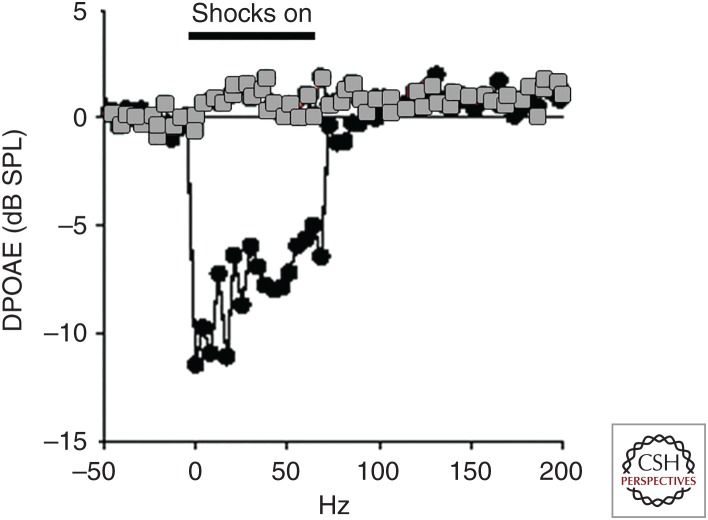
Efferent suppression of distortion product otoacoustic emissions (DPOAEs). DPOAEs measured in wild-type (black) and α10 knockout (gray) mice. DPOAE amplitude drops during electrical shocks to the medial olivocochlear (MOC) efferent neurons. Hair cells are not subject to cholinergic inhibition in the α10 knockout mouse, and efferent shocks have no effect on DPOAEs. SPL, Sound pressure level. (Figure based on data in Vetter et al. 2007.)
This electrical stimulation method is invasive and requires high-frequency electrical stimulation of the efferents for maximal effects. An alternative is to stimulate MOCs with contralateral sound. Numerous experiments have explored the effects of presenting sound to the contralateral ear on otoacoustic emission (OAE) amplitudes, and such stimulation can yield both suppressive and facilitative effects (Guinan 2014). Though there is no direct evidence that LOC neurons respond to sound, stimulation of the inferior colliculus alters auditory nerve activity. These effects have been ascribed to connections with LOC neurons (Groff and Liberman 2003). The olivocochlear neurons receive direct inputs from the auditory cortex, as well as indirect inputs from cortex via the inferior colliculus (Schofield 2011). Stimulation of auditory cortex neurons can increase or decrease cochlear activity (Dragicevic et al. 2015; Terreros and Delano 2015). Thus, olivocochlear stimulation through both reflexive (auditory nerve to cochlear nucleus to superior olivary regions) and higher-order processes (corticofugal pathways) contributes to the constellation of physiological and perceptual effects.
PERCEPTUAL EFFECTS
Hearing in Noise
Research into the perceptual effects of olivocochlear activation is confined to MOC effects. The influence of LOC function on perception is unknown. The enhanced neural representation of transient signals in noise with olivocochlear activation has long been thought to translate into improved detection and discrimination in noise; however, behavioral experiments have produced mixed results. Sectioning of the olivocochlear bundle can impair detection of sounds in noise, discrimination of intensity and frequency changes in noise, and discrimination of vertical sound location in noise under some stimulus conditions (May and McQuone 1995; Hienz et al. 1998; May et al. 2004), but the effects are variable and sometimes small. Furthermore, perceptual performance can recover with postlesion practice (May et al. 2004). Speech-in-noise performance by human listeners can be positively, negatively, or not correlated with olivocochlear suppression of OAEs elicited by presenting noise to the contralateral ear (Giraud et al. 1995; Micheyl and Collet 1996; Micheyl et al. 1997; Kumar and Vanaja 2004; de Boer and Thornton 2008; Wagner et al. 2008). The sometimes small and inconsistent effects reported in these studies cast doubt on the role of olivocochlear activation in hearing in noise. Central processing of afferent signals also plays a role in hearing in noise, and these mechanisms may blur attempts to identify associations between olivocochlear activation strength and hearing in noise performance.
Selective Attention
More consistent behavioral effects mediated by the olivocochlear system occur for tests of selective attention. In some studies, attention to a visual task decreases peripheral auditory sensitivity measured using CAPs (Oatman and Anderson 1977; Delano et al. 2007). This effect is presumed to be because of olivocochlear activation because cochlear microphonics (CMs) are increased and OAEs are suppressed when performing the task and become larger when the visual stimuli are ignored (Puel et al. 1988; Delano et al. 2007; Wittekindt et al. 2009). Selectively attending to one ear or particular sound frequencies also changes OAEs, presumably via olivocochlear mechanisms (Collet et al. 1994; Maison et al. 2001; Srinivasan et al. 2012). However, those attentional/olivocochlear effects on auditory sensitivity are quite variable across individuals, sometimes show different or no effects, and tend to be fairly small (Froehlich et al. 1990; Harkrider and Bowers 2009; Srinivasan et al. 2012). This may be because olivocochlear activation strength and changes in cochlear sensitivity are modulated by independent corticofugal mechanisms (Dragicevic et al. 2015; Terreros and Delano 2015).
Behavioral Deficits in Mutant Mouse Strains
Mutant mouse strains provide opportunities to examine the role of more specific olivocochlear manipulations. α9-nAChR knockout mice with chronically deficient MOC suppression of OHCs show normal detection of tones and intensity discrimination in noise and normal prepulse inhibition in noise (May et al. 2004; Allen and Luebke 2017). Additional experiments in α9-nAChR knockout mice show abnormal prepulse inhibition of the acoustic startle response in quiet, abnormal horizontal sound localization, abnormal temporal processing, abnormal processing of frequency changes in quiet backgrounds, and impaired selective attention (Lauer and May 2011; Terreros et al. 2016; Allen and Luebke 2017; Clause et al. 2017). Other strains with mutations affecting the MOC and LOC neurons (CGRP knockouts, α9 gain-of-function point mutation) show abnormal prepulse inhibition of the acoustic startle response (Allen and Luebke 2017). Auditory processing deficits in these mice may be attributable to abnormal development of afferent circuits (Clause et al. 2017), but experiments to determine the effects of conditional expression of mutations have not yet been performed to test this hypothesis.
PROTECTION FROM DAMAGE
Protection from Noise
Both the MOC and LOC systems protect the auditory system against damage from noise exposure. Lesions of the olivocochlear bundle generally result in impaired auditory nerve responses after acute loud noise exposure, although the effects depend on the frequency, intensity, and duration of the noise exposures, monaural or binaural stimulation, and species (Fuente 2015). Conversely, electrical stimulation of the olivocochlear bundle reduces noise-induced threshold shifts (Rajan and Johnstone 1988; Rajan 2001). The majority of these effects are attributed to the MOC system, since midline sectioning of the olivocochlear bundle affects primarily the crossed MOC fibers. Studies using mice with a gain-of-function point mutation of the α9 receptor subunit, which enhances hyperpolarization of OHCs, confirm that cholinergic modulation of OHC activity by MOC efferents contributes to noise protection (Taranda et al. 2009). Lesions specifically targeting the LOC neurons also appear to increase susceptibility to noise-induced hearing loss (Darrow et al. 2007). Impairments of the olivocochlear system also may increase susceptibility to chronic exposure to noisy environments (Lauer and May 2011; Maison et al. 2013). However, studies performed in humans have not found a consistent relationship between the strength of olivocochlear activation as estimated by contralateral suppression of OAEs and protection from noise (Fuente 2015).
Protection from Age-Related Hearing Loss
Several studies correlating contralateral suppression of OAEs with hearing thresholds have found an association between reduced olivocochlear activation and increased age-related hearing loss (Parthasarathy 2001; Kim et al. 2002; Jacobson et al. 2003; Varghese et al. 2005), although other studies have not found evidence of weakened olivocochlear feedback in older adults (Quaranta et al. 2001; Abdala et al. 2014). Weakening olivocochlear feedback with age may actually be attributed to the effects of hearing loss, since reduced afferent drive to olivocochlear neurons or damaged OHCs likely contributes to reduced suppression of OAEs (Castor et al. 1994; Tadros et al. 2005; Keppler et al. 2010; Konomi et al. 2014). Surgical lesions of the olivocochlear bundle that result in loss of peripheral olivocochlear synapses cause accelerated cochlear degeneration and hearing loss in middle age (Liberman et al. 2014). However, α9-nAChR knockout mice with chronically deficient MOC feedback do not show accelerated age-related hearing loss on a similar time course (Lauer 2017). These studies suggest that loss of olivocochlear synapses may lead to a loss of trophic factors thereby accelerating hearing loss, but that accelerated age-related hearing loss is not a necessary consequence of weakened olivocochlear feedback.
PLASTICITY (HEARING LOSS AND EXPERIENCE-DEPENDENT)
Pathology
There is evidence that synaptic reorganization of cochlear efferents occurs in certain pathological conditions. MOC terminals disappear from OHCs in C57BL/6 mice with age-related hearing loss (Fu et al. 2010). This strain also shows an increase in inhibitory olivocochlear synapses directly contacting the IHCs that is correlated with ribbon synapse loss and hearing loss (Lauer et al. 2012; Zachary and Fuchs 2015). It is uncertain whether these synapses arise from medial or lateral efferents, although they function much in the way of the efferents that contact IHCs early in development (Zachary and Fuchs 2015).
There is some evidence that sparse loss of MOC synapses may occur in cases of noise-induced hearing loss. In noise-exposed guinea pigs with no substantial loss of OHCs, reduced synaptophysin immunolabeling is observed in the midfrequency region 1 month after exposure, and this loss can be mitigated with sound conditioning (Niu et al. 2007). The effects of noise exposure on olivocochlear efferents may vary depending on the particular characteristics of the noise exposure, time after exposure, and species, but these effects have not been investigated extensively.
Perceptual Learning
Olivocochlear feedback appears to play a role in and change with perceptual learning. In ferrets, lesioning the olivocochlear bundle induces deficits in learning to use new acoustic cues for localizing sounds in the horizontal plane during unilateral conductive hearing loss (Irving et al. 2011). In humans, weaker MOC suppression of distortion product OAEs (DPOAEs) prior to training on a speech-in-noise discrimination task correlates positively with greater performance improvement (de Boer and Thornton 2008). Likewise, increased MOC strength is observed after training in listeners that showed the most perceptual improvement (de Boer and Thornton 2008). Studies have also shown increased olivocochlear suppression strength in listeners with musical training compared to listeners without musical training, providing further support for experience-dependent plasticity (Perrot and Collet 2014).
SUMMARY AND CONCLUSIONS
Studies of efferent innervation have provided fundamental insights into the highly specialized operation of the mammalian cochlea. MOC efferents suppress cochlear sensitivity and tuning by their selective innervation of OHCs, leaving the IHCs to transmit acoustic information without altering their synaptic transfer function. LOC efferents modulate the activity of type I cochlear afferents to an as-yet unknown purpose.
Synaptic inhibition of hair cells presents several surprises, employing a pharmacologically unusual and genetically distinct nAChR through which calcium entry activates associated calcium-dependent potassium channels. The potassium channels generate the predominant conductance increase, hyperpolarizing and shunting the OHC’s membrane potential. Consequently, OHC electromotility is suppressed and cochlear vibrations are smaller and less sharply tuned. MOC efferents are well driven by sound, constituting a gain control loop that can adjust the sensitivity and tuning of the cochlea to the acoustic surround. This negative feedback also can protect the cochlea against acoustic trauma.
Olivocochlear efferents play a still-to-be-explored role in the maturation of cochlear innervation. Inhibitory synapses on IHCs prior to the onset of hearing help to pattern spontaneous activity of afferent neurons that shapes the connectivity of brainstem nuclei. The role of efferent feedback on hearing itself has proven more difficult to discern. Measurements of signal discrimination or localization show mixed or no effects in different studies. This could reflect in part the possibility that efferent feedback to the cochlea is plastic, becoming stronger through use. Additionally, centrifugal modulation occurs throughout the auditory pathway so that behavioral measures are subject to a multiplicity of feedback mechanisms. Consequently, it has been difficult to associate deficits of olivocochlear neurons with pathogenesis, although there is some evidence of abnormal olivocochlear activity in listeners with tinnitus, reduced sound tolerance (hyperacusis), and central auditory processing disorders (Knudson et al. 2014). Abnormal efferent processing has been considered in a host of neurological disorders that include dysfunctional sound processing: autism, schizophrenia, migraine, selective mutism, specific language impairment, and dyslexia (Khalfa et al. 2001; Veuillet et al. 2001).
Footnotes
Editors: Guy P. Richardson and Christine Petit
Additional Perspectives on Function and Dysfunction of the Cochlea available at www.perspectivesinmedicine.org
REFERENCES
- Abdala C, Dhar S, Ahmadi M, Luo P. 2014. Aging of the medial olivocochlear reflex and associations with speech perception. J Acoust Soc Am 135: 754–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PD, Luebke A. 2017. Reflex modification audiometry reveals dual roles for olivocochlear neurotransmission. Front Cell Neurosci 11: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler RA, Kachar B, Rubio JA, Parakkal MH, Fex J. 1985. Immunocytochemical localization of choline acetyltransferase-like immunoreactivity in the guinea pig cochlea. Brain Res 338: 1–11. [DOI] [PubMed] [Google Scholar]
- Art JJ, Crawford AC, Fettiplace R, Fuchs PA. 1982. Efferent regulation of hair cells in the turtle cochlea. Proc R Soc Lond B Biol Sci 216: 377–384. [DOI] [PubMed] [Google Scholar]
- Art JJ, Fettiplace R, Fuchs PA. 1984. Synaptic hyperpolarization and inhibition of turtle cochlear hair cells. J Physiol 356: 525–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art JJ, Crawford AC, Fettiplace R, Fuchs PA. 1985. Efferent modulation of hair cell tuning in the cochlea of the turtle. J Physiol 360: 397–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J, Russell J. 1982. Effect of efferent stimulation on hair cells of the frog sacculus. J Physiol 329: 25P. [Google Scholar]
- Ballestero J, Zorrilla de San Martin J, Goutman J, Elgoyhen AB, Fuchs PA, Katz E. 2011. Short-term synaptic plasticity regulates the level of olivocochlear inhibition to auditory hair cells. J Neurosci 31: 14763–14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolome MV, Zuluaga P, Carricondo F, Gil-Loyzaga P. 2009. Immunocytochemical detection of synaptophysin in C57BL/6 mice cochlea during aging process. Brain Res Rev 60: 341–348. [DOI] [PubMed] [Google Scholar]
- Bergeron AL, Schrader A, Yang D, Osman AA, Simmons DD. 2005. The final stage of cholinergic differentiation occurs below inner hair cells during development of the rodent cochlea. J Assoc Res Otolaryngol 6: 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet C, Erostegui C, Sugasawa M, Dulon D. 1996. Acetylcholine-induced potassium current of guinea pig outer hair cells: Its dependence on a calcium influx through nicotinic-like receptors. J Neurosci 16: 2574–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. 2003. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci 23: 10832–10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce LL, Christensen MA, Warr WB. 2000. Postnatal development of efferent synapses in the rat cochlea. J Comp Neurol 423: 532–548. [DOI] [PubMed] [Google Scholar]
- Cabanillas LA, Luebke AE. 2002. CGRP- and cholinergic-containing fibers project to guinea pig outer hair cells. Hear Res 172: 14–17. [DOI] [PubMed] [Google Scholar]
- Castor X, Veuillet E, Morgon A, Collet L. 1994. Influence of aging on active cochlear micromechanical properties and on the medial olivocochlear system in humans. Hear Res 77: 1–8. [DOI] [PubMed] [Google Scholar]
- Clause A, Lauer AM, Kandler K. 2017. Mice lacking the α9 subunit of the nicotinic acetylcholine receptor exhibit deficits in frequency difference limens and sound localization. Front Cell Neurosci 11: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet L, Bouchet P, Pernier J. 1994. Auditory selective attention in the human cochlea. Brain Res 633: 353–356. [DOI] [PubMed] [Google Scholar]
- Dallos P, He DZ, Lin X, Sziklai I, Mehta S, Evans BN. 1997. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci 17: 2212–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Simons EJ, Dodds L, Liberman MC. 2006. Dopaminergic innervation of the mouse inner ear: Evidence for a separate cytochemical group of cochlear efferent fibers. J Comp Neurol 498: 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. 2007. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J Neurophysiol 97: 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins R, Keller SL, Sewell WF. 2005. Pharmacology of acetylcholine-mediated cell signaling in the lateral line organ following efferent stimulation. J Neurophysiol 93: 2541–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Thornton AR. 2008. Neural correlates of perceptual learning in the auditory brainstem: Efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J Neurosci 28: 4929–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano PH, Elgueda D, Hamame CM, Robles L. 2007. Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas. J Neurosci 27: 4146–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic CD, Aedo C, León A, Bowen M, Jara N, Terreros G, Robles L, Delano PH. 2015. The olivocochlear reflex strength and cochlear sensitivity are independently modulated by auditory cortex microstimulation. J Assoc Res Otolaryngol 16: 223–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Lenoir M. 1996. Cholinergic responses in developing outer hair cells of the rat cochlea. Eur J Neurosci 8: 1945–1952. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. 1994. α9: An acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79: 705–715. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. 2001. α10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Nat Acad Sci 98: 3501–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling MR, Sobkowicz HM, Levenick CV, Scott GL, Slapnick SM, Rose JE. 1990. Biochemical and morphological differentiation of acetylcholinesterase-positive efferent fibers in the mouse cochlea. J Elect Micros Tech 15: 123–143. [DOI] [PubMed] [Google Scholar]
- Evans MG, Lagostena L, Darbon P, Mammano F. 2000. Cholinergic control of membrane conductance and intracellular free Ca2+ in outer hair cells of the guinea pig cochlea. Cell Calcium 28: 195–203. [DOI] [PubMed] [Google Scholar]
- Eybalin M, Charachon G, Renard N. 1993. Dopaminergic lateral efferent innervation of the guinea-pig cochlea: Immunoelectron microscopy of catecholamine-synthesizing enzymes and effect of 6-hydroxydopamine. Neuroscience 54: 133–142. [DOI] [PubMed] [Google Scholar]
- Felix D, Ehrenberger K. 1992. The efferent modulation of mammalian inner hair cell afferents. Hear Res 64: 1–5. [DOI] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PW, Lall K, Liberman MC, Kujawa SG. 2015. Aging after noise exposure: Acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci 35: 7509–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A, Russell I. 1973. Efferent nerve fibres: Postsynaptic action on hair cells. Nat New Biol 243: 89–91. [PubMed] [Google Scholar]
- Fritzsch B. 1996. Development of the labyrinthine efferent system. Ann NY Acad Sci 781: 21–33. [DOI] [PubMed] [Google Scholar]
- Froehlich P, Collet L, Chanal JM, Morgon A. 1990. Variability of the influence of a visual task on the active micromechanical properties of the cochlea. Brain Res 508: 286–288. [DOI] [PubMed] [Google Scholar]
- Fu B, Le Prell C, Simmons D, Lei D, Schrader A, Chen AB, Bao J. 2010. Age-related synaptic loss of the medial olivocochlear efferent innervation. Mol Neurodegener 5: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. 1992a. Cholinergic inhibition of short (outer) hair cells of the chick’s cochlea. J Neurosci 12: 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. 1992b. A novel cholinergic receptor mediates inhibition of chick cochlear hair cells. Proc Biol Sci 248: 35–40. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Lehar M, Hiel H. 2014. Ultrastructure of cisternal synapses on outer hair cells of the mouse cochlea. J Comp Neurol 522: 717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuente A. 2015. The olivocochlear system and protection from acoustic trauma: A mini literature review. Front Syst Neurosci 9: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos R. 1956. Suppression of auditory nerve activity by stimulation of efferent fibers to the cochlea. J Neurophysiol 19: 424–437. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Collet L, Chéry-Croze S, Magnan J, Chays A. 1995. Evidence of a medial olivocochlear involvement in contralateral suppression of otoacoustic emissions in humans. Brain Res 705: 15–23. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. 2000. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science 288: 2366–2368. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Fuchs PA, Glowatzki E. 2005. Facilitating efferent inhibition of inner hair cells in the cochlea of the neonatal rat. J Physiol 566: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff JA, Liberman MC. 2003. Modulation of cochlear afferent response by the lateral olivocochlear system: Activation via electrical stimulation of the inferior colliculus. J Neurophysiol 90: 3178–3200. [DOI] [PubMed] [Google Scholar]
- Guinan JJ Jr. 2014. Cochlear mechanics, otoacoustic emissions, and medial olivocochlear efferents: Twenty years of advances and controversies along with areas ripe for new work. In Perspectives on auditory research, pp. 229–246. Springer, New York. [Google Scholar]
- Guinan JJ, Gifford ML. 1988a. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. I: Rate-level functions. Hear Res 33: 97–113. [DOI] [PubMed] [Google Scholar]
- Guinan JJ Jr, Gifford ML. 1988b. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. III: Tuning curves and thresholds at CF. Hear Res 37: 29–45. [DOI] [PubMed] [Google Scholar]
- Harkrider AW, Bowers CD. 2009. Evidence for a cortically mediated release from inhibition in the human cochlea. J Am Acad Audiol 20: 208–215. [DOI] [PubMed] [Google Scholar]
- He DZ, Dallos P. 1999. Development of acetylcholine-induced responses in neonatal gerbil outer hair cells. J Neurophysiol 81: 1162–1170. [DOI] [PubMed] [Google Scholar]
- Hienz RD, Stiles P, May BJ. 1998. Effects of bilateral olivocochlear lesions on vowel formant discrimination in cats. Hear Res 116: 10–20. [DOI] [PubMed] [Google Scholar]
- Huang LC, Thorne PR, Housley GD, Montgomery JM. 2007. Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development 134: 2925–2933. [DOI] [PubMed] [Google Scholar]
- Im GJ, Moskowitz HS, Lehar M, Hiel H, Fuchs PA. 2014. Synaptic calcium regulation in hair cells of the chicken basilar papilla. J Neurosci 34: 16688–16697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving S, Moore DR, Liberman MC, Sumner CJ. 2011. Olivocochlear efferent control in sound localization and experience-dependent learning. J Neurosci 31: 2493–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M, Kim S, Romney J, Zhu X, Frisina RD. 2003. Contralateral suppression of distortion—Product otoacoustic emissions declines with age: A comparison of findings in CBA mice with human listeners. Laryngoscope 113: 1707–1713. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ. 2005. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol 563: 177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Franz C, Knipper M, Marcotti W. 2009. Functional maturation of the exocytotic machinery at gerbil hair cell ribbon synapses. J Physiol 587: 1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eckrich T, Kuhn S, Zampini V, Franz C, Ranatunga KM, Roberts TP, Masetto S, Knipper M, Kros CJ, et al. 2011. Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nat Neurosci 14: 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Wedemeyer C, Vetter DE, Adachi R, Holley MC, Elgoyhen AB, Marcotti W. 2013. Cholinergic efferent synaptic transmission regulates the maturation of auditory hair cell ribbon synapses. Open Biol 3: 130163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PM, Parks XX, Contini D, Holt JC. 2013. A review of synaptic mechanisms of vestibular efferent signaling in turtles: Extrapolation to efferent actions in mammals. J Vestib Res 23: 161–175. [DOI] [PubMed] [Google Scholar]
- Katz E, Elgoyhen AB, Gomez-Casati ME, Knipper M, Vetter DE, Fuchs PA, Glowatzki E. 2004. Developmental regulation of nicotinic synapses on cochlear inner hair cells. J Neurosci 24: 7814–7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase T, Liberman MC. 1993. Antimasking effects of the olivocochlear reflex. I: Enhancement of compound action potentials to masked tones. J Neurophysiol 70: 2519–2532. [DOI] [PubMed] [Google Scholar]
- Keppler H, Dhooge I, Corthals P, Maes L, D’haenens W, Bockstael A, Philips B, Swinnen F, Vinck B. 2010. The effects of aging on evoked otoacoustic emissions and efferent suppression of transient evoked otoacoustic emissions. Clin Neurophysiol 121: 359–365. [DOI] [PubMed] [Google Scholar]
- Khalfa S, Bruneau N, Roge B, Georgieff N, Veuillet E, Adrien JL, Barthelemy C, Collet L. 2001. Peripheral auditory asymmetry in infantile autism. Eur J Neurosci 13: 628–632. [DOI] [PubMed] [Google Scholar]
- Kim S, Frisina DR, Frisina RD. 2002. Effects of age on contralateral suppression of distortion product otoacoustic emissions in human listeners with normal hearing. Audiol Neurootol 7: 348–357. [DOI] [PubMed] [Google Scholar]
- Klinke R, Galley N. 1974. Efferent innervation of vestibular and auditory receptors. Physiol Rev 54: 316–357. [DOI] [PubMed] [Google Scholar]
- Knudson IM, Shera CA, Melcher JR. 2014. Increased contralateral suppression of otoacoustic emissions indicates a hyperresponsive medial olivocochlear system in humans with tinnitus and hyperacusis. J Neurophysiol 112: 3197–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong JH, Adelman JP, Fuchs PA. 2008. Expression of the SK2 calcium-activated potassium channel is required for cholinergic function in mouse cochlear hair cells. J Physiol 586: 5471–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong JH, Zachary S, Rohmann KN, Fuchs PA. 2013. Retrograde facilitation of efferent synapses on cochlear hair cells. J Assoc Res Otolaryngol 14: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konomi U, Kanotra S, James AL, Harrison RV. 2014. Age related changes to the dynamics of contralateral DPOAE suppression in human subjects. J Otolaryngol Head Neck Surg 43: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rusch A. 1998. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature 394: 281–284. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. 2015. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res 330: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar UA, Vanaja CS. 2004. Functioning of olivocochlear bundle and speech perception in noise. Ear Hear 25: 142–146. [DOI] [PubMed] [Google Scholar]
- Lauer AM. 2017. Minimal effects of age and exposure to a noisy environment on hearing in α9 nicotinic receptor knockout mice. Front Neurosci 11: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, May BJ. 2011. The medial olivocochlear system attenuates the developmental impact of early noise exposure. J Assoc Res Otolaryngol 12: 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Fuchs PA, Ryugo DK, Francis HW. 2012. Efferent synapses return to inner hair cells in the aging cochlea. Neurobiol Aging 33: 2892–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Shnerson A, Pujol R. 1980. Cochlear receptor development in the rat with emphasis on synaptogenesis. Anat Embryol (Berl) 160: 253–262. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. 1986. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res 24: 17–36. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Liberman LD, Maison SF. 2014. Efferent feedback slows cochlear aging. J Neurosci 34: 4599–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioudyno M, Hiel H, Kong JH, Katz E, Waldman E, Parameshwaran-Iyer S, Glowatzki E, Fuchs PA. 2004. A “synaptoplasmic cistern” mediates rapid inhibition of cochlear hair cells. J Neurosci 24: 11160–11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipovsek M, Im GJ, Franchini LF, Pisciottano F, Katz E, Fuchs PA, Elgoyhen AB. 2012. Phylogenetic differences in calcium permeability of the auditory hair cell cholinergic nicotinic receptor. Proc Nat Acad Sci 109: 4308–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipovsek M, Fierro A, Perez EG, Boffi JC, Millar NS, Fuchs PA, Katz E, Elgoyhen AB. 2014. Tracking the molecular evolution of calcium permeability in a nicotinic acetylcholine receptor. Mol Biol Evol 31: 3250–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Timofeyev V, Li N, Rafizadeh S, Singapuri A, Harris TR, Chiamvimonvat N. 2009. α-Actinin2 cytoskeletal protein is required for the functional membrane localization of a Ca2+-activated K+ channel (SK2 channel). Proc Nat Acad Sci 106: 18402–18407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison S, Micheyl C, Collet L. 2001. Influence of focused auditory attention on cochlear activity in humans. Psychophysiology 38: 35–40. [PubMed] [Google Scholar]
- Maison SF, Adams JC, Liberman MC. 2003. Olivocochlear innervation in the mouse: Immunocytochemical maps, crossed versus uncrossed contributions, and transmitter colocalization. J Comp Neurol 455: 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Liu XP, Eatock RA, Sibley DR, Grandy DK, Liberman MC. 2012. Dopaminergic signaling in the cochlea: Receptor expression patterns and deletion phenotypes. J Neurosci 32: 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Usubuchi H, Liberman MC. 2013. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci 33: 5542–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B, McQuone SJ. 1995. Effects of bilateral olivocochlear lesions on pure-tone intensity discrimination in cats. Aud Neurosci 1: 385–400. [PMC free article] [PubMed] [Google Scholar]
- May BJ, Budelis J, Niparko JK. 2004. Behavioral studies of the olivocochlear efferent system: Learning to listen in noise. Arch Otolaryngol Head Neck Surg 130: 660–664. [DOI] [PubMed] [Google Scholar]
- McLean WJ, Smith KA, Glowatzki E, Pyott SJ. 2009. Distribution of the Na,K-ATPase α subunit in the rat spiral ganglion and organ of corti. J Assoc Res Otolaryngol 10: 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven AI, Yuhas WA, Fuchs PA. 1996. Ionic dependence and agonist preference of an acetylcholine receptor in hair cells. Aud Neurosci 2: 63–77. [Google Scholar]
- Micheyl C, Collet L. 1996. Involvement of the olivocochlear bundle in the detection of tones in noise. J Acoust Soc Am 99: 1604–1610. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Perrot X, Collet L. 1997. Relationship between auditory intensity discrimination in noise and olivocochlear efferent system activity in humans. Behav Neurosci 111: 801. [DOI] [PubMed] [Google Scholar]
- Moglie MJ, Fuchs PA, Elgoyhen AB, Goutman JD. 2018. Compartmentalization of antagonistic Ca2+ signals in developing cochlear hair cells. Proc Natl Acad Sci 115: E2095–E2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V, Maison SF, Taranda J, Haque N, Bond CT, Elgoyhen AB, Adelman JP, Liberman MC, Vetter DE. 2009. SK2 channels are required for function and long-term survival of efferent synapses on mammalian outer hair cells. Mol Cell Neurosci 40: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB Jr. 1988. Comparative anatomy of the cochlea and auditory nerve in mammals. Hear Res 34: 253–266. [DOI] [PubMed] [Google Scholar]
- Niu X, Tahera Y, Canlon B. 2007. Environmental enrichment to sound activates dopaminergic pathways in the auditory system. Physiol Behav 92: 34–39. [DOI] [PubMed] [Google Scholar]
- Oatman LC, Anderson BW. 1977. Effects of visual attention on tone burst evoked auditory potentials. Exp Neurol 57: 200–211. [DOI] [PubMed] [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. 2000. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron 26: 595–601. [DOI] [PubMed] [Google Scholar]
- Parks XX, Contini D, Jordan PM, Holt JC. 2017. Confirming a role for α9nAChRs and SK potassium channels in type II hair cells of the turtle posterior crista. Front Cell Neurosci 11: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy TK. 2001. Aging and contralateral suppression effects on transient evoked otoacoustic emissions. J Am Acad Audiol 12: 80–85. [PubMed] [Google Scholar]
- Perrot X, Collet L. 2014. Function and plasticity of the medial olivocochlear system in musicians: A review. Hear Res 308: 27–40. [DOI] [PubMed] [Google Scholar]
- Poppi LA, Tabatabaee H, Drury HR, Jobling P, Callister RJ, Migliaccio AA, Jordan PM, Holt JC, Rabbitt RD, Lim R, et al. 2017. ACh-induced hyperpolarization and decreased resistance in mammalian type II vestibular hair cells. J Neurophysiol 119: 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel JL, Bonfils P, Pujol R. 1988. Selective attention modifies the active micromechanical properties of the cochlea. Brain Res 447: 380–383. [DOI] [PubMed] [Google Scholar]
- Quaranta N, Debole S, Di Girolamo S. 2001. Effect of ageing on otoacoustic emissions and efferent suppression in humans. Audiology 40: 308–312. [PubMed] [Google Scholar]
- Rajan R. 2001. Noise priming and the effects of different cochlear centrifugal pathways on loud-sound-induced hearing loss. J Neurophysiol 86: 1277–1288. [DOI] [PubMed] [Google Scholar]
- Rajan R, Johnstone BM. 1988. Binaural acoustic stimulation exercises protective effects at the cochlea that mimic the effects of electrical stimulation of an auditory efferent pathway. Brain Res 459: 241–255. [DOI] [PubMed] [Google Scholar]
- Reijntjes DO, Pyott SJ. 2016. The afferent signaling complex: Regulation of type I spiral ganglion neuron responses in the auditory periphery. Hear Res 336: 1–16. [DOI] [PubMed] [Google Scholar]
- Robertson D, Gummer M. 1985. Physiological and morphological characterization of efferent neurones in the guinea pig cochlea. Hear Res 20: 63–77. [DOI] [PubMed] [Google Scholar]
- Rohmann KN, Wersinger E, Braude JP, Pyott SJ, Fuchs PA. 2015. Activation of BK and SK channels by efferent synapses on outer hair cells in high-frequency regions of the rodent cochlea. J Neurosci 35: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Wersinger E, McIntosh JM, Fuchs PA, Glowatzki E. 2011. Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea. J Neurosci 31: 15092–15101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Wu JS, McIntosh JM, Glowatzki E. 2016. Assessment of the expression and role of the α1-nAChR subunit in efferent cholinergic function during the development of the mammalian cochlea. J Neurophysiol 116: 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel J, Nouvian R, Gervais d’Aldin C, Pujol R, Eybalin M, Puel JL. 2001. Dopamine inhibition of auditory nerve activity in the adult mammalian cochlea. Eur J Neurosci 14: 977–986. [DOI] [PubMed] [Google Scholar]
- Ruel J, Wang J, Rebillard G, Eybalin M, Lloyd R, Pujol R, Puel JL. 2007. Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear Res 227: 19–27. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Murugasu E. 1997. Medial efferent inhibition suppresses basilar membrane responses to near characteristic frequency tones of moderate to high intensities. J Acoust Soc Am 102: 1734–1738. [DOI] [PubMed] [Google Scholar]
- Sato M, Henson MM, Smith DW. 1997. Synaptic specializations associated with the outer hair cells of the Japanese macaque. Hear Res 108: 46–54. [DOI] [PubMed] [Google Scholar]
- Sato M, Henson MM, Henson OW Jr, Smith DW. 1999. The innervation of outer hair cells: 3D reconstruction from TEM serial sections in the Japanese macaque. Hear Res 135: 29–38. [DOI] [PubMed] [Google Scholar]
- Schofield BR. 2011. Central descending auditory pathways. In Auditory and vestibular efferents, pp. 261–290. Springer, New York. [Google Scholar]
- Scholl ES, Pirone A, Cox DH, Duncan RK, Jacob MH. 2014. Alternative splice isoforms of small conductance calcium-activated SK2 channels differ in molecular interactions and surface levels. Channels 8: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DD. 2002. Development of the inner ear efferent system across vertebrate species. J Neurobiol 53: 228–250. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Mansdorf NB, Kim JH. 1996. Olivocochlear innervation of inner and outer hair cells during postnatal maturation: Evidence for a waiting period. J Comp Neurol 370: 551–562. [DOI] [PubMed] [Google Scholar]
- Smith CA, Sjostrand FS. 1961. Structure of the nerve endings on the external hair cells of the guinea pig cochlea as studied by serial sections. J Ultrastruct Res 5: 523–556. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Emmerling MR. 1989. Development of acetylcholinesterase-positive neuronal pathways in the cochlea of the mouse. J Neurocytol 18: 209–224. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Keil A, Stratis K, Carr KLW, Smith DW. 2012. Effects of cross-modal selective attention on the sensory periphery: Cochlear sensitivity is altered by selective attention. Neuroscience 223: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamataki S, Francis HW, Lehar M, May BJ, Ryugo DK. 2006. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear Res 221: 104–118. [DOI] [PubMed] [Google Scholar]
- Tadros SF, Frisina ST, Mapes F, Kim S, Frisina DR, Frisina RD. 2005. Loss of peripheral right-ear advantage in age-related hearing loss. Audiol Neurootol 10: 44–52. [DOI] [PubMed] [Google Scholar]
- Taranda J, Maison SF, Ballestero JA, Katz E, Savino J, Vetter DE, Boulter J, Liberman MC, Fuchs PA, Elgoyhen AB. 2009. A point mutation in the hair cell nicotinic cholinergic receptor prolongs cochlear inhibition and enhances noise protection. PLoS Biol 7: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terreros G, Delano PH. 2015. Corticofugal modulation of peripheral auditory responses. Front Syst Neurosci 9: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terreros G, Jorratt P, Aedo C, Elgoyhen AB, Delano PH. 2016. Selective attention to visual stimuli using auditory distractors is altered in α-9 nicotinic receptor subunit knock-out mice. J Neurosci 36: 7198–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE. 2010. Developmental regulation of spontaneous activity in the Mammalian cochlea. J Neurosci 30: 1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. 2007. The origin of spontaneous activity in the developing auditory system. Nature 450: 50–55. [DOI] [PubMed] [Google Scholar]
- Valdes-Baizabal C, Soto E, Vega R. 2015. Dopaminergic modulation of the voltage-gated sodium current in the cochlear afferent neurons of the rat. PLoS ONE 10: e0120808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese GI, Zhu X, Frisina RD. 2005. Age-related declines in distortion product otoacoustic emissions utilizing pure tone contralateral stimulation in CBA/CaJ mice. Hear Res 209: 60–67. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. 1999. Role of α9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23: 93–103. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Katz E, Maison SF, Taranda J, Turcan S, Ballestero J, Liberman MC, Elgoyhen AB, Boulter J. 2007. The α10 nicotinic acetylcholine receptor subunit is required for normal synaptic function and integrity of the olivocochlear system. Proc Nat Acad Sci 104: 20594–20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veuillet E, Georgieff N, Philibert B, Dalery J, Marie-Cardine M, Collet L. 2001. Abnormal peripheral auditory asymmetry in schizophrenia. J Neurol Neurosurg Psychiatry 70: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Frey K, Heppelmann G, Plontke SK, Zenner HP. 2008. Speech-in-noise intelligibility does not correlate with efferent olivocochlear reflex in humans with normal hearing. Acta Otolaryngol 128: 53–60. [DOI] [PubMed] [Google Scholar]
- Warr WB. 1975. Olivocochlear and vestibular efferent neurons of the feline brain stem: Their location, morphology and number determined by retrograde axonal transport and acetylcholinesterase histochemistry. J Comp Neurol 161: 159–181. [DOI] [PubMed] [Google Scholar]
- Warr WB, Guinan JJ Jr. 1979. Efferent innervation of the organ of corti: Two separate systems. Brain Res 173: 152–155. [DOI] [PubMed] [Google Scholar]
- Wedemeyer C, Zorrilla de San Martin J, Ballestero J, Gomez-Casati ME, Torbidoni AV, Fuchs PA, Bettler B, Elgoyhen AB, Katz E. 2013. Activation of presynaptic GABA(B(1a,2)) receptors inhibits synaptic transmission at mammalian inhibitory cholinergic olivocochlear-hair cell synapses. J Neurosci 33: 15477–15487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger E, McLean WJ, Fuchs PA, Pyott SJ. 2010. BK channels mediate cholinergic inhibition of high frequency cochlear hair cells. PLoS ONE 5: e13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold ML, Kiang NY. 1970. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am 48: 950–965. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. 1987. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol 57: 1002–1021. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. 1988. Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise, and with stimulation of the crossed olivocochlear bundle. Hear Res 35: 165–189. [DOI] [PubMed] [Google Scholar]
- Wittekindt A, Gaese BH, Kössl M. 2009. Influence of contralateral acoustic stimulation on the quadratic distortion product f2–f1 in humans. Hear Res 247: 27–33. [DOI] [PubMed] [Google Scholar]
- Wu JS, Vyas P, Glowatzki E, Fuchs PA. 2018. Opposing expression gradients of calcitonin-related polypeptide α (Calca/Cgrpα) and tyrosine hydroxylase (Th) in type II afferent neurons of the mouse cochlea. J Comp Neurol 526: 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Goutman JD, Pyott SJ, Glowatzki E. 2017. mGluR1 enhances efferent inhibition of inner hair cells in the developing rat cochlea. J Physiol 595: 3483–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas WA, Fuchs PA. 1999. Apamin-sensitive, small-conductance, calcium-activated potassium channels mediate cholinergic inhibition of chick auditory hair cells. J Comp Physiol A 185: 455–462. [DOI] [PubMed] [Google Scholar]
- Zachary SP, Fuchs PA. 2015. Re-emergent inhibition of cochlear inner hair cells in a mouse model of hearing loss. J Neurosci 35: 9701–9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary S, Nowak N, Vyas P, Bonanni L, Fuchs PA. 2018. Voltage-gated calcium influx modifies cholinergic inhibition of inner hair cells in the immature rat cochlea. J Neurosci 38: 5677–5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla de San Martin J, Pyott S, Ballestero J, Katz E. 2010. Ca2+ and Ca2+-activated K+ channels that support and modulate transmitter release at the olivocochlear efferent-inner hair cell synapse. J Neurosci 30: 12157–12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. 2002. Short-term synaptic plasticity. Ann Rev Physiol 64: 355–405. [DOI] [PubMed] [Google Scholar]