Abstract.
A variety of growth factors promote the complex multistep process of angiogenesis. The mitogenic activity of vascular endothelial growth factors (VEGFs) and placental growth factors (PlGFs), known as cytokines acting predominantly on endothelial cells, was tested on human umbilical vein endothelial cells (HUVEC) and microvascular endothelial cells (MIEC) and compared with the potency of the universally acting basic fibroblast growth factor (FGF‐2). The cells were seeded at different cell numbers and incubated with various doses of growth factors for a period of 24–72 h in culture medium ± serum. Proliferation was determined by measuring the optical density after staining the cells with the tetrazolium salt WST‐1.
VEGF 121 and VEGF 165 increased the number of HUVEC and MIEC at low and high seeding densities various doses and incubation times. The efficiency of FGF‐2 was less pronounced at high seeding densities of the cells under serum‐free conditions. PlGF‐1 and PlGF‐2 stimulated mitogenesis on HUVEC only at low cell numbers and after a short incubation time by 125 ± 3% and 102 ± 5% (P < 0.001), respectively. Longer incubation times with the lower seeding density in the absence of FCS did not induce a significant stimulatory effect of the PlGFs. MIEC responded stronger to all growth factors. In particular under serum free conditions, PlGF‐1 and PlGF‐2 effectively stimulated cell proliferation by 247 ± 54% (P < 0.01) and 288 ± 40% (P < 0.05) at low cell numbers, and by 81 ± 13% (P < 0.05) and 49 ± 13% (P < 0.01), respectively, at high cell numbers. The addition of fetal calf serum caused a reduced proliferative response of all growth factors on both cell types related to the controls. In conclusion, MIEC and HUVEC differ in their proliferative response to VEGFs, PlGFs and FGF‐2.
Introduction
Angiogenesis, the formation of new blood vessels, plays an important role in many physiological processes. A variety of peptide growth factors, in particular the heparin‐binding growth factors, promote this complex multistep process, which includes endothelial cell proliferation in vitro and in vivo (Kumar et al. 1998). The only growth factors known to act almost exclusively on endothelial cells (besides their reactivity on placental trophoblast) are the vascular endothelial growth factors (VEGFs) and the placental growth factors (PlGFs) (review Neufeld et al. 1999, review Torry et al. 1999). VEGFs and PlGFs are members of a dimeric growth factor subfamily with homologous sequences to platelet derived growth factor (Ferrara et al. 1992; Terman et al. 1992; DiSalvo et al. 1995). VEGFs and PlGFs exist as multiple, alternatively spliced isoforms that may constitute various homodimers as well as heterodimers via interchain disulphide bridge formations (Ferrara et al. 1991; Hauser & Weich 1993; Maglione et al. 1993). The free soluble protein isoforms VEGF 121, VEGF 165, PlGF 131/149 (PlGF‐1) and PlGF 201/219 (PlGF‐3) are assumed to induce mitosis of endothelial cells. The highly basic proteins VEGF 145, VEGF 189, VEGF 206 and PlGF152/170 (PlGF‐2) are bound to heparin‐containing proteoglycans on the cell surface, basement membrane or extracellular matrix and rather act as vascular permeability factors (Ferrara et al. 1991; Hauser & Weich 1993; Maglione et al. 1993; Cao et al. 1997). VEGF mRNA and protein are expressed in diverse cell types of mesenchymal and epithelial origin of several organs (Ferrara et al. 1991), whereas the expression of PlGF mRNA and protein is mainly restricted to placental tissues and human umbilical vein endothelial cells (HUVEC) (Hauser & Weich 1993; Khalig et al. 1999; Torry et al. 1999).
The high affinity receptors for VEGFs are KDR (kinase insert domain‐containing receptor) and flt‐1 (Fms‐like tyrosine kinase) proteins. The localization of KDR and flt‐1 mRNA is restricted to vascular endothelial and trophoblast cells (Neufeld et al. 1999; Torry et al. 1999). KDR is regarded as the major regulator of vasculogenesis and angiogenesis (Millauer et al. 1993; 1999b, 1999a) and transduces signals for mitogenicity, chemotaxis, actin reorganization, mobilization of intracellular Ca2+ and changes of gross morphology of the cell. Flt‐1 does not signal any of these effects in response to VEGF, despite its ability to bind VEGF with higher affinity than KDR (Waltenberger et al. 1994). PlGFs bind with high affinity to flt‐1, but not to KDR receptors and appear to have weak growth stimulatory effects on endothelial cells.
Most of the studies into mitogenic effects of endothelial growth factors have been carried out on HUVECs, but they produced varying results. Low doses of VEGF 165 were shown to induce a three‐ to fourfold increase in the final cell count of HUVEC, whereas the major isoform of the PlGF family, PlGF‐2, caused only a 10–20% growth stimulation at high concentrations (Park et al. 1994). Other studies reported about a 40% lower efficiency of PlGF‐2 as compared to VEGF (Sawano et al. 1996). PlGF‐1 seemed to have negligible effects on HUVEC proliferation when cells were exposed to PlGF‐1 for a long period of time (3–7 days) in the presence of high serum concentrations (Park et al. 1994; Sawano et al. 1996; Cao et al. 1997). In contrast, short‐term exposure (48 h) to PlGF‐1 at low serum concentrations resulted in a dose dependent increase in HUVEC proliferation, but with significantly weaker mitogenic potency than VEGF 165 (Ziche et al. 1997). In very recent studies PlGF‐1 and ‐2 induced the migration of endothelial cells, but had, if any, either weak stimulating or even inhibiting effects on proliferation (Migdal et al. 1998; Khalig et al. 1999).
Endothelial cells are morphologically and functionally heterogeneous with the greatest differences between those from the macro‐ and microcirculation as documented in a variety of tissues (review Garlanda & Dejana 1997). In the human full term placenta this heterogeneity was reflected by different antigenic properties and glycosylation patterns between macro‐ and microvascular endothelial cells (1994, 1993). The mitogenic activity of endothelial growth factors is tissue‐specific and differs with the location of the endothelial cell (Millauer et al. 1994; Waltenberger et al. 1994; Seetharam et al. 1995). Human dermal microvascular endothelial cells (MIEC) and HUVEC differ in their dose–response characteristic towards VEGF (Gupta et al. 1997).
Different experimental conditions are likely to account for opposing effects of growth factors on the same cell type, as was shown on HUVEC, and results on cells from the macro‐ and microcirculation cannot be compared at all. The diversity of parameters obviously affecting the activity of endothelial cell mitogens calls for a detailed and systematic analysis. The present study tested the hypothesis that different experimental conditions account for the inconsistent data. Therefore, we concentrated on well‐known confounding factors, which have varied among the studies, i.e. concentration of cytokines, presence or absence of serum, duration of incubation as well as seeding density. Because of the well known heterogeneity of endothelial cells we compared the proliferative effects of the most frequently used VEGF‐and PlGF isoforms on HUVEC and MIEC. In addition the universally acting basic fibroblast growth factor (FGF‐2), an 18kd heparin binding protein monomer, was included in the study because it stimulates the proliferation of a variety of cell types regardless of their origin from the macro‐ or microcirculation (for review see Szebenyi & Fallon 1999).
MATERIALS AND METHODS
Cell culture
HUVEC and MIEC were purchased from BioWhittaker‐Clonetics (Verviers, Belgium) and cultured in BioWhittaker‐Clonetics complete medium® containing 5% (v/v) dialysed fetal bovine serum, bovine brain extract (18 µg/ml), rhEGF (10 ng/ml), hydrocortisone (1.0 µg/ml), gentamycin (50 mg/ml) and amphotericin B (50 ng/ml). All experiments were carried out with the same serum batch. Cells were only used up to the fifth passage to avoid phenotypic drift. BioWhittaker‐Clonetics basal medium® (modified MCDB 131 formulation) without supplements but with gentamycin and amphotericin B was used for the proliferation assays.
Characterization of the cells
For each passage the identity of the endothelial cells was tested with monoclonal von Willebrand factor antibody (Dakopatts, Glostrup, Denmark) and Ulex europaeus lectin (Sigma, Taufkirchen, Germany). Both are standard markers for endothelial cells. HUVEC and MIEC were grown and stained on chamber slides (Lab‐Tek, Nalgene Nunc International, Naperville, USA). At confluence, the chamber slides were washed in HBSS (Life Technologies Gibco, Wien, Austria), fixed in acetone for 3 min at room temperature and stained as previously described (Lang et al. 1993).
The cells were further characterized by measuring LDL uptake: acetylated low density lipoprotein labelled with 1,1′‐dioctadecyl – 3,3,3′,3′‐tetramethylindocarbo‐cyanine perchlorate (DiI‐Ac‐LDL) (Biomedical Technologies, Stoughton MA, USA) was diluted to 10 µg/ml in the culture medium, added to the living cells and incubated for 4 h at 37 °C. The culture medium was removed and the cells were washed in PBS (three times). The cells were fixed in 3% formaldehyde/PBS for 20 min at room temperature, rinsed in distilled water (5 s) and mounted in glycerol/PBS (90%/10%). Stained slides were examined using a Zeiss Axiophot microscope. According to these measurements more than 99% of the cells were viable endothelial cells at seeding.
Cell proliferation assay
HUVEC and MIEC were seeded at a density of 3 × 103 and 6 × 103 cells/well on 96 well microtiter plates, which had been precoated with 1% (v/v) gelatin (Sigma) in HBSS for 1 h at 37 °C. Two hundred microlitres basal medium were added with or without 2.5% fetal calf serum and either without (control) or with 1, 10, 50, 100 ng/ml of VEGF 121, VEGF 165, PlGF‐1, PlGF‐2 and FGF‐2 (Strathmann Biotech, Hannover, Germany). The concentration of the cytokines was within the range applied in previous experiments (Park et al. 1994; Sawano et al. 1996; Cao et al. 1997; Ziche et al. 1997; Migdal et al. 1998).
The mitogenic activity of the growth factors was determined by a colourimetric assay based on formazan dye formation (WST‐1, Boehringer Mannheim, Mannheim, Germany), which directly correlates with the number of metabolically active cells in the culture. After incubation of the cells for a period of 24, 48 and 72 h, 20 µl/well of the reagent WST‐1 were added and incubated for 1 h at 37 °C. An increase in the number of viable cells resulted in an increase in the overall activity of mitochondrial dehydrogenases in the sample with an ensuing increase in formazan dye formation. The formazan dye was quantified by measuring the optical density of the dye solution at 450 nm with a scanning multiwell spectrophotometer (Spectramax 250, MWG‐Biotech, Germany) using 630 nm as the internal reference. In pilot experiments the optical density correlated with the number of both cell types under two different medium conditions (Fig. 1). Cell numbers smaller than 1000 did not correlate (not shown). Therefore, cell numbers of greater than 1000 were used throughout the study. All results in the study were based on at least five parallel measurements each time and repeated up to five independent experiments.
Figure 1.
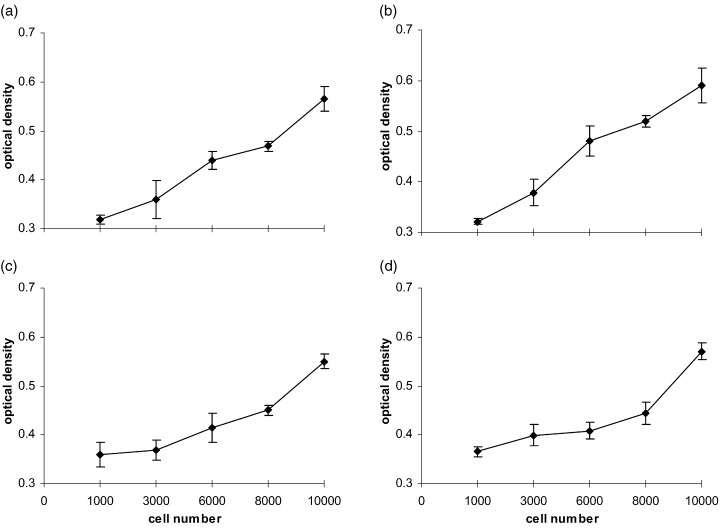
Correlation between the number of HUVEC (a, b) and MIEC (c, d) with the optical density of the WST‐1 reaction product measured after 24 h in basal medium with FCS (a, c) and complete medium with FCS (b, d).
Data analysis and statistics
Effect is defined as the growth factor‐induced increment in optical density exceeding controls expressed relative to the increase of the controls over the culture time. Data are presented as medians (± SEM) of the percentage of control. Statistical comparisons between groups were performed using the Wilcoxon rank sum test or one‐way anova followed by a post hoc test (Neuman‐Keuls or Dunnett’s), as appropriate. Differences among medians were considered significant when P < 0.05.
RESULTS
In pilot experiments cell proliferation was measured at various serum concentrations and seeding densities. Finally, serum concentration was adjusted so as to result in 40% and 50% confluency (visual inspection) of MIEC and HUVEC after 48 h in basal culture medium without growth factors at a seeding density of 6000 cells. In complete medium (containing supplements for the optimal growth of endothelial cells) after 72 h a confluency of 70% and 100% (visual inspection) was reached in MIEC and HUVEC, respectively.
At a seeding density of 6000 cells/well and in serum‐containing medium the proliferation in the presence of the growth factors was similar to that in untreated cultures at 24 h (HUVEC, MIEC) and 48 h (HUVEC) and therefore likely due to a normal mitotic increase in cell number probably owing to serum effects. On MIEC the proliferative activity of the cytokines was observed already after 48 h (data not shown), but with maximal effects after 72 h. Untreated cultures exhibited a significant increase in cell number up to 72 h in the presence of serum except for only a small increase in MIEC at low seeding density. In the absence of serum only HUVEC proliferated (Fig. 2). In FCS absence no loss of cellular adherence to the gelatine coated plates was observed.
Figure 2.
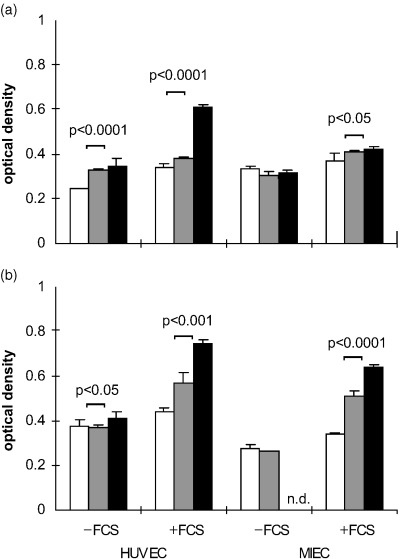
Influence of seeding density and FCS on the growth characteristics of HUVEC and MIEC in the absence of cytokines. (a) 3000 and (b) 6000 cells/well were seeded in the presence or absence of FCS and the optical density of the WST‐1 reaction product was measured after 24 h (□), 48 h (▒) and 72 h (▪). In the presence of serum all control cultures exhibited a significant increase in cell density, whereas in the absence of serum only HUVEC proliferated. n.d. not determined.
After 72 h a significant mitogenic effect of some growth factors was observed in both cell types in the presence of FCS at high seeding densities (Fig. 3). In HUVEC (Fig. 3a), maximum stimulatory effects of VEGF 121, VEGF 165 and FGF‐2 were obtained with 100 ng/ml (VEGF 121 37 ± 3%, FGF‐2 34 ± 3%, P < 0.001, VEGF 165 16 ± 5%, P < 0.05). PlGF‐1 caused no proliferative response and 100 ng/ml PlGF‐2 inhibited cell proliferation (−4 ± 5%, P < 0.01). MIEC (Fig. 3b) were more sensitive to VEGF 121 and VEGF 165, which achieved maximum effects at lower concentrations (10 ng/ml 46 ± 7% and 50 ng/ml 26 ± 3%, respectively, P < 0.001) than in HUVECs. FGF‐2 increased the proliferation in a dose‐dependent manner with maximum effects at 100 ng/ml (49 ± 9%, P < 0.001). PlGF‐1 induced a weak proliferative response at various concentrations (1 ng/ml 13 ± 6%, 100 ng/ml 11 ± 5%, P < 0.01), but PlGF‐2 was only mitogenic at 0.1 ng/ml (10 ± 5%, P < 0.05). The mitogenic potencies of VEGF 121, VEGF 165 and FGF‐2 (100 ng/ml) were similar in both cell types. Among the PlGFs only PlGF‐1 was mitogenic on MIEC whereas in HUVEC PlGF‐1 and ‐2 were not mitogenic (Fig. 3c).
Figure 3.
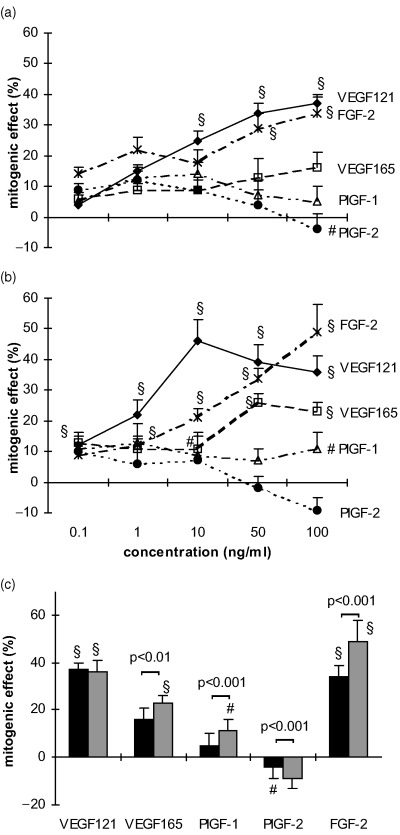
Dose‐dependent effects of cytokines on HUVEC (a) and MIEC (b) expressed as percentage increase over untreated controls. Six‐thousand cells/well were incubated for 72 h in basal medium with 2.5% FCS containing various amounts (0.1–100 ng/ml) of VEGF 121, VEGF 165, PlGF‐1, PlGF‐2 and FGF‐2. (c) Comparison of the mitogenic effects of the cytokines at a concentration of 100 ng/ml between HUVEC (▪) and MIEC (▒). At this concentration VEGF 121, VEGF 165 and FGF‐2 are potent cell mitogens, PlGF‐1 and PlGF‐2 did not produce a significant effect under these conditions, except for a weak proliferative effect of PlGF‐1 on MIEC. *P < 0.05, #P < 0.01, §P < 0.001 vs. untreated control.
The incubation of HUVEC and MIEC (6000 cells/well) in serum‐free basal medium with 100 ng/ml of the respective growth factor for 24 h (Fig. 4a) and 48 h (Fig. 4b) resulted in more pronounced effects than in the presence of serum, and revealed differences in the kinetics of the cytokine effects. 1–50 ng/ml of the cytokines induced a similar mitogenic response on both cell types under these conditions (data not shown). In HUVEC pronounced proliferative responses were found after 24 h culture with VEGF 121 (219 ± 21%, P < 0.001) and VEGF 165 (148 ± 21%, P < 0.01), whereas the FGF‐2 effect was weaker (76 ± 14%, P < 0.01). PlGF‐1 and ‐2 did not stimulate proliferation under this condition. After 48 h only VEGF 121 (176 ± 27%, P < 0.001) and VEGF 165 (123 ± 23%, P < 0.05) stimulated HUVEC. MIEC responded to all tested cytokines after 24 h, except for FGF‐2. VEGF 121 and VEGF 165 induced a proliferation of 105 ± 14% and of 86 ± 8% (P < 0.01), respectively. Under serum‐free conditions PlGF‐2 became mitogenic in MIEC (Fig. 3a,b) in contrast to serum presence (Fig. 3c). In HUVEC the cytokine‐induced effects were greater (P < 0.05) after 24 h than after 48 h, whereas in MIEC the kinetics were reversed. Culture of MIEC for 72 h in serum‐free medium caused a gradually loss of viability.
Figure 4.
Kinetics of the cytokine effects on HUVEC (▪) and MIEC (▒) expressed as percentage increase over untreated controls. Six‐thousand cells/well were incubated for 24 h (a) and 48 h (b) in serum‐free basal medium in the presence or absence (control) of 100 ng/ml of the respective growth factor. In general, the response of MIEC increases, whereas that of HUVEC decreases from 24 to 48 h. *P < 0.05, #P < 0.01, §P < 0.001 vs. untreated control.
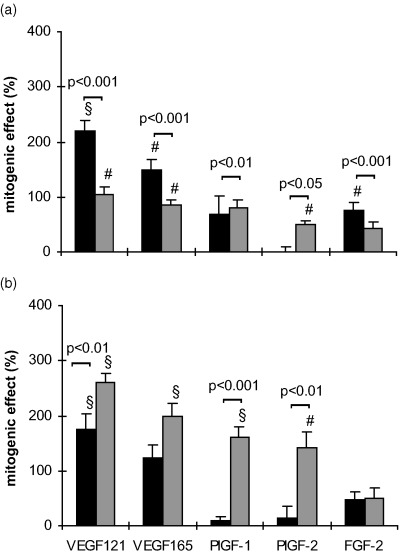
When the seeding density was reduced to 3000 cells/well the mitogenic effects of the cytokines (100 ng/ml) after 24 h without FCS were different from those with 6000 cells/well in both cell types (Fig. 5). At this low seeding density also the PlGFs became mitogenic in HUVECs with effects similar to those of the VEGFs. Except for VEGF 165 the effects on MIEC were in general more pronounced than in HUVEC (Fig. 5). The addition of 2.5% FCS to basal medium containing 100 ng/ml of the respective cytokine for 24 h caused a reduction in the mitogenic effect of PlGFs and FGF‐2 in both cell types at a seeding density of 3000 cells/well (Fig. 5b).
Figure 5.
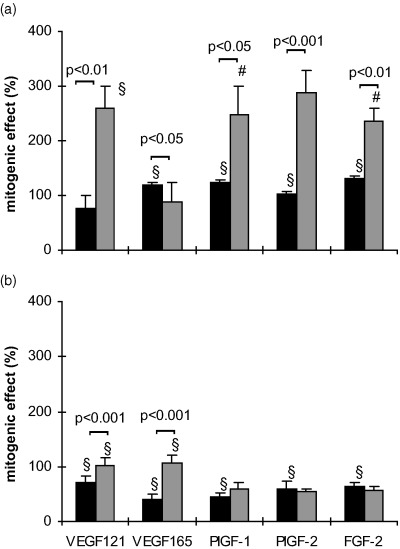
Serum‐dependent effects of the cytokines on HUVEC (▪) and MIEC (▒) expressed as percentage increase over untreated controls. Three thousand cells/well were incubated for 24 h in serum‐free basal medium (a) or in medium with FCS (b), in the presence or absence (control) of 100 ng/ml of the respective growth factor. The proliferative response of MIEC to growth factors is more dependent on serum presence than that of HUVEC. Note that at this seeding density PlGF‐1 and PlGF‐2 elicited a mitogenic effect also in HUVEC *P < 0.05, #P < 0.01, §P < 0.001 vs. untreated control.
DISCUSSION
Cell proliferation was quantified by the tetrazolium salt WST‐1, which is reduced by mitochondrial dehydrogenases to a soluble and intensely coloured formazan. Its concentration, and hence optical density, depends on the number of metabolically active cells. These assays can be carried out in a microculture format and are therefore widely used to determine cellular growth (Marshall, Goodwin & Holt 1995).
The response of HUVEC to growth factors and inhibitors has been more widely characterized than that of any other endothelium (Bicknell 1993), nevertheless available information about the mitogenic effects of VEGFs and PlGFs is contradictory. The only agreement reached so far is the higher mitogenic potency of VEGFs as compared to PlGFs, probably due to the interaction of both growth factors with their type III receptor‐tyrosine kinases.
HUVEC proliferated well in serum‐containing medium regardless of the seeding density even in the absence of the endothelial growth factors and, thus, seem to be less demanding in their selective requirements than MIEC. Even serum‐starvation for 72 h did not lead to a growth arrest. In contrast MIEC are less ‘robust’ and require the presence of some growth factors present either in serum or added to the culture medium. Whether this reflects distinct differences in the intracellular machinery regulating cell cycle progression is unknown but would deserve further investigation.
In the present study high doses of both VEGF 121 and VEGF 165 effectively stimulated proliferation under serum‐free conditions, high cell seeding densities and during an incubation time of 24 h on both cell types. However, the reactivity of the PlGFs clearly differed between both cell lines. The MIEC responded to high doses of PlGF‐1 and ‐2, whereas HUVEC did not respond, although the corresponding flt‐1 receptor was localized on both cell types (Nomura et al. 1995; Detmar et al. 1997). Maximum effects of the growth factors related to the untreated control were achieved on HUVEC after 24 h and on MIEC after 48 h. This could indicate a lower efficiency of the cytokine‐induced stimulatory action on serum‐starved HUVEC compared to MIEC. The presence of serum delayed the maximum growth factor response in HUVEC to 72 h, whereas it did not change the kinetics of response in MIEC. This difference in the kinetics may reflect that MIEC are more specific in their reaction to cytokines (Bicknell 1993).
After 72 h a significant mitogenic effect of some growth factors was observed on both cell types at high seeding densities. Increasing amounts of VEGF 121 and VEGF 165 lead to an increase of the mitogenic effect on HUVEC, whereas PlGF‐1 and ‐2 failed to induce proliferation. On MIEC maximum effects of VEGF 121 were achieved at lower concentrations and PlGF‐1 induced a weak proliferative response. Apart from this both cell types similarly responded to the growth factors under this conditions.
The more uniform effects of PlGFs, if any, as compared to the VEGFs may reflect differential binding to and activation of their receptors. VEGFs bind with higher affinity to flt‐1 than to the KDR receptor, resulting at first in effects not related to proliferation and became only active after binding to the obviously more essential Kdr receptor. Trophoblast growth is limited by VEGF mediated NO release via flt‐1 receptor activation (Ahmed et al. 1997) indicating a growth suppressive function of the VEGF/flt‐1 complex. PlGFs do not bind to KDR receptors, but PlGF/flt‐1 interactions induce a weak proliferative response (Khalig et al. 1996; Sawano et al. 1996). A 10–20 fold molar excess of PlGF seemed to potentiate the mitogenic activity and vascular permeability action of low‐dosed VEGF in vitro and in vivo, probably because of a competitive displacement of VEGF from flt‐1 by PlGF, which may result in an increase in VEGF binding to the more relevant KDR receptor (Park et al. 1994; Sawano et al. 1996).
The most abundant isoforms VEGF 121, VEGF 165, PlGF‐1, PlGF‐2 and their receptors were shown to be colocalized in HUVEC and MIEC (Hauser & Weich 1993; Nomura et al. 1995; Detmar et al. 1997; Yonekura et al. 1999). It can be speculated that PlGFs modulate VEGF action by an autocrine mechanism. Alternatively, the formation of heterodimers between KDR and flt‐1 might confer new properties or ligand specificities upon these receptors (Hauser & Weich 1993).
FGF‐2, expressed widely during embryogenesis and in tissues of the human fetus, is a potent endothelial cell mitogen and has angiogenic activity in vivo. In accordance with our data, FGF‐2 was reported to be more potent in stimulating HUVEC proliferation than VEGF 165 at conditions of high cell numbers, serum concentration (10% FCS) and after 72 h of incubation (Yoshida, Anad‐Apte & Zetter 1996). Under serum‐free conditions FGF‐2 was mitogenic predominantly at low seeding densities. At high seeding densities HUVEC responded to FGF‐2 only after 24 h but MIEC did not respond at all. Interestingly, renal microvascular endothelial cells did not show any proliferative response to FGF‐2 also under similar conditions (Khalig et al. 1999), suggesting that the absence of effect on microvascular endothelial cells is a general phenomenon.
The seeding density of the cells is one of the most important parameter when studying mitogenic effects of any growth factors on any cell type. The present study clearly confirmed this for both HUVEC and MIEC. Therefore, the seeding density is one parameter confounding studies into mitogenic effects of growth factors on endothelial cells, and may account for some of the opposing results published in the literature. Cells at low seeding densities are in the cell cycle and are more sensitive to growth factors, probably as a result of the lower self‐stimulatory interactions between the cells.
After addition of serum, i.e. when the cells are in a proliferating state, the cell‐specific differences of HUVEC and MIEC in their mitogenic response to growth factors are reduced. In general, cells in serum‐free medium are more sensitive to growth factors compared to cells incubated in medium containing fetal calf serum. The smaller differences in cytokine sensitivity between the cell types in the presence of FCS could be the consequence of the reduced overall sensitivity under these conditions. This, in turn, could be explained by other factors, such as the mitogenic effect of serum masking part of the cytokine effects, or the survival promoting effect of the cytokines.
In FCS absence no loss of cell adherence to the surface of the plates was observed. This differs from the apoptosis in HUVEC induced by serum deprivation (Gerber et al. 1998), but is likely the result of different matrices (Fukai et al. 1998) on which the cells had been cultured, i.e. gelatine (this study) and plastic (Gerber et al. 1998). VEGF and FGF‐2 inhibit apoptosis and delay senescence in serum‐free cultured HUVEC, whereas flt‐1‐specific ligands such as PlGF or an flt‐1 selective VEGF mutant did not promote the survival of serum‐starved primary human macrovascular endothelial cells (Gerber et al. 1998). This appears to be a cell‐specific phenomenon, because in MIEC FGF‐2 did not prevent apoptosis in contrast to VEGF (Watanabe & Dvorak 1997). In our study the mitogenic effects induced by the VEGFs were less serum‐dependent compared with the PlGFs, which are effective mainly under serum‐free conditions, i.e. when the cells are in G0. Therefore, it can be speculated that PlGFs act as competence factors stimulating the G0/G1 transition, whereas the VEGFs with their cytokine sensitivity mainly in the presence of FCS may predominantly act as progression factors thereby stimulating the G1/S transition. The latter notion is in line with recently published data demonstrating a VEGF‐induced entry of endothelial cells in the S‐phase of their cell cycle, an effect which was mediated by PI3‐kinase (Thakker et al. 1999). The fact that in FCS absence HUVEC responded to the PlGFs only at low cell density, i.e. when the cells were in the cell cycle, further corroborates above hypothesis.
Throughout our study VEGF 121 was more potent than VEGF 165. A similar result was found on MIEC using low serum conditions (2.5% FCS) (Birkenhäger et al. 1996). In two other studies, however, a higher activity of VEGF 165 compared to VEGF 121 was found, but under higher serum conditions than here (20% and 5% FCS) (Soker et al. 1997) or on different types of endothelial cells (Keyt et al. 1996). Therefore, the discrepancies in the literature about the relative potencies of VEGF 121 vs. VEGF 165 may be the result of different serum concentrations or cellular models used.
Collectively, all cytokines tested can act as endothelial cell mitogens under certain conditions, which vary among the cytokines and between the type of endothelial cell, i.e. whether they have been derived from the macro‐ or microvasculature. This differential reactivity of macro‐ and microvascular endothelial cells to VEGFs and PlGFs was noted particularly in the absence of serum. Addition of fetal calf serum to the culture medium reduces the cell‐specific differences of HUVEC and MIEC in their proliferative response to growth factors. MIEC are more sensitive to changing cell culture conditions than HUVEC and generally stronger respond to the cytokines. This lower flexibility of the MIEC to changing ambient conditions may be a further indication of their highly specialized function. However, one has to keep in mind that some of the effects found here were very small though statistically significant. It remains to be demonstrated in further experiments with different endpoints whether these in vitro effects reflect in vivo effects of biological significance.
Acknowledgements
It is a honour to dedicate this work to Prof. W. Burkl on the occasion of his 80th birthday.
The authors are grateful to Christian Caluba for excellent technical assistance.
This work was supported by grants P10900, P13321 (to Gernot Desoye) and by grant P13721 (to Tom Hahn) of the Austrian Science Foundation, Vienna, and by grant 6230 (to Gernot Desoye) of the Jubilee Fund, Austrian National Bank, Vienna.
References
- Ahmed A, Dunk C, Kniss D, Wilkes M (1997) Role of VEGF receptor‐1 (flt‐1) in mediating calcium‐dependent nitric oxide release and limiting DNA synthesis in human trophoblast cells. Lab. Invest 76, 779. [PubMed] [Google Scholar]
- Bicknell R (1993) Physiological Aspects. Heterogeneity of the endothelial cell. Behring Inst. Mitt 92, 1. [PubMed] [Google Scholar]
- Birkenhäger R, Schneppe B, Röckl W, Wilting J, Weich HA, McCarthy JEG (1996) Synthesis and physiological activity of heterodimers comprising different splice forms of vascular endothelial growth factor and placenta growth factor. Biochem. J. 316, 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YH, Ji WDR, Qi P, Rosin A, Cao YM (1997) Placenta growth factor: Identification and characterization of a novel isoform generated by RNA alternative splicing. Biochem. Biophys. Res. Commun 235, 493DOI: 10.1006/bbrc.1997.6813 [DOI] [PubMed] [Google Scholar]
- Cunningham SA, Tran TM, Arrate MP, Bjercke R, Brock TA (1999b) KDR activation is crucial for VEGF165‐mediated Ca2+ mobilization in human umbilical vein endothelial cells. Am. J. Physiol. 276, C176. [DOI] [PubMed] [Google Scholar]
- Cunningham SA, Tran TM, Arrate MP, Brock TA (1999a) Characterization of vascular endothelial cell growth factor interactions with the kinase insert domain‐containing receptor tyrosine kinase. A real time kinetic study. J. Biol. Chem 274, 18421. [DOI] [PubMed] [Google Scholar]
- Detmar M, Brown LF, Berse B et al. (1997) Hypoxia regulates the expression of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptors in human skin. J. Invest. Dermatol. 108, 263. [DOI] [PubMed] [Google Scholar]
- DiSalvo J, Bayne ML, Conn G et al. (1995) Purification and characterization of a naturally occurring vascular endothelial growth factor. placenta growth factor heterodimer. J. Biol. Chem 270, 7717. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Houk K, Jakeman L, Leung DW (1992) Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocrine Rev. 13, 18. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Houk K, Jakeman L, Winer J, Leung DW (1991) The vascular endothelial growth factor family of polypeptides. J. Cell. Biochem. 47, 211. [DOI] [PubMed] [Google Scholar]
- Fukai F, Mashimo A, Akiyama K, Goto T, Tanuma S, Katayama T (1998) Modulation of apoptotic cell death by extracellular matrix proteins and a fibronectin‐derived antiadhesive peptide. Exp Cell Res. 242, 92DOI: 10.1006/excr.1998.4076 [DOI] [PubMed] [Google Scholar]
- Garlanda C & Dejana E (1997) Heterogeneity of endothelial cells. Specific markers. Arterioscler. Thromb. Vasc. Biol. 17, 1193. [DOI] [PubMed] [Google Scholar]
- Gerber HP, McMurtrey A, Kowalski J et al. (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′‐kinase Akt signal transduction pathway – Requirement for Flk‐1/KDR activation. J. Biol. Chem 273, 30336. [DOI] [PubMed] [Google Scholar]
- Gupta K, Ramakrishnan S, Browne PV, Solovey A, Hebbel RP (1997) A novel technique for culture of human dermal microvascular endothelial cells under either serum‐free or serum‐supplemented conditions: Isolation by panning and stimulation with vascular endothelial growth factor. Exp. Cell Res. 230, 244DOI: 10.1006/excr.1996.3421 [DOI] [PubMed] [Google Scholar]
- Hauser S & Weich HA (1993) A heparin‐binding form of placenta growth factor (PlGF‐2) is expressed in human umbilical vein endothelial cells and in placenta. Growth Factors 9, 259. [DOI] [PubMed] [Google Scholar]
- Keyt BA, Berleau LT, Nguyen HV et al. (1996) The carboxyl‐terminal domain (111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J. Biol. Chem 271, 7788–7795. [DOI] [PubMed] [Google Scholar]
- Khalig A, Dunk C, Jiang J et al. (1999) Hypoxia down‐regulates placenta growth factor expression: molecular evidence for ‘placental hyperoxia’ in intrauterine growth restriction. Lab. Invest 79, 151. [PubMed] [Google Scholar]
- Khalig A, Li XF, Shams M et al. (1996) Localization of placenta growth factor (PLGF) in human term placenta. Growth Factors 13, 243. [DOI] [PubMed] [Google Scholar]
- Kumar R, Yoneda J, Bucana CD, Fidler IJ (1998) Regulation of distinct steps of angiogenesis by different angiogenic molecules. Intern. J. Oncol. 12, 749. [DOI] [PubMed] [Google Scholar]
- Lang I, Hahn T, Dohr G, Skofitsch G, Desoye G (1994) Heterogeneous histochemical reaction pattern of the lectin Bandeiraea (Griffonia) simplicifolia with blood vessels of human full term placenta. Cell Tissue Res. 278, 433. [DOI] [PubMed] [Google Scholar]
- Lang I, Hartmann M, Blaschitz A, Dohr G, Skofitsch G, Desoye G (1993) Immunohistochemical evidence for the heterogeneity of maternal and fetal vascular endothelial cells in human full‐term placenta. Cell Tissue Res. 274, 211. [DOI] [PubMed] [Google Scholar]
- Maglione D, Guerriero V, Viglietto G et al. (1993) Two alternative mRNAs coding for the angiogenic factor, placental growth factor (PLGF) are transcribed from a single gene of chromosome 14. Oncogene 8, 925. [PubMed] [Google Scholar]
- Marshall NJ, Goodwin CJ, Holt SJ (1995) A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul 5, 69. [PubMed] [Google Scholar]
- Migdal M, Huppertz B, Tessler S et al. (1998) Neuropilin‐1 is a placenta growth factor‐2 receptor. J. Biol. Chem 273, 22272. [DOI] [PubMed] [Google Scholar]
- Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A (1994) Glioblastoma growth inhibited in vivo by a dominant‐negative flk‐1 mutant. Nature 367, 576. [DOI] [PubMed] [Google Scholar]
- Millauer B, Wizimann‐Voos S, Schnurch H et al. (1993) High affinity VEGF binding and developmental expression suggests flk‐1 as a major regulator of vasculogenesis and angiogenesis. Cell 72, 835. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 13, 9. [PubMed] [Google Scholar]
- Nomura M, Yamagishi S, Harada S et al. (1995) Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia‐induced proliferation of endothelial cells and pericytes. J. Biol. Chem 270, 28316. [DOI] [PubMed] [Google Scholar]
- Park JE, Chen HH, Winer J, Houck KA, Ferrara N (1994) Placenta growth factor – Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt‐1 but not to Flk‐1/KDR. J. Biol. Chem 269, 25646. [PubMed] [Google Scholar]
- Sawano A, Takahashi T, Yamaguchi S, Aonuma M, Shibuya M (1996) Flt‐1 but not KDR/flk‐1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differentiation 7, 213. [PubMed] [Google Scholar]
- Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M (1995) A unique signal transduction from flt tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene 10, 135. [PubMed] [Google Scholar]
- Soker S, Gollamudi‐Payne S, Fidder H, Charmahelli H, Klagsbrun M (1997) Inhibition of vascular endothelial growth factor (VEGF)‐induced endothelial cell proliferation by a peptide corresponding to the exon 7‐encoded domain of VEGF 165 . J. Cell. Biol. 272, 31582. [DOI] [PubMed] [Google Scholar]
- Szebenyi G & Fallon JF (1999) Fibroblast growth factors as multifunctional signaling factors. Int. Rev. Cytol. 185, 45. [DOI] [PubMed] [Google Scholar]
- Terman BI, Dougher‐Vermazen M, Carrion ME et al. (1992) Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun 187, 1579. [DOI] [PubMed] [Google Scholar]
- Thakker GD, Hajjar DP, Muller WA, Rosengart TK (1999) The role of phosphatidylinositol 3‐kinase in vascular endothelial growth factor signaling. J. Biol. Chem 274, 10002. [DOI] [PubMed] [Google Scholar]
- Torry DS, Ahn H, Barnes EL, Torry RJ (1999) Placenta growth factor: potential role in pregnancy. Am. J. Reprod. Immunol. 41, 79. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Claesson‐Welsh L, Sieghalin A, Shibuya M, Heldin CH (1994) Different signal transduction properties of kdr and flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem 269, 26988. [PubMed] [Google Scholar]
- Watanabe Y & Dvorak HF (1997) Vascular permeability factor vascular endothelial growth factor inhibits anchorage– disruption‐induced apoptosis in microvessel endothelial cells by inducing scaffold formation. Exp. Cell Res. 223, 340DOI: 10.1006/excr.1996.0089 [DOI] [PubMed] [Google Scholar]
- Yonekura H, Sakurai S, Liu X et al. (1999) Placenta growth factor and vascular endothelial growth factor B and C expression in microvascular endothelial cells and pericytes. J.Biol. Chem 274, 35172. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Anad‐Apte B, Zetter BR (1996) Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth Factors 13, 57. [DOI] [PubMed] [Google Scholar]
- Ziche M, Maglione D, Ribatti D et al. (1997) Placenta growth factor‐1 is chemotactic, mitogenic, and angiogenic. Lab Invest 76, 517. [PubMed] [Google Scholar]


