Abstract
Objective
Roles of bone morphogenetic proteins (BMPs) on osteogenesis of human adipose‐derived stem cells (hASCs) remain ambiguous. In this study, we evaluated in vitro and in vivo functional characteristics of BMPs of different dimerization types, with the aim of determining osteoinductive efficiency of heterodimeric BMP‐2/7 on osteogenesis of hASCs.
Materials and methods
We explored osteoinductive effects of three different BMPs by using cell DNA assay, alkaline phosphatase (ALP) activity assay, alizarin red staining and mineralization assay, and real‐time PCR, in vitro. Also, we examined ectopic bone formation in nude mice by using soft X‐ray, histomorphometric and immunohistochemical analyses in vivo.
Results
In our dose–response study, we showed that BMPs with both dimerization types did not induce in vitro osteogenesis of hASCs without osteogenic medium (OM). In the presence of OM, BMPs significantly enhanced hASC osteogenesis in a dose‐dependent manner. In in vivo experiments, our analyses indicated that BMPs promoted osteogenesis of hASCs without in vitro osteogenic induction. However, both in vitro and in vivo, there were no significant differences in hASC osteogenic induction between heterodimeric and homodimeric BMPs.
Conclusions
Heterodimeric BMP‐2/7 significantly promoted osteogenesis of hASCs in vitro and in vivo. However, BMP‐2/7 was not found to be a stronger inducer of osteogenesis compared to homodimeric either BMP‐2 or BMP‐7.
Introduction
Compared to bone marrow‐derived stem cells (BMSCs), human adipose‐derived stem cells (hASCs) show promising potential as a cell source for bone tissue engineering, due to their easy accessibility, high yield efficiency and low donor‐site morbidity 1, 2.
Bone morphogenetic proteins (BMPs) have long been introduced into the field of bone tissue engineering. In particular, BMP‐2 and BMP‐7 have been approved for clinical use in the United States, Europe and Australia 3. It is, therefore, conceivable to be able to apply exogenous BMPs to promote osteogenesis of hASCs. However, direct effects of exogenous BMPs on hASC osteogenic differentiation are still controversial. Several isotypes of BMP have been demonstrated to play paramount roles on hASC osteogenesis 4, 5, 6, 7, 8, whereas other studies have shown that exogenous BMP‐2 was unable to influence osteogenic fate of hASCs in vitro 9 or in vivo 10. It seems as if ability of exogenous BMPs to promote osteogenic differentiation of hASCs is highly dependent on numerous factors, such as BMP type, concentration, differentiation medium and administration time point 2. Consequently, tremendous amounts of investigation is still needed to modify application pattern of BMPs to facilitate their optimal promoting effects on hASC osteogenesis.
One such effort is to adopt different BMPs. Generally, most mature BMP molecules are composed of two monomers linked by a disulphide bridge. When they are derived from the same BMP member, the BMP molecule is termed homodimeric BMP 11. Hitherto, almost all our knowledge in this aspect has mainly been based on homodimeric BMPs. In contrast, heterodimeric BMPs consist of two monomers that are derived from different BMP members 12. Application potential of heterodimeric BMPs is promising, as they exhibit higher efficiency at inducing osteogenesis of murine cells 13, 14, 15, 16, 17, 18, 19, 20, 21. Yet, up to now there have been no reports related to any biofunction of BMP‐2/7 on human mesenchymal stem cells.
In the present study, we have aimed to unveil functions of heterodimeric BMP‐2/7 on hASC osteogenic differentiation, by comparing inducing efficiency of heterodimeric BMP‐2/7 with homodimeric BMP‐2 and BMP‐7 in vitro and in vivo.
Materials and methods
All materials were purchased from Sigma‐Aldrich (St. Louis, MO, USA) unless otherwise stated. Dulbecco's modified Eagle's medium (DMEM), foetal bovine serum (FBS) and antibiotics were purchased from Gibco (Grand Island, NY, USA).
Ethics statement
hASCs from three different healthy human donors were purchased from ScienCell (7510; ScienCell, San Diego, CA, USA). The protocol used was approved by the Ethics Committee of the Peking University Health Science Center, Beijing, China (Permit Number: LA2014233). All surgery was performed under sodium pentobarbital anaesthesia, and all efforts were made to minimize animal suffering.
Cell culture
hASCs were cultured in proliferation medium (PM) containing fresh DMEM + 10% (v/v) FBS + antibiotics (100 U/ml penicillin G and 100 μg/ml streptomycin) at 37 °C, in an incubator, atmosphere of 95% air, 5% CO2 and 100% relative humidity.
BMP concentration selection assay
To prepare stock BMP solutions, three different lyophilized BMPs were reconstituted at 10 μg/ml in sterile 4 mm hydrochloric acid (HCL) containing 0.1% bovine serum albumin (BSA) according to the manufacturer's instructions (R&D Systems, Inc., Minneapolis, MN, USA). To assess hASC osteogenic differentiation in response to the variety of concentrations of different exogenous BMPs (5, 50, 100 and 200 ng/ml) and to identify optimal BMP concentration, dose–response assays of alkaline phosphatase (ALP) activities were performed as described in “ALP activity assay” and “Real‐time quantitative PCR analysis” sections below. Administration of exogenous BMPs was in the presence of PM or osteogenic medium (OM) containing PM + 100 nm dexamethasone (DEX) + 0.2 mm ascorbic acid + 10 mm β‐glycerophosphate (β‐GP).
Proliferation and osteogenic differentiation of hASCs stimulated by BMPs in vitro
Experimental design
Fourth passage cells were used for the following experiments, and all experiments were repeated three times using hASCs from the three donors. hASCs were seeded into 12‐well plates at a density of 5 × 104, and were seeded into six‐well plates at a density of 105 for the studies below. Each group was performed in triplicate. Based on results of “BMP concentration selection assay”, OM + 200 ng/ml BMPs were selected as experimental groups for in vitro experiments. hASCs were exposed to the following treatments: (i) negative control medium (PM); (ii) positive control medium (OM); (iii) OM with 200 ng/ml BMP‐2; (iv) OM with 200 ng/ml BMP‐7; (v) OM with 200 ng/ml BMP‐2/7.
Cell proliferation assay
The hASCs were seeded in 12‐well plates and divided into five groups as above. To investigate their proliferation in response to different BMPs, numbers of cells were calculated by content of cell DNA after stimulation for 1, 4 and 7 days according to the manufacturer's protocol (CyQUANT® Cell Proliferation Assay Kit, Life Technologies, Carlsbad, CA, USA).
Cell differentiation assay
The hASCs were seeded in 12‐well plates and divided into five groups as above. ALP staining was performed on 4th and 7th days of osteoinduction as described in detail previously 22. After 2, 4, 7 and 14 days induction, ALP activity was determined using an ALP kit according to the manufacturer's protocol, and normalized to total protein content, as described previously 22. To assess mineralization, alizarin red staining was performed on days 21 and 28 after stimulation. Cells were washed three times in phosphate‐buffered saline (PBS), fixed in ethanol for 30 min, and then stained with alizarin red at room temperature. To quantitatively determine matrix calcification, alizarin red was destained using 10% cetylpyridinium chloride in 10 mm sodium phosphate for 30 min. Absorbance of released alizarin red was measured at 562 nm. Final mineralization levels in each group were normalized to total protein concentrations obtained from duplicate plates 22.
Real‐time quantitative PCR analysis
hASCs were seeded in six‐well plates and divided into five groups as above. After 2, 4, 7 and 14 days osteoinduction, total RNA was isolated according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA) and 2 μg RNA aliquots were reverse‐transcribed according to the manufacturer's instructions (Roche, Basel, Switzerland). Real‐time quantitative PCR assays were performed using Power SYBR Green PCR Master Mix and an ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, CA, USA). Primers for Runt‐related transcription factor 2 (RUNX2), ALP, collagen 1A1 (COL‐1A1), osteopontin (OPN) and osteocalcin (OC) were synthesized by Invitrogen and are listed in Table 1. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as internal standard 23.
Table 1.
Sequences of primers used for real‐time PCR
| Genes | Forward primer | Reverse primer |
|---|---|---|
| RUNX2 | ACTACCAGCCACCGAGACCA | ACTGCTTGCAGCCTTAAATGACTCT |
| ALP | ATGGGATGGGTGTCTCCACA | CCACGAAGGGGAACTTGTC |
| COL‐1A1 | GTGCCAAGGGTCTGACTGGAA | ATCACACCAGCCTGACCACG |
| OPN | ACCCTGACCCATCTCAGAAGCA | CTTGGAAGGGTCTGTGGGGCTA |
| OC | AGCCACCGAGACACCATGAGA | GGCTGCACCTTTGCTGGACT |
| GAPDH | AAGGTCGGAGTCAACGGATTTG | TCCTGGAAGATGGTGATGGGAT |
Subcutaneous transplantation in nude mice
Preparation of β‐tricalcium phosphate (β‐TCP) scaffolds containing BMPs
β‐TCP (Bicon, Boston, MA, USA) was used for the scaffold. Based on the in vitro results, we demonstrated that BMP‐2/7 exhibited osteoinductive effects similar to BMP‐2, whereas inducing efficiency of BMP‐7 was slightly weaker. Thus, the β‐TCP + BMP‐2 + hASCs complex was used as positive control group for in vivo experiments. Four groups were used to conduct the in vivo study: (I) β‐TCP only (blank control); (II) β‐TCP + hASCs; (III) β‐TCP + BMP‐2 + hASCs; (IV) β‐TCP + BMP‐2/7 + hASCs. For groups III and IV, 500 μl BMP‐2‐containing or BMP‐2/7‐containing suspension was absorbed on to β‐TCP (40 mg) respectively. According to our previous experiments 24, final loading of BMPs was approximately 400 ng per β‐TCP scaffold. The hASCs were cultured in PM for 1 week before the in vivo study, when they (4 × 105 cells) were resuspended directly into PM and then seeded on the β‐TCP scaffold.
Animal experiments
6‐week‐old male BALB/c homozygous nude (nu/nu) mice were purchased from Peking University Experimental Animal Center, and given 1 week to acclimatise to the housing facility. During housing, animals were monitored twice daily for health status during which no adverse events were observed. At the beginning of the in vivo experiment, the nude mice were anaesthetized with sodium pentobarbital. To avoid contamination from one group to another, four respective enclosed transplantation sites were prepared by means of haemostatic forceps in dorsal subcutaneous spaces. Subsequently, the four groups of β‐TCP complexes were implanted aseptically into the four different sites (n = 6 per group).
Analyses of bone formation in vivo
As described in our previous study 25, specimens of each group were harvested 4 weeks after in vivo implantation, and animals in each group were sacrificed by CO2 asphyxiation. Soft X‐ray examinations were used to evaluate mineral density and relative grey scale was determined by Image J software (National Institutes of Health, Bethesda, MD,USA). Bone constructs were fixed in 4% paraformaldehyde and then decalcified for 10 days in 10% EDTA (pH 7.4). Following decalcification, specimens were dehydrated and subsequently embedded in paraffin wax. 5 μm tissue sections were stained with haematoxylin and eosin (HE) and Masson's trichrome. Osteogenesis was evaluated by immunohistochemical analysis for OC (primary antibody purchased from Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Statistical analysis
Data were analysed using SPSS software (Chicago, IL, USA) and statistical analysis of was performed by one‐way analysis of variance (ANOVA). Post hoc testing for multiple comparisons was carried out using the Fisher LSD test. When variance was not homogeneous, Kruskal–Wallis testing was used, followed by the Nemenyi test for multiple comparisons. For all tests, statistical significances were accepted for P‐values lower than 0.05.
Results
BMP concentration selection assay
Our results showed that BMPs were unable to exert their osteoinductive effects in the presence of PM (Figs. 1, 2, 3), whereas they could induce osteogenic differentiation of hASCs in the presence of OM (Figs. 1, 2, 3). Moreover, osteoinductive effect of BMPs was positively correlated to concentrations. However, inducing effects of 50 ng/ml and 100 ng/ml BMPs were slightly higher than OM, whereas 200 ng/ml seemed to be an optimal concentration. Thus, we selected 200 ng/ml to perform in vitro assays. However, there were no significant differences in osteoinductive effects on hASCs between the three BMPs (Figs. 1, 2, 3), which needed to be verified further.
Figure 1.
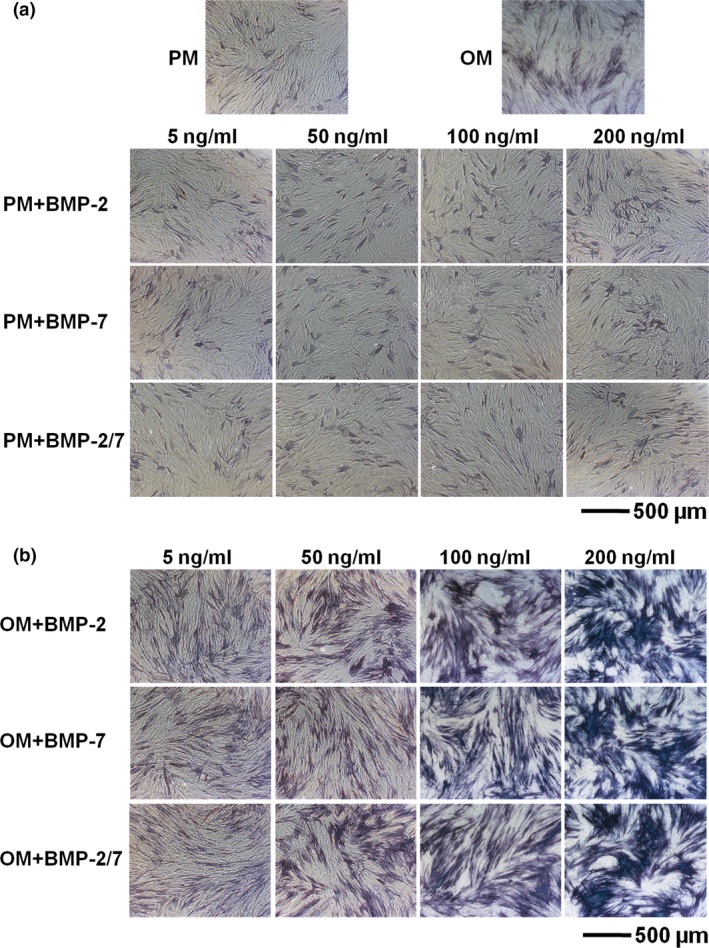
ALP staining of hASC s induced by BMP s in presence of either PM or OM . (a) hASCs induced by BMPs in presence of PM did not express ALP. (b) hASCs induced by BMPs in the presence of OM stained ALP positively by 7 days of osteoinduction. However, at the same concentration level, there were no obvious differences between the three BMPs. hASCs, human adipose‐derived stem cells; ALP, alkaline phosphatase; PM, proliferation medium; OM, osteogenic medium; BMP, bone morphogenetic protein.
Figure 2.
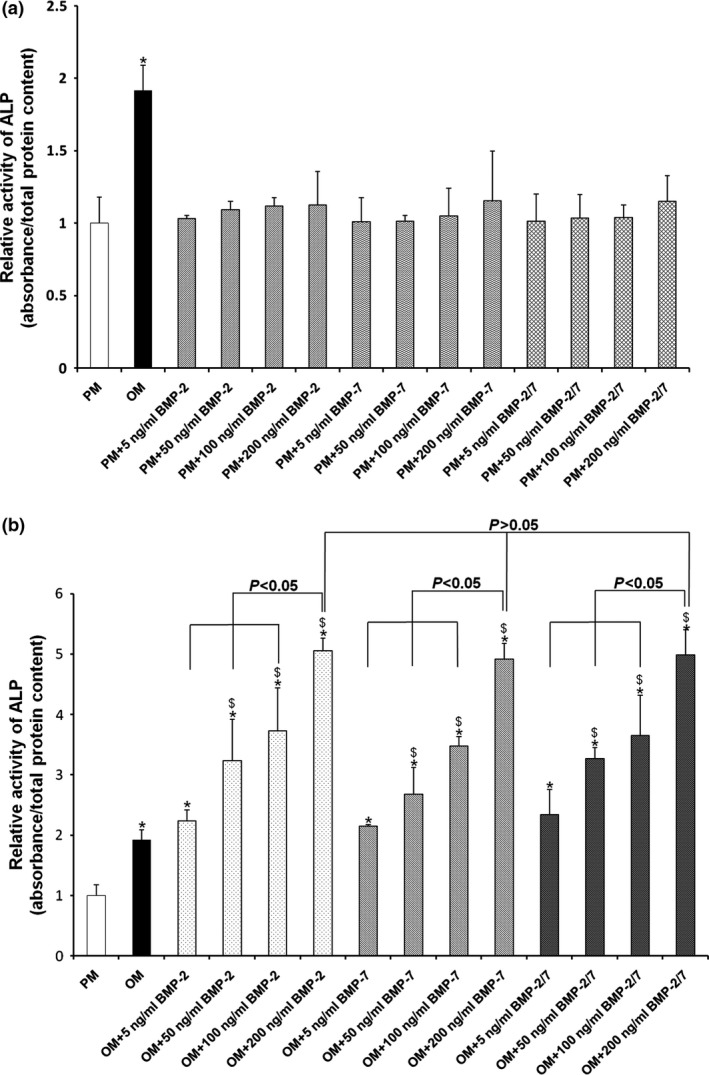
ALP activities in hASC s induced by BMP s in presence of either PM or OM . (a) BMPs were unable to promote ALP activity in hASCs in the presence of PM. (b) BMPs significantly promoted hASC ALP activity in the presence of OM after 7 days osteoinduction. Moreover, the osteoinductive effect positively correlated to concentrations of BMPs. However, at the same concentration level, there were no significant differences between the three BMPs. *P < 0.05 compared to PM, $ P < 0.05 compared to OM. hASCs, human adipose‐derived stem cells; ALP, alkaline phosphatase; PM, proliferation medium; OM, osteogenic medium; BMP, bone morphogenetic protein.
Figure 3.
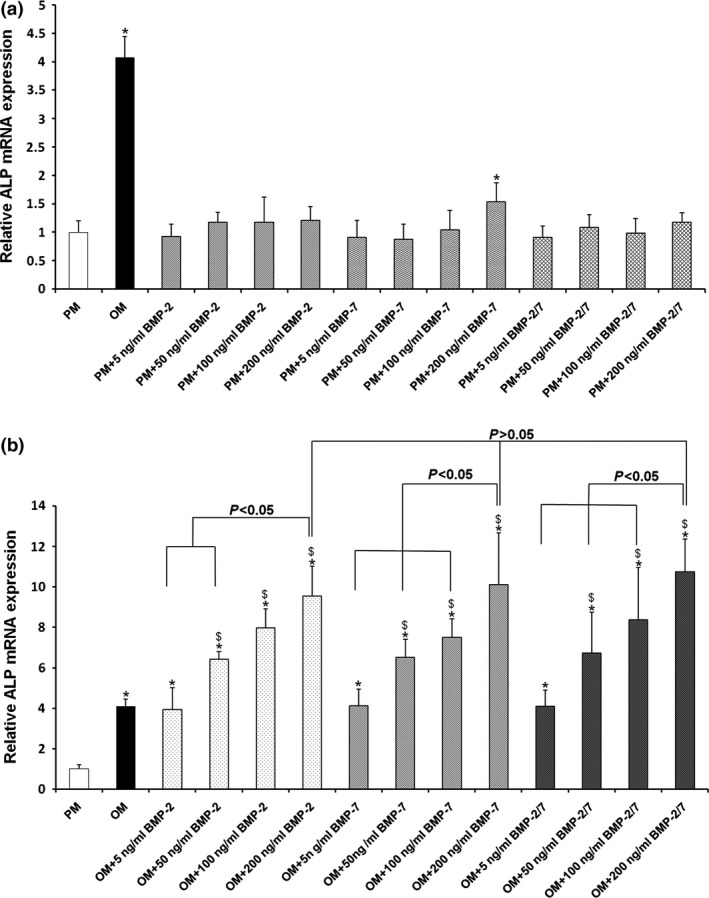
ALP gene expression in hASC s induced by BMP s in presence of either PM or OM . (a) BMPs were unable to elevate ALP gene expression in hASCs in the presence of PM. (b) BMPs significantly elevated ALP gene expression in hASCs in the presence of OM after 7 days osteoinduction. Furthermore, inducing efficiency positively correlated with concentrations of BMPs. However, at the same concentration level, there were no significant differences between the three BMPs. *P < 0.05 compared to PM, $ P < 0.05 compared to OM. hASCs, human adipose‐derived stem cells; ALP, alkaline phosphatase; PM, proliferation medium; OM, osteogenic medium; BMP, bone morphogenetic protein.
Comparison of proliferative capacity of hASCs stimulated by BMPs
BMP‐2/7 and BMP‐2 promoted cell proliferation in the initial stages, and there were no differences compared to PM on day 7 (P > 0.05); whereas, BMP‐7 continuously promoted cell proliferation (P < 0.05) (Fig. 4).
Figure 4.
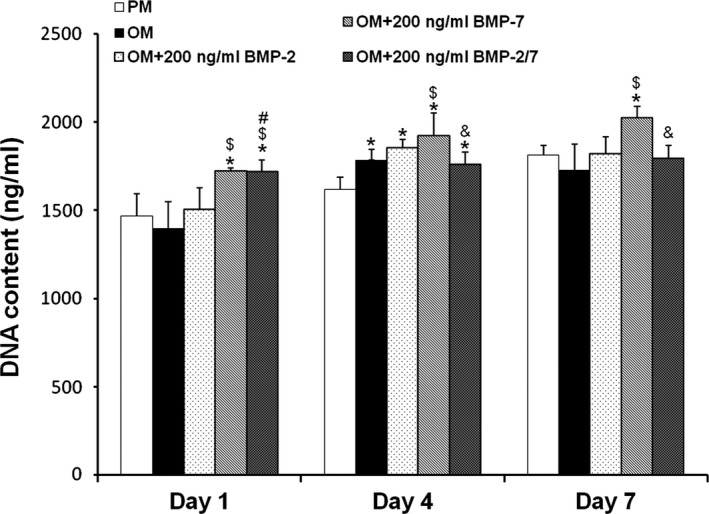
DNA contents of hASC s induced by different BMP s at days 1, 4 and 7. BMP‐2/7 and BMP‐2 promoted cell proliferation at initial stages, and there were no significant differences compared to PM on day 7 (P > 0.05); whereas, BMP‐7 promoted cell proliferation continuously (P < 0.05). *P < 0.05 compared to PM, $ P < 0.05 compared to OM, # P < 0.05 compared to OM + 200 ng/ml BMP‐2, & P < 0.05 compared to OM + 200 ng/ml BMP‐7. hASCs, human adipose‐derived stem cells; PM, proliferation medium; OM, osteogenic medium; BMP, bone morphogenetic protein.
Comparison of osteogenic potential of hASCs stimulated by BMPs in vitro
Positive results for ALP staining confirmed osteoinductive effects of the BMPs (Fig. 5a). However, there were no notable differences between the three BMPs by either days 4 or 7. ALP activity assay revealed that the BMPs significantly enhanced ALP activity compared to PM and OM (P < 0.05) (Fig. 5b). BMP‐2/7 and BMP‐2 seemed to exert a similar effect (P > 0.05), whereas inducing effect of BMP‐7 was slightly weaker; however, the differences were not significant on days 4 and 14 (P > 0.05).
Figure 5.
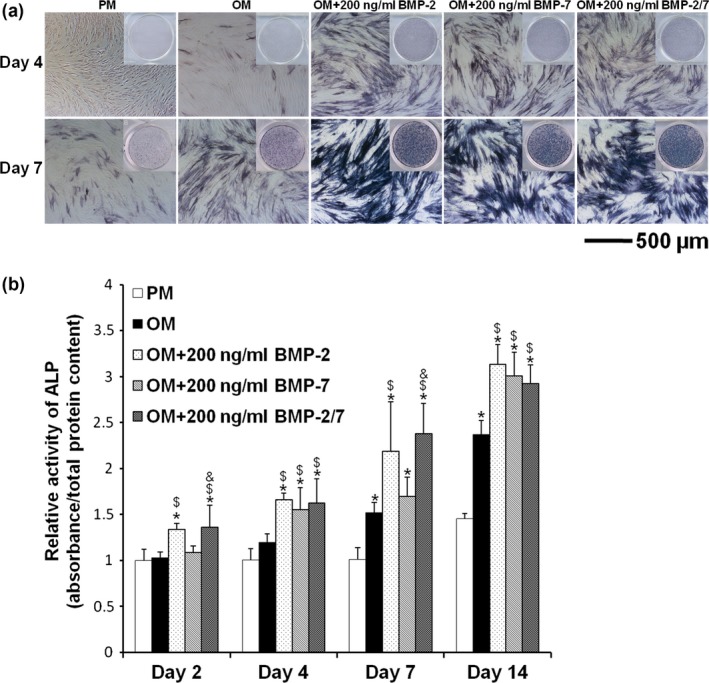
ALP staining and activity of hASC s induced by different BMP s in vitro . (a) After 4 and 7 days induction, positive results for ALP staining confirmed the osteoinductive effect of BMPs. However, there were no notable differences between the three BMPs. (b) After 2, 4, 7 and 14 days induction, ALP activities showed that BMPs significantly enhanced ALP activities compared to PM and OM (P < 0.05). However, there were no significant differences between the three BMPs (P > 0.05). *P < 0.05 compared to PM, $ P < 0.05 compared to OM, & P < 0.05 compared to OM + 200 ng/ml BMP‐7. hASCs, human adipose‐derived stem cells; ALP, alkaline phosphatase; PM, proliferation medium; OM, osteogenic medium; BMP, bone morphogenetic protein.
Alizarin red staining and mineralization assays demonstrated that hASC cell matrix calcifications were significantly elevated after 21 and 28 days induction by BMPs, compared to PM and OM (P < 0.05). However, there were no significant differences between the three BMPs (P > 0.05) (Fig. 6a,b).
Figure 6.
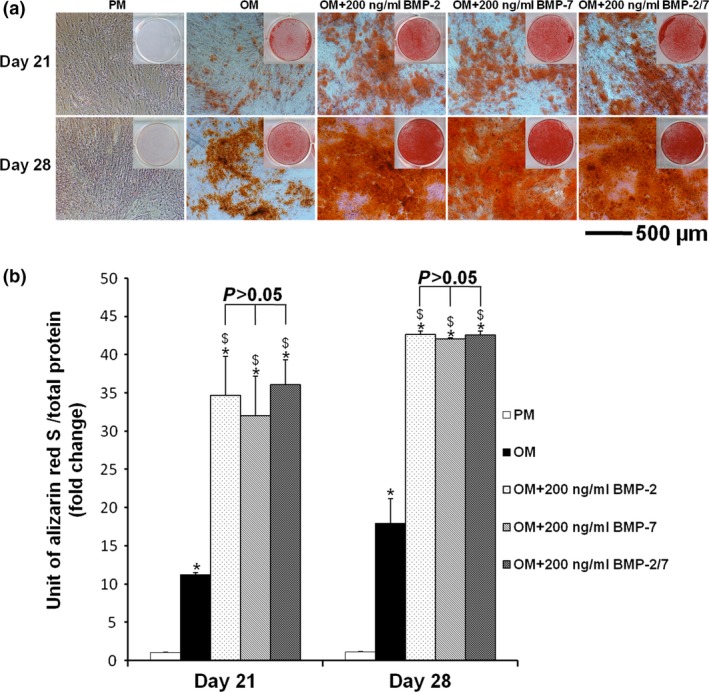
Alizarin red staining and mineralization assay of hASC s induced by different BMP s in vitro . (a) After 21 and 28 days induction, positive results for alizarin red staining confirmed the osteoinductive effect of BMPs. However, there were no notable differences between the three BMPs. (b) After 21 and 28 days induction, mineralization assays demonstrated that hASC matrix calcifications were significantly enhanced by BMPs compared to PM and OM (P < 0.05). However, there were no significant differences between the three BMPs (P > 0.05). *P < 0.05 compared to PM, $ P < 0.05 compared to OM, & P < 0.05 compared to OM + 200 ng/ml BMP‐7. hASCs, human adipose‐derived stem cells; PM, proliferation medium; OM, osteogenic medium; BMP, bone morphogenetic protein.
Comparison of osteogenesis‐associated gene expression in hASCs stimulated by BMPs
After 2 days induction with BMPs, expression of osteogenic genes (RUNX2, ALP, COL‐1A1) was significantly up‐regulated compared to PM and OM (P < 0.05). Moreover, the expression levels were continually elevated from day 2 to day 7 (Fig. 7). After 7 and 14 days induction, expression of OPN and OC genes was significantly up‐regulated in BMP groups compared to PM and OM groups (P < 0.05) (Fig. 7). However, there were no significant differences (P > 0.05) in mRNA levels of these genes in hASCs induced by the three types of BMPs (Fig. 7).
Figure 7.
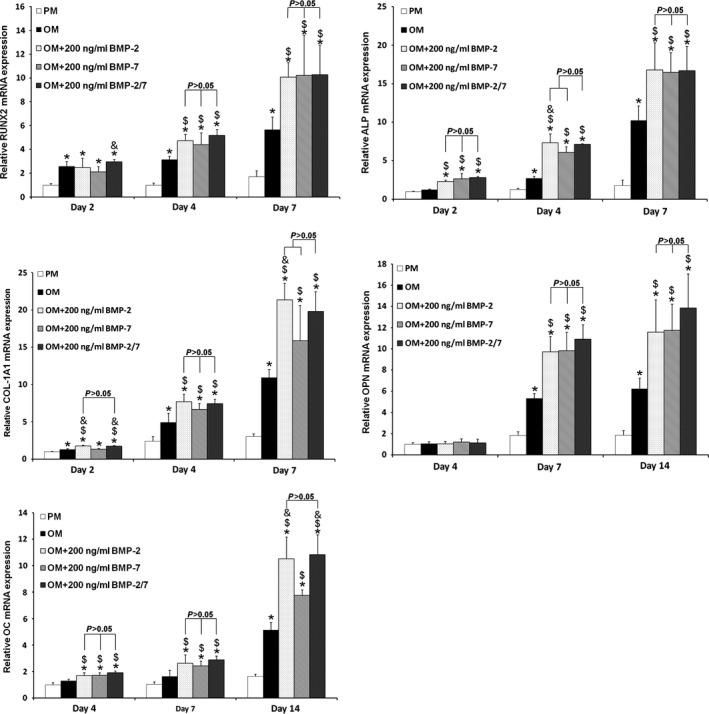
Osteogenesis‐associated gene expressions of hASC s induced by different BMP s. There were no significant differences in mRNA levels of RUNX2,ALP,COL‐1A1,OPN or OC after osteoinduction by the three BMPs at 2, 4, 7 or 14 days (P > 0.05). *P < 0.05 compared to PM, $ P < 0.05 compared to OM, & P < 0.05 compared to OM + 200 ng/ml BMP‐7. hASCs, human adipose‐derived stem cells; PM, proliferation medium; OM, osteogenic medium; BMP, bone morphogenetic protein.
Comparison of in vivo bone formation capability of hASCs induced by BMPs
Four weeks after in vivo transplantation, soft X‐ray examination revealed that group III (β‐TCP + BMP‐2 + hASCs) and group IV (β‐TCP + BMP‐2/7 + hASCs) complexes had formed bone‐like tissues with relatively higher density than control groups (Fig. 8a). However, there was no significant difference on the grey scale between these two groups (P > 0.05) (Fig. 8b).
Figure 8.
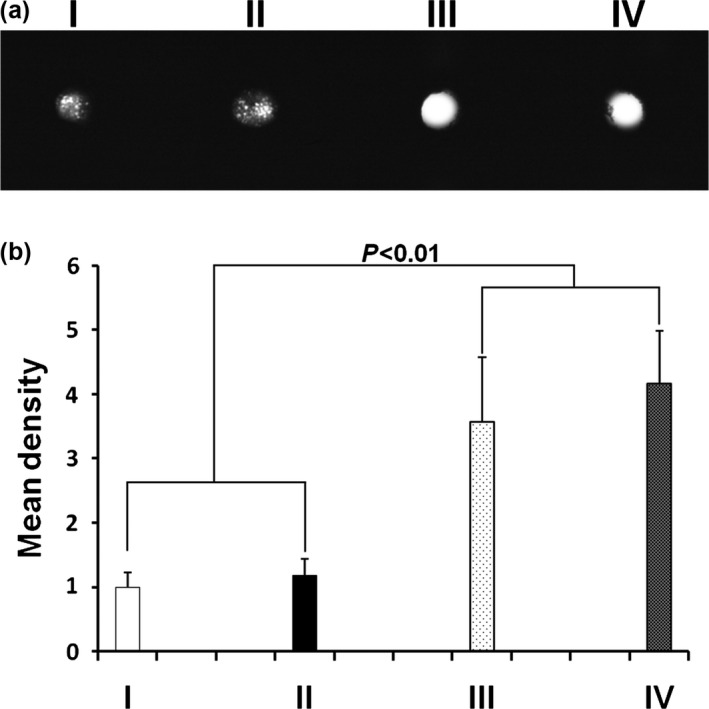
Soft X‐ray examination of β‐ TCP + BMP s + hASC complex implanted into nude mice for 4 weeks. (a) After 4‐week in vivo transplantation, soft X‐ray radiography showed that β‐TCP + BMP‐2 + hASC and β‐TCP + BMP‐2/7 + hASC complexes formed bone‐like tissues with relatively higher density than control groups. (b) Relative grey scales were determined by Image J software. Mean density of β‐TCP + BMPs + hASC complexes was significantly higher than control groups (P < 0.01). However, there was no significant difference between these two groups (P > 0.05). (I) β‐TCP only (blank control); (II) β‐TCP + hASCs; (III) β‐TCP + BMP‐2 + hASCs; (IV) β‐TCP + BMP‐2/7 + hASCs. hASCs, human adipose‐derived stem cells; β‐TCP, β‐tricalcium phosphate; BMP, bone morphogenetic protein.
HE staining showed that in groups III and IV, numbers of cells were significantly lower and eosinophilic bone‐like tissues were found in the extracellular matrix (ECM) around the scaffold materials (Fig. 9a). However, there was no notable difference between the two groups. In control groups, cells were numerous and there were no typical bone‐like structures in the ECM (Fig. 9a).
Figure 9.
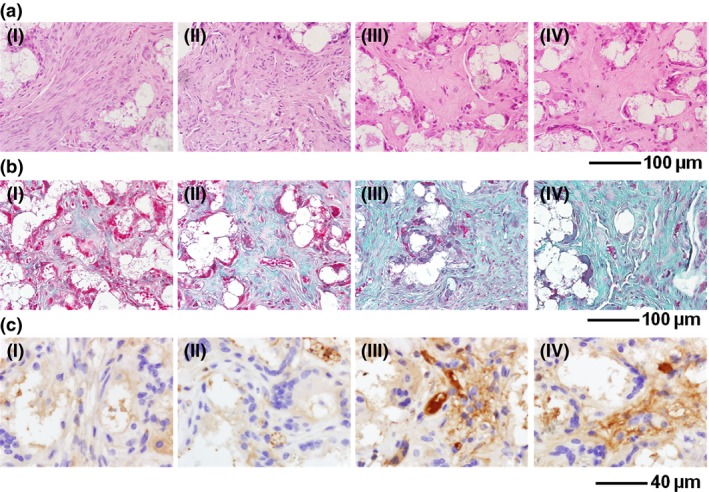
Histological and immunohistochemical analyses of β‐ TCP + BMP s + hASC complexes implanted into nude mice for 4 weeks. (a) HE staining revealed eosinophilic bone‐like tissues in group III and group IV animals after 4‐weeks implantation, whereas there were no typical bone‐like structures in the control groups. (b) Masson's trichrome staining showed strong positive results in groups III and IV. Higher expression of collagen could be observed compared to the control groups. (c) Immunohistochemical staining showed higher expression of OC in groups III and IV compared to control groups. (I) β‐TCP only (blank control); (II) β‐TCP + hASCs; (III) β‐TCP + BMP‐2 + hASCs; (IV) β‐TCP + BMP‐2/7 + hASCs. hASCs, human adipose‐derived stem cells; β‐TCP, β‐tricalcium phosphate; BMP, bone morphogenetic protein.
Masson's trichrome staining illustrated strong positive results in groups III and IV. More expression of collagen was observed in ECM compared to control groups (Fig. 9b). However, similar to HE staining, no notable difference were observed between the two groups. There was only a small amount of collagen expression in group II (β‐TCP + hASCs) complex, and no obviously positive results were found in the blank control group (Fig. 9b).
Immunohistochemical staining showed that osteogenic marker OC was highly expressed in groups III and IV. Moreover, the two groups had much higher expression of OC than the control group. However, there was no significant difference in expression level between groups III and IV (Fig. 9c).
Discussion
Gradually, BMPs have been found to play more pleiotropic roles in promoting differentiation of pluripotent stem cells to different lineages, such as osteogenesis and adipogenesis 5, 26, 27. This phenomenon raises the question of whether BMPs can cause any certain differentiation direction in hASCs or not. In the absence of OM, irrespective of concentrations or dimerization type, BMPs did not significantly promote ALP activity in vitro (Figs. 1, 2, 3). In contrast, in the presence of OM, BMPs significantly enhanced hASC osteogenesis (Figs. 1, 2, 3). This result strongly suggests that BMPs did not commit hASCs to the osteogenic lineage, but enhanced osteogenic differentiation after committment. Even in the presence of OM, the effect of BMPs was controversial. Some previous studies have shown positive effects 5, 6, 7, 8, whereas others were completely negative 9. One recent study indicated that even under the same culture conditions, exogenous BMP‐2 promoted hASC osteogenesis from two donors, but inhibited it in hASCs from a further six donors, revealing donor variation 28. These results indicate that osteogenic efficacy of hASCs under certain osteogenic induction conditions varies largely between different donors.
In our concentration selection test, the promoting effect of BMPs on hASC osteogenic differentiation had increasing dose‐dependent pattern. At 200 ng/ml, all three selected BMPs exhibited the highest effects for promoting ALP activity. On the basis of these results, we determined to use 200 ng/ml in the remaining tests. At the same concentration level, there were no significant differences in ALP activities after stimulation by BMPs. Consistently, the following ALP staining and quantitative assay, alizarin red staining and mineralization assay, and osteogenic gene expressions also showed no significant differences between these three BMPs. These results were not consistent with previous findings. Heterodimeric BMP has been frequently shown to be more potent than homodimeric BMPs in inducing osteogenesis of murine cells 13, 14, 15, 16, 17, 18, 21. In previous studies, BMP‐2/7 showed a different dose‐dependent pattern from BMP‐2 and BMP‐7; BMP‐2/7 started to take effect at 5 ng/ml and reached a plateau at 50 ng/ml; whereas, BMP‐2 or BMP‐7 started to take effect at 50 ng/ml and continued to increase osteogenic differentiation as the dose increased 19. However, in our study, induction of osteogenesis by BMP‐2/7 at these low effective concentrations (5–50 ng/ml) disappeared. Thus, the mechanism for this phenomenon needs to be further investigated.
In the subsequent in vivo experiments, β‐TCP +BMPs + hASCs complexes caused formation of ectopic bone structures, which suggests that in vitro pre‐osteoinduction was not a requirement for in vivo bone formation. In contrast to their in vitro performance, BMPs induced hASC osteogenesis in vivo without OM; however, the mechanisms that account for their different performances remain to be unveiled.
Consistent with the in vitro findings, BMP‐2/7 did not result in superior osteogenesis of hASCs compared to BMP‐2 in the in vivo model. Our previous data have already corroborated that BMP‐2/7 combined with pure scaffolds significantly accelerates and enhances regeneration of new bone compared to BMP‐2 and BMP‐7, in the mini pig 24. This finding suggests that BMP‐2/7 was superior in inducing in vivo osteogenesis. However, caution needs to be taken when extrapolating the data to human cells. In the previous studies, BMP‐2 was less efficient in inducing osteogenesis of human BMSCs compared to BMSCs from rats and mice 29. In addition, Carpenter et al. have indicated that combined AdBMP‐2 and ‐7 gene transfer did not have any greater effect than using single AdBMP on osteogenic differentiation of human BMSCs 30. Species‐specific differences may account for different sensitivities to BMP‐2/7 between murine cells and hASCs. Furthermore, distinct BMP signalling patterns may be in underlying molecular mechanisms. Further investigation is needed in the future to provide a definitive answer to this question.
Selection of BMP concentration range in this study was mainly based on results of previous studies 19. According to earlier reports, osteoinductive efficiency of exogenous BMP‐2/7 reaches its maximum at 200 ng/ml and significantly decreases by 250 ng/ml. Besides this, inducing effects of exogenous BMP‐2 and BMP‐7 also reached plateaux stages at 200 ng/ml. Although these results came from different cell lines, it has still provided a reference point for us. Furthermore, BMPs of higher concentration may not seem cost‐effective in future clinical usage, which is very important for clinical translation. More importantly, we mainly wished to determine whether BMP‐2/7 could exhibit superior osteoinductive effects compared to BMP‐2 and BMP‐7 over a relatively low concentration range. Consequently, we used maximum BMP concentration of 200 ng/ml. In addition, β‐TCP was used as scaffold for in vivo transplantation due to its appropriate mechanical properties and good biological compatibility 22. Furthermore, to evaluate in vivo osteoinductive effects of BMPs more directly, numbers of hASCs loaded on to scaffolds was much lower than in our previous studies 22.
Limitations of this study are that a broader concentration range could be considered to completely exhibit characteristics of heterodimeric BMP‐2/7 in vitro, and evaluation of bone formation in vivo could be performed at additional time points.
Conflicts of interest
None.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (2011/81170937 to Prof. Dr. Y. Zhou), the Program for New Century Excellent Talents in University from Ministry of Education (NCET‐11‐0026), and the Construction Program for National Key Clinical Specialty from National Health and Family Planning Commission of China (2011).
References
- 1. Levi B, Longaker MT (2011) Concise review: adipose‐derived stromal cells for skeletal regenerative medicine. Stem Cells 29, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang X, Guo J, Zhou Y, Wu G (2014) The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose‐derived stem cells. Tissue Eng. Part B Rev. 20, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reddi AH (2005) BMPs: from bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 16, 249–250. [DOI] [PubMed] [Google Scholar]
- 4. Jeon O, Rhie JW, Kwon IK, Kim JH, Kim BS, Lee SH (2008) In vivo bone formation following transplantation of human adipose‐derived stromal cells that are not differentiated osteogenically. Tissue Eng. Part A 14, 1285–1294. [DOI] [PubMed] [Google Scholar]
- 5. Levi B, Hyun JS, Nelson ER, Li S, Montoro DT, Wan DC et al (2011) Nonintegrating knockdown and customized scaffold design enhances human adipose‐derived stem cells in skeletal repair. Stem Cells 29, 2018–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mizrahi O, Sheyn D, Tawackoli W, Kallai I, Oh A, Su S et al (2013) BMP‐6 is more efficient in bone formation than BMP‐2 when overexpressed in mesenchymal stem cells. Gene Ther. 20, 370–377. [DOI] [PubMed] [Google Scholar]
- 7. Zeng Q, Li X, Beck G, Balian G, Shen FH (2007) Growth and differentiation factor‐5 (GDF‐5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat‐derived stromal cells in vitro. Bone 40, 374–381. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Salleeh F, Beatty MW, Reinhardt RA, Petro TM, Crouch L (2008) Human osteogenic protein‐1 induces osteogenic differentiation of adipose‐derived stem cells harvested from mice. Arch. Oral Biol. 53, 928–936. [DOI] [PubMed] [Google Scholar]
- 9. Zuk P, Chou YF, Mussano F, Benhaim P, Wu BM (2011) Adipose‐derived stem cells and BMP2: part 2. BMP2 may not influence the osteogenic fate of human adipose‐derived stem cells. Connect. Tissue Res. 52, 119–132. [DOI] [PubMed] [Google Scholar]
- 10. Chou YF, Zuk PA, Chang TL, Benhaim P, Wu BM (2011) Adipose‐derived stem cells and BMP2: part 1. BMP2‐treated adipose‐derived stem cells do not improve repair of segmental femoral defects. Connect. Tissue Res. 52, 109–118. [DOI] [PubMed] [Google Scholar]
- 11. Bessa PC, Casal M, Reis RL (2008) Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts). J. Tissue Eng. Regen. Med. 2, 1–13. [DOI] [PubMed] [Google Scholar]
- 12. Guo J, Wu G (2012) The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 23, 61–67. [DOI] [PubMed] [Google Scholar]
- 13. Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA et al (1996) Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors 13, 291–300. [DOI] [PubMed] [Google Scholar]
- 14. Wang EA, Rosen V, Cordes P, Hewick RM, Kriz MJ, Luxenberg DP et al (1988) Purification and characterization of other distinct bone‐inducing factors. Proc. Natl Acad. Sci. USA 85, 9484–9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sampath TK, Coughlin JE, Whetstone RM, Banach D, Corbett C, Ridge RJ et al (1990) Bovine osteogenic protein is composed of dimers of OP‐1 and BMP‐2A, two members of the transforming growth factor‐beta superfamily. J. Biol. Chem. 265, 13198–13205. [PubMed] [Google Scholar]
- 16. Zhu W, Rawlins BA, Boachie‐Adjei O, Myers ER, Arimizu J, Choi E et al (2004) Combined bone morphogenetic protein‐2 and ‐7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. J. Bone Miner. Res. 19, 2021–2032. [DOI] [PubMed] [Google Scholar]
- 17. Zhao M, Zhao Z, Koh JT, Jin T, Franceschi RT (2005) Combinatorial gene therapy for bone regeneration: cooperative interactions between adenovirus vectors expressing bone morphogenetic proteins 2, 4, and 7. J. Cell. Biochem. 95, 1–16. [DOI] [PubMed] [Google Scholar]
- 18. Koh JT, Zhao Z, Wang Z, Lewis IS, Krebsbach PH, Franceschi RT (2008) Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration. J. Dent. Res. 87, 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng Y, Wu G, Zhao J, Wang L, Sun P, Gu Z (2010) rhBMP2/7 heterodimer: an osteoblastogenesis inducer of not higher potency but lower effective concentration compared with rhBMP2 and rhBMP7 homodimers. Tissue Eng. Part A 16, 879–887. [DOI] [PubMed] [Google Scholar]
- 20. Bi W, Gu Z, Zheng Y, Wang L, Guo J, Wu G (2013) Antagonistic and synergistic effects of bone morphogenetic protein 2/7 and all‐trans retinoic acid on the osteogenic differentiation of rat bone marrow stromal cells. Dev. Growth Differ. 55, 744–754. [DOI] [PubMed] [Google Scholar]
- 21. Myllyla RM, Haapasaari KM, Lehenkari P, Tuukkanen J (2014) Bone morphogenetic proteins 4 and 2/7 induce osteogenic differentiation of mouse skin derived fibroblast and dermal papilla cells. Cell Tissue Res. 355, 463–470. [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Zhao Y, Zhang X, Chen T, Zhao X, Ma GE et al (2013) Flow cytometric cell sorting and in vitro pre‐osteoinduction are not requirements for in vivo bone formation by human adipose‐derived stromal cells. PLoS ONE 8, e56002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Zhang X, Jin X, Fan C, Ye H, Ou M et al (2014) Bi‐functionalization of a calcium phosphate‐coated titanium surface with slow‐release simvastatin and metronidazole to provide antibacterial activities and pro‐osteodifferentiation capabilities. PLoS ONE 9, e97741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Zheng Y, Zhao J, Liu T, Gao L, Gu Z et al (2012) Low‐dose rhBMP2/7 heterodimer to reconstruct peri‐implant bone defects: a micro‐CT evaluation. J. Clin. Periodontol. 39, 98–105. [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Zhou Y, Feng H, Ma GE, Ni Y (2008) Injectable tissue‐engineered bone composed of human adipose‐derived stromal cells and platelet‐rich plasma. Biomaterials 29, 3338–3345. [DOI] [PubMed] [Google Scholar]
- 26. Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM et al (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim HJ, Im GI (2009) Combination of transforming growth factor‐beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue‐derived mesenchymal stem cells. Tissue Eng. Part A 15, 1543–1551. [DOI] [PubMed] [Google Scholar]
- 28. Lee SY, Lee JH, Kim JY, Bae YC, Suh KT, Jung JS (2014) BMP2 increases adipogenic differentiation in the presence of dexamethasone, which is inhibited by the treatment of TNF‐alpha in human adipose tissue‐derived stromal cells. Cell. Physiol. Biochem. 34, 1339–1350. [DOI] [PubMed] [Google Scholar]
- 29. Osyczka AM, Diefenderfer DL, Bhargave G, Leboy PS (2004) Different effects of BMP‐2 on marrow stromal cells from human and rat bone. Cells Tissues Organs. 176, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carpenter RS, Goodrich LR, Frisbie DD, Kisiday JD, Carbone B, McIlwraith CW et al (2010) Osteoblastic differentiation of human and equine adult bone marrow‐derived mesenchymal stem cells when BMP‐2 or BMP‐7 homodimer genetic modification is compared to BMP‐2/7 heterodimer genetic modification in the presence and absence of dexamethasone. J. Orthop. Res. 28, 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]


