Abstract
Abstract. Objectives: Experiments were conducted to evaluate whether or not bovine supramammary lymph node extract (LNE) could support cell proliferation when it was substituted for bovine growth serum (BGS) in cell culture media. Materials and Methods: Two different preparations of LNE were tested. The first yielded protein concentration of 3 mg/mL and the second contained 27 mg/mL protein. Three cell lines (MDA‐MB‐435, MAC‐T and 1C6) were used in serum starvation assays to evaluate LNE. Cell proliferation assays were used to determine growth stimulation in the presence of LNE, and short‐term or rapid adaptation cultures were evaluated for LNE effects on cell survival. Results: Heat‐inactivated preparation 1 supported cell proliferation as well as or better (12–39%) than BGS following 2 days of serum starvation in culture. The second lymph node preparation provided a stimulatory effect (263–702% greater than BGS across all cell lines) following serum starvation at 2.7 and 5.4 mg/mL protein supplementation. A gradual adaptation process with lymph node supplementation into media maintained cell population growth on a short‐term basis. However, once cells were trypsinized or scraped and re‐seeded into 2.7 mg/mL LNE protein containing media, cells were unable to re‐adhere, leaving them detached, and eventually appearing to be dead. Conclusions: Substitution of BGS with LNE protein dramatically stimulated cells to proliferate, but did not allow for rapid cell population growth adaptation in vitro.
INTRODUCTION
The bovine supramammary lymph node is located on the dorso‐caudal surface of the udder (Bradley et al. 2001), and is a potential source of bioactive proteins for cell culture. Lacasse et al. (1996) reported mitogenic activity of bovine mammary gland lymph on bovine mammary epithelial cells (MAC‐T) and mammary fibroblasts. In another study, MAC‐T cells were targets for mitogenic effects of mammary tissue extracts from prepubertal heifers raised to high‐ or low rate of gain (Berry et al. 2003), and the mammary tissue extracts were observed to have mitogenic activity on these cells. Weber et al. (1999) found 5% culture supplementation with mammary extract stimulated more [3H]‐thymidine incorporation into DNA of cells, than 10% foetal bovine serum or 100 ng/mL insulin‐like growth factor‐I (IGF‐I). The IGF‐I effect observed with mammary extracts is a combination of blood borne (from the liver) and mammary tissue‐produced IGF‐I; however, locally produced IGF‐I in the mammary gland can be sufficient enough to support growth and development of mammary tissue (Akers 2006). Weber et al. (2000) also tested mammary tissue extracts on bovine primary mammary epithelial cells and investigated whether or not exogenous growth hormone or feeding level influenced mitogenic activity. When growth hormone was administered, high‐fed compared to low‐fed heifers had an increased in vitro [3H]‐thymidine incorporation into cultured mammary epithelial cells in response to mammary tissue extract supplementation, indicating a stimulus for growth factor production in mammary tissue.
Rather than using mammary tissue or lymph, as in previous studies, here, we chose to use the supramammary LNE; as an immunological organ, this lymph node is a rich source of a variety of cytokines, chemokines and growth factors (Gorewit et al. 1993; Waller et al. 2003). The supramammary lymph nodes are easy to recover, allowing one to obtain large quantities of tissue. This study was an initial attempt to examine the effects of bovine supramammary LNE as a potential cell culture supplement to induce cell proliferation.
MATERIALS AND METHODS
Cell culture
Human breast cancer MDA‐MB‐435 cells (a gift from Georgetown University, Lombardi Cancer Center, Washington, DC, USA) were thawed and initially cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% BGS (HyClone, Logan, UT, USA), 1% penicillin/streptomycin, 1% amino acids, 1.1 mg/mL sodium pyruvate, and 4.0 mm l‐glutamine. MAC‐T cells (a bovine mammary epithelial cell line; Huynh et al. 1991) were cultured in DMEM containing 10% BGS, 1% penicillin/streptomycin, and 4.0 mm l‐glutamine. 1C6 hybridoma B‐cells (our laboratory) were cultured with DMEM, containing 10% bovine calf serum, 1% penicillin/streptomycin, 1.1 mg/mL sodium pyruvate, 23.8 mg/mL HEPES, 100 µL 2‐mercaptoethanol and 4.0 mm l‐glutamine. All cell lines were maintained in 75 cm2 flasks and incubated at 37 °C in a humidified, water‐jacketed CO2 (5%) incubator.
Lymph node preparations
Supramammary lymph nodes of both beef and dairy cows were obtained from Brown Packing (Gaffney, SC, USA). The isolated lymph nodes were prepared according to one of two methods.
Lymph node preparation 1
Initially lymph nodes were trimmed of fat with scissors and were homogenized in a food‐grade blender with 10 mL of phosphate‐buffered saline (PBS) for every five lymph nodes; the extract was then centrifuged for 30 min at 26 500 g. Supernatant was recovered and centrifuged again for 30 min at 32 500 g. Twenty millilitres of PBS were added to every 10 mL of extract and filter‐sterilized with serum Acrodisc (0.2 µm) syringe filters. The extract was then heat‐inactivated at 60 °C for 60 min. This lymph node preparation yielded a protein concentration of 3 mg/mL by the Warburg and Christian (1942) method.
Lymph node preparation 2
Lymph nodes were trimmed of fat with scissors and were processed through a Hobart meat grinder until moderately homogenous. The homogenate was placed in freezer bags at –80 °C for 2 days. Frozen lymph node homogenate was then crushed into small pieces using pestle and mortar, and was lyophilized for approximately seven days in a VirTis freeze dryer (SP Industries Inc., Warminster, PA, USA); it was then ground into a fine powder using a small food processor. Five grams of the powder was weighed and mixed with PBS in a tared centrifuge tube, to reach a total weight of 50 g. The solution was incubated at room temperature for 20 min and was then centrifuged for 15 min at 739 g. Supernatant was removed and heat‐inactivated at 60 °C for 60 min. The solution was then centrifuged for 30 min at 7000 g and filtre sterilized (0.2 µm) with a Nalgene bottle‐top filter into a sterile container. This preparation yielded a protein concentration of 27 mg/mL by the Warburg and Christian (1942) method.
Cell proliferation assay
The CyQuant assay (Jones et al. 2001) was used to determine DNA synthesis and cell proliferation through direct DNA staining with the CyQuant GR fluorescent dye. CyQuant GR dye and 20× cell lysis buffer were purchased from Invitrogen (Carlsbad, CA, USA). Cells were trypsinized with HyQ® Trypsin (0.25%) and centrifuged for 5 min at 400 g. The supernatant was removed and cells were re‐suspended in approximately 2 mL of medium. Cells were counted and added to a 96‐well plate with 1 × 104 cells/well and held overnight in a CO2 incubator, to adhere. The next day all media were removed and wells were rinsed twice with 100 µL sterile PBS. Appropriate media were then added to the wells without serum and were incubated for 2 days for the starvation phase of the assay. Lymph node preparations 1 and 2 were used in the CyQuant assay. MDA‐MB‐435 cells were treated with both preparations, and MAC‐T and 1C6 cells were treated with only lymph node preparation 2. After 3 days with the different treatments, plates were inverted and blotted. The CyQuant GR dye/cell lysis buffer contained 1.2 mL of 20× lysis buffer, 22.8 mL nuclease‐free distilled water, and 60 µL of CyQuant GR dye. Two hundred microlitres of dye/lysis buffer were added to all wells. Plates were mixed gently and were incubated for 2–5 min, with covers, to protect them from light. Plates were read on a BioTek Synergy HT plate reader at excitation of 480 and emission of 520 nm. Fluorescence intensity of each well was recorded.
Sodium dodecyl sulphate‐polyacrylamide gel electrophoresis
Bovine growth serums (BGS), as well as lymph node preparation 2, were run on an SDS‐PAGE (sodium dodecyl sulphate‐polyacrylamide gel electrophoresis) 12% Tris‐HCl gel. Samples were denatured with SDS‐PAGE sample buffer (100 mm Tris, 2% SDS, 5%β‐mercaptoethanol and 15% glycerol) and boiled for 5 min. Five microlitres of each sample were loaded on a 30‐µg/mL basis with sample buffer. The gel was run at 120 V for 1 h.
Rapid adaptation growth assay
Cells (MDA‐MB‐435, MAC‐T and 1C6) were cultured to nearly 100% confluence (day 1) in standard medium containing non‐diluted 10% serum (6.6 mg/mL BGS or BCS protein) in 6‐well plates. Once confluent, all media were removed and 2.5% (0.675 mg/mL protein) lymph node supplementation was added along with 7.5% serum (4.95 mg/mL BGS or BCS protein). Once medium was removed from the wells of the 6‐well plate, Azure II dye was added and photomicrographs were taken using an inverted microscope fitted with a digital camera. Day 2 represents 2.5% lymph node supplementation, day 3 is 5.0% (1.35 mg/mL protein) lymph node media, day 4 is 7.5% (2.025 mg/mL protein) lymph node media, and day 5 is 10.0% (2.7 mg/mL protein) lymph node media. Detachment of cells resulting in floating cells, cell debris, and standard attachment and spreading, were characteristics of cells observed in the cultures.
Statistical analysis
CyQuant assay data were analysed using a SAS program (Statistical Analysis System, Cary, NC, USA). The CyQuant experiments were completely random designs with factorial arrangements of treatments. Variability between treatments was determined with an anova procedure using PROC GLM with α = 0.05. Differences between treatment means were further separated by post‐analysis t‐tests and were adjusted with the Dunn–Sidak method to control for erroneous means’ separations.
RESULTS
Serum starvation assays
Serum starvation assays were run with both 3 and 27 mg/mL LNE. Results are expressed as proliferation indices (PI = fluorescence intensity of supplemented media/fluorescence intensity of 0% supplemented media). A proliferation index above 1 is indicative of cell proliferation greater than the control group (0 mg/mL serum or LNE protein).
Figure 1a represents proliferation of MDA‐MB‐435 cells with lymph node preparation 1 (3 mg/mL). This figure indicates that heat‐inactivated extract supported cell proliferation better than both non‐heat‐inactivated LNE and BGS, at all concentrations of supplementation. BGS best supported cell proliferation at 0.075 mg/mL protein, followed by a decline in proliferation at 0.15–0.6 mg/mL protein supplementation. Non‐heat‐inactivated LNE did not support cell proliferation at any percentage, as evidenced by the apparent inhibitory level of cell proliferation.
Figure 1.
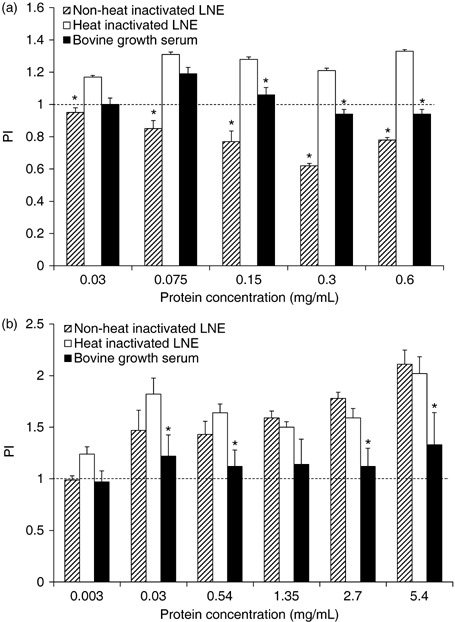
Proliferation indices (PI) of MDA‐MB‐435 human breast cancer cells in a serum starvation assay with lymph node extract (LNE) of preparation 1 (a) and preparation 2 (b). Following 2‐day starvation, cells were cultured in media containing non‐heat‐inactivated LNE (striped bars), heat‐inactivated LNE (white bars), and bovine growth serum (black bars) at different protein concentrations of supplementation. Data are expressed as means ± SEM. Means are significantly different (P = 0.05) from respective heat‐inactivated means within protein concentrations. Dashed line is the PI (1.0) of cells cultured with 0 mg/mL bovine growth serum or LNE protein.
Lymph node preparation 2 (27 mg/mL) was used in a similar fashion as described above (Fig. 1b). Heat‐inactivated and non‐heated LNE from this preparation did not differ in ability to support population growth of MDA‐MB‐435 cells, following serum starvation. However, these two LNE preparations proved to be better at stimulating cell population growth when compared to BGS supplementation. As concentration of both LNE sources increased to 2.7 and 5.4 mg/mL protein, positive effects of these LNE preparations were much better than BGS.
Proliferation assays with lymph node preparation 2, comparing the response of three different cell lines, did not include non‐heat‐inactivated LNE. Little difference in overall influence of either LNE on population growth of MDA‐MB‐435 cells was observed, but visual clarity of heat‐inactivated LNE was better than for non‐heated LNE. Heat‐inactivated LNE appeared more like blood serum, although its protein composition varied from that of BGS (Fig. 2). The BGS sample primarily had albumin and γ‐globulin present, while heat‐inactivated LNE had multiple types of protein, apparent from the sample's appearance on SDS‐PAGE.
Figure 2.
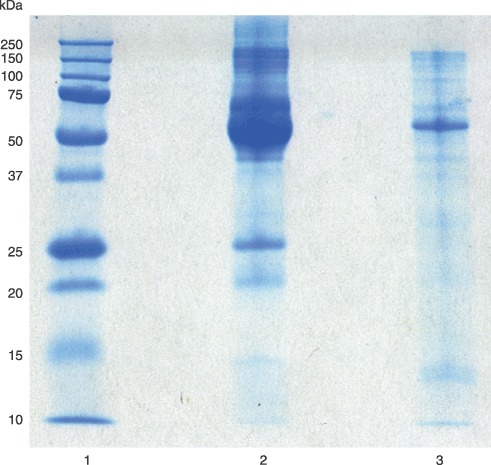
Sodium dodecyl sulphate‐polyacrylamide gel electrophoresis of bovine growth serum (BGS) and lymph node extract (LNE) from lymph node preparation 2. Lane 1, protein standards; lane 2, BGS; and lane 3, LNE.
MDA‐MB‐435, MAC‐T and 1C6 cells were cultured with lymph node preparation 2 (Fig. 3). BGS maintained MDA‐MB‐435 cell proliferation (Fig. 3a) at or slightly above controls, from 0.27 to 1.35 mg/mL protein with a drop at 2.7 and 5.4 mg/mL protein supplementation. Heat‐inactivated LNE maintained cell proliferation below the control from 0.27 to 1.35 mg/mL protein supplementation; however, MDA‐MB‐435 cells exhibited a dramatic increase in proliferation (PI > 3.0) with heat‐inactivated LNE at 2.7 and 5.4 mg/mL protein supplementation. This latter effect of heat‐inactivated LNE resulted in a significant supplementation source, by supplementation concentration interaction (P ≤ 0.0001).
Figure 3.
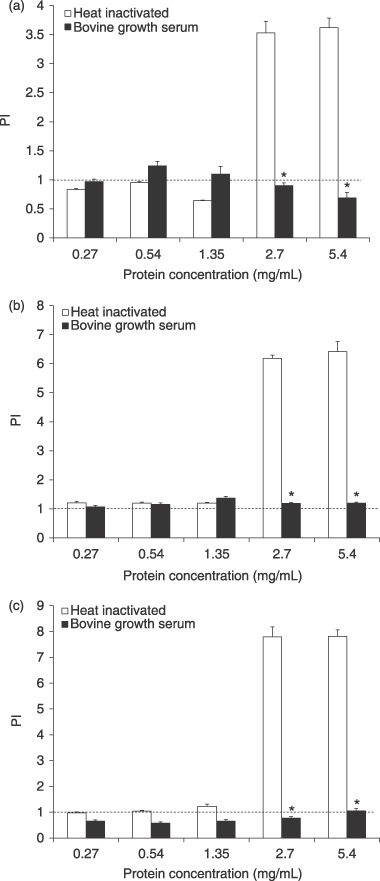
Proliferation indices (PI) of MDA‐MB‐435 human breast cancer (a), MAC‐T bovine mammary epithelial (b), and 1C6 hybridoma B (c) cells in a serum starvation assay with lymph node extract (LNE) of preparation 2. Following 2‐day starvation, cells were cultured in media containing heat‐inactivated LNE (white bars), and bovine growth serum (black bars) at different protein concentrations of supplementation. Data are expressed as means ± SEM. Means are significantly different (P ≤ 0.05) from respective heat‐inactivated means within protein concentrations. Dashed line is the PI (1.0) of cells cultured with 0 mg/mL bovine growth serum or LNE protein.
MAC‐T cell proliferation was significantly different between BGS and heat‐inactivated LNE cultured cells at 2.7 and 5.4 mg/mL protein supplementations (Fig. 3b) with LNE providing a large stimulatory effect at higher concentrations. BGS maintained cell population growth at, or slightly above, the control throughout all protein supplementation concentrations. Heat‐inactivated LNE supported cell proliferation slightly above the control from 0.27 to 1.35 mg/mL protein supplementation. Similar to the results with MDA‐MB‐435 cells, MAC‐Ts had a dramatic increase in proliferation (PI > 6) at 2.7 and 5.4 mg/mL protein supplementation, leading to a significant interaction effect (P ≤ 0.0001).
1C6 cell proliferation was significantly different between BGS and heat‐inactivated LNE (Fig. 3c). BGS maintained cell proliferation slightly below the control at 0.27, 0.54, 1.35 and 2.7 mg/mL protein supplementations, and right at the control level for 5.4 mg/mL protein. Heat‐inactivated LNE maintained cell proliferation at or above the control at 0.27, 0.54 and 1.35 mg/mL protein supplementations. In common with MDA‐MB‐435 and MAC‐T cells, 1C6 cells also had a dramatic increase in proliferation (PI > 7) at 2.7 and 5.4 mg/mL protein supplementation and a significant interaction for supplementation source and concentration of supplementation (P ≤ 0.0001).
Rapid adaptation growth assays
Photomicrographs of azure II dyed MDA‐MB‐435, MAC‐T and 1C6 cells in 6‐well culture plates are to be seen in Fig. 4. Day 1 for all cell lines represents confluence without any LNE media (Fig. 4a,f,k). Day 2 represents 2.5% LNE media addition; day 3: 5.0% of LNE media addition; day 4: 7.5% LNE media addition; and day 5: 10.0% LNE medium. MDA‐MB‐435 (Fig. 4a–e) and MAC‐T (Fig. 4f–j) cells maintained confluence and anticipated morphology through day 5. 1C6 cells did not maintain confluence like MDA‐MB‐435 and MAC‐T cells. On days 1 (Fig. 4k) and 2 (Fig. 4l) cells were confluent; but with 5.0% lymph node media addition, on day 3 (Fig. 4m), 1C6 cells changed shape and decreased in confluence. These cells changed in appearance by day 3 and continued to change morphology on days 4 (Fig. 4n) and 5 (Fig. 4o) with a noticeable decrease in confluence on day 5 when 2.7 mg/mL LNE protein was present in culture.
Figure 4.
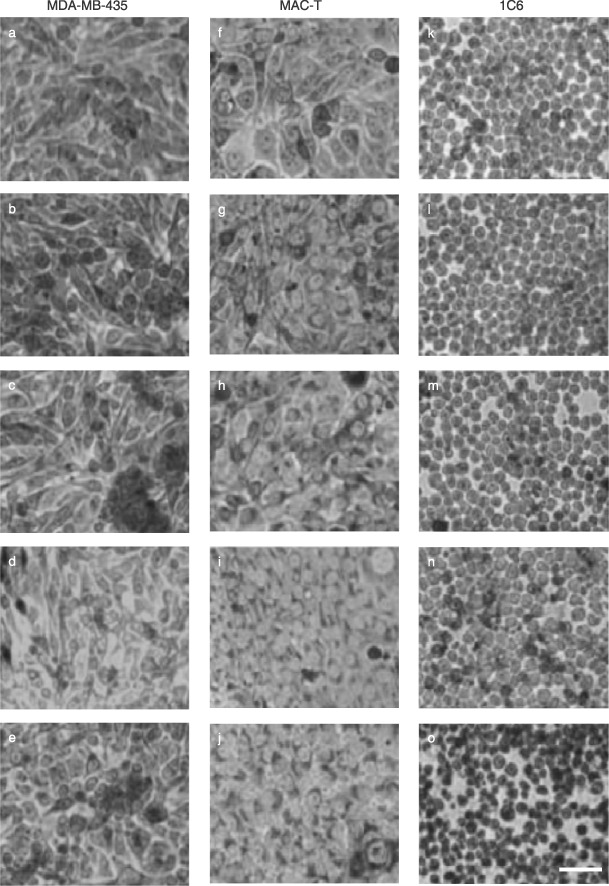
Photomicrographs of cells subjected to rapid adaptation assay with lymph node extract (LNE) following initial adherence for 24 h with bovine growth serum (BGS) containing media. MDA‐MB‐435 human breast cancer cells (a–e), MAC‐T bovine mammary epithelial cells (f–j), and 1C6 hybridoma B‐cells (k–o) were provided with increasing concentrations of LNE in place of BGS, daily. Day 0 (a, f, k): 0 mg/mL LNE; day 1 (b, g, l): 0.675 mg/mL LNE, day 2 (c, h, m): 1.35 mg/mL LNE, day 3 (d, i, n): 2.025 mg/mL LNE, and day 4 (e, j, o): 2.7 mg/mL LNE. Bar = 20 µm.
Although the MDA‐MB‐435 and MAC‐T cells survived well through the short supplementation exchange from BGS to LNE; once they were trypsinized and reseeded into LNE supplemented media, cells died within one additional day of culture. As noted above, 1C6 cells began to appear distressed in culture with increasing LNE supplementation; and when scraped and re‐seeded, these cells were unable to re‐adhere and appeared to be dead the following day.
DISCUSSION
The question of whether or not substitution of standard bovine sera supplementation with LNE could induce proliferation and population growth of three cell lines was addressed with a set of experiments. Cells serum‐starved over a period of 2 days followed by LNE addition for 3–4 days of culture tested the influence of LNE on cell proliferation. Non‐heat‐inactivated LNE of the first extract preparation did not support cells at any level of supplementation (Fig. 1a), and appeared to inhibit cell proliferation. This was thought to be due to the presence of complement proteins in the extract. BGS and other bovine serums used in cell culture are routinely heat‐inactivated to remove complement, which is a heat‐sensitive factor (Carroll 2004). Presence of complement could lead to lysis of cells, thereby contributing to decrease of their proliferation potential. Thus, heat inactivation of extracts was employed in order to eliminate this possible effect on the cultured cells. The follow‐up assay conducted with preparation 2 protein extract, however, provided an extract source that did not exhibit negative influence on proliferation of the cells in the serum starvation assay (Fig. 1b). Complement might have been a consideration in the first preparation, which was a much cruder approach, but the second preparation provided a source of factors that did not exhibit an inhibiting effect on cell population growth. Visual examination of both heated and non‐heated extract did reveal that the overall appearance and clarity of the heated extract was more satisfactory for cell culture supplementation. Heating led to precipitation of insoluble components of the extract that apparently did not affect proliferation of the cells.
The effect LNE has on cells may be due to a number of growth promoting factors present in the extract. A variety of cytokines (IL‐6, IL‐13, IL‐4, IL‐12, IL‐10) is present in lymph nodes (Zou et al. 1997) and may provide the proliferative effect seen on the cells. IGFs have been detected in lymph draining supramammary lymph nodes (Lacasse et al. 1996) and may also be contributing to the cell population growth. Although growth factors are present in BGS, the amount may not be sufficient to provide a stimulatory response, but rather only provide maintenance of population size. Albumin is a major component of serum, and it is a carrier protein for cholesterol and fatty acids for membrane synthesis by cells (Washburn et al. 1978), which is clearly important for cell population growth and maintenance. IGF‐I and IGF‐II have both been identified in BGS (Honegger & Humbel 1986). There may be similarities in protein content between bovine serum and LNE, but the actual concentrations of these growth promoting factors may define maintenance versus stimulation. Additionally, bovine serum contains a complex assortment of different substances, many of which are still undetermined, which is clearly the case with LNE at this time.
Much of what is understood about serum supplementation is anecdotal, but many cell lines in culture still require the growth supporting effect of bovine serum. Most cells in an organism rarely come in direct contact with blood plasma; however, the use of serum to grow cells has not been overly questioned. Scientists assume cells need serum and initially culture cells with it. Although different serum replacement supplements are commercially available, serum remains in high demand as an essential ingredient in media for many cell lines in culture today.
The ability to culture cells in the LNE‐supplemented media over an extended period of time without BGS supplementation would be the eventual objective in deciding whether or not to use the extract as a substitute. Rapid adaptive population growth assay was performed with three cell lines (MDA‐MB‐435, MAC‐T and 1C6). Rather than continuing to passage cells with different percentage supplementations of the LNE, cells remained adhered and were not passaged until complete replacement had been achieved. As the LNE was stepwise added to media with BGS still present in lowering concentrations, MDA‐MB‐435 and MAC‐T cells appeared to adapt well to LNE presence. The 1C6 cells did not thrive as well and began to shrink by day 3 in culture. Morphology of the 1C6 cells changed with crenation and loss of rounded appearance. When all three lines were trypsinized or scraped and re‐seeded into new flasks, cells were unable to adhere and appeared to die.
Inability to re‐adhere may be due to the ‘stimulation effect’ of LNE. Cells exposed to LNE were being stimulated with greater concentrations of bioactive proteins than cells are normally accustomed to, with BGS (see Fig. 2), which is primarily composed of albumin and globulin components. Abundance of bioactive proteins in the extract may provide multiple mitogenic signals that would not favour nor influence cell adherence. Weber et al. (1999) observed mitogenic activity in bovine mammary epithelial cells cultured with bovine mammary extracts. Cell population growth with the mammary extract application was double that found after treatment with IGF‐I or foetal bovine serum alone. IGF‐I supported cell population growth significantly better than basal medium (0%), even at 3 µg/mL, implying that low concentrations of growth factors can support/sustain cell replication. While high protein supplementations with the LNE were mitogenic, lower protein concentrations might sustain cells in culture with submitogenic levels of growth factors.
Although alternatives to sera are available, various cell lines require specific factors (e.g. insulin, growth hormone, IGF‐I and IGF‐II, epithelium growth factor, tumour growth factor‐α) that may not be obtained from sources other than serum. An optimal replacement would include an abundance of growth promoting factors that can support numerous cell types in culture. Considering the presence of interstitial fluid around cells in the tissue environment, use of a protein‐rich tissue extract may offer an alternate to serum. Interstitial fluid drains from tissues to enter the lymphatics and become the major fluid component of lymph. As lymph fluid moves through the lymphatic circulation and lymph nodes, it becomes enriched with lymph factors (immunoglobulins, cytokines, growth and differentiation factors) prior to the lymph mixing with blood (Gorewit et al. 1993; Waller et al. 2003). Therefore, recovered tissue extracts from lymph nodes appear to possess a rich mixture of factors that can support proliferation of cells in culture. Additional studies are required to determine the utility of the LNE for maintenance of cells in long‐term culture, which entails extensive adaptation over long periods of time (on the order of months). Of importance for use of LNE would be the identification of component‐bioactive proteins in the extract. Currently, discovery and recovery of proteins through separation technology and proteomics is leading to isolation of moderately and low‐abundant proteins in body fluids such as serum. These proteins occur there at low concentrations, but possess potent biological activity. Identification and testing of these proteins could lead to opportunities to formulate and refine culture media supplements for improved cell culture methodologies. Partitioning of LNE for these moderately and low‐abundant proteins should yield defined supplements for serum replacement in culture.
AKNOWLEDGEMENTS
The authors would like to thank M. Dimmick Owens, J. Campbell, N. Korn and J. Andre for their technical assistance. This work was supported by the Fats and Proteins Research Foundation through the Animal Co‐Products Research and Education Center at Clemson University.
REFERENCES
- Akers RM (2006) Major advances associated with hormone and growth factor regulation of mammary growth and lactation in dairy cows. J. Dairy Sci. 89, 1222–1234. [DOI] [PubMed] [Google Scholar]
- Berry SDK, Weber Nielsen MS, Sejresen K, Pearson RE, Boyle PL, Akers RM (2003) Use of immortalized bovine mammary epithelial cell line (MAC‐T) to measure the mitogenic activity of extracts from heifer mammary tissue: effects of nutrition and ovariectomy. Domest. Anim. Endocrinol. 25, 245–253. [DOI] [PubMed] [Google Scholar]
- Bradley KJ, Bradley AJ, Barr FJ (2001) Ultrasonographic appearance of the superficial supramammary lymph nodes in lactating dairy cattle. Vet. Rec. 148, 497–501. [DOI] [PubMed] [Google Scholar]
- Carroll MC (2004) The complement system in regulation of adaptive immunity. Nat. Immunol. 5, 981–986. [DOI] [PubMed] [Google Scholar]
- Gorewit RC, Ostensson K, Astrom G, Svennerstenz. K (1993) Flow and composition of afferent mammary gland lymph. J. Dairy Sci. 76, 1539–1543. [DOI] [PubMed] [Google Scholar]
- Honegger A, Humbel RE (1986) Insulin‐like growth factors I and II in fetal and adult bovine serum. J. Biol. Chem. 261, 569–575. [PubMed] [Google Scholar]
- Huynh HT, Robitaille G, Turner JB (1991) Establishment of bovine mammary epithelial cells (MAC‐T): an in vitro model for bovine lactation. Exp. Cell Res. 197, 191–199. [DOI] [PubMed] [Google Scholar]
- Jones LJ, Gray M, Yue ST, Haugland RP, Singer VL (2001) Sensitive determination of cell number using the CyQUANT cell proliferation assay. J. Immunol. Methods 254, 85–98. [DOI] [PubMed] [Google Scholar]
- Lacasse P, Block E, Turner J, Woodward T, Couture Y, Petitclerc D (1996) Evolution of IGF‐I, PGE‐II, and mitogenic activity of bovine primary lymph during the dry period and lactogenesis. J. Dairy Sci. 79, 21746–21753. [DOI] [PubMed] [Google Scholar]
- Waller PK, Colditz. IG , Lun S, Ostensson K (2003) Cytokines in mammary lymph and milk during endotoxin‐induced bovine mastitis. Res. Vet. Sci. 74, 31–36. [DOI] [PubMed] [Google Scholar]
- Warburg O, Christian W (1942) Isolation and crystallization of enolase. Biochem. Z. 310, 386–421. [Google Scholar]
- Washburn LR, Hughes JH, Somerson NL (1978) Mycoplasma growth factors in bovine serum fraction. J. Bacteriol. 135, 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MS, Purup S, Vestergaard M, Akers RM, Sejrsen K (2000) Nutritional and somatotropin regulation of the mitogenic response of mammary cells to mammary tissue extracts. Domest. Anim. Endocrinol. 18, 159–164. [DOI] [PubMed] [Google Scholar]
- Weber MS, Purup S, Vestergaard M, Ellis SE, Scndergard‐Anderson JS, Akers RM, Sejrsen K (1999) Contribution of insulin‐like growth factor (IGF)‐I and IGF‐binding protein‐3 to mitogenic activity in bovine mammary extracts and serum. J. Endocrinol. 161, 365–373. [DOI] [PubMed] [Google Scholar]
- Zou W, Lackner A, Simon M, Durand‐Gasselin I, Galanaud P, Desroseires R, Dominique E (1997) Early cytokine and chemokine gene expression in lymph nodes of macaques infected with Simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J. Virol. 71, 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]


