Abstract
Abstract. Cells of the central nervous system were once thought to be incapable of regeneration. This dogma has been challenged in the last decade with studies showing new, migrating stem cells in the brain in many rodent injury models and findings of new neurones in the human hippocampus in adults. Moreover, there are reports of bone marrow‐derived cells developing neuronal and vascular phenotypes and aiding in repair of injured brain. These findings have fuelled excitement and interest in regenerative medicine for neurological diseases, arguably the most difficult diseases to treat. There are numerous proposed regenerative approaches to neurological diseases. These include cell therapy approaches in which cells are delivered intracerebrally or are infused by an intravenous or intra‐arterial route; stem cell mobilization approaches in which endogenous stem and progenitor cells are mobilized by cytokines such as granulocyte colony stimulatory factor (GCSF) or chemokines such as SDF‐1; trophic and growth factor support, such as delivering brain‐derived neurotrophic factor (BDNF) or glial‐derived neurotrophic factor (GDNF) into the brain to support injured neurones; these approaches may be used together to maximize recovery. While initially, it was thought that cell therapy might work by a ‘cell replacement’ mechanism, a large body of evidence is emerging that cell therapy works by providing trophic or ‘chaperone’ support to the injured tissue and brain. Angiogenesis and neurogenesis are coupled in the brain. Increasing angiogenesis with adult stem cell approaches in rodent models of stroke leads to preservation of neurones and improved functional outcome. A number of stem and progenitor cell types has been proposed as therapy for neurological disease ranging from neural stem cells to bone marrow derived stem cells to embryonic stem cells. Any cell therapy approach to neurological disease will have to be scalable and easily commercialized if it will have the necessary impact on public health. Currently, bone marrow‐derived cell populations such as the marrow stromal cell, multipotential progenitor cells, umbilical cord stem cells and neural stem cells meet these criteria the best. Of great clinical significance, initial evidence suggests these cell types may be delivered by an allogeneic approach, so strict tissue matching may not be necessary. The most immediate impact on patients will be achieved by making use of the trophic support capability of cell therapy and not by a cell replacement mechanism.
INTRODUCTION
Until recently, cells of the brain and central nervous system were thought to be incapable of regeneration. In 1928, Ramon y Cajal wrote, ‘In adult centres, the nerve paths are something fixed, ended, immobile. Everything may die, nothing may be regenerated.’ However, even Ramon y Cajal left it open to future generations to change ‘this harsh decree’. Vertebrates such as the urodele amphibians show a remarkable plasticity of the central nervous system, able to regenerate tails and limbs (Brockes 1997; Brockes & Kumar 2005), but in mammals and man, the central nervous system shows much less capacity for repair. This has been attributed to limited neurogenesis, the presence of active inhibitors of axonal regeneration associated with myelin such as Nogo A, and with glial scarring. (Schwab 2004; Yiu & He 2006). Recent challenges have been made to the dogma that the central nervous system in man is incapable of regeneration. In 1998, Eriksson et al. reported new neurones born in the dentate gyrus of the hippocampus of human adults, indicating that neurogenesis occurred throughout life here. On record to indicate this, in 2006, a patient who had been in a minimally conscious state for 19 years began to show marked improvement in his speech and language with accompanying magnetic resonance imaging and computed tomography–positron emission tomography evidence of axonal growth and central nervous system remodeling (Voss et al. 2006).
Nevertheless, of all organs, the central nervous system shows the most limited regeneration and recovery after injury. This biological fact accounts for the devastating nature of many neurological diseases where recovery is incomplete and major disability often results. In mammals, neurogenesis in vivo is restricted to the subgranular zone‐dentate gyrus of the hippocampus and the subventricular zone‐olfactory bulb. In man, neurogenesis is restricted to the dentate gyrus of the hippocampus. Elegant studies with 14C dating of neuronal DNA that exploit changes in environmental background atmospheric 14C levels from above ground nuclear weapon testing in the 1950s, do not reveal any evidence of significant neurogenesis in cortical areas outside the hippocampus (Bhardwaj et al. 2006). Neurones in the human cortex are all generated by birth and there does not appear to be any significant birth of new neurones throughout life. Restriction of neurogenesis to the hippocampus and subventricular zone appears to be related to the local microenvironment; astrocytes in the neurogenic zones secrete factors and cytokines that promote neural differentiation whereas astrocytes in non‐neurogenesis regions of the brain secrete inhibitory factors (Barkho et al. 2006).
While regeneration and repair is limited in the central nervous system of mammals, there is evidence of neurogenesis and projections of these new neurones to distant brain regions, in the mouse after very focused injury (Magavi et al. 2000; Chen et al. 2004). When apoptosis was induced in level VI corticothalamic neurones in the mouse, new neurones were generated as evidenced by bromodeoxyuridine incorporation; these neurones sent projections to the thalamus (Magavi et al. 2000). In a similar model of induced apoptosis of corticospinal motor neurones in the mouse, migrating neuroblasts entered the cortex and differentiated into mature pyramidal cells in layer V, and some sent projections to the spinal cord. These studies demonstrate that during focused injury, neurogenesis can occur in the mammalian brain although it does not occur in normal circumstances. Moreover, these new neurones can send axonal projections to distant brain regions such as the thalamus and to the spinal cord. One regenerative strategy is to stimulate these endogenous repair processes.
NEURAL STEM CELLS
Somewhat surprisingly, recent evidence suggests that the neural stem and progenitor cell in vivo in the brain and spinal cord is an astroglial cell (Doetsch et al. 1999; Alvarez‐Buylla et al. 2002; Doetsch 2003). In man, neurogenesis is restricted largely to the dentate gyrus of the hippocampus, neural stem cells (NSC) can be isolated from other (non‐hippocampal) regions of the developing and adult human brain (Svendsen et al. 1997; Svendsen et al. 1999; Vescovi & Snyder 1999). Expansion of NSC in vitro is dependent upon culture with epidermal growth factor and fibroblast growth factor‐2. Multipotent astroglial neural progenitors have also been isolated from adult human brain and expanded in culture (Walton et al. 2006). These adult human neural progenitors are derived from multiple forebrain regions, co‐express glial markers and nestin, and are highly expandable, being maintained for more than 60 population doublings with minimal senescence in culture and without evidence of immortalizing mutations. This high expandability equates to one of these progenitor cells theoretically forming enough cells for 4 × 107 brains. These adult human neural progenitors generate both neuronal and glial cells in vitro, are genetically modifiable, and when transplanted into rodent brains incorporate and adopt neuronal and glial phenotypes.
As an expandable cell population, NSC possess therapeutic potential. One of their important characteristics is their ability to migrate and ‘home’ towards distant areas of injury (Kabos et al. 2003; Imitola et al. 2004). This homing is mediated by SDF‐1 that is up‐regulated in astrocytes and endothelial cells in injured tissue and by the receptor for SDF‐1, CXCR4, expressed on NSC (Imitola et al. 2004). NSC can also differentiate into neurones and glial cells. They have been grafted into a rat Parkinson's disease animal model where they survive, differentiate into neurones and glial cells, express tyrosine hydroxylase, and ameliorate neurological deficits (Svendsen et al. 1996; Svendsen et al. 1997). Yet, perhaps the major therapeutic effect of NSC is their ability to rescue injured and dysfunctional endogenous neurones and serve as a chaperone cells (Ourednik et al. 2002). NSCs have been utilized as cellular delivery vehicles for growth factors such as glial‐derived neurotrophic factor (GDNF). As it is a large protein, GDNF penetrates the blood–brain barrier poorly. However, human neural progenitor cells (NPC) have been genetically modified to release glycosylated GDNF in vitro under an inducible promoter system and then transplanted into the striatum of rats, following partial lesion of the dopamine system. The cells survived, secreted GDNF for 8 weeks and partially ameliorated the deficit. Transplantation was also successful in aged primates as human NPC secreted GDNF for 3 months (Behrstock et al. 2006). A similar strategy has been used successfully in a rodent model of amyotrophic lateral sclerosis where genetically modified human NPC survived, integrated, and released GDNF in the spinal cord of SOD1 (G93A) rats (Klein et al. 2005).
OLFACTORY ENSHEATHING CELLS
Olfactory receptor neurones are continuously replaced throughout life in mammals. Situated at the interface between the peripheral and central nervous system, they send axonal projections into the olfactory bulb. The cell that ensheaths these axons has unique characteristics, sharing features of Schwann cells and astrocytes; it is known as an olfactory ensheathing cell (OEC) (Doucette 1984, 1991, 1995).These OECs survive transplantation into the nervous system and in models of dorsal spinal cord transaction, bridge the transected zone, myelinate axons, and improve locomotor activity (Doucette 1995; Sasaki et al. 2004). Xenotransplanted OECs also have been shown to effectively remyelinate regions of injured spinal cord in a non‐human primate model (Radtke et al. 2004). Recent enthusiasm for OEC treatment as a cellular therapy has been fuelled by two recent phase I trials of autologous OEC in spinal cord injury patients, which has demonstrated safety and feasibility (Feron et al. 2005; Lima et al. 2006).
BONE MARROW AND BRAIN
A fundamental dogma of developmental biology has been lineage restriction, the concept that germ layer boundaries are respected and are not crossed. This dogma has been challenged with findings that bone marrow‐derived cells (from the embryonic mesoderm) can generate neurones (from the embryonic ectoderm). This may not be that surprising as the neurone is the default program in stem cell differentiation (Munoz‐Sanjuan & Brivanlou 2002). Bone marrow contains a heterogeneous population of cells and notably contains at least two stem cell populations: haematopoietic stem cells and mesenchymal stem cells (MSC). Under specific conditions of cell culture, MSC, also termed marrow stromal cells, can adopt a neuronal phenotype (Woodbury et al. 2000, 2002; Bossolasco et al. 2005; Cho et al. 2005; Guo et al. 2005); local environment and the extracellular matrix influence and drive MSC differentiation. For example, elasticity and rigidity of the extracellular collagen matrix determines the phenotype of MSC; when grown on soft matrices MSC adopt a neuronal phenotype while on a hard matrix a bone phenotype (Engler et al. 2006).
More surprisingly, a number of studies has reported that bone marrow cells have generated neurones in vivo (Brazelton et al. 2000; Mezey et al. 2000). These experiments utilized bone marrow chimaeras in mice in which genetically tagged bone marrow was tracked into the brain with colocalization of donor bone marrow and neuronal markers. These findings generated controversy when other investigators could not reproduce them (Wagers et al. 2002), a situation probably explained by different transplanted bone marrow populations (whole, unfractionated bone marrow versus haematopoietic stem cells) and tissue processing (Mezey et al. 2003b). In addition, using bromodeoxyuridine as a marker of proliferating cells can lead to erroneous conclusions as donor cells are phagocytosed by host inflammatory response cells and the leaked bromodeoxyuridine can be transferred to host cells. This can be misleadingly interpreted as differentiation of these cells into neurones (Coyne et al. 2006). Autopsy studies in humans that underwent gender mismatched bone marrow transplantation confirmed that bone marrow derived cells had adopted a neuronal phenotype in the brain (Mezey et al. 2003a; Cogle et al. 2004). Fusion of bone marrow cells with general somatic cells may account for some of the findings of bone marrow cell‐neuronal transdifferentiation (Terada et al. 2002). In the brain, bone marrow cells fuse with Purkinje cells (Alvarez‐Dolado et al. 2003; Weimann et al. 2003); this mechanism of cell fusion may rescue injured Purkinje cells. A further explanation for the apparent generation of neurones from bone marrow cells has been advanced by Kucia et al. (2005) with their findings of tissue‐committed stem cells. In this concept, the bone marrow contains a heterogeneous population of these that might have neuronal, glial, endothelial, or cardiac fate. During injury and stress, these cells may be mobilized into the blood and then circulate/migrate and home to an SDF‐1 gradient in an injured organ.
BONE MARROW‐DERIVED CELL THERAPIES
A number of bone marrow‐derived stem and progenitor cell types show therapeutic promise and represent cell therapy that is likely to have the earliest impact in neurological diseases. As cell therapy products, they generate no ethical concerns, they are easily obtainable from bone marrow or placental tissue, and are highly expandable and scalable. Blood banking is already a well‐established technique and such a protocol with blood and bone marrow derived cells is a logical commercial and technical extension. MSC can be isolated from bone marrow (and other tissues) and are able to differentiate into cartilage, bone, and adipose tissue at least. They can be induced to express a neuronal phenotype in vitro and engraft and migrate in the brain (Azizi et al. 1998). Importantly, MSC also secrete cytokines and trophic factors that support other cell types (Caplan & Dennis 2006), and they have shown efficacy in preclinical animal models of stroke, head trauma, and multiple sclerosis (Chopp & Li 2002; Zhang et al. 2005). The therapeutic effect of human MSC in rodent models of cerebral ischaemia is enhanced when they are modified with growth factor encoding genes such as brain‐derived neurotrophic factor and placental growth factor (Nomura et al. 2005; Liu et al. 2006). MSC are also anti‐inflammatory, immunomodulatory and immune privileged, allowing them to be potentially used in allogeneic transplantation (Aggarwal & Pittenger 2005; Beyth et al. 2005). However, there is some evidence that MSC may elicit an immune response in MHC mismatched recipients, so caution needs to be exercised (Eliopoulos et al. 2005). If MSC can be used in allogeneic transplantation, this would represent a major advantage for cell therapy, as it may permit isolation from healthy donors (versus autologous) and provide an effective ‘off the shelf’ product.
A subset of MSC, that copurify with them, are multipotent adult progenitor cells (MAPC) isolated by Verfaillie and colleagues (Jiang et al. 2002a,2002b, 2003). MAPCs are pluripotent, differentiating into cell types of all three germ layers including neurones. Moreover, they generate all tissues when injected into blastocysts but have not been reported to form teratomas. MAPC can be isolated from human and rodent bone marrow as well as from other tissues such as brain and muscle (Jiang et al. 2002b). MAPC are highly expandable and represent a promising form of cell therapy; they are effective in animal models of neonatal hypoxic‐ischaemic encephalopathy, suggesting that they may have a role in treating children with some forms of cerebral palsy (Yasuhara et al. 2006a,b). We have also found them to be effective by intravenous delivery in rodent models of cerebral ischaemia with a time window of up to 7 days.
Numerous investigators have isolated and cultured pluripotential cells from human umbilical cord blood (Kogler et al. 2004; McGuckin et al. 2005); these cells express Oct3/4 and have a wide differentiation potential, making them attractive for regenerative therapies. Other groups have derived a clonogenic non‐immortalized neural stem cell‐like line from CD34 negative adherent umbilical cord blood (Sun et al. 2005; Buzanska et al. 2006); moreover, Kucia et al. (2006) have isolated very small embryonic stem cells (VSEL) from both bone marrow and umbilical cord blood. These cells are pluripotential, express stage specific embryonic antigen‐1 (SSEA‐1) and Oct3/4, and display characteristics of embryonic stem cells.
REGENERATIVE THERAPEUTIC APPROACHES TO NEUROLOGICAL DISEASE
Stem cell and regenerative approaches to neurological diseases can be divided into a number of categories depending upon the target neurological disease. Certain approaches work better in some categories than in others. First, there are neurological diseases caused by acute injury. Timing of the insult is known, the cascade of injury peaks within hours and is mostly is complete within 24 h (Fig. 1). These diseases include stroke, spinal cord injury, neonatal hypoxia‐ischaemic encephalopathy or perinatal asphyxia and traumatic brain injury. These diseases all produce major morbidity and mortality. Stroke is the third leading cause of death in the USA (second leading cause of death in the world) and is the leading cause of disability among adults in the USA. Since the cascade of injury is mostly complete within 24–48 h, neuroprotection to be effective, must be started within hours of the injury. One of the reasons for failure of neuroprotective treatments for stroke has been the need to start treatment early, within 3–6 h of the onset of ischaemia; this has proven difficult in clinical practice. Later treatment is restorative, aiming to promote repair processes such as angiogenesis, neurogenesis, and synaptogenesis. The window for this is not precisely known but it may be limited to 1 week or 1 month; Fig. 2 shows the impact of therapeutic approach plotted against the time it should be initiated. Neurorestorative treatment can commence later than neuroprotective treatment and therefore many more stroke patients could potentially benefit.
Figure 1.
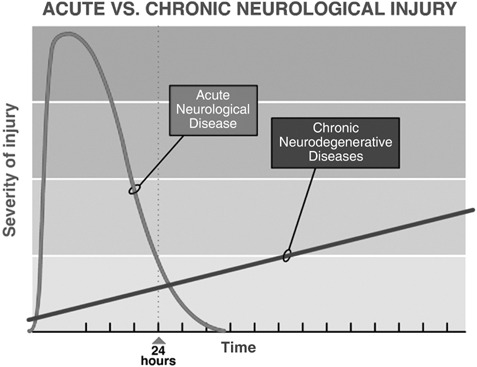
Graph depicting the timing of injury in acute neurological diseases such as stroke, spinal cord injury and traumatic brain injury where most of the injury cascade is completed with 24 h; in chronic neurodegenerative diseases such as Parkinson's and Huntington's, the disease process has been ongoing for many months and even years before symptoms appear and continues to inexorably progress.
Figure 2.
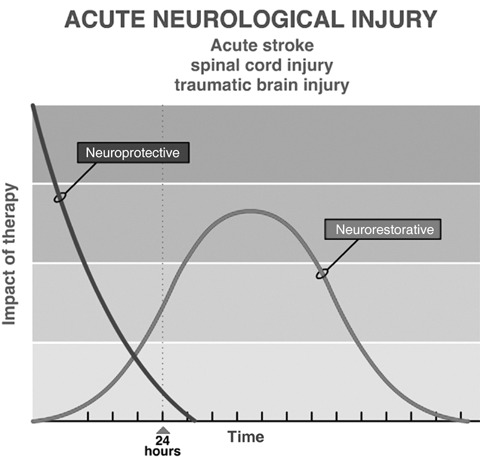
In acute neurological diseases, therapy needs to be started within hours or at most 24–48 h to have a neuroprotective effect and reduce tissue damage from the insult. A later start of therapy is targeted to neurorestorative processes such as angiogenesis, neurogenesis, and syntaptogenesis.
A second category is the chronic neurodegenerative diseases. In these, timing of onset is not known but only the time over which the disease clinically manifests itself; there is a period over which the process is active but clinical symptoms have not yet become apparent. The pathological process of cell death and injury continues slowly but inexorably (Fig. 1); this class of disease includes Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, and Alzheimer's disease, in some of these there is loss of specific cell populations. In Parkinson's disease there is degeneration and loss of dopaminergic neurones in the substantia nigra (SNc), pars compacta (SNC) and other dopaminergic and noradrenergic nuclei, while in amyotrophic lateral sclerosis there is specific loss of motor neurones. For these, stem cell therapies may have both a neuroprotective effect (reduce neuronal apoptosis) and a neurorestorative effect (promote neurogenesis, axonal growth, and synaptogenesis) over time (Fig. 3).
Figure 3.
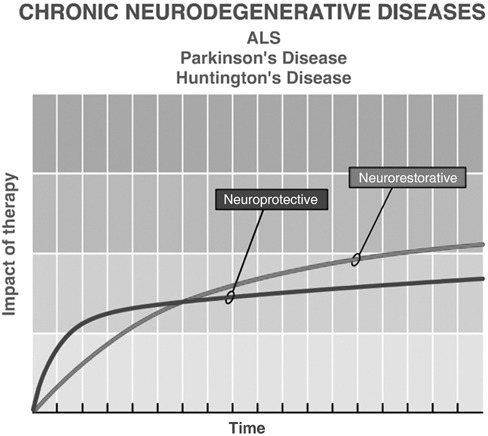
In chronic neurodegenerative diseases, cells continue to die, often by apoptosis, so that treatments are needed with a neuroprotective effect. In addition, a neurorestorative benefit is simultaneously possible.
The third category of neurological disease is composed of the chronic inflammatory and immunologically mediated conditions such as multiple sclerosis. Multiple sclerosis is a relapsing/remitting disease more common in women than men and represents the major cause of neurological disability among young adults in most Western nations. While chronic inflammation plays an important role early in the disease, later there is a large degenerative component with axonal degeneration (Trapp et al. 1999).
The fourth category includes genetic diseases that present in children. These result from inborn errors of metabolism where an enzyme is missing leading to abnormal storage of glycolipids or proteins in lysosomes, or there is a failure to make normal myelin. These diseases include neuronal ceroid lipofuscinosis, the mucopolysaccharidoses, and the leucodystrophies such as adenoleucodystrophy, globoid cell leucodystophy (Krabbe's disease), and metachromatic leucodystrophy. Stem cell and neuroregenerative approaches make use of six broad approaches that can be used alone or in combination as shown in Table 1.
Table 1.
Stem cell and regenerative approaches
| A. Cell therapy |
| Example: Deliver a cell such as mesenchymal stem cell, multipotent adult progenitor cell, or umbilical cord stem cell intravenously or by stereotactic injection into the brain in stroke; transplantation of olfactory ensheathing cell in spinal cord injury |
| B. Combination of cell and gene therapy |
| Example: Transplantation of neural stem cell expressing transgene such as glial‐derived neurotrophic factor for Parkinson's disease |
| C. Enhance endogenous neurogenesis and repair |
| Examples: Erythropoietin, granulocyte stimulating factor (G‐CSF), brain‐derived neurotrophic factor in stroke; use of dopamine D3 agonists in Parkinson's disease |
| D. Allogeneic stem cell transplantation |
| Example: Unrelated human umbilical cord transplant in Krabbe's disease, Hurler's disease |
| E. Autologous hemtopoietic stem cell transplantation |
| Example: Autologous HSCT in patients with early multiple sclerosis |
| F. Inhibiting myelin‐associated inhibitors of axonal regeneration |
| Example: Nogo inhibitors in stroke, spinal cord injury |
While this review is not exhaustive, the most promising stem cell and regenerative approaches for a representative disease from each of the above categories is presented.
Acute injury: stroke
Ischaemic stroke (cerebral ischaemia) involves destruction of multiple cell types including neurones, astrocytes, oligodendrocytes, endothelial cells, and pericytes. Therefore, the regenerative strategy will need to restore not only neural elements but supportive structures such as blood vessels. There is evidence of an endogenous repair mechanism that operates after cerebral ischaemia; nestin for example, is up‐regulated in astrocytes (Duggal et al. 1997; Li & Chopp 1999). In rodents, subventricular progenitor cells proliferate after middle cerebral artery occlusion and cerebral ischaemia, and migrate to the striatum where they contribute to formation of striatal medium‐sized spiny neurones and glial cells (Arvidsson et al. 2002). However, most of these neuroblasts then undergo apoptosis and die; also there is no evidence of new neurones in the cortex despite it being injured in this model as well. Subventricular progenitor cells continue to migrate to the striatum for at least 4 months after injury. This is directed by a gradient of SDF‐1 up‐regulated in the ischaemic tissue and CXCR4 expressed on the migrating neuroblasts (Thored et al. 2006). SDF‐1 is up‐regulated for at least 1 month after cerebral ischaemia in astrocytes and in endothelial cells, and serves to direct the migration of bone marrow‐derived cells involved in tissue repair (Hill et al. 2004). Thus, there is evidence of an endogenous repair mechanism operating after cerebral ischaemia, however, it is insufficient for recovery of function.
Stimulating this endogenous response with trophic factors is an attractive therapeutic target and is most likely to have the earliest impact in stroke. Cytokines with trophic effects on bone marrow cells (haematopoietic cytokines) are already in clinical trial in stroke (Bath & Sprigg 2006), these haematopoietic growth factors combine both neuroprotective and neurorestorative effects in the brain, making them ideal agents. G‐CSF is an FDA‐approved drug used to mobilize CD34 cells for bone marrow transplantation and to treat neutropenia after cancer chemotherapy. It is effective in rodent stroke models with a therapeutic window extending for 24 h (Shyu et al. 2004). The GCSF receptor is dramatically up‐regulated on neurones during cerebral ischaemia (Schneider et al. 2005) and it has direct effects upon neurones, reducing neuronal apoptosis and stimulating endogenous neural progenitors (Schneider et al. 2005). A further mechanism of action for GCSF in stroke is mobilizing bone marrow derived stem cells to participate in neurogenesis and angiogenesis (Kawada et al. 2006), similarly, erythropoietin is neuroprotective in rodent models of cerebral ischaemia (Brines et al. 2000). Erythropoietin also has a restorative effect when administered 24 h after a stroke, improving functional outcome and stimulating angiogenesis and neurogenesis (Wang et al. 2004); it has been shown to be safe and effective in one small clinical trial in stroke patients with an 8 h window (Ehrenreich et al. 2002). One safety issue with erythropoietin, however, is the rise in haematocrit associated with its use; this has been avoided by the development of carbamylated erythropoietin that is devoid of an erythropoietic effect, but maintains the neuroprotective effect (Doggrell 2004).
The first attempt at cell therapy for stroke involved intracerebral transplantation of human hNT cells, neuronal like cells derived from a teratocarcinoma line (NT2‐N) exposed to retinoic acid (Borlongan et al. 1998; Kondziolka et al. 2000). Twelve stroke patients with a basal ganglia stroke and stable motor deficit were transplanted between 6 months and 6 years after the stroke. No tumour formation ensued and PET scanning indicated increased cell uptake in 6 of 11 patients. A subsequent small randomized early phase II study showed no functional benefit from transplantation but there were no major safety issues occurred (Kondziolka et al. 2005). Presently, the UK biotechnology company, ReNeuron, is organizing a phase I trial of a conditionally transformed (c‐myc) human neural line cells in patients with stroke. The proprietary technology (c‐mycERTAM) used to achieve conditional growth control is a fusion protein consisting of a growth promoting gene, c‐myc, and a hormone receptor that is regulated by a synthetic drug, 4‐hydroxy‐tamoxifen; in a rodent stroke model this line was beneficial (Pollock et al. 2006).
One of the obstacles to neural transplantation in stroke is development of a cystic cavity in the tissue. Such a cavity prevents transplanted cells from having an adequate blood supply and matrix upon which to anchor. In a neonatal hypoxic‐ischaemic model, where a large cavity forms, neural stem cells (transformed mouse cerebellar granule cell line) were seeded on a biodegradable scaffold and were then transplanted into the cavity (Park et al. 2002). The NSC scaffold altered the trajectory and complexity of host cortical neurites while donor‐derived neurones were capable of directed, target‐appropriate neurite outgrowth. The scaffold, which degraded later, enhanced reciprocal interactions between host and donor cells. This approach of combining stem cells with a biodegradable scaffold is an attractive therapeutic strategy in ischaemic stroke in adults also, where cavities often form.
Bone marrow‐derived cells, notably, MSC are a promising form of cell therapy for stroke. Much of the preclinical work for this approach has been performed by Chopp and colleagues. MSC improve functional outcome in a dose–response fashion in rodent middle cerebral artery occlusion models, when applied intracerebrally, intra‐arterially, or intravenously (Chen et al. 2001a, b; Li et al. 2001a). Intravenous transplantation was effective at improving functional outcome even when given as late as 1 month after the insult (Shen et al. 2006). There is no reduction of infarct size; instead there is a neurorestorative effect with increases in angiogenesis, neurogenesis and synaptogenesis in the MSC‐treated groups (Chen et al. 2003a,b). The mechanism of action is not direct cell replacement; rather, MSC act as a trophic factory, elaborating a host of trophic and growth factors (Chopp & Li 2002). A major advantage of MSC and other bone marrow‐derived stem cells is their effectiveness in stroke after intravenous delivery. There is already one reported phase I trial of autologous MSC in ischaemic stroke (Bang et al. 2005). Thirty patients were randomly assigned in the study, five receiving MSC and 25 serving as controls. Autologous MSC were delivered intravenously at a dose of 1 × 108 within 1 month of the index ischaemic stroke. The treatment proved feasible and safe and there was a trend towards functional improvement. Combining MSC with a transgene for a trophic factor may enhance the therapeutic effect of MSC. MSC expressing a transgene for placental derived growth factor or for brain‐derived neurotrophic factor were more effective than MSC alone in reducing infarct volume and improving functional outcome in a rodent permanent middle cerebral artery occlusion model. In these studies, the MSC were intravenously infused at either 3 or 6 h after onset of ischaemia so these studies were measuring a neuroprotective effect more than a restorative effect (Nomura et al. 2005; Liu et al. 2006).
Intravenous and intrastriatal delivery of human umbilical cord blood (HUCB) stem cells are also reported to be effective in animal models of cerebral ischaemia (Chen et al. 2001c; Borlongan et al. 2004; Taguchi et al. 2004; Vendrame et al. 2004), intravenous route appearing to be more effective than intrastriatal delivery (Willing et al. 2003). The intravenous time window extends to at least 48 h post‐stroke (Newcomb et al. 2006); however, not all groups have found that intravenously delivered HUCB are effective in improving the outcome and few delivered cord blood cells were seen in the brains of rodents with stroke (Makinen et al. 2006). Cord blood‐derived CD34 cells improved functional outcome and increased angiogenesis and associated neurogenesis when delivered intravenously 48 h after a cortical occlusion in mice (Taguchi et al. 2004). The key element in success appeared to be the cells’ ability to promote angiogenesis and entry into the central nervous system of intravenously delivered HUCB does not appear to be a prerequisite for their beneficial effects (Borlongan et al. 2004), and they may also act on the immune system and promote an immunosuppression. When intravenously transplanted into rodents with MCA occlusion, cord blood cells prevented the reduction of spleen size and in splenic CD8+ T cells seen in this model and also increased the anti‐inflammatory cytokine, IL‐10 (Vendrame et al. 2006).
The multipotent adult progenitor cells subset of MSC are also effective in a rodent stroke therapy model. Intracerebral transplantation of MAPCs 1 week after cortical stroke resulted in improved sensorimotor function. Although some of the transplanted cells expressed neuronal markers, the small number suggested that improvement was from a trophic effect of the transplanted cells on host brain (Zhao et al. 2002). MAPCs delivered intravenously improve functional outcome given as late as 1 week after cerebral ischaemia.
From preclinical studies of cell transplantation in animal stroke models, a number of principles emerge. Presently, the strongest preclinical data is for MSC therapy followed by umbilical cord stem cell therapy. These are attractive as they are effective when delivered intravenously and have time windows of at least 48 h, and in some studies, a window of 1 month. This is critical as the only effective treatment for a stroke, tissue plasminogen activator has a 3‐h window, resulting in its use in only approximately 2% of stroke patients in the USA and even less in many parts of the world. An intravenous delivery route is also practical and allows the therapy to be provided in community hospitals. MSC and umbilical cord stem cells are also scalable and easily commercialized.
Cell therapy in stroke does not depend upon cell replacement as the transplanted cells rarely acquire a neuronal phenotype. The transplants appear to work by a trophic effect upon host brain and stimulate endogenous repair processes. MSC and umbilical cord stem cells appear to have an anti‐inflammatory and also immunosuppressive effect which may, in addition, be protective and restorative. However, it is not clear whether any new neurones generated after a stroke would establish functional connections (Lindvall & Kokaia 2006), neither is it clear whether neurogenesis after stroke occurs in humans. Repairing a structure as complex as the brain with a lifetime of accumulated learning and synaptic connections is a difficult target and any predictions of improvement with cell therapy should be tempered with caution.
Chronic neurodegenerative disease: Parkinson's disease
Parkinson's disease is a chronic neurodegenerative type due largely to loss of dopaminergic neurones in the SNc, but other non‐dopaminergic neurones are also involved. The condition has always been an attractive target for cell therapy and tissue transplantation because of the specific loss of one cell population, however, has proved more difficult to treat than expected. Early scientific enthusiasm accompanied reports of open label trials where foetal mesencepahilic dopaminergic neurones from aborted foetuses seemed to improve the motor disability in patients (Lindvall et al. 1992; Hauser et al. 1999; Brundin et al. 2000). Some of these grafts survived in the brains of transplanted patients (Hauser et al. 1999) but two randomized, controlled studies failed to show much benefit, particularly in older patients (Freed et al. 2001; Olanow et al. 2003); also there were troublesome dyskinesias (abnormal involuntary movements) reported in both studies. These poor and less than expected results combined with the ethical and logistical concerns over using aborted foetuses led to abandonment of this approach.
Other cell sources for dopaminergic neurones have been sought. Embryonic stem cells can be differentiated into dopaminergic neurones in culture and transplantation of undifferentiated embryonic stem cells into the striatum in a rodent model of Parkinson's disease led to differentiation into dopaminergic neurones and improvement in motor deficit (Bjorklund et al. 2002), one of the concerns though of transplanting such undifferentiated cells is the possible formation of teratomas. Transplantation of human embryonic stem cell‐derived neural progenitors into rodent striatum led to partial behavioural recovery in a rodent Parkinson's disease model but the cells did not acquire a full dopaminergic phenotype in the rodent brain, leading the investigators to conclude that differentiation into midbrain dopaminergic neurones should take place before transplantation (Ben‐Hur et al. 2004). In a 1 methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) primate model of Parkinson's disease, transplantation of embryonic stemm cell‐derived highly enriched dopaminergic neurones improved functional deficit (Takagi et al. 2005) but less than 1% of the dopaminergic neurones that were transplanted, survived until 14 weeks. Nuclear transfer has also been proposed as a source of embryonic stem cells, custom made for an individual. In a mouse mode of Parkinson's disease, nuclear transfer‐derived embryonic stem cells that were predifferentiated into dopaminergic neurones corrected behavioural and motor deficit (Barberi et al. 2003).
Dopaminergic neurones derived from adult stem cells are an attractive alternative to embryonic stem cell‐derived cells as they avoid the ethical problems associated with embryonic stem cells. In culture, MSC can be induced to differentiate into cells with many characteristics of dopaminergic neurones (Guo et al. 2005) expressing genes characteristic of them (Kramer et al. 2006). MSC transplanted intrastriatally in a mouse MPTP model, survived, expressed tyrosine hydroxylase activity, and promoted functional improvement (Li et al. 2001b).
Stimulating endogenous neurogenesis is an attractive approach to Parkinson's disease therapy. Activating the dopamine D3 receptor increases neurogenesis in the substantia nigra in animal models. In a 6 hydroxydopamine model, a dopamine D3 agonist restored dopaminergic cell counts in the SNc, improved striatal innervation from these cells and improved locomotor activity (Van Kampen & Eckman 2006). Using this approach early in disease development is an attractive therapy, as drugs with dopamine D3 agonist activity are already available.
Glial cell line‐derived neurotrophic factor (GDNF) was purified and cloned in 1993 and was found to have a relatively specific effect on preserving and differentiating dopaminergic neurones in culture (Lin et al. 1993). Methods of gene delivery of GDNF into the substantia nigra were developed that included both adenoviral and lentiviral vectors for testing in animal models. Replication‐defective adenoviral vector encoding human GDNF injected into the substantia nigra reduced dopaminergic cell loss in a 6 hydroxydopamine model in the rat (Choi‐Lundberg et al. 1997) and lentiviral vector delivery of GDNF in an MPTP non‐human primate model of Parkinson's disease led to extensive and sustained expression, completely prevented nigrostriatal degeneration, and improved functional outcome. (Kordower et al. 2000) These promising findings in animal models led to phase I open label trials in Parkinson's disease patients, many with promising and encouraging results (Patel et al. 2005; Slevin et al. 2005). In these trials, GDNF was continuously infused in the striatum with a catheter. However, in a randomized controlled study on 34 Parkinson's disease patients of bilateral intraputamenal infused GDNF, no significant improvement was seen in the GDNF group, there were serious device‐related issues and there were concerns about cerebellar toxicity in primates administered large doses of GDNF (Lang et al. 2006). Delivery of GDNF by continuous catheter infusion is technically difficult and a cell therapy approach where GDNF would delivered via NPC is an attractive alternative. Human NPC genetically modified to release GDNF with an inducible promoter, have been transplanted into rat and non‐human primate striatum and have survived and expressed GDNF for prolonged periods without evidence of tumour formation (Behrstock et al. 2006).
Parkinson's disease has proven more difficult to treat with stem cells than initially thought. Unregulated release of dopamine by transplanted dopamine‐producing stem cells in the striatum will likely lead to less than desired motor control and unwanted dyskinesias. Reconstituting a regulated dopaminergic pathway from the stem cells to the striatum is a daunting task; stimulating endogenous repair mechanisms and preventing further neuronal loss will have the most immediate impact. Moreover, Parkinson's disease involves more than dopaminergic degeneration in the SNc so that more than mesencephalic transplantation will be needed.
Inflammatory and immunologically driven disease: multiple sclerosis
Multiple sclerosis is a chronic inflammatory and immunologically driven demyelinating disease with either a relapsing/remission or progressive clinical course. Early in the disease, inflammation plays an important role while later the course is axonal degeneration, and degenerative mechanisms predominate (Trapp et al. 1999; Ludwin 2006).
Of all stem cell treatments for multiple sclerosis, autologous bone marrow transplantation is the most successful and advanced. The rationale for its use in this and in other severe autoimmune diseases is to ‘reset’ the immune system and to regenerate an antigen‐naïve immune system from the patient's own HSC (Burt 1997; Burt et al. 2005). First proposed and carried out in 1995, the protocols for autologous bone marrow transplantation for multiple sclerosis have undergone revision based on early experience (Burt et al. 2005). Due to high toxicity, previous myeloablative conditioning regimens were changed to non‐myeloablative and lymphoablative regimens. In the early experience, patients with chronic progressive disease did not respond; this has shifted the focus to patients with early disease, likely to have a major inflammatory component. Non‐myeloablative HSCT is being tested in a large multinational and multicentre trial, the Multiple Sclerosis International Stem (MIST) Cell Trial (Burt et al. 2005).
A further cell therapy approach to treating multiple sclerosis and neuroinflammatory diseases aims to remyelinate the central nervous system using NSC (Pluchino et al. 2003). In a mouse model for multiple sclerosis, experimental allergic encephalomyelitis, both intravenous and intraventiculocerebral delivery of syngeneic NPC led to remyelination, reduction of astrogliosis and near abolishment of functional deficits in the mice. Some of the donor cells developed neural markers and stimulated host oligodendrocyte progenitors to proliferate. In a mouse model of chronic multiple sclerosis where there are remissions and exacerbations of disease activity, intravenous injection of syngeneic neurosphere‐derived multipotent NPC led to perivascular accumulation of undifferentiated NPC in areas of inflammation (Pluchino et al. 2005). In this undifferentiated state, the NPC exhibited an immunosuppressive and anti‐inflammatory effect, triggering apoptosis in infiltrating encephalotigenic T cells and reducing further disease activity. These studies in multiple sclerosis animal models collectively suggest that stem cells may have a potent anti‐inflammatory effect when placed in a proinflammatory environment and a direct repair function (remyelination) when placed in a neurodegenerative environment (Uccelli et al. 2006).
An important principle has emerged: a common property of stem cells is their anti‐inflammatory and immunosuppressive effect in neurological diseases where there is a prominent inflammatory component. MSC and marrow stromal cells are also effective in the experimental allergic encephalomyelitis model when delivered intravenously (Zappia et al. 2005; Zhang et al. 2005). They induce T‐cell hyporesponsiveness and blunt the inflammatory response. These data suggest that the anti‐inflammatory effect of NSC and MSC might be best exploited early in multiple sclerosis when there is a prominent inflammatory component. In this role, their directed migration and collection in the perivascular space may allow them to blunt the inflammatory response.
Paediatric inborn errors of metabolism
These diseases are devastating, progressive and often fatal. They generally result from genetic lack of a critical enzyme that leads to lysosomal storage of an abnormal protein or glycolipid. The aim of the therapeutic strategy is to replace the missing enzyme in the central nervous system. Advances have been made in treating many of these diseases with enzyme replacement thanks to the orphan drug acts in the USA and Europe. The most notable success has been with Gaucher's disease (Wraith 2006). However, treatment with enzyme replacement remains expensive, lifelong, and not fully effective in many of the diseases. Moreover, many of these enzymes penetrate the blood–brain barrier poorly and recovery or stabilization of neurological function is generally poor. Gene therapy approaches have met with some success in animal models (Passini et al. 2006).
There is a long record of allogeneic haematopoietic stem cell transplantation for lysosomal storage diseases and the leucodystrophies with more than 500 cases reported (Krivit 2004). Proof of principle of this approach has been the amelioration of many neurological signs and symptoms in these patients, some not requiring further medication. Bone marrow transplantation has transformed the treatment of globoid cell leucodystrophy (Krabbe's disease), a disease that results from a deficiency of galactocerebrosidase. The infantile form leads to progressive cognitive and motor disability and death at a young age. There is an accumulation of galactocerebroside in myelin‐producing cells in both the central (oligodendrocytes) and peripheral nervous system (Schwann cells). In five patients with globoid cell leucodystrophy, the neurological deterioration was reversed in four and did not appear in one after bone marrow transplantation. All had normal leucocyte levels of galactocerebrosidase (the deficient enzyme) and magnetic resonance imaging showed a favourable signal change in three, indicating normal myelination in the brain (Krivit et al. 1998). In a set of elegant experiments, oligodendrocytes deficient in galactocerebrosidase (GALC) from the twitcher mouse were transplanted into a shiverer mouse, a mouse deficient in myelin. These GALC deficient oligodendrocytes took up galactocerebosidease from the shiverer brain and myelinated axons. These findings illustrate that enzyme presented or supplied to the brain may be taken up by these enzyme‐deficient oligodendrocytes and they can function normally as myelin producing cells (Kondo et al. 2005).
The mechanism of neurological improvement in lysosomal storage diseases with bone marrow transplantation likely involves infiltration of microglial cells derived from the donor's bone marrow into the brain with partial replacement of the missing enzyme (Krivit et al. 1995). Microglial cells in the brain are at least partially derived from the haematopoietic stem cell (Eglitis & Mezey 1997; Priller et al. 2001; Asheuer et al. 2004; Hess et al. 2004); during injury, there is an increase in the brain of microglial cells derived from the bone marrow. In a mouse model of GM1 gangliosidosis, a lysosomal storage disease caused by deficiency of beta galactosidase, transplantation with bone marrow that carries a transgene for beta galactosidase corrects the enzymatic deficiency in the brain, reverses accumulation of storage material, reduces neuronal apoptosis and produces functional improvements. The bone marrow cells are attracted into specific brain regions such as the thalamus by the up‐regulation of SDF‐1, a consequence of the inflammatory response associated with the chronic neurodegeneration (Sano et al. 2005).
As transplant donors can often be difficult to find, investigators have resorted to using unrelated umbilical cord stem cell transplants. This has been successful in a number of lysosomal diseases. In 20 children with Hurler's syndrome this technique led to reversal of enzyme deficiency in the blood (iduronidase) in 17 of the children who survived for up to 905 days, and there was significant neurocognitive improvement in the children (Staba et al. 2004). In Krabbe's disease, transplantation with unrelated umbilical cord blood led to normal blood galactocerebrosidase levels and near normal development in those chidren transplanted before the onset of symptomatic disease, but had little effect if the transplant occurred after onset, demonstrating the need for early transplantation (Escolar et al. 2005).
Neuronal ceroid lipofuscinoses are a family of progressive and devastating neurological diseases characterized by seizures, psychomotor deterioration, visual loss, and death. There is abnormal storage of fluorescent lipofuscin and ceroid in neuronal and in non‐neuronal cells. The infantile form results from a genetic mutation (CNL1) coding for an enzyme, palmitoyl‐protein thioesterase (PPT1), a soluble lipase that cleaves fatty acids from cysteine residues on proteins, during degradation of lysosomal proteins. (Lu & Hofmann 2006).This condition has received attention as the result of the US Food and Drug Administration's approval of an investigational new drug for HuCNS‐SC, proprietary human neural stem cells (Taupin 2006). The company Stem Cells Inc. is beginning a phase I trial of these cells in infantile and late infantile neuronal ceroid lipofuscinosis.
CONCLUSIONS
Neurological diseases are most difficult to treat and cure. This reflects limited neurogenesis in the human central nervous system, the presence of active inhibitors associated with myelin such as Nogo, and the inhibiting effect of glial scarring. Nevertheless, there are endogenous repair processes that represent the best target for therapy. Stimulating these with growth factor treatment is likely to have the most immediate impact. For maximal benefit, myelin‐associated inhibitors such as Nogo may need to be blocked and inhibited. The notion of direct cell replacement with exogenously administered cells is overly simplistic. The mechanism of the benefit of cell therapies upon neurological function involves trophic or chaperone effects on endogenous neurones, astrocytes, and blood vessels. This trophic effect appears to be a characteristic of stem cells. Even transplanted human embryonic germ cells appear to work by a trophic effect on endogenous neurones rather than by direct cell replacement in repairing the spinal cord (Kerr et al. 2003). The effectiveness of cell therapy will be more related to the capacity to secrete trophic and growth factors and an anti‐inflammatory effect rather than by the ability to differentiate into neurones and integrate directly into a complex nervous system. The relative advantages and disadvantages of the currently proposed cell therapies is shown in Table 2.
Table 2.
Proposed cell therapies for neurological diseases
| Cell type | Diseases a | Safety | Ethical concerns | Scalability |
|---|---|---|---|---|
| Mesenchymal stem cells – allogeneic | Stroke Multiple sclerosis | Very good; possible issues with rejection | None | Excellent |
| Autologous marrow stromal cells | Stroke (phase I trial) Traumatic brain injury | Excellent | None | Poor |
| Umbilical cord | Stroke | Very good | None | Very good to excellent |
| Multipotent adult progenitor cells | Stroke Neonatal hypoxic – ischemic | Very good | None | Excellent |
| Neural stem cells | Parkinson's disease Experimental allergic encephalomyelitis (multiple sclerosis) Stroke | Good but largely unknown | Minor (depends on source) | Moderate |
| Olfactory ensheathing cells | Spinal cord injury | Very good (autologous) | None | Good |
| Embryonic stem cells | Spinal cord injury Parkinson's disease | Teratomas if undifferentiated | Major (if embryo destroyed) | Poor at present |
| Embryonic stem cells from nuclear transfer | Parkinson's disease (mouse model) | Teratomas if undifferentiated | Major | Poor |
These are diseases where preclinical data are strongest for cell type or where phase I studies are in progress/completed.
Both authors have received grant support from Athersys, Inc, Cleveland, Ohio.
REFERENCES
- Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822. [DOI] [PubMed] [Google Scholar]
- Alvarez‐Buylla A, Seri B, Doetsch F (2002) Identification of neural stem cells in the adult vertebrate brain. Brain Res. Bull. 57, 751–758. [DOI] [PubMed] [Google Scholar]
- Alvarez‐Dolado M, Pardal R, Garcia‐Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez‐Buylla A (2003) Fusion of bone‐marrow‐derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968–973. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970. [DOI] [PubMed] [Google Scholar]
- Asheuer M, Pflumio F, Benhamida S, Dubart‐Kupperschmitt A, Fouquet F, Imai Y, Aubourg P, Cartier N (2004) Human CD34+ cells differentiate into microglia and express recombinant therapeutic protein. Proc. Natl. Acad. Sci. USA 101, 3557–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi SA, Stokes D, Augelli BJ, Digirolamo C, Prockop DJ (1998) Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats – similarities to astrocyte grafts. Proc. Natl. Acad. Sci. USA 95, 3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G (2005) Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 57, 874–882. [DOI] [PubMed] [Google Scholar]
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L (2003) Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat. Biotechnol. 21, 1200–1207. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X (2006) Identification of astrocyte‐expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 15, 407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath PM, Sprigg N (2006) Colony stimulating factors (including erythropoietin, granulocyte colony stimulating factor and analogues) for stroke. Cochrane Database Syst Rev. 3, CD005207. [DOI] [PubMed] [Google Scholar]
- Behrstock S, Ebert A, McHugh J, Vosberg S, Moore J, Schneider B, Capowski E, Hei D, Kordower J, Aebischer P, Svendsen CN (2006) Human neural progenitors deliver glial cell line‐derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther. 13, 379–388. [DOI] [PubMed] [Google Scholar]
- Ben‐Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, Reubinoff BE (2004) Transplantation of human embryonic stem cell‐derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells 22, 1246–1255. [DOI] [PubMed] [Google Scholar]
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J (2005) Human mesenchymal stem cells alter antigen‐presenting cell maturation and induce T‐cell unresponsiveness. Blood 105, 2214–2219. [DOI] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork‐Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisen J (2006) From the Cover: Neocortical neurogenesis in humans is restricted to development. Proc. Natl. Acad. Sci. USA 103, 12564–12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund LM, Sanchez‐Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O (2002) Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc. Natl. Acad. Sci. USA 99, 2344–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Hadman M, Sanberg CD, Sanberg PR (2004) Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 35, 2385–2389. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR (1998) Transplantation of cryopreserved human embryonal carcinoma‐derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp. Neurol. 149, 310–321. [DOI] [PubMed] [Google Scholar]
- Bossolasco P, Cova L, Calzarossa C, Rimoldi SG, Borsotti C, Deliliers GL, Silani V, Soligo D, Polli E (2005) Neuro‐glial differentiation of human bone marrow stem cells in vitro . Exp. Neurol. 193, 312–325. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Rossi FM, Keshet GI, Blau HM (2000) From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290, 1775–1779. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, De Lanerolle NC, Cerami C, Itri LM, Cerami A (2000) Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc. Natl. Acad. Sci. USA 97, 10526–10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP (1997) Amphibian limb regeneration: rebuilding a complex structure. Science 276, 81–87. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A (2005) Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science 310, 1919–1923. [DOI] [PubMed] [Google Scholar]
- Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, Kupsch A, Crabb L, Odin P, Gustavii B, Bjorklund A, Brooks DJ, Marsden CD, Oertel WH, Quinn NP, Rehncrona S, Lindvall O (2000) Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson's disease. Brain 123 (7), 1380–1390. [DOI] [PubMed] [Google Scholar]
- Burt RK (1997) Immune ablation and hematopoietic stem cell rescue for severe autoimmune diseases (SADS). Cancer Treat. Res. 77, 317–332. [DOI] [PubMed] [Google Scholar]
- Burt RK, Cohen B, Rose J, Petersen F, Oyama Y, Stefoski D, Katsamakis G, Carrier E, Kozak T, Muraro PA, Martin R, Hintzen R, Slavin S, Karussis D, Haggiag S, Voltarelli JC, Ellison GW, Jovanovic B, Popat U, McGuirk J, Statkute L, Verda L, Haas J, Arnold R (2005) Hematopoietic stem cell transplantation for multiple sclerosis. Arch. Neurol. 62, 860–864. [DOI] [PubMed] [Google Scholar]
- Buzanska L, Jurga M, Domanska‐Janik K (2006) Neuronal differentiation of human umbilical cord blood neural stem‐like cell line. Neurodegener. Dis. 3, 19–26. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE (2006) Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 98, 1076–1084. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M (2001a) Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J. Neurol. Sci. 189, 49–57. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M (2001b) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32, 1005–1011. [DOI] [PubMed] [Google Scholar]
- Chen J, Magavi SS, Macklis JD (2004) Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc. Natl. Acad. Sci. USA 101, 16357–16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez‐Ramos J, Chopp M (2001c) Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32, 2682–2688. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M (2003a) Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 73, 778–786. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M (2003b) Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ. Res. 92, 692–699. [DOI] [PubMed] [Google Scholar]
- Cho KJ, Trzaska KA, Greco SJ, McArdle J, Wang FS, Ye JH, Rameshwar P (2005) Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin‐1 α. Stem Cells 23, 383–391. [DOI] [PubMed] [Google Scholar]
- Choi‐Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC (1997) Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science 275, 838–841. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y (2002) Treatment of neural injury with marrow stromal cells. Lancet Neurol. 1, 92–100. [DOI] [PubMed] [Google Scholar]
- Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, Scott EW (2004) Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet 363, 1432–1437. [DOI] [PubMed] [Google Scholar]
- Coyne TM, Marcus AJ, Woodbury D, Black IB (2006) Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells 24, 2483–2492. [DOI] [PubMed] [Google Scholar]
- Doetsch F (2003) The glial identity of neural stem cells. Nat. Neurosci. 6, 1127–1134. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia‐Verdugo JM, Alvarez‐Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716. [DOI] [PubMed] [Google Scholar]
- Doggrell SA (2004) A neuroprotective derivative of erythropoietin that is not erythropoietic. Expert Opin. Invest. Drugs 13, 1517–1519. [DOI] [PubMed] [Google Scholar]
- Doucette JR (1984) The glial cells in the nerve fiber layer of the rat olfactory bulb. Anat. Rec. 210, 385–391. [DOI] [PubMed] [Google Scholar]
- Doucette R (1991) PNS‐CNS transitional zone of the first cranial nerve. J. Comp. Neurol. 312, 451–466. [DOI] [PubMed] [Google Scholar]
- Doucette R (1995) Olfactory ensheathing cells: potential for glial cell transplantation into areas of CNS injury. Histol. Histopathol. 10, 503–507. [PubMed] [Google Scholar]
- Duggal N, Schmidt‐Kastner R, Hakim AM (1997) Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain Res. 768, 1–9. [DOI] [PubMed] [Google Scholar]
- Eglitis MA, Mezey E (1997) Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc. Natl. Acad. Sci. USA 94, 4080–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Ruther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Siren AL (2002) Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 8, 495–505. [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J (2005) Allogeneic marrow stromal cells are immune rejected by MHC class I‐ and class II‐mismatched recipient mice. Blood 106, 4057–4065. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork‐Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317. [DOI] [PubMed] [Google Scholar]
- Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J (2005) Transplantation of umbilical‐cord blood in babies with infantile Krabbe's disease. N. Engl. J. Med. 352, 2069–2081. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, Geraghty T, Mackay‐Sim A (2005) Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain 128, 2951–2960. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, Dumouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S (2001) Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N. Engl. J. Med. 344, 710–719. [DOI] [PubMed] [Google Scholar]
- Guo L, Yin F, Meng HQ, Ling L, Hu‐He TN, Li P, Zhang CX., Yu S, Duan DS, Fan HX (2005) Differentiation of mesenchymal stem cells into dopaminergic neuron‐like cells in vitro . Biomed. Environ. Sci. 18, 36–42. [PubMed] [Google Scholar]
- Hauser RA, Freeman TB, Snow BJ, Nauert M, Gauger L, Kordower JH, Olanow CW (1999) Long‐term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch. Neurol. 56, 179–187. [DOI] [PubMed] [Google Scholar]
- Hess DC, Abe T, Hill WD, Studdard AM, Carothers J, Masuya M, Fleming PA, Drake CJ, Ogawa M (2004) Hematopoietic origin of microglial and perivascular cells in brain. Exp. Neurol. 186, 134–144. [DOI] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin‐Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ (2004) SDF‐1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 63, 84–96. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ (2004) Directed migration of neural stem cells to sites of CNS injury by the stromal cell‐derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA 101, 18117–18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz‐Gonzalez XR, Reyes M, Lenvik T, Lund T, Du Blackstad MJ, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002a) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM (2002b) Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp. Hematol. 30, 896–904. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Henderson D, Blackstad M, Chen A, Miller RF, Verfaillie CM (2003) Neuroectodermal differentiation from mouse multipotent adult progenitor cells. Proc. Natl. Acad. Sci. USA 100 (Suppl. 1), 11854–11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabos P, Ehtesham M, Black KL, JS (2003) Neural stem cells as delivery vehicles. Expert Opin. Biol. Ther. 3, 759–770. [DOI] [PubMed] [Google Scholar]
- Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T (2006) Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow‐derived neuronal cells. Circulation 113, 701–710. [DOI] [PubMed] [Google Scholar]
- Kerr DA, Llado J, Shamblott MJ, Maragakis NJ, Irani DN, Crawford TO, Krishnan C, Dike S, Gearhart JD, Rothstein JD (2003) Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J. Neurosci. 23, 5131–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, Svendsen CN (2005) GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum. Gene Ther. 16, 509–521. [DOI] [PubMed] [Google Scholar]
- Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, Greschat S, Knipper A, Bender J, Degistirici O, Gao J, Caplan AI, Colletti EJ, Almeida‐Porada G, Muller HW, Zanjani E, Wernet P (2004) A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J. Exp. Med. 200, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Wenger DA, Gallo V, Duncan ID (2005) Galactocerebrosidase‐deficient oligodendrocytes maintain stable central myelin by exogenous replacement of the missing enzyme in mice. Proc. Natl. Acad. Sci. USA 102, 18670–18675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, Decesare S, Jovin T, Zafonte R, Lebowitz J, Flickinger JC, Tong D, Marks MP, Jamieson C, Luu D, Bell‐Stephens T, Teraoka J (2005) Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J. Neurosurg. 103, 38–45. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, Decesare S, Elder EM, McGrogan M, Reitman MA, Bynum L (2000) Transplantation of cultured human neuronal cells for patients with stroke. Neurology 55, 565–569. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P (2000) Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 290, 767–773. [DOI] [PubMed] [Google Scholar]
- Kramer BC, Woodbury D, Black IB (2006) Adult rat bone marrow stromal cells express genes associated with dopamine neurons. Biochem. Biophys. Res. Commun. 343, 1045–1052. [DOI] [PubMed] [Google Scholar]
- Krivit W (2004) Allogeneic stem cell transplantation for the treatment of lysosomal and peroxisomal metabolic diseases. Springer Semin. Immunopathol. 26, 119–132. [DOI] [PubMed] [Google Scholar]
- Krivit W, Shapiro EG, Peters C, Wagner JE, Cornu G, Kurtzberg J, Wenger DA, Kolodny EH, Vanier MT, Loes DJ, Dusenbery K, Lockman LA (1998) Hematopoietic stem‐cell transplantation in globoid‐cell leukodystrophy. N. Engl. J. Med. 338, 1119–1126. [DOI] [PubMed] [Google Scholar]
- Krivit W, Sung JH, Shapiro EG, Lockman LA (1995) Microglia: the effector cell for reconstitution of the central nervous system following bone marrow transplantation for lysosomal and peroxisomal storage diseases. Cell Transplant. 4, 385–392. [DOI] [PubMed] [Google Scholar]
- Kucia M, Ratajczak J, Ratajczak MZ (2005) Are bone marrow stem cells plastic or heterogenous – that is the question. Exp. Hematol. 33, 613–623. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba‐Surma E, Majka M, Ratajczak J, Ratajczak MZ (2006) A population of very small embryonic‐like (VSEL) CXCR4(+) SSEA‐1(+) Oct–4+ stem cells identified in adult bone marrow. Leukemia 20, 857–869. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M (2006) Randomized controlled trial of intraputamenal glial cell line‐derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 59, 459–466. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Wang L, Lu M, Chopp M (2001a) Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 56, 1666–1672. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M (2001b) Intracerebral transplantation of bone marrow stromal cells in a 1‐methyl‐4‐phenyl‐1–3,6‐tetrahydropyridine mouse model of Parkinson's disease. Neurosci. Lett. 316, 67–70. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M (1999) Temporal profile of nestin expression after focal cerebral ischemia in adult rat. Brain Res. 838, 1–10. [DOI] [PubMed] [Google Scholar]
- Lima C, Pratas‐Vital J, Escada P, Hasse‐Ferreira A, Capucho C, Peduzzi JD (2006) Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J. Spinal. Cord Med. 29, 191–203; discussion 204–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line‐derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z (2006) Stem cells for the treatment of neurological disorders. Nature 441, 1094–1096. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Widner H, Rehncrona S, Brundin P, Odin P, Gustavii B, Frackowiak R, Leenders KL, Sawle G, Rothwell JC, Björklund A, Marsden CD (1992) Transplantation of fetal dopamine neurons in Parkinson's disease: one‐year clinical and neurophysiological observations in two patients with putaminal implants. Ann. Neurol. 31, 155–165. [DOI] [PubMed] [Google Scholar]
- Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD (2006) Neuroprotection by PlGF gene‐modified human mesenchymal stem cells after cerebral ischaemia. Brain 129 (Pt10), 2734–2745. Epub August 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JY, Hofmann SL (2006) Thematic review series: lipid posttranslational modifications. Lysosomal metabolism of lipid‐modified proteins. J. Lipid. Res. 47, 1352–1357. [DOI] [PubMed] [Google Scholar]
- Ludwin SK (2006) The pathogenesis of multiple sclerosis: relating human pathology to experimental studies. J. Neuropathol. Exp. Neurol. 65, 305–318. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD (2000) Induction of neurogenesis in the neocortex of adult mice. Nature 405, 951–955. [DOI] [PubMed] [Google Scholar]
- Makinen S, Kekarainen T, Nystedt J, Liimatainen T, Huhtala T, Narvanen A, Laine J, Jolkkonen J (2006) Human umbilical cord blood cells do not improve sensorimotor or cognitive outcome following transient middle cerebral artery occlusion in rats. Brain Res. 1123, 207–215. [DOI] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Baradez MO, Navran S, Zhao J, Urban R, Tilton R, Denner L (2005) Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif. 38, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR (2000) Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290, 1779–1782. [DOI] [PubMed] [Google Scholar]
- Mezey E, Key S, Vogelsang G, Szalayova I, Lange GD, Crain B (2003a) Transplanted bone marrow generates new neurons in human brains. Proc. Natl. Acad. Sci. USA 100, 1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Nagy A, Szalayova I, Key S, Bratincsak A, Baffi J, Shahar T (2003b) Comment on ‘Failure of bone marrow cells to transdifferentiate into neural cells in vivo’. Science 299, 1184; author reply 1184. [DOI] [PubMed] [Google Scholar]
- Muñoz‐Sanjuán I, Brivanlou AH (2002) Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 3, 271–280. [DOI] [PubMed] [Google Scholar]
- Newcomb JD, Ajmo CT Jr, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE (2006) Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant. 15, 213–223. [DOI] [PubMed] [Google Scholar]
- Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD (2005) I.V. infusion of brain‐derived neurotrophic factor gene‐modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience 136, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB (2003) A double‐blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann. Neurol. 54, 403–414. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY (2002) Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 20, 1103–1110. [DOI] [PubMed] [Google Scholar]
- Park KI, Teng YD, Snyder EY (2002) The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 20, 1111–1117. [DOI] [PubMed] [Google Scholar]
- Passini MA, Dodge JC, Bu J, Yang W, Zhao Q, Sondhi D, Hackett NR, Kaminsky SM, Mao Q, Shihabuddin LS, Cheng SH, Sleat DE, Stewart GR, Davidson BL, Lobel P, Crystal RG (2006) Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis. J. Neurosci. 26, 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS (2005) Intraputamenal infusion of glial cell line‐derived neurotrophic factor in PD: a two‐year outcome study. Ann. Neurol. 57, 298–302. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G (2003) Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 422, 688–694. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G (2005) Neurosphere‐derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 436, 266–271. [DOI] [PubMed] [Google Scholar]
- Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, Dong Z, Hodges H, Price J, Sinden JD (2006) A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp. Neurol. 199, 143–155. [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez‐Klett F, Prass K, Bechmann I, De Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U (2001) Targeting gene‐modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat. Med. 7, 1356–1361. [DOI] [PubMed] [Google Scholar]
- Radtke C, Akiyama Y, Brokaw J, Lankford KL, Wewetzer K, Fodor WL, Kocsis JD (2004) Remyelination of the nonhuman primate spinal cord by transplantation of H‐transferase transgenic adult pig olfactory ensheathing cells. FASEB J. 18, 335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S (1928) Degeneration and Regeneration of the Nervous System. London: Oxford University Press. [Google Scholar]
- Sano R, Tessitore A, Ingrassia A, D’Azzo A (2005) Chemokine‐induced recruitment of genetically modified bone marrow cells into the CNS of GM1‐gangliosidosis mice corrects neuronal pathology. Blood 106, 2259–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Lankford KL, Zemedkun M, Kocsis JD (2004) Identified olfactory ensheathing cells transplanted into the transected dorsal funiculus bridge the lesion and form myelin. J. Neurosci. 24, 8485–8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schabitz WR (2005) The hematopoietic factor G‐CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Invest. 115, 2083–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME (2004) Nogo and axon regeneration. Curr. Opin. Neurobiol. 14, 118–124. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, Lu M, Raginski K, Vanguri P, Smith A, Chopp M (2006) Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J. Cereb. Blood Flow Metab. 27, 6–13. [DOI] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, Li H (2004) Functional recovery of stroke rats induced by granulocyte colony‐stimulating factor‐stimulated stem cells. Circulation 110, 1847–1854. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B (2005) Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line‐derived neurotrophic factor. J. Neurosurg. 102, 216–222. [DOI] [PubMed] [Google Scholar]
- Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison‐Thacker J, Wood S, Wenger DA, Rubinstein P, Hopwood JJ, Krivit W, Kurtzberg J (2004) Cord‐blood transplants from unrelated donors in patients with Hurler's syndrome. N. Engl. J. Med. 350, 1960–1969. [DOI] [PubMed] [Google Scholar]
- Sun W, Buzanska L, Domanska‐Janik K, Salvi RJ, Stachowiak MK (2005) Voltage‐sensitive and ligand‐gated channels in differentiating neural stem‐like cells derived from the nonhematopoietic fraction of human umbilical cord blood. Stem Cells 23, 931–945. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Caldwell MA, Ostenfeld T (1999) Human neural stem cells: isolation, expansion and transplantation. Brain Pathol. 9, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen CN, Caldwell MA, Shen J, Ter Borg MG, Rosser AE, Tyers P, Karmiol S, Dunnett SB (1997) Long‐term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp. Neurol. 148, 135–146. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Clarke DJ, Rosser AE, Dunnett SB (1996) Survival and differentiation of rat and human epidermal growth factor‐responsive precursor cells following grafting into the lesioned adult central nervous system. Exp. Neurol. 137, 376–388. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T (2004) Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest. 114, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N (2005) Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J. Clin. Invest. 115, 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P (2006) HuCNS‐SC (StemCells). Curr. Opin. Mol. Ther. 8, 156–163. [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW (2002) Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416, 542–545. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O (2006) Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells 24, 739–747. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Ransohoff R, Rudick R (1999) Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr. Opin. Neurol. 12, 295–302. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Zappia E, Benvenuto F, Frassoni F, Mancardi G (2006) Stem cells in inflammatory demyelinating disorders: a dual role for immunosuppression and neuroprotection. Expert Opin. Biol. Ther. 6, 17–22. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Eckman CB (2006) Dopamine D3 receptor agonist delivery to a model of Parkinson's disease restores the nigrostriatal pathway and improves locomotor behavior. J. Neurosci. 26, 7272–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE (2004) Infusion of human umbilical cord blood cells in a rat model of stroke dose‐dependently rescues behavioral deficits and reduces infarct volume. Stroke 35, 2390–2395. [DOI] [PubMed] [Google Scholar]
- Vendrame M, Gemma C, Pennypacker KR, Bickford PC, Davis Sanberg C, Sanberg PR, Willing AE (2006) Cord blood rescues stroke‐induced changes in splenocyte phenotype and function. Exp. Neurol. 199, 191–200. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Snyder EY (1999) Establishment and properties of neural stem cell clones: plasticity in vitro and in vivo . Brain Pathol. 9, 569–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss HU, Uluc AM, Dyke JP, Watts R, Kobylarz EJ, McCandliss BD, Heier LA, Beattie BJ, Hamacher KA, Vallabhajosula S, Goldsmith SJ, Ballon D, Giacino JT, Schiff ND (2006) Possible axonal regrowth in late recovery from the minimally conscious state. J. Clin. Invest. 116, 2005–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL (2002) Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297, 2256–2259. [DOI] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Chen HX, Chang LJ, Roper SN, Scheffler B, Steindler DA (2006) Derivation and large‐scale expansion of multipotent astroglial neural progenitors from adult human brain. Development 133, 3671–3681. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M (2004) Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 35, 1732–1737. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Johansson CB, Trejo A, Blau HM (2003) Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat. Cell Biol. 5, 959–966. [DOI] [PubMed] [Google Scholar]
- Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez‐Ramos J, Sanberg PR (2003) Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J. Neurosci. Res. 73, 296–307. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Reynolds K, Black IB (2002) Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J. Neurosci. Res. 69, 908–917. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 61, 364–370. [DOI] [PubMed] [Google Scholar]
- Wraith JE (2006) Limitations of enzyme replacement therapy: current and future. J. Inherit. Metab. Dis. 29, 442–447. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J, Deans R, Hess DC, Carroll JE, Borlongan CV (2006a) Transplantation of cryopreserved human bone marrow‐derived multipotent adult progenitor cells for neonatal hypoxic‐ischemic injury: targeting the hippocampus. Rev. Neurosci. 17, 215–225. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Yu G, Xu L, Mays RW, Kovach J, Deans RJ, Hess DC, Carroll JE, Borlongan CV (2006b) Behavioral and histological characterization of intrahippocampal grafts of human bone marrow‐derived multipotent progenitor cells in neonatal rats with hypoxic‐ischemic injury. Cell Transplant. 15, 231–238. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A (2005) Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T‐cell anergy. Blood 106, 1755–1761. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M (2005) Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp. Neurol. 195, 16–26. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC (2002) Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. 174, 11–20. [DOI] [PubMed] [Google Scholar]


