Abstract
Abstract. The purpose of this study was to examine the effect of recombinant human keratinocyte growth factor (rHuKGF) on clinically manifest acute oral mucositis. The animal model utilized in this investigation was ventral tongue epithelium of C3H/Neu mice. In a first experiment, graded single doses were applied in order to define dose effect and time course of acute mucosal ulceration, as a clinically relevant endpoint. Irradiation was given to a 3 × 3 mm2 test field in the centre of the ventral tongue with 25 kV X‐rays. A single dose of 18 Gy, i.e. a dose after which ulceration is expected in more than 99% of the animals, was applied in subsequent experiments. In the study group of 20 animals, rHuKGF was applied at a daily dose of 5 mg/kg subcutaneously from the time of first diagnosis of ulcer for a maximum of 5 days. In the control group, phosphate‐buffered saline was used as a placebo. The time course of ulceration, i.e. individual ulcer duration, was analysed in both the control group without rHuKGF and the study group. Irradiation with graded single doses yielded an ED50 of 11.5 ± 0.7 Gy (logit analysis). In responding animals, the latent time to first diagnosis of ulceration and the individual ulcer duration were independent of dose. Mean latency (± standard deviation) was 10.5 ± 0.5 days, mean ulcer duration 3.9 ± 0.6 days for doses 11, 13 and 16 Gy. After a dose of 18 Gy, 39 animals developed ulceration after a mean latency of 9.3 ± 0.3 days (control and KGF‐treated). The average ulcer duration was 4.2 ± 0.9 days in the placebo group and 4.8 ± 0.8 days in the KGF group (P = 0.02). We conclude that when rHuKGF treatment is delayed until radiation‐induced ulcers are manifest, the therapeutic activity previously reported with other treatment schedules was not observed and there was a slight prolongation of duration of ulceration. These data suggest that during tumour radiotherapy, effective rHuKGF therapy schedules should include administration before the onset of ulcerative mucositis.
Keywords: growth factors, keratinocyte growth factor, mouse model, oral mucositis, oral ulceration, radiation reaction
Introduction
Keratinocyte growth factor (KGF) is a member of the fibroblast growth factor family (FGF‐7). KGF is almost exclusively synthesized and released by fibroblasts and other mesenchymal cells. Target cells for this growth factor, expressing the specific receptor, are epithelial cells in a variety of tissues. These include epidermis and hair follicles, oral and gastrointestinal epithelium, corneal epithelium, lung epithelium, urothelium, and prostate epithelium (Rubin et al. 1995). Hence, KGF is thought to be a paracrine mediator of mesenchymal‐epithelial communication.
The specific KGF receptor is a tyrosine kinase, which is a splice variant of the fgfr‐2 gene. Activation of the receptor by KGF induces a variety of responses, which include stimulation of epithelial proliferation and modification of migration and differentiation processes. KGF plays a predominant role in wound healing. In mouse dermis, a marked increase in transcriptional activity was observed (Werner et al. 1992; Werner et al. 1994; Werner et al. 1996; Werner & Munz 1998). Systemic treatment with exogenous KGF induces hyperproliferation in various epithelia, eventually resulting in organ hyperplasia (Rubin et al. 1995). Topical application stimulates wound healing in various animal models (Staiano‐Coico et al. 1993).
Oral epithelium is a typical turnover tissue, with a well‐tuned balance between cell production in the germinal layer and cell loss via differentiation and mechanical stress at the surface. Radiation injury in these tissues results in impairment of proliferation, and as a consequence, results in a cellular supply insufficient to the postmitotic, functional cell layers. Cell loss is dependent on the natural turnover rate, but is independent of radiation exposure, and therefore continues at its normal rate after irradiation. Cells present at the time of irradiation undergo near normal differentiation (Dörr et al. 1996; Liu et al. 1996). The radiation‐induced imbalance between cell production and loss eventually results in complete cellular depletion. In skin and mucosae this reaction manifests as denudation and ulceration, associated with a significant impairment of the epithelial barrier.
In recent studies (2000a, 2000b; Dörr 2001; Dörr et al. 2001) in mouse oral mucosa, a marked mucoprotective effect of rHuKGF was demonstrated if the growth factor was administered before or after single dose or fractionated irradiation, or during fractionated radiotherapy. However, in all these studies, KGF was given before the onset of a clinically manifest response of the mucosa. The present study was initiated in order to investigate the effect of rHuKGF on a clinically present radiation‐induced ulceration in the mouse tongue model.
Materials and methods
Animals and housing
For all experiments, female mice of the C3H/Neu strain, bred in the colony of the Medical Faculty Carl Gustav Carus, Technical University of Dresden, were used. The animals were bred and housed under specified pathogen‐free (SPF) conditions with controlled humidity (30‐50%) and temperature (21‐24 °C). A 12‐h light/12‐h dark rhythm with lights on between 06.00 and 18.00 h was maintained.
The mice were kept in Macrolon® cages on sawdust bedding (Sniff 3/4, Altrogge, Lage, Germany). Standard mouse diet (Altromin 1326, Altrogge) and filtered city tap water from standard perspex drinking bottles were provided ad libitum.
Irradiation technique
The technique and set‐up for local administration of radiation to the ventral tongue surface has been reported elsewhere (Dörr et al. 2000a, ; Dörr, Brankovic & Hartmann 2000).
In brief, a test area of the lower tongue surface was irradiated with a DARPAC 150‐MC device (Forward Raytech Ltd, UK), which was operated at 25 kV with a tube current of 20 mA. The beam filter was 0.3 mm Al. The dose rate at the focus‐to‐skin distance of 15 cm was 3.78 Gy/min, resulting in an irradiation time of 4.76 min for a dose of 18 Gy. The dose rate was checked regularly during the standard procedures for radiotherapy units and found to be constant, which allowed for definition of the dose by adjustment of the irradiation time.
For irradiation, the mice were immobilized with sodium pentobarbitone (Narcoren®, Rhone Merieux) at a dose of about 60 mg/kg, administered intraperitoneally. The animals were placed in the central bore (diameter 2.5 cm) of a prewarmed aluminium block (∼35 °C) in a supine position. The tongue was guided through a hole (diameter 3 mm) in the roof of the block by means of a forceps, and the upper surface fixed to the block by double adhesive tape. The head then was supported by a polystyrene wedge in order to avoid local hypoxia by traction at the base of the tongue.
The treatment area was defined by a 3 × 3 mm2 window in an aluminium plate (thickness: 1 mm), which was positioned over the central portion of the tongue in order to shield tip, margins and base of the ventral tongue.
Recombinant human keratinocyte growth factor (rHuKGF)
Recombinant human KGF was kindly provided by AMGEN Inc., U.S.A. The growth factor was produced in Escherichia coli and purification to homogeneity was achieved by conventional chromatography. Tests for endotoxins were performed by AMGEN Inc.
The lyophylisate was dissolved in the diluent provided by the company to a final concentration of 5.0 mg/mL. This solution was further diluted in sterile phosphate buffered saline (PBS) to a final concentration of 1 mg/mL. The solution was newly prepared immediately before injection. The injection volume was 0.1‐0.12 mL per mouse for a dose of 5 mg/kg per injection; administration was subcutaneously. Dosage and route of administration were based on previous studies in mice (2000a, 2000b; Dörr 2001; Dörr et al. 2001; Farrell et al. 1998; Farrell et al. 1999).
Experimental design
Dose‐effect study.
In a first experiment, graded single doses of 6, 9, 11, 13 or 16 Gy were applied to the lower tongue surface of 8 animals in each dose group.
Single dose irradiation, KGF versus placebo treatment.
A single dose of 18 Gy was applied to the ventral tongue surface in order to induce ulceration in a total of 40 animals. Scoring of the tongue response was performed daily from day 5 after irradiation until complete re‐epithelialization of all ulcers was observed (day 16). Phosphate‐buffered saline (PBS) or rHuKGF was injected daily from the day of first diagnosis of ulcer until re‐epithelialization. KGF was applied at a dose of 5 mg/kg subcutaneously for each injection, corresponding to a daily volume of 0.1‐0.12 mL. PBS injection volumes were 0.1 mL/day. When ulcers lasted for more than 5 days, a maximum of five injections was given.
Follow‐up, end‐point, and statistical analysis
In both experimental groups, scoring of radiation‐induced changes in the tongue epithelium was done daily from day 5 until complete re‐epithelialization. For this, animals were immobilized by ultra‐short anaesthesia with Methohexitone (Brevimytal®, Lilly) at a dose of ∼40 mg/kg intraperitoneally (Dörr & Weber‐Frisch 1999), and the tongue was fixed carefully with forceps. Scoring was performed under a cold light source. The frequency of responding animals, latent time and, most importantly, the duration of the individual ulcers were assessed.
Dose‐response analysis in experiment 1 was done by probit analysis assuming a normal log distribution, which results in ED50 values and their standard deviation σ (SAS Institute 1990). In addition, P‐values for the effect of dose on ulcer induction were calculated, based on the slope of the regression line of the probit curve.
For analyses of the effect of KGF on ulcer duration, a two‐sample t‐test was used (SPSS 1999).
Results
Dose‐effect study
The dose‐effect curve for ulcer induction by single dose irradiation is shown in Fig. 1. The ED50‐value for single dose irradiation alone was 11.5 ± 0.7 Gy. This is in good agreement with results from previous studies with the same animal model (2000a, 2000b; Dörr 2001; Dörr et al. 2001; Dörr & Weber‐Frisch 1995a, 1995b). The P‐value for dose‐dependence of ulcer incidence was 0.006.
Figure 1.
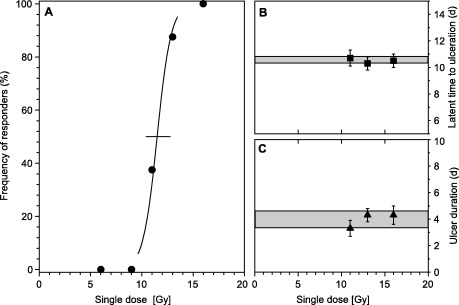
Dose‐response and time course of mucosal ulceration. Panel A, dose‐effect curve, generated by logit analysis, for the frequency of animals developing ulcer. The error bar represents the 95% confidence interval of the ED50. Panel B, latent time to ulceration, i.e. time between irradiation and first diagnosis of ulcer. Error bars represent ±1 standard deviation (SD). The shaded area indicates the mean value ±1 SD for all responding animals. Panel C, ulcer duration. Data points represent the mean value of all responders in the individual dose groups; error bars display 1 SD. The shaded area illustrates the mean value of all responding animals ±1 SD.
The time course of ulceration is also illustrated in Fig. 1. Neither ulcer duration nor latent time was dose‐dependent. The ulcers on average lasted for 3.9 ± 0.6 days (mean ± SD), with a range from 2 to 5 days. Mean latent times to first diagnosis were 10.5 ± 0.5 days. The treatment was well tolerated, similar to previous studies (2000a, 2000b; Dörr 2001; Dörr et al. 2001; Dörr & Weber‐Frisch 1995a, 1995b), and no acute morbidity was observed.
Placebo or KGF treatment
Single dose irradiation with 18 Gy induced ulceration in 39/40 animals; one animal in the KGF group did not develop an ulcer. The average latent time to ulceration was 9.3 ± 0.5 days and 9.2 ± 0.4 days in the placebo and the KGF group, respectively (P = 0.7). Overall latency was 9.3 ± 0.3 days.
Ulcer duration is illustrated in Fig. 2. In the PBS control group, mean ulcer duration was 4.2 ± 0.9 days, with a range from 3 to 5 days, and was not significantly different from the duration in the dose‐effect study (P = 0.35). In the KGF group, ulcer duration was 4.8 ± 0.8 days, with a range from 3 to 6 days. Ulcer duration in the KGF group was significantly longer than in the placebo group (P = 0.0218).
Figure 2.
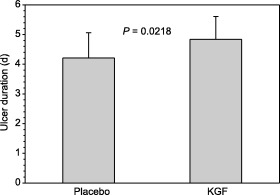
Ulcer duration in placebo and KGF treated animals. The bars represent the mean values of individual ulcer duration in 19 animals treated with PBS as a placebo and in 20 animals treated with KGF. Placebo or KGF were administered daily from the day of first diagnosis of ulcer until clinical re‐epithelialization, or to a maximum of five injections if ulcers persisted longer than 5 days. Error bars represent 1 SD
Discussion
A number of prophylactic or therapeutic measures for the amelioration of acute mucosal responses to radiation have been tested in animal models as well as in patients (Dörr et al. 1997; Marks 1997; Dörr & Riesenbeck 2000). Most of these are antiseptic or antibiotic mouthwashes, aimed at a reduction of secondary infections. In addition, stimulation of the immune system has been introduced, e.g. by G‐CSF or GM‐CSF. Other treatment approaches included the application of growth factors, like interleukin‐1 [Zaghloul et al. 1994], epidermal growth factor (Potten et al. 1995; Sonis et al. 1992), tumour necrosis factor‐α (Potten et al. 1995), transforming growth factor‐α (Guttenberger et al. 1991; Potten et al. 1995), insulin‐like growth factor (IGF) I and II [Potten et al. 1995), acidic fibroblast growth factor (Potten et al. 1995) or transforming growth factor‐β (Sonis et al. 1994). However, so far no effective treatment has been identified in clinical studies.
KGF is known to stimulate proliferation of epithelial cells, including those of the mucosal lining in the upper and lower gastrointestinal tract and skin. A radioprotective potential for rHuKGF in these tissues has already been demonstrated for epithelia of the oral cavity, oesophagus and intestine (2000a, 2000b; Dörr 2001; Dörr et al. 2001; Farrell et al. 1998; Farrell et al. 1999). In all these studies, rHuKGF has been administered before the onset of the clinical radiation effects, even when administered after the cessation of radiation therapy.
The present study was initiated to determine the mucoprotective potential of KGF applied after development of the acute radiation response in oral mucosa in order to test the healing potential of rHuKGF once ulcers are manifest. The studies were performed in mouse tongue epithelium, using ulceration as the most clinically relevant endpoint.
The results of the dose‐effect study, both dose‐dependence and time course of the response, were in excellent agreement with earlier investigations in the same model (Dörr et al. 2000, 2000a, 2000b; Dörr 2001; Dörr et al. 2001).
In order to test for modification of a manifest erosive response of the mucosa to irradiation, a single dose of 18 Gy, expected to induce ulcers in almost all of the animals, was applied. Ulcer frequency was 97.5% (39/40). The latent time to ulceration was not significantly different from that in the dose‐effect study. In the placebo group, ulcer duration was 4.2 days, which is in agreement with that in the dose‐effect study. However, KGF resulted in slightly but significantly longer ulcer duration of 4.8 days (P = 0.02).
In conclusion, in these experiments rHuKGF does not ameliorate a manifest ulcerative response of oral epithelium. This is in contrast to previous studies where various schedules of rHuKGF administration were observed to enhance radiotolerance of the oral epithelium dramatically in the same model. As these studies all involved treatment prior to ulcer formation, even when applied postradiation, it may be that a schedule involving preulcer rHuKGF therapy is required for maximum therapeutic benefit. Hence, in clinical studies, rHuKGF administration should also include pretreatment and possibly cease before focal or confluent mucositis is expected. Usually, the latter is seen about 9 days after a dose of 20 Gy was applied (Van der Schueren et al. 1990), i.e. in the third week of conventional radiotherapy with 5 × 2 Gy/week.
Acknowledgements
All experiments were performed according to the current animal welfare legislation with permission of Regierungspräsidium Dresden. The authors are grateful to Mrs Dorothee Pfitzmann for skilful technical assistance.
References
- Dörr W (2001) Modification of acute radio (chemo) therapy effects in squamous epithelia by keratinocyte growth factor. Radiother. Oncol. 60 (Suppl. 2), S8. [Google Scholar]
- Dörr W, Brankovic K, Hartmann B (2000) Repopulation in mouse oral mucosa: changes in the effect of dose fractionation. Int. J. Radiat. Biol. 76, 383. [DOI] [PubMed] [Google Scholar]
- Dörr W, Dölling‐Jochem I, Baumann M, Herrmann T (1997) The therapeutic management of radiogenic oral mucositis. Strahlenther. Onkol. 173, 183. [DOI] [PubMed] [Google Scholar]
- Dörr W, Emmendörfer H, Weber‐Frisch M (1996) Tissue kinetics in mouse tongue mucosa during daily fractionated radiotherapy. Cell Prolif. 29, 495. [DOI] [PubMed] [Google Scholar]
- Dörr W, Lacmann A, Noack R, Spekl K, Rex K, Farrell CL (2000a) Modulation of radiation effects in tissues by keratinocyte growth factor (KGF) In: Heinemann G, Müller W‐U, eds. Strahlenbiologie und Strahlenschutz. Individuelle Strahlenempfindlichkeit und Ihre Bedeutung für Den Strahlenschutz, Vol. I Köln: TÜV‐Verlag GmbH; p. 209. [Google Scholar]
- Dörr W, Noack R, Spekl K, Farrell CL (2000b) Amelioration of radiation‐induced oral mucositis by keratinocyte growth factor (rhKGF): experimental studies. Int. J. Radiat. Oncol. Biol. Phys. 46, 729. [Google Scholar]
- Dörr W, Noack R, Spekl K, Farrell CL (2001) Modification of oral mucositis by keratinocyte growth factor: single radiation exposure. Int. J. Radiat. Biol. 77, 341. [DOI] [PubMed] [Google Scholar]
- Dörr W, Riesenbeck D (2000) Mundhöhle In: Dörr W, Zimmermann JS, Seegenschmiedt MH, eds. Nebenwirkungen in der Radioonkologie, p. 130 Munich: Urban and Vogel. [Google Scholar]
- Dörr W, Weber‐Frisch M (1995a) Effect of changing weekly dose on accelerated repopulation during fractionated irradiation of mouse tongue mucosa. Int. J. Radiat. Biol. 67, 577. [DOI] [PubMed] [Google Scholar]
- Dörr W, Weber‐Frisch M (1995b) Repopulation response of mouse oral mucosa during unconventional radiotherapy protocols. Radiother. Oncol. 37, 230. [DOI] [PubMed] [Google Scholar]
- Dörr W, Weber‐Frisch M (1999) Short‐term immobilisation of mice by methohexitone. Laboratory Anim. 33, 35. [DOI] [PubMed] [Google Scholar]
- Farrell CL, Bready JV, Rex KL, Chen JN, Dipalma CR, Whitcomb KL, Yin S, Hill DC, Wiemann B, Starnes CO, Havill AM, Lu ZN, Aukerman SL, Pierce GF, Thomason A, Potten CS, Ulich TR, Lacey DL (1998) Keratinocyte growth factor protects mice from chemotherapy and radiation‐induced gastrointestinal injury and mortality. Cancer Res. 58, 933. [PubMed] [Google Scholar]
- Farrell CL, Rex KL, Kaufman SA, Di Palma CR, Chen JN, Scully S, Lacey DL (1999) Effects of keratinocyte growth factor in the squamous epithelium of the upper aerodigestive tract of normal and irradiated mice. Int. J. Radiat. Biol. 75, 609. [DOI] [PubMed] [Google Scholar]
- Guttenberger R, Feng Y, Hunter N, Ang KK, Price R (1991) Effects of TGF‐α on radiation response in lip and jejunal mucosa in mice In: Chapman JD, Dewey WC, Whitmore GF, eds. Radiation Research: a Twentieth‐Century Perspective, Vol. I, p. 151 San Diego‐New York‐Boston: Academic Press Inc. [Google Scholar]
- Liu K, Kasper M, Trott KR (1996) Changes in keratinocyte differentiation during accelerated repopulation of the irradiated mouse epidermis. Int. J. Radiat. Biol. 69, 763. [DOI] [PubMed] [Google Scholar]
- Marks JE (1997) Mucosal protectants and their application for head and neck chemoirradiation. Curr. Opin. Oncol. 9, 267. [DOI] [PubMed] [Google Scholar]
- Potten CS, Owen G, Hewitt D, Chadwick CA, Hendry H, Lord BI, Woolford LB (1995) Stimulation and inhibition of proliferation in the small intestinal crypts of the mouse after in vivo administration of growth factors. Gut 36, 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JS, Bottaro DB, Chedid M, Miki T, Ron D, Cheon HG, Taylor WG, Fortney E, Sakata H, Finch PW, La Rochelle WJ (1995) Keratinocyte growth factor. Cell Biol. Int. 19, 399. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc Cary NC (1990) SAS/STAT User S Guide, Version 6, p. 892.
- Sonis ST, Costa JWJ, Evitts SM, Lindquist LE, Nicolson M (1992) Effect of epidermal growth factor on ulcerative mucositis in hamsters that receive cancer chemotherapy. Oral Surg. Oral Med. Oral Pathol. 74, 749. [DOI] [PubMed] [Google Scholar]
- Sonis ST, Lindquist L, Van Vugt A, Stewart AA, Stam K, Qu Gy, Iwata KK, Haley JD (1994) Prevention of chemotherapy‐induced ulcerative mucositis by transforming growth factor beta 3. Cancer Res. 54, 1135. [PubMed] [Google Scholar]
- SPSS Inc (1999) Base 9.0 Applications Guide, p. 103.
- Staiano‐Coico L, Krueger JG, Rubin JS, Dlimi S, Vallat VP, Valentino L, Fahey T III, Hawes A, Kingston G, Madden MR (1993) Human keratinocyte growth factor effects in a porcine model of epidermal wound healing. J. Exp. Med. 178, 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Schueren E, Van en Bogaert W, Vanuytsel L, Van Limbergen E (1990) Radiotherapy by multiple fractions per day (MFD) in head and neck cancer: Acute reactions of skin and mucosa. Int. J. Radiat. Oncol. Biol. Phys. 19, 301. [DOI] [PubMed] [Google Scholar]
- Werner S, Brauchle M, Hübner G, Frank S, Smola H (1996) Function of keratinocyte growth factor in wound healing. Zbl. Chir. 121 (Suppl.), 20. [PubMed] [Google Scholar]
- Werner S, Munz B (1998) Molecular biology contributions to wound healing and practical applications. Langenbecks Arch. Chir. Kongressband (Suppl.) 15, 678. [PubMed] [Google Scholar]
- Werner S, Peters KG, Longaker MT, Fuller PF, Banda MJ, Williams LT (1992) Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc. Natl. Acad. Sci. USA 89, 6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, Williams LT (1994) The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science 266, 819. [DOI] [PubMed] [Google Scholar]
- Zaghloul MS, Dorie MJ, Kallman RF (1994) Interleukin 1 increases thymidine labeling index of normal tissues of mice but not the tumor. Int. J. Radiat. Oncol. Biol. Phys. 29, 805. [DOI] [PubMed] [Google Scholar]


