Abstract
Objectives
The aim of this study was to investigate effects of mineral trioxide aggregate (MTA) on odonto/osteogenic differentiation of bone marrow stromal cells (BMSCs) from craniofacial bones.
Materials and methods
Craniofacial BMSCs were isolated from rat mandible and effects of MTA on their proliferation, differentiation and MAPK pathway involvement were subsequently investigated, in vitro. MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐2,5‐tetrazoliumbromide) assay was performed to evaluate proliferation of the MTA‐treated cells. Alkaline phosphatase (ALP) activity, alizarin red staining, real‐time reverse transcription polymerase chain reaction and western blot assays were used to assess differentiation capacity as well as MAPK pathway involvement.
Results
0.02 mg/ml MTA‐treated BMSCs had significantly higher ALP activity and formed more mineralized nodules than the untreated group. Odonto/osteoblastic marker genes/proteins (Alp, Runx2/RUNX2, Osx/OSX, Ocn/OCN and Dspp/DSP respectively) in MTA‐treated cells were remarkably upregulated compared to untreated ones. Mechanistically, phosphorylated Jun N‐terminal kinase (P‐JNK) and phosphorylated extracellular regulated protein kinases (P‐ERK) in MTA‐treated BMSCs increased significantly in a time‐dependent manner, while inhibition of JNK and ERK MAPK pathways dramatically blocked MTA‐induced odonto/osteoblastic differentiation, as indicated by reduced ALP levels, weakened mineralization capacity and downregulated levels of odonto/osteoblastic marker genes (Alp, Runx2, Osx, Ocn and Dspp).
Conclusion
Mineral trioxide aggregate promoted odonto/osteogenic capacity of craniofacial BMSCs via JNK and ERK MAPK signalling pathways.
Introduction
In clinical practice, endodontists often face young people with permanent teeth suffering from periapical inflammation, which can be treated either by apexification or pulp revascularization 1, 2 (instead of more traditional root canal therapy) due to their incompletely developed and open mouth‐shaped apical foramina. The biomaterial and its interaction with apical cells are two major factors behind the success of apexification or revascularization, which play paramount roles during continuous formation of calcified tissues in the root canal system. Potential cell candidates residing in the apical tissue of young permanent teeth, which are responsible for hard‐tissue regeneration, may include at least three types of stem/progenitor cell, stem cells from the apical papilla, periodontal ligament stem cells and bone marrow stromal cells (BMSCs) from jaw bones 3, 4, 5. Some studies have reported that bone marrow‐derived cells may communicate with dental tissues and ultimately become tissue‐specific mesenchymal progenitor/stem cells, to maintain pulp homeostasis 5. Thus, it is believed that BMSCs from apical alveolar bone marrow may also play an important role in repair of root apical foramina due to their capacity for multipotential differentiation, and easy attachment to biomaterials via blood flow in root canals during pulp revascularization. However, up to now interactions between biomaterials and BMSCs in the root canal system have remained unexplored.
Mineral trioxide aggregate (MTA) has been widely used in clinical apexification/pulp revascularization and MTA‐treated cases have presented excellent outcomes in endodontic practice due to MTA low cytotoxicity, good biocompatibility and competent inducibility over more traditional materials such as Ca(OH)2 6. Some studies have revealed that patients with apical inflammation successfully achieved complete new root development with the help of MTA, and blood flow introduction in the root canal system 1, 5. Recently, investigators have indicated that MTA promoted proliferation and differentiation of dental pulp stem cells 7, and induced differentiation of human periodontal ligament fibroblasts 1, 4, 5, 8. Moreover, MTA can assist cell adhesion, expansion and migration of bone marrow‐derived mesenchymal stem cells 8. However, little is known concerning effects of MTA on craniofacial BMSCs, which certainly participate in MTA‐based pulp revascularization within the root canal system via influx of blood flow.
In this study, we explored whether MTA could exert inductive influence on craniofacial BMSCs via MAPK pathways. To test this hypothesis, BMSCs were isolated from rat mandibles and treated with MTA‐conditioned media. Their odonto/osteogenic capacity and intracellular MAPK pathways were evaluated in vitro. Our findings revealed for the first time that MTA can enhance odonto/osteogenic differentiation of craniofacial BMSCs using Jun N‐terminal kinase (JNK) and extracellular regulated protein kinases (ERK) MAPK pathways.
Materials and methods
Cell isolation and culture
For preparation of BMSCs, 6 (4‐week old) female Sprague‐Dawley rats from the Experimental Animal Centre of Nanjing Medical University were euthanased by overdose of pentobarbital. Animals were treated according to the animal experimental guidelines approved by the Animal Experiment Committee of Nanjing Medical University. BMSCs from mandibles were isolated and identified as previously described 9 and isolated ones were then cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco, Life Technologies, Grand Island, NY, USA) supplemented with 10% foetal bovine serum (Hyclone, Logan, UT, USA), 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C in 5% CO2. Culture media were changed every 3 days and cells were routinely observed using phase‐contrast microscopy (Olympus, Shanghai, China). Their mesenchymal nature was confirmed by immunostaining with anti‐Stro‐1 antibody (1:100, Millipore, Bedford, MA, USA, Catalog number: mab4315). BMSCs at passages 2–4 were used in the following experiments.
CD marker analysis
For surface epitope expression analysis, 106 cells/well were first Fc‐blocked by treatment with 1 mg normal IgG for 20 min at room temperature, and subsequently stained with the following fluorochrome‐conjugated rabbit anti‐rat antibodies: CD29‐APC, CD34‐FITC, CD45‐PE and CD105‐FITC (all from BD Biosciences, San Jose, CA, USA). Cells were incubated with various combinations of antibodies for 20 min at room temperature in the dark, before washing twice in PBS (0.01 mol/l) and analysis using BD FACSCalibur (BD Biosciences).
Preparation of MTA‐conditioned media
ProRoot MTA (Dentsply, Tulsa, OK, USA) was mixed with sterile water according to the manufacturer's instructions, dried for 24 h and ground to fine powder. The powder was filtered through a 45 μm strainer, mixed with DMEM at a concentration of 200 mg/ml (200 mg MTA:1 ml DMEM), vortexed until completely suspended and incubated for 1 week at 37 °C to obtain the bioactive ingredients in MTA. Then, MTA supernatant collected from 200 mg MTA powder was filtered through a 2.5‐μm strainer and mixed with fresh routine culture media to obtain MTA‐conditioned media at different concentrations (0.002, 0.02, 0.2, 2 and 20 mg/ml) according to the powder/media (W/V) ratios. Cells were treated every other day with freshly prepared MTA‐conditioned media.
MTT assay
MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐2,5‐tetrazoliumbromide) assay was performed to investigate capacity for cell proliferation. Third passage cells were seeded into 96‐well plates (Nunc, Thermo Fisher Scientific Inc., South Logan, UT, USA) 2 × 103 cells/well, for 24 h. They were starved in serum‐free media for a further 24 h, then treated with MTA‐conditioned media at different concentrations. After 0, 1, 3, 5 and 7 days co‐culture, 20 μl fresh MTT solution (5 mg/ml; Sigma‐Aldrich, St. Louis, MO, USA) was added into the wells of each group and incubated for four more hours at 37 °C. Culture medium was removed and formazan crystals were solubilized using 150 μl/well dimethyl sulphoxide (DMSO; Sigma‐Aldrich). Optical density (OD) values were measured at 490 nm using a microtiter plate reader (Titertek, Helsinki, Finland). Data were described as mean ± SD; this experiment was repeated six times.
Alkaline phosphatase (ALP) activity and alizarin red staining
To screen the optimal and effective concentration of MTA‐conditioned medium, BMSCs were seeded into the 96‐well plates (Nunc) at 2 × 103 cells/well and cultured in DMEM containing 10% foetal bovine serum or MTA‐conditioned medium at concentrations of 0.002, 0.02, 0.2, 2 and 20 mg/ml respectively. On day 3 and day 5, ALP activity of each group was measured using an ALP kit (Biosino Bio‐technology & Science Inc., Beijing, China) and normalized on the basis of equivalent protein concentrations. According to the ALP results, 0.02 mg/ml MTA solution was considered and selected to be the optimal inductive medium for subsequent experiments. Then, cells were respectively cultured in DMEM and 0.02 mg/ml MTA‐conditioned medium for 14 days and alizarin red staining was performed to evaluate mineralization capacity of MTA‐treated BMSCs as previously described 10, 11.
To further determine roles of JNK and ERK MAPK pathways in MTA‐mediated differentiation of BMSCs, U0126 (specific ERK inhibitor; Sigma) and SP600125 (identified JNK inhibitor; Sigma) were respectively used 30 min prior to MTA treatment to block cellular ERK and JNK pathways. Cells were respectively cultured in 0.02 mg/ml MTA, 0.02 mg/ml MTA + 10 μm U0126, 0.02 mg/ml MTA + 10 μm SP600125 and 0.02 mg/ml MTA + 10 μm U0126 + 10 μm SP600125. ALP assay and alizarin red staining were then performed as mentioned earlier, respectively at day 5 and day 14. Results are presented as mean ± SD; this experiment was repeated three times.
Real‐time reverse transcription polymerase chain reaction (Real‐time RT‐PCR)
To assess odonto/osteogenic effects of MTA on treated and untreated BMSCs, by MTA for 3 and 7 days, cells respectively were collected. Furthermore, to evaluate JNK and ERK pathways during differentiation of the cells, those treated with 0.02 mg/ml MTA, 0.02 mg/ml MTA + 10 μm U0126, 0.02 mg/ml MTA + 10 μm SP600125 and 0.02 mg/ml MTA + 10 μm U0126 + 10 μm SP600125 were collected. Total RNA of each sample was extracted by adding TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions, and concentration was measured using a Protein/RNA calculator. mRNA was reverse transcribed into cDNA using PrimeScript RT Master Mix Kit (TaKaRa Biotech., Dalian, China). Real‐time RT‐PCR was performed using SYBR® Premix Ex Taq™ kit (TaKaRa Biotech.) and ABI 7300 real‐time PCR system. Primers used in this experiment are listed in Table 1. Gapdh was used as an internal control and expression of odonto/osteoblastic genes was calculated using the 2−ΔΔCt method as previously reported 12. Data are expressed as mean ± SD of three independent experiments.
Table 1.
Sense and antisense primers for the real‐time reverse transcription‐polymerase chain reaction
| Genes | Primers | Sequences (5′–3′) |
|---|---|---|
| Alp | Forward | CGAGCAGGAACAGAAGTTTGC |
| Reverse | GAATCCGACCCACGGAGG | |
| Runx2 | Forward | AATGCCTCCGCTGTTATG |
| Reverse | TTCTGTCTGTGCCTTCTTG | |
| Osx | Forward | GCCTACTTACCGTGACTTT |
| Reverse | GCCCACTATTGCCAACTGC | |
| Ocn | Forward | AAGCCCAGCGACTCTGAGTCT |
| Reverse | CCGGAGTCTATTCACCACCTTACT | |
| Dspp | Forward | TGACAGCAAGGACAGCAC |
| Reverse | GGGGTTCTCTGCTCTAATC | |
| Gapdh | Forward | GAAGGCAGCCCTGGTAACC |
| Reverse | ATGGTGGTGAAGACGCCAGTA |
Western blotting
To investigate odonto/osteogenic differentiation of BMSCs after stimulation by MTA, MTA‐treated cells at day 3 or day 7 were collected. To measure expression of MAPK pathway proteins, cells were collected after MTA treatment for 0, 30, 60 and 90 min. Samples were lysed in RIPA lysis buffer (Beyotime, Nanjing, China) containing 1 mm phenylmethylsulphonyl fluoride (PMSF; Beyotime) according to the manufacturer's instructions. Protein concentrations were measured using the Bradford protein assay. Thirty microgram protein per lane was loaded on 10% SDS‐PAGE gel for electrophoresis, then transferred to 0.22 μm PVDF membranes (Millipore) at 300 mA for 1 h, in blotting apparatus (Bio‐Rad, Hercules, CA, USA). Membranes were blocked in blocking solution (5% w/v skimmed milk, 0.01 M PBS, 0.1% Tween‐20) at room temperature for 2 h, and incubated with primary antibodies [DSP, 1:500, Santa Cruz, Dallas, TX, USA (catalog number: sc‐33587); RUNX2, 1:1000, Abcam, Hong Kong, China (catalog number: ab76956); OSX, 1:1000, Abcam (catalog number: ab22552); OCN, 1:1000, Abcam (catalog number: ab13418); P38, 1:1000, Bioworld, Minneapolis, MN, USA (catalog number: BS3566); P‐P38, 1:1000, Bioworld (catalog number: BS4766); JNK, 1:1000, Bioworld (catalog number: BS3630); P‐JNK, 1:1000, Bioworld (catalog number: BS4322); ERK, 1:1000, Bioworld (catalog number: BS3627); P‐ERK, 1:1000, Bioworld (catalog number: BS6377); β‐ACTIN, 1:1000, Bioworld (catalog number: BS1002)] overnight at 4 °C. Finally, membranes were washed three times in PBST for 10 min followed by incubation in secondary antibody (1:10 000; Boster, Wuhan, China) at 37 °C for 1 h, visualized by SuperSignal West Pico Chemiluminescent Substrate (Thermo, Rockford, IL, USA) and exposed to Kodak X‐ray films. β‐actin served as internal control in this experiment, which was repeated three times.
Statistical analysis
The two‐sample t‐test was performed to compare means of two independent samples. For multiple comparisons between experimental groups and control group, Dunnett's test was used to check significant differences. Two‐tailed P < 0.05 were considered statistically significant. All statistical analysis was performed using spss 13.0 software (SPSS Inc., Chicago, IL, USA).
Results
Effects of MTA on proliferation of BMSCs
Bone marrow stromal cells at passage 2 were fibroblast‐ or spindle‐like (Fig. 1a) with positive expression of Stro‐1 (Fig. 1b), CD29 and CD105 (Fig. 1d), while not expressing CD34 and CD45 (Fig. 1d). MTT results (Fig. 1e) showed that there was no significant difference in OD values bewteen the control group and MTA groups at different concentrations, whereas OD values in the 20 mg/ml group were significantly lower compared to the control group (P < 0.01). Growth curves (Fig. 1f) indicated no statistical difference between control and the 0.02 mg/ml MTA group between day 0 and day 11.
Figure 1.
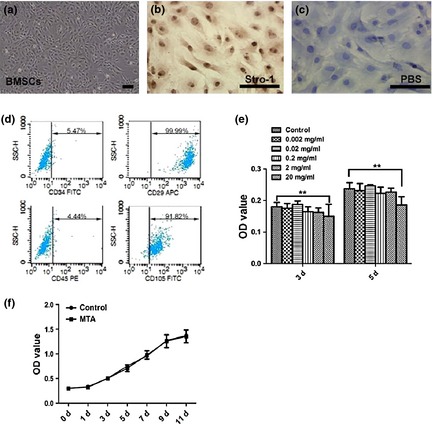
Proliferation features of mineral trioxide aggregate ( MTA )‐treated bone marrow stromal cells ( BMSC s). (a) Spindle‐like BMSCs at passage 2. (b) BMSCs were immunopositive for Stro‐1. (c) PBS served as a negative control. (d) Flow cytometry diagrams demonstrate the positive expression of mesenchymal markers (CD105, CD29) and negative expression of hematopoietic markers (CD45, CD34). (e) MTT results at day 3 and day 5. (f) Growth curve of BMSCs after MTA treatment at the concentration of 0.02 mg/ml. Values are mean ± SD, n = 6, **P < 0.01. Scale bars = 100 μm.
Effect of MTA on odonto/osteogenic differentiation of BMSCs
Odonto/osteogenic potential was measured respectively by ALP activity, alizarin red staining, real‐time RT‐PCR and western blot assay. On day 3 and day 5, ALP levels in 0.02 mg/ml MTA group were highest among all groups and were significantly higher than those of the control group (P < 0.01 or P < 0.05), whereas ALP activities in 20 mg/ml MTA group were significantly lower (P < 0.01) compared to the control group (Fig. 2a). Thus, 0.02 mg/ml was selected to be the optimal concentration of MTA for the following experiments, according to ALP activity and proliferative features of the BMSCs. Alizarin red staining revealed that in the 0.02 mg/ml MTA group, cells formed more calcified nodules than those in the control group (Fig. 2b).
Figure 2.
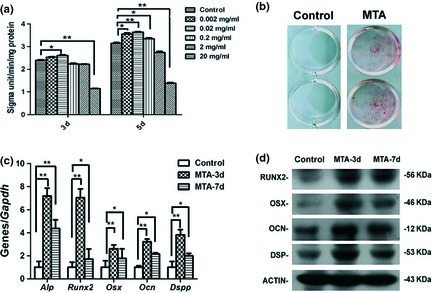
Odonto/osteogenic differentiation of mineral trioxide aggregate ( MTA )‐treated bone marrow stromal cells ( BMSC s). (a) Alkaline phosphatase activities of BMSCs after MTA treatment (0.02 mg/ml) at day 3 and day 5. Values are mean ± SD, n = 6, *P < 0.05, **P < 0.01. (b) Alizarin red staining in BMSCs after 14 days of treatment by 0.02 mg/ml MTA‐conditioned media. (c) Real‐time RT‐PCR for the odonto/osteogenic genes (Alp, Runx2, Osx, Ocn and Dspp) in MTA‐treated BMSCs at day 3 and day 7. Gapdh was used as an internal control. Values are mean ± SD. **2−ΔΔCt > 2, P < 0.01; *1 < 2−ΔΔCt < 2, P < 0.01 (n = 3). (d) Western blot analyses for the odonto/osteogenic proteins (RUNX2, OSX, OCN and DSP) in MTA‐treated BMSCs at day 3 and day 7. ACTIN served as an internal control.
Real‐time RT‐PCR assay showed that odonto/osteogenic genes (Alp, Runx2, Osx, Ocn and Dspp) in the BMSCs were significantly upregulated (P < 0.01) after MTA treatment (Fig. 2c). Western blotting results demonstrated that odonto/osteogenic proteins (RUNX2, OSX, OCN and DSP) were also enhanced in MTA‐treated cells on day 3 and day 7, in comparison to the control group (Fig. 2d).
MTA enhanced odonto/osteogenic capacity of BMSCs by activating JNK and ERK MAPK pathways
To explore MAPK pathway involvement in MTA‐treated BMSCs, MAPK signalling proteins were measured after MTA treatment. Levels of p38 and P‐p38 had almost no change during treatment, while expression of P‐JNK gradually increased from 0 to 60 min then reduced by 90 min. Expression of P‐ERK increased by 30 min and gradually decreased again by 60 and 90 min (Fig. 3a). Ratio of phosphorylated to unphosphorylated forms of proteins confirmed that expression of P‐p38 was not affected by MTA treatment (Fig. 3b), whereas expressions of P‐JNK (Fig. 3c) and P‐ERK (Fig. 3d) were significantly enhanced (P < 0.01) after MTA treatment.
Figure 3.
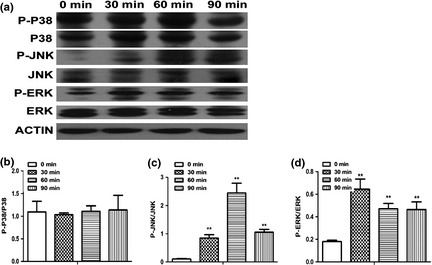
Activation of Jun N‐terminal kinase ( JNK ) and extracellular regulated protein kinases ( ERK ) MAPK pathways in mineral trioxide aggregate ( MTA )‐treated bone marrow stromal cells. (a) The expression of MAPK pathway proteins at different time points. (b) The ratio of phosphorylated to unphosphorylated form of P38 (P‐P38/P38). (c) The ratio of phosphorylated‐JNK (P‐JNK) to unphosphorylated JNK. (d) The ratio of phosphorylated‐ERK (P‐ERK) to ERK. Values are mean ± SD, n = 3, **P < 0.01.
To further confirm the role of JNK and ERK MAPK pathways during MTA‐induced differentiation of BMSCs, U0126 (specific ERK inhibitor) and SP600125 (specific JNK inhibitor) were respectively used to block ERK and JNK pathways. As shown in Fig. 4a and 4b, both P‐ERK and P‐JNK were downregulated after 30 min treatment with the inhibitors. Real‐time RT‐PCR results demonstrated that odonto/osteogenic genes (Alp, Runx2, Osx, Ocn and Dspp) in U0126 + MTA, SP600125 + MTA and U0126 + SP600125 + MTA groups were significantly downregulated compared to MTA‐treated counterparts (Fig. 4c; P < 0.01). Similarly, ALP levels in U0126 + MTA, SP600125 + MTA and U0126 + SP600125 + MTA groups were clearly downregulated (Fig. 4d; P < 0.01) in comparison to the MTA group. Alizarin red staining revealed that mineralized nodules in U0126 + MTA, SP600125 + MTA and U0126 + SP600125 + MTA groups were also remarkably lower compared to the MTA group (Fig. 4e).
Figure 4.
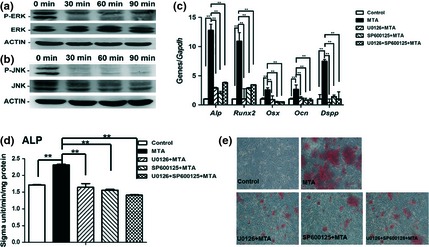
Effects of inhibitors of Jun N‐terminal kinase ( JNK ) and extracellular regulated protein kinases ( ERK ) MAPK pathway on mineral trioxide aggregate ( MTA )‐induced differentiation of bone marrow stromal cells ( BMSC s). (a) The expression of P‐ERK in BMSCs treated by 10 μm U0126 at different time points. (b) The levels of P‐JNK in BMSCs treated by 10 μm SP600125 at different time points. (c) The odonto/osteogenic genes (Alp, Runx2, Osx, Ocn and Dspp) respectively in different groups (Control, MTA, 10 μm U0126 + MTA, 10 μm SP600125 + MTA and U0126 + SP600125 + MTA). Values are mean ± SD. **2−ΔΔCt > 2, P < 0.01 (n = 3). (d) Alkaline phosphatase levels of BMSCs after 5 days of culture in different groups (0.02 mg/ml MTA, 10 μm U0126 + 0.02 mg/ml MTA, 10μm SP600125 + 0.02 mg/ml MTA and 10 μm U0126 + 10 μm SP600125 + 0.02 mg/ml MTA). Values are mean ± SD, n = 6, *P < 0.05, **P < 0.01. (e) Alizarin red staining in BMSCs after 14 days of treatment respectively by 0.02 mg/ml MTA, 10 μm U0126 + 0.02 mg/ml MTA,10 μm SP600125 + 0.02 mg/ml MTA and 10 μm U0126 + 10 μm SP600125 + 0.02 mg/ml MTA.
Discussion
Mineral trioxide aggregate is a type of bioactive material that has previously been involved in diverse endodontic procedures including pulpotomy, root‐end filling, root perforation repair, apexification and apexogenesis 13, 14. Inductive effects of MTA on target cells are of great importance during MTA‐based apexification or pulp revascularization. To date, little knowledge has been available concerning effects of MTA on craniofacial BMSCs. These have the potential to differentiate into odonto/osteoblasts and to participate in root development as well as in regeneration of periapical tissues (alveolar bone, periodontium and cementum) 5, 15, 16, 17.
In the present study, we prepared MTA‐conditioned media according to previous studies, with some modifications 7. A volume of 0.02 mg/ml MTA solution was decided to be the optimal concentration to trigger differentiation of craniofacial BMSCs, while high concentration of MTA (20 mg/ml) significantly diminished proliferative activity of the cells due to higher basic pH values and overdose of inorganic salts 18.
Mineral trioxide aggregate‐treated BMSCs presented stronger odonto/osteoblastic differentiation capacity than untreated ones, as indicated by increased ALP activity, enhanced calcification and upregulated expression of odonto/osteoblastic markers (Alp, Runx2/RUNX2, Osx/OSX and Ocn/OCN, and Dspp/DSP) in vitro. Alp/ALP, Runx2/RUNX2, Osx/OSX, and Ocn/OCN are very important factors involved in either early stage or later stage osteogenic differentiation and odontogenesis 19, 20. ALP is known to be associated with bone metabolism and osteoblast differentiation, and its activity is one of the most frequently used indicators of osteoblastic differentiation. In addition, ALP activity in the subodontoblastic layer is highest in dental pulp tissue closely related to dentin formation, and has also been considered to be a suitable marker of odontogenic differentiation 12, 21. Runx2 is an essential factor for bone formation, expressed in the early stages of bone development continuing to be present throughout later phases of osteogenesis. RUNX2 is also expressed in preodontoblasts and odontoblasts 22. Osx acts downstream of Runx2 and is required in osteogenic differentiation in early and later stages of osteogenesis 19, 23. OCN is synthesized by osteoblasts and odontoblasts, and is a well‐known marker of osteogenic differentiation and mineralization in late stages of bone formation 24. Dspp and DSP are odontoblast‐specific markers that usually appear in dentin or predentin structures 25, 26. They are involved in nucleation and modulation of the hydroxyapatite mineral phase during dentin calcification 27. Together, the above findings demonstrated that MTA can trigger odontogenic potential and accelerate osteogenic differentiation of BMSCs.
Mechanistically, MTA can cause upregulation of P‐JNK and P‐ERK in a time‐dependent manner, activate the MAPK pathway and enhance differentiation of BMSCs. These inductive actions were dramatically reduced by specific inhibitors of MAPK (U0126 and SP600125), indicating that MTA regulates odonto/osteogenic differentiation of BMSCs via the MAPK pathway. The MAPK pathway includes three paralleled mechanisms, p38, ERK1/2 and JNK1/2/3, all of which have been reported to play an important role in regulating proliferation, ostogenic and odontogenic differentiation of various cell types, for example, dental pulp stem cells, periodontal ligament stem cells, dental papilla cells, perichondral cells and BMSCs) 28, 29, 30.
In addition, many factors can trigger the MAPK pathway to modify cell phenotypes 31, 32. As a mixture of different kinds of inorganic salts (Ca2+, iron and inorganic phosphate), MTA can affect activity of the MAPK pathway by different approaches 33, 34. Calcium can activate ERK and p38 MAPK pathways through changes in intracellular calcium signalling 35, 36, while iron supplementation can prevent cytotoxicity and apoptosis induced by activation of the ERK MAPK pathway 34. Moreover, inorganic phosphate can activate the MAPK signalling pathway in different cell types 33, 37, 38. Some studies have proven that ERK in the MAPK pathway can converge with PI3K/PDK‐1/Akt pathway downstream effectors and upregulate expression of ALP, RUNX2, OSX and OCN, which subsequently stimulate odonto/osteoblastic differentiation at different stages 39, 40, 41.
In conclusion, 0.02 mg/ml MTA‐conditioned media can initiate odontogenic potential and promote osteogenic differentiation of craniofacial BMSCs by activation of JNK and ERK MAPK signalling pathways, although more extensive studies are required to investigate other pathway mechanisms embedded in MTA‐mediated differentiation of BMSCs.
Acknowledgements
The authors thank Dr Ruoning Wang for his help in improving the language in this manuscript. This study was supported by National Natural Science Foundation of China (no. 81371144), Natural Science Foundation of Jiangsu Province (no. BK20131392), the Medical Elitist Project of Jiangsu Province (no. RC2011140) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, no. 2011‐137).
References
- 1. Ding RY, Cheung GS, Chen J, Yin XZ, Wang QQ, Zhang CF (2009) Pulp revascularization of immature teeth with apical periodontitis: a clinical study. J. Endod. 35, 745–749. [DOI] [PubMed] [Google Scholar]
- 2. Forghani M, Parisay I, Maghsoudlou A (2013) Apexogenesis and revascularization treatment procedures for two traumatized immature permanent maxillary incisors: a case report. Restor. Dent. Endod. 38, 178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang GT (2008) A paradigm shift in endodontic management of immature teeth: conservation of stem cells for regeneration. J. Dent. 36, 379–386. [DOI] [PubMed] [Google Scholar]
- 4. Yan P, Yuan Z, Jiang H, Peng B, Bian Z (2010) Effect of bioaggregate on differentiation of human periodontal ligament fibroblasts. Int. Endod. J. 43, 1116–1121. [DOI] [PubMed] [Google Scholar]
- 5. Zhou J, Shi S, Shi Y, Xie H, Chen L, He Y et al (2011) Role of bone marrow‐derived progenitor cells in the maintenance and regeneration of dental mesenchymal tissues. J. Cell. Physiol. 226, 2081–2090. [DOI] [PubMed] [Google Scholar]
- 6. Hashiguchi D, Fukushima H, Nakamura M, Morikawa K, Yasuda H, Udagawa N et al (2011) Mineral trioxide aggregate solution inhibits osteoclast differentiation through the maintenance of osteoprotegerin expression in osteoblasts. J. Biomed. Mater. Res. A 96, 358–364. [DOI] [PubMed] [Google Scholar]
- 7. Zhao X, He W, Song Z, Tong Z, Li S, Ni L (2012) Mineral trioxide aggregate promotes odontoblastic differentiation via mitogen‐activated protein kinase pathway in human dental pulp stem cells. Mol. Biol. Rep. 39, 215–220. [DOI] [PubMed] [Google Scholar]
- 8. D'Anto V, Di Caprio MP, Ametrano G, Simeone M, Rengo S, Spagnuolo G (2010) Effect of mineral trioxide aggregate on mesenchymal stem cells. J. Endod. 36, 1839–1843. [DOI] [PubMed] [Google Scholar]
- 9. Zhang P, Men J, Fu Y, Shan T, Ye J, Wu Y et al (2012) Contribution of SATB2 to the stronger osteogenic potential of bone marrow stromal cells from craniofacial bones. Cell Tissue Res. 350, 425–437. [DOI] [PubMed] [Google Scholar]
- 10. Yu J, He H, Tang C, Zhang G, Li Y, Wang R et al (2010) Differentiation potential of STRO‐1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 11, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karin M (2009) NF‐kappaB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 1, a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang S, Mu J, Fan Z, Yu Y, Yan M, Lei G et al (2012) Insulin‐like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res. 8, 346–356. [DOI] [PubMed] [Google Scholar]
- 13. Parirokh M, Torabinejad M (2010) Mineral trioxide aggregate: a comprehensive literature review – part III: clinical applications, drawbacks, and mechanism of action. J. Endod. 36, 400–413. [DOI] [PubMed] [Google Scholar]
- 14. Wigler R, Kaufman AY, Lin S, Steinbock N, Hazan‐Molina H, Torneck CD (2013) Revascularization: a treatment for permanent teeth with necrotic pulp and incomplete root development. J. Endod. 39, 319–326. [DOI] [PubMed] [Google Scholar]
- 15. Tsumanuma Y, Iwata T, Washio K, Yoshida T, Yamada A, Takagi R et al (2011) Comparison of different tissue‐derived stem cell sheets for periodontal regeneration in a canine 1‐wall defect model. Biomaterials 32, 5819–5825. [DOI] [PubMed] [Google Scholar]
- 16. Miranda SC, Silva GA, Hell RC, Martins MD, Alves JB, Goes AM (2011) Three‐dimensional culture of rat BMMSCs in a porous chitosan‐gelatin scaffold: a promising association for bone tissue engineering in oral reconstruction. Arch. Oral Biol. 56, 1–15. [DOI] [PubMed] [Google Scholar]
- 17. Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J. Dent. Res. 88, 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masuda‐Murakami Y, Kobayashi M, Wang X, Yamada Y, Kimura Y, Hossain M et al (2010) Effects of mineral trioxide aggregate on the differentiation of rat dental pulp cells. Acta Histochem. 112, 452–458. [DOI] [PubMed] [Google Scholar]
- 19. Chen S, Gluhak‐Heinrich J, Wang YH, Wu YM, Chuang HH, Chen L et al (2009) Runx2, osx, and dspp in tooth development. J. Dent. Res. 88, 904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fei L, Wang C, Xue Y, Lin K, Chang J, Sun J (2012) Osteogenic differentiation of osteoblasts induced by calcium silicate and calcium silicate/beta‐tricalcium phosphate composite bioceramics. J. Biomed. Mater. Res. B Appl. Biomater. 100, 1237–1244. [DOI] [PubMed] [Google Scholar]
- 21. Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P et al (2011) Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 56, 709–721. [DOI] [PubMed] [Google Scholar]
- 22. Komori T (2010) Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 339, 189–195. [DOI] [PubMed] [Google Scholar]
- 23. Baek WY, Lee MA, Jung JW, Kim SY, Akiyama H, de Crombrugghe B et al (2009) Positive regulation of adult bone formation by osteoblast‐specific transcription factor osterix. J. Bone Miner. Res. 24, 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bai Y, Matsuzaka K, Hashimoto S, Kokubu E, Wang X, Inoue T (2010) Formation of bone‐like tissue by dental follicle cells co‐cultured with dental papilla cells. Cell Tissue Res. 342, 221–231. [DOI] [PubMed] [Google Scholar]
- 25. Wu G, Deng ZH, Fan XJ, Ma ZF, Sun YJ, Ma DD et al (2009) Odontogenic potential of mesenchymal cells from hair follicle dermal papilla. Stem Cells Dev. 18, 583–589. [DOI] [PubMed] [Google Scholar]
- 26. Nakashima M, Tachibana K, Iohara K, Ito M, Ishikawa M, Akamine A (2003) Induction of reparative dentin formation by ultrasound‐mediated gene delivery of growth/differentiation factor 11. Hum. Gene Ther. 14, 591–597. [DOI] [PubMed] [Google Scholar]
- 27. Suzuki S, Haruyama N, Nishimura F, Kulkarni AB (2012) Dentin sialophosphoprotein and dentin matrix protein‐1: two highly phosphorylated proteins in mineralized tissues. Arch. Oral Biol. 57, 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang H, Liu S, Zhou Y, Tan J, Che H, Ning F et al (2012) Natural mineralized scaffolds promote the dentinogenic potential of dental pulp stem cells via the mitogen‐activated protein kinase signaling pathway. Tissue Eng. Part A 18, 677–691. [DOI] [PubMed] [Google Scholar]
- 29. Kang Y, Kim S, Bishop J, Khademhosseini A, Yang Y (2012) The osteogenic differentiation of human bone marrow MSCs on HUVEC‐derived ECM and beta‐TCP scaffold. Biomaterials 33, 6998–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S et al (2012) Insulin‐like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem. Cell Biol. 137, 513–525. [DOI] [PubMed] [Google Scholar]
- 31. Li X, Zhang R, Luo D, Park SJ, Wang Q, Kim Y et al (2005) Tumor necrosis factor alpha‐induced desumoylation and cytoplasmic translocation of homeodomain‐interacting protein kinase 1 are critical for apoptosis signal‐regulating kinase 1‐JNK/p38 activation. J. Biol. Chem. 280, 15061–15070. [DOI] [PubMed] [Google Scholar]
- 32. Lin SJ, Shyue SK, Hung YY, Chen YH, Ku HH, Chen JW et al (2005) Superoxide dismutase inhibits the expression of vascular cell adhesion molecule‐1 and intracellular cell adhesion molecule‐1 induced by tumor necrosis factor‐alpha in human endothelial cells through the JNK/p38 pathways. Arterioscler. Thromb. Vasc. Biol. 25, 334–340. [DOI] [PubMed] [Google Scholar]
- 33. Minashima T, Small W, Moss SE, Kirsch T (2012) Intracellular modulation of signaling pathways by annexin A6 regulates terminal differentiation of chondrocytes. J. Biol. Chem. 287, 14803–14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Q, Luo W, Zhang W, Dai Z, Chen Y, Chen J (2007) Iron supplementation protects against lead‐induced apoptosis through MAPK pathway in weanling rat cortex. Neurotoxicology. 28, 850–859. [DOI] [PubMed] [Google Scholar]
- 35. Chi J, Zhu Y, Fu Y, Liu Y, Zhang X, Han L et al (2012) Cyclosporin A induces apoptosis in H9c2 cardiomyoblast cells through calcium‐sensing receptor‐mediated activation of the ERK MAPK and p38 MAPK pathways. Mol. Cell. Biochem. 367, 227–236. [DOI] [PubMed] [Google Scholar]
- 36. Wang S, Han HM, Pan ZW, Hang PZ, Sun LH, Jiang YN et al (2012) Choline inhibits angiotensin II‐induced cardiac hypertrophy by intracellular calcium signal and p38 MAPK pathway. Naunyn Schmiedebergs Arch Pharmacol. 385, 823–831. [DOI] [PubMed] [Google Scholar]
- 37. Bergwitz C, Rasmussen MD, DeRobertis C, Wee MJ, Sinha S, Chen HH et al (2012) Roles of major facilitator superfamily transporters in phosphate response in Drosophila. PLoS One 7, e31730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suzuki A, Palmer G, Bonjour JP, Caverzasio J (2001) Stimulation of sodium‐dependent inorganic phosphate transport by activation of Gi/o‐protein‐coupled receptors by epinephrine in MC3T3‐E1 osteoblast‐like cells. Bone 28, 589–594. [DOI] [PubMed] [Google Scholar]
- 39. Qiao M, Shapiro P, Kumar R, Passaniti A (2004) Insulin‐like growth factor‐1 regulates endogenous RUNX2 activity in endothelial cells through a phosphatidylinositol 3‐kinase/ERK‐dependent and Akt‐independent signaling pathway. J. Biol. Chem. 279, 42709–42718. [DOI] [PubMed] [Google Scholar]
- 40. Celil AB, Campbell PG (2005) BMP‐2 and insulin‐like growth factor‐I mediate Osterix (Osx) expression in human mesenchymal stem cells via the MAPK and protein kinase D signaling pathways. J. Biol. Chem. 280, 31353–31359. [DOI] [PubMed] [Google Scholar]
- 41. Granero‐Molto F, Myers TJ, Weis JA, Longobardi L, Li T, Yan Y et al (2011) Mesenchymal stem cells expressing insulin‐like growth factor‐I (MSCIGF) promote fracture healing and restore new bone formation in Irs1 knockout mice: analyses of MSCIGF autocrine and paracrine regenerative effects. Stem Cells 29, 1537–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]


