Abstract
Abstract. Objectives: In this study, we investigated the potential of umbilical cord blood stem cell lineages to produce C‐peptide and insulin. Materials and methods: Lineage negative, CD133+ and CD34+ cells were analyzed by flow cytometry to assess expression of cell division antigens. These lineages were expanded in culture and subjected to an established protocol to differentiate mouse embryonic stem cells (ESCs) toward the pancreatic phenotype. Phase contrast and fluorescence immunocytochemistry were used to characterize differentiation markers with particular emphasis on insulin and C‐peptide. Results: All 3 lineages expressed SSEA‐4, a marker previously reported to be restricted to the ESC compartment. Phase contrast microscopy showed all three lineages recapitulated the treatment‐dependent morphological changes of ESCs as well as the temporally restricted expression of nestin and vimentin during differentiation. After engineering, each isolate contained both C‐peptide and insulin, a result also obtained following a much shorter protocol for ESCs. Conclusions: Since C‐peptide can only be derived from de novo synthesis and processing of pre‐proinsulin mRNA and protein, we conclude that these results are the first demonstration that human umbilical cord blood‐derived stem cells can be engineered to engage in de novo synthesis of insulin.
INTRODUCTION
Stem cells hold tremendous promise for the future of regenerative medicine (Gerecht‐Nir & Itskovitz‐Eldor 2004; Kume 2005). Remarkable advances have been made in the engineering of embryonic stem cells (ESC) into mesoderm, endoderm and ectoderm developmental lineages (Brimble et al. 2004; D’Amour et al. 2005). Cells expressing markers of these stages have been further engineered to produce cardiac (Xue et al. 2005), neuronal, pancreatic (Kumar et al. 2003), haematopoietic (Suzuki & Nakano 2001), alveolar epithelial (Ali et al. 2002) and hepatic progenitors that perform many of the functions necessary to provide physiological rescue of pathological cells and tissues in many diseases. This demonstration of pluripotency of ESCs has provided important insights into cell and tissue engineering. Most notably relevant to the engineering of functional equivalents of pancreatic, islet‐like, glucose‐responsive, insulin‐producing cells to treat diabetes, ESCs have been directed along the endodermal lineage (Lumelsky et al. 2001; Stafford & Prince 2002; Segev et al. 2004; Shi et al. 2005). While several reports showed insulin‐containing cells (Lumelsky et al. 2001; Segev et al. 2004; Shi et al. 2005), the observation that such cells could accumulate insulin from culture medium has brought additional criteria to successful production and demonstration of insulin‐producing cells (Rajagopal et al. 2003; Hansson et al. 2004).
Umbilical cord blood (UCB) stem cells provide a parallel alternative to ESCs, while capitalizing on the wealth of knowledge in the ESC field (Schulze et al. 2005). The multi‐potency of UCB and adult stem cells (ADSC), like ESCs, was demonstrated by differentiation into haematopoietic, neuronal and cardiomyogenic progenitor stem cells (Abuljadayel 2003). UCB stem cells and ADSCs have been used successfully in treating myocardial infarction using bone marrow‐derived stem cells and in haematotherapy using UCB stem cells (Stamm et al. 2003; Cohen & Nagler 2004; Perin & Silva 2004). ADSCs were also described from ductal pancreatic epithelial cells (Rao et al. 1990; Bonner‐Weir et al. 1993; Grapin‐Botton 2005). Furthermore, stem cells have tremendous potential specifically in diabetes (Bonner‐Weir 2003; Street et al. 2003; Hussain & Theise 2004; Kodama & Faustman 2004; Lechner 2004; Yamada & Kojima 2005; Roche et al. 2006). Indeed, there are reports of both endogenous, somatic progenitor stem cells (Gao et al. 2003; Booth & Holland 2004; Dor et al. 2004; Seaberg et al. 2004; Weir & Bonner‐Weir 2004) and cells that can transdifferentiate into islets (Burke & Tosh 2005; Di Gioacchino et al. 2005; Yalniz & Pour 2005), reflecting an ongoing challenge of identifying optimal sources for these cells (Bock 2004; Choi et al. 2004; Habener 2004; Hayek 2004). Complex signals, which can be produced by the embryonic mouse pancreas, are required for ESC differentiation to the insulin‐producing phenotype (Brolen et al. 2005).
We previously reported (Forraz et al. 2002a; 2004, 2003a, 2003b) the isolation, expansion and engineering of UCB‐derived stem cell lineages of various degrees of multi‐potency into many types of functional progenitor phenotypes including haematopoietic, neuronal and hepatic (2002b, 2004; McGuckin et al. 2004). Using multi‐parametric phenotyping, we demonstrated that UCB contained a complex succession of heterogeneous stem and progenitor cell groups up‐ and down‐regulating a range of surface antigens including CD133, CD34, CD38, CD7 and CD90 as they differentiated (Forraz et al. 2002a; McGuckin et al. 2003b).
With the goal of engineering stem cells from human UCB for cell replacement therapy in type 1 diabetes (Giannoukakis & Robbins 2002), it was important to test the potential of various lineages for differentiation towards the islet‐like lineage (Kato & Tsunoda 1993). We report here the engineering of CD34+, CD133+ and lineage‐negative (CD133−/CD34−) stem cells to a C‐peptide producing, insulin‐containing phenotype. These populations represent increasingly primitive lineages that were expanded in cell culture under the direction of specific cytokines and then subjected to a protocol reported to differentiate ESCs to an insulin‐containing, pancreatic‐like phenotype (Lumelsky et al. 2001; Segev et al. 2004). These cells exhibited temporal expression of markers reported to characterize development of the pancreatic lineage. Ultimately, the cells contained C‐peptide that ensured that they engaged in de novo synthesis and processing of pre‐proinsulin mRNA, contained proper functional pro‐insulin proteolytic processing machinery, and that the insulin they contained at least partly derived from de novo synthesis with an untested contribution of insulin via uptake from the media. This is the first example of engineering human UCB stem cells to contain the phenotypic repertoire that might provide an avenue to treat type 1 diabetes.
MATERIALS AND METHODS
Isolation of human UCB stem cell lineages
Umbilical cord blood units were collected from normal microbiologically screened and ethically cleared donors after caesarean sections and diluted upon collection with phosphate‐buffered saline (PBS; Sigma‐Aldrich, St. Louis, MO, USA) supplemented with 0.6% acid citrate dextrose formula‐A acid anticoagulant (Sigma‐Aldrich) and bovine serum albumin (0.5% fraction V, Sigma‐Aldrich) at pH 7.4 (ACD‐A). Four volumes of diluted cord blood units were overlaid onto one volume of research grade Ficoll‐Paque solution (d: 1.077 g/cm3, Amersham Biosciences, Uppsala, Sweden) following the manufacturer's instructions. Following centrifugation at 400 g for 30 min at room temperature, the mononuclear layer was removed, washed twice in ACD‐A buffer, and used for further purification. Primitive lineage‐restricted or lineage‐negative stem cells were purified by sequential immunomagnetic depletion as previously reported (Forraz et al. 2004; McGuckin et al. 2004) by blocking non‐specific Fc receptors with human gammaglobulins (2% in PBS) followed by sequential immunomagnetic depletion with antibodies to mouse antihuman antiglycophorin A (Dakocytomation, Carpentaria, CA, USA) and then a cocktail of antibodies to CD45, CD33 and CD7 (all from Autogenbioclear, Wiltshire, UK). CD34+ or CD133+ cells were isolated as previously described (McGuckin et al. 2003b) by positive selection with colloidal super‐paramagnetic MACS MicroBeads conjugated to monoclonal mouse antihuman CD34 or CD133 (Miltenyi Biotec Inc., Bergisch Gladbach, Germany) antibodies.
Immunohistochemistry
Cells in suspension were spotted directly onto microscope slides or collected using a Cytospin, air‐dried, fixed in 4% paraformaldehyde, washed with PBS, and treated with 70% ethanol. Adherent cells were similarly fixed directly on cover slips or chamber slides. For staining insulin, C‐peptide, nestin and vimentin, non‐specific Fc receptors were blocked with 2% human γ‐globulin in PBS solution (4 °C, 20 min; Sigma‐Aldrich). Antihuman insulin mouse monoclonal immunoglobulin G (IgG) (Sigma) was detected with fluorescein isothiocyanate (FITC)‐conjugated goat antimouse IgG (Santa Cruz, CA, USA). Antihuman C‐peptide rabbit IgG (Acris Antibodies GmbH, Hiddenhausen, Germany) and antihuman nestin rabbit IgG (Chemicon Int., Temecula, CA, USA) were detected with FITC‐conjugated sheep antirabbit IgG (Chemicon Int.). Antihuman vimentin mouse monoclonal IgG (Chemicon Int.) was detected with Texas Red‐conjugated goat antimouse (StemCell Technologies, Vancouver, Canada). For SSEA‐4 staining, blocking was performed in 0.1% saponin, 0.1% NaN3, 2% foetal calf serum in PBS with 1 : 50 dilutions of primary and secondary antibodies. Antihuman SSEA‐4 rat IgG3 (Chemicon Int.) was detected with FITC‐conjugated IgG (Santa Cruz). Nuclei were stained with 4′,6‐diamidino‐2‐phenylindole (DAPI)‐containing Vectashield mounting medium (Vector Laboratories Inc., Burlingame, CA, USA). Fluorescence microscopy was performed on a Nikon Eclipse 80i (Tokyo, Japan) and confocal imaging on a Zeiss LSM 510 UV Meta laser scanning confocal microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany).
Flow cytometry
Approximately 100 000 cells were incubated at 4 °C for 30 min with antihuman CD34‐FITC, antihuman CD38‐FITC (both from StemCell Technologies), or antihuman CD133‐PE (Miltenyi Biotec Inc., Bergisch Gladbach, Germany). Negative controls were incubated with mouse IgG isotype control FITC or PE (StemCell Technologies). Cells were centrifuged, washed, fixed with 0.5% paraformaldehyde and kept refrigerated until flow cytometry analysis within 1 week using a BD FACSCanto (Becton Dickinson Biosciences, Rockville, MD, USA) with excitation/emission for FITC at 488/530 and PE at 488/585 nm.
Cytokine‐induced expansion
Umbilical cord blood stem cells (104 cells/ml) were cultured in tissue culture microflasks (Nunc, Rochester, NY, USA) at 37 °C, 5% CO2 humidified atmosphere in Iscove's‐modified Dulbecco's medium (IMDM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% foetal bovine serum (FBS) (Es‐CultTM‐Tested FBS, StemCell Technologies Inc.) in the presence of the human recombinant cytokines (Pepro Tech Inc., Rocky Hill, NJ, USA) thrombopoietin (10 ng/ml), flt3‐ligand (50 ng/ml) and c‐kit ligand (20 ng/ml).
Directed engineering towards the pancreatic phenotype
Expanded UCB‐derived stem cells were treated essentially as previously described (Lumelsky et al. 2001) following the four‐step protocol provided by the manufacturer (StemCell Technologies). Step 1: expanded human cord blood stem cells were seeded at 1 × 105 cells/ml in ES differentiation medium, containing 15% FBS, 0.1 mm nonessential amino acids, 2 mm l‐glutamine, 1 mm methyl B‐D‐thiogalactoside (MTC) and Dulbecco's modified Eagle's medium (DMEM) high glucose. After incubation for 2 days at 37 °C, media was replaced and incubation continued for an additional 2 days. Step 2: in order to produce nestin‐positive cells, media was changed to serum‐free insulin, transferrin, selenium (ITS) in basal medium‐A and incubation continued for 6 days with media changed every 2 days. Step 3: cells were then harvested, seeded in serum‐free pancreatic proliferation medium, containing N2 supplement‐A, B27 supplements and 25 ng/ml basic fibroblast growth factor (bFGF) in basal medium‐A, and incubated for 6 days with media changed every 2 days. Step 4: cells were differentiated into insulin‐ and C‐peptide‐containing cells by incubation for 6 days in pancreatic differentiation medium containing N2 supplement‐A, B27 supplements and 10 mm nicotinamide in basal medium‐A with serum.
Rapid directed engineering towards the pancreatic phenotype
Lineage‐negative cells were plated into ultra low attachment plates in 10% FBS in DMEM with cytokines for 24–48 h, then transferred to Laboratory‐Tek Chamber slides (Nalge Nunc Int., Rochester, NY, USA) according to a published protocol (Shi et al. 2005). After at least 2 h in slide chambers, 100 ng/ml activin A (Sigma) was added for 24 h. Cells were then transferred to 10% FBS in DMEM for 6–8 h followed by addition of 1 µm All‐trans retinoic acid (Sigma) for an additional 24 h (Step 1). Media was changed to 10% FBS in DMEM with 10 ng/ml bFGF (Sigma) for 3–5 days (Step 2). Finally, cells were cultured in DMEM/F12 with N2 supplement, B27 supplement (all from Gibco‐BRL), 1 µg/ml laminin (Sigma), 10 ng/ml bFGF and 10 mm nicotinamide (Sigma) for 3–5 days.
RESULTS
Initial characterization of cell surface markers was important to assess stage of differentiation, purity and reproducibility of yields from original blood volume and buffy coat mononuclear cells. Flow cytometry analysis shown in Fig. 1, indicated lineage‐negative isolates (left) contained barely detectable levels of CD133+, CD34+, or CD38+ markers. Typical yields were ~100 000 cells from 40 ml of cord blood or 0.1–0.4% of the mononuclear cells. CD133+ isolates (middle) were all positive for both CD133 and CD34 markers with most cells also expressing CD38. These cells were typically present in ~20‐fold higher abundance than lineage‐negative cells. CD34+ isolates (right) were all positive for CD34 and CD133 and mostly positive for CD38. All three lineages contained highly birefringent, small, round cells that were morphologically indistinguishable from each other (data not shown).
Figure 1.
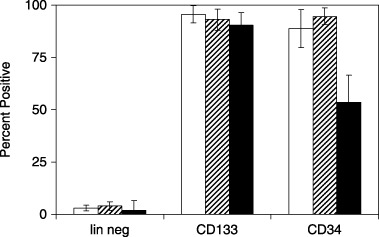
Flow cytometry analysis of lineages from umbilical cord blood stem cells. Freshly isolated stem cells from the indicated lineages were analyzed by flow cytometry for expression of CD133 (open bars), CD34 (horizontal hatched bars) and CD38 (filled bars). Data represent means and standard errors of the mean for at least three independent isolations.
Because we recently reported that lineage negative isolates express SSEA‐4 (McGuckin et al. 2005), previously considered to be unique to ESCs, all three isolates used in this study were further characterized for expression of this marker. Figure 2 illustrates that lineage‐negative, CD133+ and CD34 isolates all express SSEA‐4.
Figure 2.
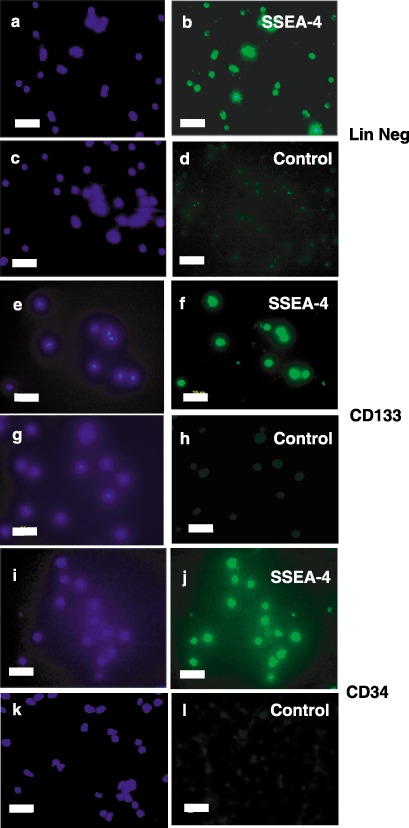
Immunocytochemical characterization of SSEA‐4 expression in stem cell lineages. Freshly isolated lineage negative (Panels a–d), CD133+ (Panels e–h) or CD34+ (Panels i–l) cells were stained with antihuman SSEA‐4 immunoglobulin G (IgG) (Panels b, f and j) or control IgG (Panels d, h and l) followed by fluorescein isothiocyanate (FITC)‐conjugated second antibodies (Panels b, d, f, h, j and l). Nuclei were then visualized by staining with 4′,6‐diamidino‐2‐phenylindole (DAPI) (Panels a, c, e, g, i and k). Fluorescence micrographs are representative of at least three independent isolations. Scale bars, 25 µm.
After expansion in recombinant cytokines with serum, the three lineages were next treated according to a protocol published for directed differentiation of human ESCs to the pancreatic, insulin‐synthesizing phenotype. Shown in Fig. 3 are phase contrast micrographs, representative of all three lineages, that demonstrate the morphological changes seen during the differentiation process of lineage‐negative isolates. Multi‐cellular aggregates or clusters (Panel A), similar to the embryoid bodies formed by ESCs, formed during culture in differentiation medium in Step 1. Subsequent culture in serum‐free media led to disaggregation and attachment of cells to the culture dish in Step 2 (Panel B). Expansion of pancreatic precursor cells induced by bFGF in Step 3 resulted in large, elongate cells (Panel C). Finally, removal of bFGF and inclusion of nicotinamide in Step 4 produced cells with long, narrow processes or pseudopodia, which appeared to contact adjacent cells (Panel D). Identical morphological responses were seen during differentiation of CD133+ and CD34+ cells (data not shown).
Figure 3.
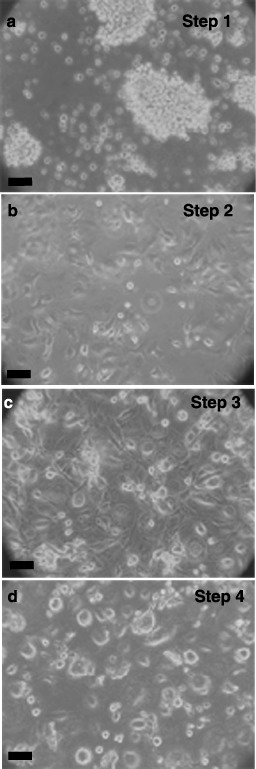
Differentiation of cord blood stem cells. Lineage‐negative cells were expanded for 1 week and then differentiated as described in ‘Methods’ and cell morphology shown with phase contrast microscopy. Step 1 (4 days, Panel a), aggregation in differentiation medium. Step 2 (6 days, Panel b), production of nestin‐positive, attached cells in serum‐free medium. Step 3 (6 days, Panel c), proliferation towards pancreatic lineage with basic fibroblast growth factor (bFGF). Step 4 (6 days, Panel d), differentiation to insulin‐ and C‐peptide‐containing cells by nicotinamide. Phase contrast micrographs are representative of at least five experiments. Scale bars, 25 µm.
Because human ESCs express nestin and vimentin during engineered differentiation along the pancreatic lineage, we tested lineage‐negative cells for expression of these markers. After culture in serum‐free medium in Step 2, immunocytochemistry in Fig. 4 demonstrated expression of vimentin (Panel B) and nestin (Panel C). Similar temporal expression patterns of these differentiation markers were seen for CD133+ and CD34+ cells (data not shown).
Figure 4.
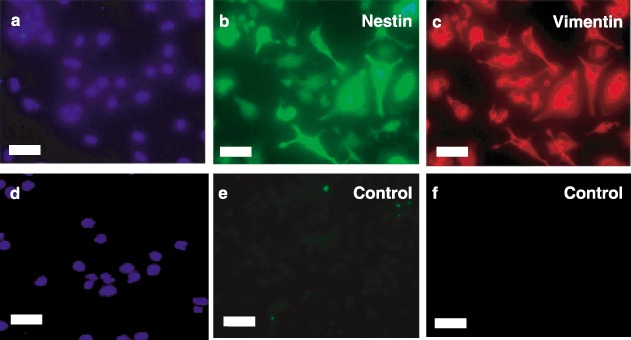
Immunocytochemical characterization of nestin and vimentin expression. Lineage‐negative stem cells after serum‐free induced attachment in Step 2 were fixed and stained with antihuman nestin IgG (Panel b), antihuman vimentin immunoglobulin G (IgG) (Panel c) or control IgG (Panels e and f). Cells were then exposed to fluorescein isothiocyanate (FITC) (Panels b and e) – or Texas Red (Panels c and f) – conjugated secondary antibodies and nuclei stained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (Panels a and d). Fluorescent micrographs are representative of at least three experiments. Scale bars, 25 µm.
We next tested for expression of key markers for the pancreatic phenotype. While insulin is necessary for our long‐term goals of stem cell therapy for type 1 diabetes, insulin is present in the cell culture medium and can be taken up from the medium. Thus, it is necessary to test for the presence of C‐peptide, which can only be derived from de novo synthesis of pro‐insulin and proper proteolytic processing. Towards this end, immunocytochemistry for both insulin and C‐peptide was performed. Figure 5 shows that differentiated CD34+ (Fig. 5a), CD133+ (Fig. 5b) and lineage‐negative (Fig. 5c) isolates were all capable of producing C‐peptide and containing insulin. This demonstrates that some, if not all, of the insulin in these cells is derived from de novo synthesis. Laser scanning confocal microscopy of lineage‐negative cells showed that the punctate nuclei were surrounded by an extensive cytoplasm in these differentiated cells (Fig. 5d).
Figure 5.
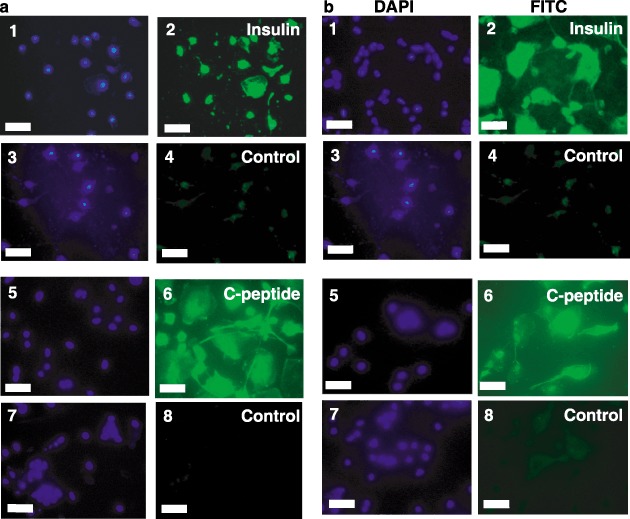
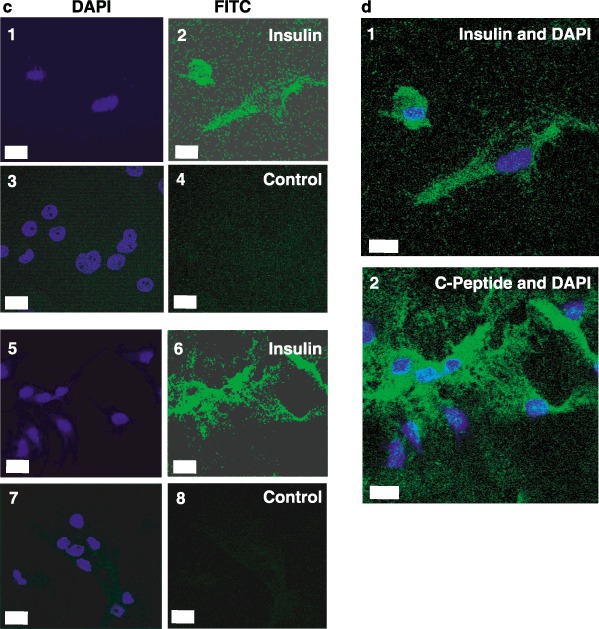
Insulin and C‐peptide staining of differentiated stem cells. CD34+ (Panel a), CD133+ (Panel b) and lineage‐negative (Panel c) cells were differentiated as described in ‘Methods.’ Cells were stained with anti‐insulin immunoglobulin G (IgG) (subpanel 2), anti‐C‐peptide IgG (subpanel 6), or control IgGs followed by fluorescein isothiocyanate (FITC)‐conjugated secondary antibodies (subpanels 4 and 8). Nuclei were then stained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (subpanels 1, 3, 5 and 7). Images were captured by fluorescence microscopy (Panels A and B; Scale bars, 25 µm) or laser scanning confocal microscopy (Panel c; Scale bars, 10 µm). Merged images of DAPI and insulin (Panel d, subpanel 1) or DAPI and C‐peptide (Panel d, subpanel 2) were captured by laser scanning confocal microscopy (Scale bars, 10 µm). Micrographs are representative of at least three experiments.
Finally, we tested the potential of human lineage‐negative stem cells to produce C‐peptide with an independent, more rapid engineering protocol utilizing distinct factors for expansion and differentiation. Cells were expanded by treatment with Activin A followed by treatment with all‐trans retinoic acid in the presence of serum. After several days in bFGF, differentiation was then induced by laminin and nicotinamide in the final step. Figure 6 illustrates that this substantially different protocol resulted in differentiation to pancreatic β cell‐like precursors that produce insulin and C‐peptide. On a practical note, this latter protocol was much shorter, requiring only 9 days compared to 22 days for the former protocol.
Figure 6.
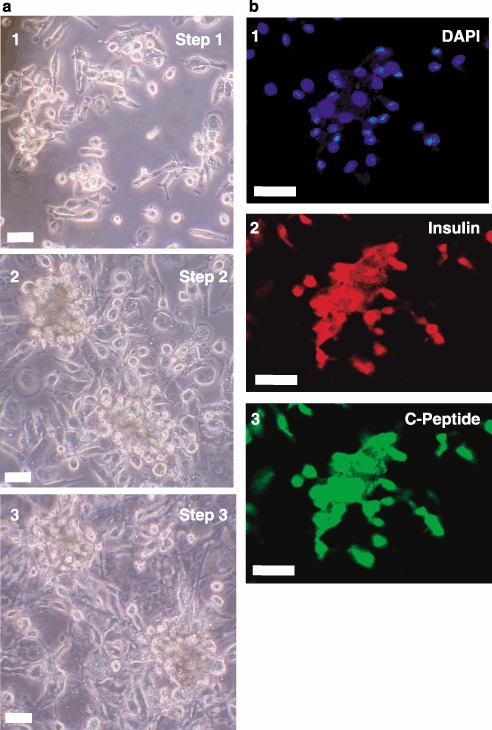
Insulin and C‐peptide staining after a 9‐day differentiation protocol. Phase contrast microscopy is shown in Panel (a) for lineage‐negative cells treated with Activin A followed by retinoic acid (Step 1, subpanel 1), basic fibroblast growth factor (bFGF) (Step 2, subpanel 2) and finally, laminin, nicotinamide and bFGF (Step 3, subpanel 3). Differentiated cells were visualized by fluorescence immunocytochemistry in Panel (b) for insulin (subpanel 2) and C‐peptide (subpanel 3). Nuclei were stained with 4’,6‐diamidino‐2‐phenylindole (DAPI) (subpanel 1). Micrographs are representative of three experiments. Scale bars, 25 µm.
DISCUSSION
We recently reported the novel discovery, isolation and expansion of human UCB‐derived, embryonic‐like stem cells (CBE) in the lineage‐negative compartment (McGuckin et al. 2005). These cells expressed many markers unique to ESCs and formed cellular aggregates that resembled embryoid bodies formed by ESCs. We expanded CBEs in two‐dimensional culture and engineered them in three‐dimensional culture to form hepatic progenitors of the definitive endodermal lineage. Because pancreatic β cells form developmentally from the definitive endoderm, distinct from other endodermal derivatives (Lu et al. 2001; Robb & Tam 2004), we hypothesized that CBEs, and perhaps less primitive lineages could be differentiated into insulin‐producing cells.
To test this hypothesis, we isolated lineages from the UCB stem cell compartment that differed in the extent of development and subjected them to a protocol reported to differentiate human ESCs to the pancreatic lineage of insulin production (Lumelsky et al. 2001). Lineage‐negative, CD133+, and CD34+ isolates all recapitulated the temporal protein expression patterns of nestin and vimentin shown for ESCs, as well as the morphological changes. Most importantly, at the end of this protocol, all three lineages contained C‐peptide and insulin.
The presence of insulin in cells clearly does not, by itself, reflect insulin production. In fact, several reports of stem cells differentiated to insulin‐producing cells have been questioned (Rajagopal et al. 2003; Hansson et al. 2004). One of the confounding problems is that insulin, which is present in high concentrations in the serum typically used for the expansion and differentiation of stem cells, can be taken up and concentrated within many cell types. The next critical level of evidence for de novo insulin synthesis derives from the presence of C‐peptide that has become the standard for demonstrating that cells do indeed synthesize insulin (Blyszczuk et al. 2004; Blyszczuk & Wobus 2004; Kania et al. 2004; Segev et al. 2004; Shi et al. 2005). In contrast to insulin, C‐peptide can only come from the endogenous, de novo synthesis of pre‐proinsulin mRNA, translation of the pre‐proinsulin protein and properly regulated proteolytic processing to produce mature insulin and C‐peptide. This is the first demonstration of human cord blood stem cells producing insulin.
To independently confirm that stem cells could be differentiated to produce insulin, a second, entirely distinct, protocol was followed (Shi et al. 2005). Indeed, using Activin A and all‐trans retinoic acid followed by laminin, bFGF and nicotinamide, lineage‐negative cells were engineered through the same temporal protein expression patterns and morphological changes yielding cells that contained both insulin and C‐peptide. This further confirms the multi‐potency of cord blood stem cells to become pancreatic‐like, insulin‐producing cells.
Finally, in the initial characterization of isolates from different stem cell compartments representing different stages of development, we tested for expression of SSEA‐4, a marker previously thought to be specific for ESCs we recently reported on lineage‐negative cells. While confirming SSEA‐4 on lineage‐negative cells, surprisingly, CD133+ and CD34+ isolates also expressed this rare antigen. This immunocytochemical localization has been confirmed by flow cytometry (data not shown). Furthermore, expression of this ‘primitive’ consensus ESC marker has now been confirmed in cord blood mononuclear cells (Zhao et al. 2006). Together, these studies confirm that SSEA‐4 is expressed outside the ESC lineage in multiple stem cell lineages such as CD133 and CD34 during haematopoietic differentiation.
ACKNOWLEDGEMENTS
This work was supported by the McCoy Foundation (LAD, RGT, JAC, RJU), the Clayton Foundation for Research (LAD, RGT, RJU), and Jack Currie. The authors are deeply indebted to the staff of the Perinatal Research Division at University of Texas Medical Branch for excellent assistance in collection of human umbilical cord blood.
REFERENCES
- Abuljadayel IS (2003) Induction of stem cell‐like plasticity in mononuclear cells derived from unmobilised adult human peripheral blood. Curr. Med. Res. Opin. 19, 355–375. [DOI] [PubMed] [Google Scholar]
- Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE (2002) Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 8, 541–550. [DOI] [PubMed] [Google Scholar]
- Blyszczuk P, Asbrand C, Rozzo A, Kania G, St‐Onge L, Rupnik M, Wobus AM (2004) Embryonic stem cells differentiate into insulin‐producing cells without selection of nestin‐expressing cells. Int. J. Dev Biol. 48, 1095–1104. [DOI] [PubMed] [Google Scholar]
- Blyszczuk P, Wobus AM (2004) Stem cells and pancreatic differentiation in vitro . J. Biotechnol. 113, 3–13. [DOI] [PubMed] [Google Scholar]
- Bock T (2004) The source (S) for new pancreatic beta cells in adult life. Bioessays 26, 1156–1159. [DOI] [PubMed] [Google Scholar]
- Bonner‐Weir S (2003) Stem cells in diabetes: what has been achieved. Horm. Res. 60 (Suppl. 3), 10–12. [DOI] [PubMed] [Google Scholar]
- Bonner‐Weir S, Baxter LA, Schuppin GT, Smith FE (1993) A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes 42, 1715–1720. [DOI] [PubMed] [Google Scholar]
- Booth HA, Holland PW (2004) Eleven daughters of NANOG. Genomics 84, 229–238. [DOI] [PubMed] [Google Scholar]
- Brimble SN, Zeng X, Weiler DA, Luo Y, Liu Y, Lyons IG, Freed WJ, Robins AJ, Rao MS, Schulz TC (2004) Karyotypic stability, genotyping, differentiation, feeder‐free maintenance, and gene expression sampling in three human embryonic stem cell lines derived prior to August 9, 2001. Stem Cells Dev. 13, 585–597. [DOI] [PubMed] [Google Scholar]
- Brolen GK, Heins N, Edsbagge J, Semb H (2005) Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin‐producing beta‐cell‐like cells. Diabetes 54, 2867–2874. [DOI] [PubMed] [Google Scholar]
- Burke ZD, Tosh D (2005) Therapeutic potential of transdifferentiated cells. Clin. Sci. (Lond.) 108, 309–321. [DOI] [PubMed] [Google Scholar]
- Choi Y, Ta M, Atouf F, Lumelsky N (2004) Adult pancreas generates multipotent stem cells and pancreatic and nonpancreatic progeny. Stem Cells 22, 1070–1084. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Nagler A (2004) Umbilical cord blood transplantation – how, when and for whom? Blood Rev. 18, 167–179. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 23, 1534–1541. [DOI] [PubMed] [Google Scholar]
- Di Gioacchino G, Di Campli C, Zocco MA, Piscaglia AC, Novi M, Santoro M, Santoliquido A, Flore R, Tondi P, Pola P, Gasbarrini G, Gasbarrini A (2005) Transdifferentiation of stem cells in pancreatic cells: state of the art. Transplant. Proc. 37, 2662–2663. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA (2004) Adult pancreatic beta‐cells are formed by self‐duplication rather than stem‐cell differentiation. Nature 429, 41–46. [DOI] [PubMed] [Google Scholar]
- Forraz N, Pettengell R, Deglesne PA, McGuckin CP (2002a) AC133+ umbilical cord blood progenitors demonstrate rapid self‐renewal and low apoptosis. Br. J. Haematol. 119, 516–524. [DOI] [PubMed] [Google Scholar]
- Forraz N, Pettengell R, McGuckin CP (2002b) Haemopoietic and neuroglial progenitors are promoted during cord blood ex vivo expansion. Br. J. Haematol. 119, 888. [DOI] [PubMed] [Google Scholar]
- Forraz N, Pettengell R, McGuckin CP (2004) Characterization of a lineage‐negative stem‐progenitor cell population optimized for ex vivo expansion and enriched for LTC‐IC. Stem Cells 22, 100–108. [DOI] [PubMed] [Google Scholar]
- Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T (2003) Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 52, 2007–2015. [DOI] [PubMed] [Google Scholar]
- Gerecht‐Nir S, Itskovitz‐Eldor J (2004) The promise of human embryonic stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 18, 843–852. [DOI] [PubMed] [Google Scholar]
- Giannoukakis N, Robbins PD (2002) Gene and cell therapies for diabetes mellitus: strategies and clinical potential. Biodrugs 16, 149–173. [DOI] [PubMed] [Google Scholar]
- Grapin‐Botton A (2005) Ductal cells of the pancreas. Int. J. Biochem. Cell Biol. 37, 504–510. [DOI] [PubMed] [Google Scholar]
- Habener JF (2004) A perspective on pancreatic stem/progenitor cells. Pediatr. Diabetes 5 (Suppl. 2), 29–37. [DOI] [PubMed] [Google Scholar]
- Hansson M, Tonning A, Frandsen U, Petri A, Rajagopal J, Englund MC, Heller RS, Hakansson J, Fleckner J, Skold HN, Melton D, Semb H, Serup P (2004) Artifactual insulin release from differentiated embryonic stem cells. Diabetes 53, 2603–2609. [DOI] [PubMed] [Google Scholar]
- Hayek A (2004) In search of endocrine progenitor/stem cells in the human pancreas. Pediatr. Diabetes 5 (Suppl. 2), 70–74. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Theise ND (2004) Stem‐cell therapy for diabetes mellitus. Lancet 364, 203–205. [DOI] [PubMed] [Google Scholar]
- Kania G, Blyszczuk P, Wobus AM (2004) The generation of insulin‐producing cells from embryonic stem cells – a discussion of controversial findings. Int. J. Dev. Biol. 48, 1061–1064. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tsunoda Y (1993) Totipotency and pluripotency of embryonic nuclei in the mouse. Mol. Reprod. Dev. 36, 276–278. [DOI] [PubMed] [Google Scholar]
- Kodama S, Faustman DL (2004) Routes to regenerating islet cells: stem cells and other biological therapies for type 1 diabetes. Pediatr. Diabetes 5 (Suppl. 2), 38–44. [DOI] [PubMed] [Google Scholar]
- Kumar M, Jordan N, Melton D, Grapin‐Botton A (2003) Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev. Biol. 259, 109–122. [DOI] [PubMed] [Google Scholar]
- Kume S (2005) Stem‐cell‐based approaches for regenerative medicine. Dev. Growth Differ. 47, 393–402. [DOI] [PubMed] [Google Scholar]
- Lechner A (2004) Stem cells and regenerative medicine for the treatment of type 1 diabetes: the challenges lying ahead. Pediatr. Diabetes 5 (Suppl. 2), 88–93. [DOI] [PubMed] [Google Scholar]
- Lu CC, Brennan J, Robertson EJ (2001) From fertilization to gastrulation: axis formation in the mouse embryo. Curr. Opin. Genet. Dev. 11, 384–392. [DOI] [PubMed] [Google Scholar]
- Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R (2001) Differentiation of embryonic stem cells to insulin‐secreting structures similar to pancreatic islets. Science 292, 1389–1394. [DOI] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Allouard Q, Pettengell R (2004) Umbilical cord blood stem cells can expand hematopoietic and neuroglial progenitors in vitro . Exp. Cell Res. 295, 350–359. [DOI] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Baradez MO, Lojo‐Rial C, Wertheim D, Whiting K, Watt SM, Pettengell R (2003a) Colocalization analysis of sialomucins CD34 and CD164. Stem Cells 21, 162–170. [DOI] [PubMed] [Google Scholar]
- McGuckin CP, Forraz N, Baradez MO, Navran S, Zhao J, Urban R, Tilton R, Denner L (2005) Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif. 38, 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuckin CP, Pearce D, Forraz N, Tooze JA, Watt SM, Pettengell R (2003b) Multiparametric analysis of immature cell populations in umbilical cord blood and bone marrow. Eur J. Haematol. 71, 341–350. [DOI] [PubMed] [Google Scholar]
- Perin EC, Silva GV (2004) Stem cell therapy for cardiac diseases. Curr. Opin. Hematol. 11, 399–403. [DOI] [PubMed] [Google Scholar]
- Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA (2003) Insulin staining of ES cell progeny from insulin uptake. Science 299, 363. [DOI] [PubMed] [Google Scholar]
- Rao MS, Yeldandi AV, Reddy JK (1990) Stem cell potential of ductular and periductular cells in the adult rat pancreas. Cell Differ. Dev. 29, 155–163. [DOI] [PubMed] [Google Scholar]
- Robb L, Tam PP (2004) Gastrula organiser and embryonic patterning in the mouse. Semin. Cell Dev. Biol. 15, 543–554. [DOI] [PubMed] [Google Scholar]
- Roche E, Enseat‐Wase R, Reig JA, Jones J, Leon‐Quinto T, Soria B (2006) Therapeutic potential of stem cells in diabetes. Handb. Exp. Pharmacol. 174, 147–167. [PubMed] [Google Scholar]
- Schulze M, Fandrich F, Ungefroren H, Kremer B (2005) Adult stem cells – perspectives in treatment of metabolic diseases. Acta Gastroenterol. Belg. 68, 461–465. [PubMed] [Google Scholar]
- Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, Van Der Kooy D (2004) Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 22, 1115–1124. [DOI] [PubMed] [Google Scholar]
- Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz‐Eldor J (2004) Differentiation of human embryonic stem cells into insulin‐producing clusters. Stem Cells 22, 265–274. [DOI] [PubMed] [Google Scholar]
- Shi Y, Hou L, Tang F, Jiang W, Wang P, Ding M, Deng H (2005) Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three‐step approach with activin A and all‐trans retinoic acid. Stem Cells 23, 656–662. [DOI] [PubMed] [Google Scholar]
- Stafford D, Prince VE (2002) Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr. Biol. 12, 1215–1220. [DOI] [PubMed] [Google Scholar]
- Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schumichen C, Nienaber CA, Freund M, Steinhoff G (2003) Autologous bone‐marrow stem‐cell transplantation for myocardial regeneration. Lancet 361, 45–46. [DOI] [PubMed] [Google Scholar]
- Street CN, Rajotte RV, Korbutt GS (2003) Stem cells: a promising source of pancreatic islets for transplantation in type 1 diabetes. Curr. Top. Dev. Biol. 58, 111–136. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Nakano T (2001) Development of haematopoietic cells from embryonic stem cells. Int. J. Hematol. 73, 1–5. [DOI] [PubMed] [Google Scholar]
- Weir GC, Bonner‐Weir S (2004) Beta‐cell precursors – a work in progress. Nat Biotechnol. 22, 1095–1096. [DOI] [PubMed] [Google Scholar]
- Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Tomaselli GF, Li RA (2005) Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell‐based pacemakers. Circulation 111, 11–20. [DOI] [PubMed] [Google Scholar]
- Yalniz M, Pour PM (2005) Are there any stem cells in the pancreas? Pancreas 31, 108–118. [DOI] [PubMed] [Google Scholar]
- Yamada S, Kojima I (2005) Regenerative medicine of the pancreatic beta cells. J. Hepatobiliary Pancreat Surg. 12, 218–226. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang H, Mazzone T (2006) Identification of stem cells from human umbilical cord blood with embryonic and hematopoietic characteristics. Exp. Cell Res. 312, 2454–2464. [DOI] [PubMed] [Google Scholar]


