Abstract
Objectives: Enamel matrix proteins (EMPs) have been demonstrated to promote periodontal regeneration. However, effects of EMPs on human alveolar osteoblasts (hAOBs), up to now, have still been unclear. The purpose of this study was to investigate influence of EMPs on proliferation, differentiation and attachment of hAOBs in vitro.
Materials and methods: EMPs were extracted using the acetic acid method, hAOBs were obtained and cultured in vitro. Cell proliferation, alkaline phosphatase (ALP) activity, mRNA expression of osteogenic markers and cell attachment were measured in the absence and in the presence of EMPs (50, 100 and 200 μg/ml).
Results: EMPs increased proliferation of hAOBs; however, they inhibited ALP activity and mRNA expression of osteogenic markers (collagen I, ALP, runt‐related protein 2, osteocalcin, bone sialoprotein and osteopontin). Meanwhile, EMPs hindered hAOBs’ attachment. These effects occurred in EMPs concentration‐dependent manner.
Conclusions: These results indicate that EMPs may inhibit osteoblastic differentiation and attachment to prevent ankylosis and allow other cell types to regenerate periodontal tissues.
Introduction
Enamel matrix proteins (EMPs) secreted by Hertwig’s epithelial root sheath, play an important role in periodontal and tooth root development (1). Enamel matrix derivative (EMD) is a commercial product of enamel protein extracts from porcine tooth buds. Influence of EMPs has been demonstrated in clinical and experimental studies. Clinical investigations have shown that EMD can promote periodontal pocket depth and attachment level (2, 3, 4). From experimental results, it has been observed that EMD induces formation of acellular cementum, periodontal ligament and alveolar bone, which suggested that EMD might enhance periodontal regeneration (5, 6). However, a variety of research studies has indicated that EMD fails to promote new bone formation around titanium implants in rabbit and dog models (7, 8). Periodontal ligament cells (PDLCs), gingival fibroblasts, osteoblasts and gingival epithelial cells are involved in the process of periodontal repair. Currently it is thought that PDLCs and osteoblasts are helpful for periodontal regeneration.
Some research surveys have focused on effects of EMPs on PDLCs and osteoblasts and in vitro EMD stimulates PDLC proliferation, attachment, spreading, alkaline phosphate (ALP) activity and bone nodule formation (9, 10, 11, 12). However, effects of EMD on osteogenic cells seems to depend on cell type and culture system. According to work by Schwartz et al., EMD stimulated proliferation, but not differentiation of 2T9 cells, inhibited proliferation and stimulated differentiation of human osteoblast‐like osteosarcoma cell line MG63, increased cell proliferation and differentiation of human osteoblast NHOst cells (13). In murine osteoblastic cells (MC3T3‐E1), EMD promoted cell proliferation and increased expression of osteogenic markers (bone sialoprotein and osteopontin) (14, 15), did not change runt‐related protein 2 expression (15), but inhibited osteocalcin expression (16). Song et al. demonstrated that new cell cementum‐like tissue formed along EMP‐treated root slices, which indicated that EMPs induced differentiation of porcine bone marrow stromal cells into cementoblasts (17). In primary culture, EMD prevented rat calvaria cells from osteoblastic differentiation (18). Thus, to date, effects of EMPs on osteoblasts are controversial.
As a part of periodontal tissue, human alveolar bone plays an important role in periodontal regeneration and dental implantation. Over life as a whole, alveolar bone in maxillae and mandibles continuously remodels, due to tooth eruption, forces of mastication and periodontal inflammation. Although alveolar bone appears to resemble skeletal bone, it has specific characteristics (including origination from ectomesenchyme) and formation by intramembranous ossification (19, 20, 21). As human alveolar osteoblasts (hAOBs) could directly reflect periodontal physiological conditions, a primary culture system is useful to elucidate effects of EMPs on alveolar osteoblasts and explain clinical and experimental results. The purpose of this study was to investigate effects of EMPs on hAOBs by determining cell proliferation, ALP activity, mRNA expression of osteogenesis‐associated genes and cell adhesion.
Materials and methods
Reagents
Hank’s balanced saline (HBS), α‐minimum essential medium (α‐MEM) with 2 mmol/ml l‐glutamine, fetal bovine serum (FBS), antibiotics‐antimycotics (Gibco‐BRL, Gaithersburg, MD, USA); 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide (MTT), dimethyl sulphoxide (DMSO), ascorbic acid, β‐glycerophosphate, dexamethasone, Triton X‐100, r‐nitrophenol phosphate in 2‐amino‐2‐methyl‐1‐propanol buffer, silver nitrate, sodium thiosulphate, bovine serum albumin (BSA) (Sigma‐Aldrich, St Louis, MO, USA); Trizol reagent, SuperScriptTM III First‐strand Synthesis System (Invitrogen, Carlsbad, CA, USA); SYBR® Premix Ex TaqTM real‐time PCR kit (TaKaRa Biotechnology Co, Dalian, China) were used in this study. EMPs were extracted from porcine tooth buds and dissolved in acid water (5 mm acetic acid).
Subjects
Approval for human tissue specimens was obtained from the Committee of Ethics in Research of School of Medicine, Shanghai Jiao Tong University. Informed consent was obtained from every dental patient who was undergoing an oral surgical procedure. Discarded alveolar bone was collected from these dental operations. All donors were young adults (five women and four men, aged from 22 to 36 years), non‐smokers, of normal healthy condition without systemic disease, but with clinical periodontal inflammation. Sites from which alveolar bone was collected were not inflammed.
Cell culture
The method to explants such cultures has been described previously (22). In brief, alveolar bone explants were washed in sterile HBS, cleaned of adherent soft tissues, minced into small pieces and cultured in α‐MEM supplemented with 10% FBS, antibiotics‐antimycotics (100 U/ml penicillin G, 100 mg/ml streptomycin sulphate and 0.25 mg/ml amphotericin B). They were then incubated in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. Cells were not collected and serially passaged until ∼80% confluence was achieved. Subsequently, cells at 2nd–3rd passage were used in all experiments.
Preparation of porcine enamel matrix proteins
Porcine EMPs were extracted according to previously described methods (17). In brief, dental germs were dissected freshly from jaws of 6‐month‐old pigs. Porcine EMPs were extracted from unmineralized enamel matrix using acetic acid, and then lyophilized. Total lyophilized powder was dissolved in 5 mm acetic acid at a concentration of 4 mg/ml, sterilized by filtration and stored at −80 °C.
Cell viability assay
Cells were seeded at 4 × 104/ml in 96‐well plates (Corning Inc., Corning, NY, USA) and cultured in α‐MEM containing 10% FBS. After 24 h, media were replaced with various concentrations of EMPs (50, 100 and 200 μg/ml) in six duplicate wells. In the control group, EMPs solvent was used. Culture media were changed every 3 days and cells were re‐stimulated with EMPs. Cell viability was determined by adding MTT at concentration of 0.5 mg/ml. After being incubated at 37 °C for 4 h, MTT was converted into purple formazan product by viable cells only. Medium was then decanted and DMSO was used to dissolve formazan salts. Finally, absorbance was measured at 490 nm.
Cell population growth assay
Cells were plated in 24‐well culture plates (Corning Inc.) at density of 2 × 104 cells/well in 2 ml of α‐MEM supplemented with 10% FBS. Cells were allowed to attach and spread for 24 h, and media were replaced with various concentrations of EMPs (50, 100 and 200 μg/ml) in quadruplicate. After treatments with four experimental conditions for 0, 1, 3, 5, 7 and 9 days, cells were harvested with 0.05% trypsin and 0.02% EDTA in PBS, and counted using Z2™ Coulter Counter® Cell and Particle Counter (Beckman Coulter Inc, Fullerton, CA, USA). Data are expressed as number of cells.
Determination of ALP activity
Cells were cultured in α‐MEM containing 10% FBS, 50 mmol/ml ascorbic acid, 10 nmol/ml dexamethasone and 10 mmol/ml β‐glycerophosphate. They were incubated in humidified atmosphere of 95% air, 5% CO, at 37 °C for 7 days. Differentiation media were changed every 2–3 days. Cells were seeded as cell suspension (at 4 × 104/ml) in 24‐well plates (Corning Inc.). After 24 h, media were replaced with or without various concentrations of EMPs (0, 50, 100 and 200 μg/ml). Triplicate cultures were set up in each group. ALP activity was determined in cell lysates. After treatment with EMPs for 0, 4, 8, 12, 16 and 20 days, cells were harvested, washed twice in PBS and lysed with 1% Triton X‐100 at 4 °C for 1 h. Replicate aliquots from each lysate were added the substrate r‐nitrophenol phosphate in 2‐amino‐2‐methyl‐1‐propanol buffer and incubated at 37 °C for 1 h in dark. Then, 0.5 N NaOH was added to stop the reaction. Amount of ALP activity was measured by reading absorbance at 405 nm on a Microplate Reader (Bio‐Tek, Winooski, VT, USA). ALP activities were normalized by cell protein content and expressed as OD value/h.mg protein.
Reverse transcriptase real‐time polymerase chain reaction analysis
After 7 and 14 days in EMP‐treated media (0, 50, 100 and 200 μg/ml) as above, in six‐well plates (Corning Inc.), RNA was isolated using Trizol reagent according to the manufacturer’s instructions. RNA was reverse transcribed into cDNA using SuperScriptTM III First‐strand Synthesis System according to the manufacturer’s instructions. Real‐time polymerase chain reaction (PCR) was performed in triplicate for mRNA expression of osteogenic markers using SYBR® Premix Ex TaqTM real‐time PCR kit according to manufacturer’s instructions. Gene‐specific primers used were: forward AGGGCCAAGACGAAGACATC and reverse AGATCACGTCATCGCACAACA for collagen 1 (Col 1); forward ACCATTCCCACGTCTTCACATTTG and reverse AGACATTCTCTCGTTCACCGCC for ALP; forward ATGAGAGCCCTCACACTCCTCG and reverse GTCAGCCAACTCGTCACAGTCC for osteocalcin (OCN); forward CTGGCACAGGGTATACAGGGTTAG and reverse ACTGGTGCCGTTTATGCCTTG for bone sialoprotein (BSP); forward TTGCAGCCTTCTCAGCCAA and reverse GGAGGCAAAAGCAAATCACTG for osteopontin (OPN); forward TCTGGCCTTCCACTCTCAGT and reverse GACTGGCGGGGTGTAAGTAA for runt‐related protein 2 (Runx2); forward GCACCGTCAAGGCTGAGAAC and reverse ATGGTGGTGAAGACGCCAGT for glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH). The following conditions were used for amplification: pre‐denaturation 95 °C for 30 s, denaturation at 95 °C for 5 s, followed by annealing and extension at 58–60 °C for 30 s by 40 cycles. GAPDH served as an internal control.
Cell attachment assay
The method modified from Hebert (23) was performed to test for cell adhesion. In brief, 24‐well culture plates (Corning Inc.) were coated with 1 ml/well PBS containing EMPs (0, 50, 100 and 200 μg/ml) at 4 °C. There were six duplicate wells in each group. After 24 h, plates were washed three times in PBS, and 0.02% BSA in a‐MEM without FBS and antibiotics was used to block non‐specific binding sites for 1 h at 37 °C. Cells were seeded at density of 2.4 × 105 cells/well in EMP‐coated plates and incubated for 1.5, 3.5 and 6.5 h at 37 °C in 5% CO2. After incubation, unattached cells were gently removed with 1 ml PBS. Attached cells were harvested with trypsin and counted in a Beckman Coulter Counter as described above. Cells in uncoated plates were used as negative control. Data are expressed as percentage of attached cells compared to initial cells.
Statistical analysis
Each experiment was repeated at least twice. Real‐time data analysis was performed by relative quantification using the 2−ΔΔCT method, according to Livak and Schmittgen (24). Gene expression was normalized by gene expression of GAPDH and then described as x‐fold expression over control.
Data were statistically analysed using analysis of variance (ANOVA). Differences at P < 0.05 were considered statistically significant.
Results
To reveal effects of EMPs on human alveolar osteoblasts (hAOBs), we detected cell proliferation, differentiation and adhesion after hAOBs were treated with EMPs.
First, the effect of EMPs on human alveolar osteoblast proliferation was assessed by MTT assay and cell population growth assay. EMPs significantly increased viability and replication of hAOBs when compared to negative controls (P < 0.05), in a concentration‐dependent manner in EMPs‐treated groups (1, 2). Although cell viability decreased gradually in all groups after 8 days, it was still higher in EMP‐treated groups than in controls (P < 0.05) (Fig. 1).
Figure 1.
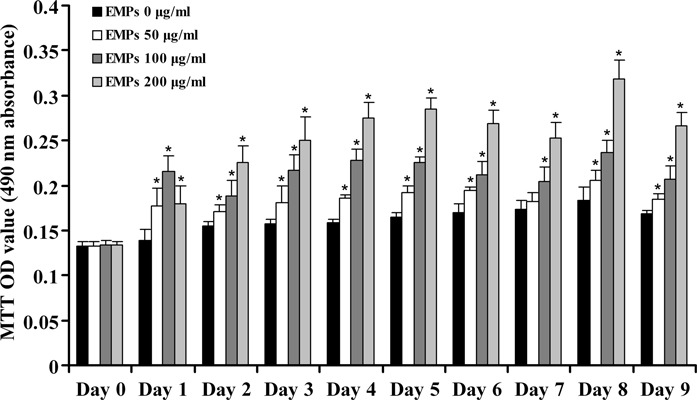
MTT assay. Human alveolar osteoblast viability by MTT assay during continuous 9‐day culture. Results are presented as means ± SD. *Statistically significant compared to control: P < 0.05.
Figure 2.
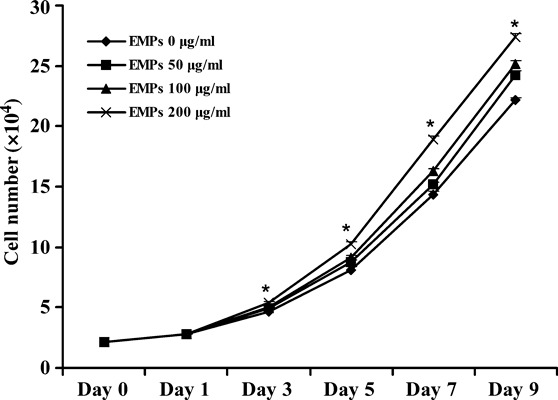
Cell population growth assay. Human alveolar osteoblast growth by counting cell number. Results are presented as means ± SD. *Statistically significant compared to control: P < 0.05.
Human alveolar bone plays an important role in periodontal tissue repair; hence, we detected osteogenic differentiation of hAOBs after being treated with EMPs. Effects of EMPs on ALP activity was explored. At the beginning of culture, there was no difference in ALP activity between all groups (P > 0.05). However, ALP activities significantly decreased in EMPs‐treated groups compared to controls at 4, 8, 12, 16 and 20 days, especially in the 200 μg/ml EMP‐treated group (P < 0.05) (Fig. 3).
Figure 3.
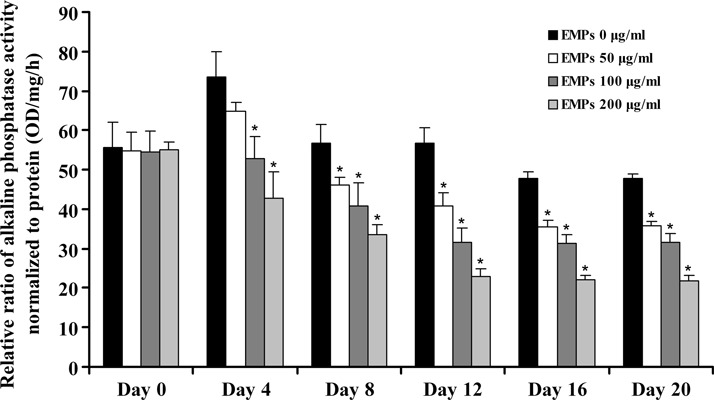
Alkaline phosphatase activity assay. Comparison of human alveolar osteoblast ALP between different EMP‐treated groups. *Statistically significant compared to control: P < 0.05.
Furthermore, we measured mRNA expression of osteogenic markers with real‐time PCR. There was no significant difference in mRNA expression of Col 1 among all groups on day 7 (P > 0.05); 200 μg/ml EMP suppressed Col I expression on day 14 (P < 0.05) although only a slight, not significant, decrease in Col I expression was found in 50 and 100 μg/ml EMP groups (P > 0.05) (Fig. 4a). EMPs suppressed ALP in a concentration‐dependent manner throughout the experimental period, as shown in Fig. 4b (P < 0.05). Figure 4c shows that EMPs inhibited Runx 2 mRNA expression on day 14 in a concentration‐dependent manner (P < 0.05); however, only 200 μg/ml EMP had this inhibitory effect on mRNA expression of Runx 2 on day 7 (P < 0.05). At early stages, OCN expression was very low in all groups, which showed no significant difference between them (P > 0.05); however, EMPs markedly suppressed mRNA expression of OCN on day 14 (P < 0.05) (Fig. 4d). EMPs stimulated mRNA expression of OPN on day 7 (P < 0.05), but mRNA levels of OPN decreased at late stages in EMP‐treated groups (P < 0.05) (Fig. 4e). On day 7, only 200 μg/ml EMPs obviously decreased BSP expression (P < 0.05); furthermore, inhibitory effects of EMPs were more obvious in a concentration‐dependent manner on day 14 (P < 0.05) (Fig. 4f).
Figure 4.
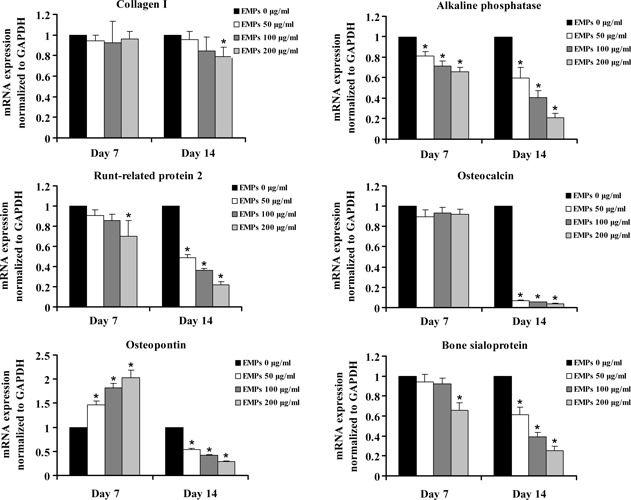
Real time quantity PCR. mRNA expression of Col I, ALP, Runx2, OCN, OPN and in hAOBs incubated with EMPs. Gene expression was normalized to GAPDH and is presented as percentage of relative expression level in untreated cells at the same time points. ALP, alkaline phosphatase; BSP, bone sialoprotein; Col I, collagen type I; OCN, osteocalcin; OPN, osteopontin; Runx2, Runt‐related protein 2. *Statistically significant compared to control: P < 0.05.
Cell adhesion plays an important role in wound tissue repair. Attachment of hAOBs appeared to be inhibited in a concentration‐dependent manner after application of EMPs (Fig. 5). At 1.5 h, 50 μg/ml EMPs increased hAOB attachment compared to controls (P < 0.05), but at 3.5 and 6.5 h, cell attachment in the 50 μg/ml EMP‐treated groups was significantly lower than in controls (P < 0.05); 100 and 200 μg/ml EMPs inhibited cell attachment at any time point (P < 0.05) (Fig. 5).
Figure 5.
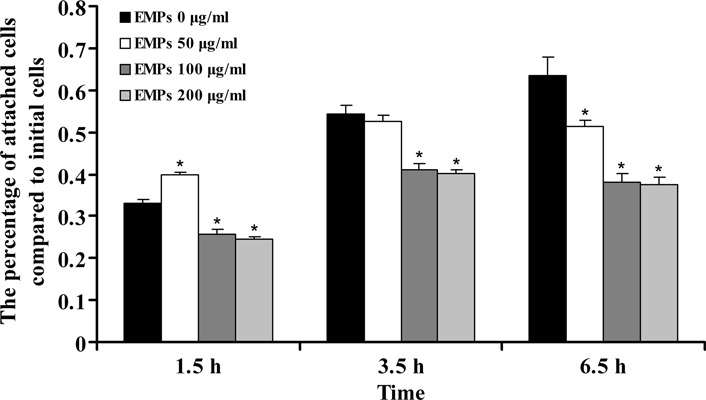
Cell attachment assay. Comparison of hAOB attachment to EMP‐coated surface at different concentrations. Data are expressed as percentage of attached cells compared to initial cells. Mean values are shown with standard deviation. *Statistically significant compared to control: P < 0.05.
Discussion
In this study, we demonstrated the effects of EMPs on proliferation, differentiation and attachment of human alveolar osteoblasts.
Cell proliferation plays an important role in the wound healing process, which enhances cell population over a wounded space at early stages and is a prerequisite for tissue formation at later stages. Some studies have shown that EMPs enhance various activities of different cell types. EMD at a concentration of 100 μg/ml increases proliferation of MC3T3‐E1 and rat bone marrow stromal cells (14, 25); Guida has indicated that EMD (12.5, 25 and 50 μg/ml) enhanced proliferation of human bone marrow stromal cells (hBMSCs) (26). In our previous study, EMPs (50, 100, 200 and 300 μg/ml) promoted proliferation of hBMSCs (27); however, the effect of 300 μg/ml EMP was lower than that of 200 μg/ml EMP. According to our previous results, we chose concentrations of EMPs (50, 100 and 200 μg/ml) in this study. hAOBs treated with EMPs (50, 100 and 200 μg/ml) increased hAOB proliferation in a time and dose‐dependent manner. Although mechanisms of how EMPs promote proliferation is not clear, enhancement of cell proliferation is clearly crucial for wound healing.
Although EMPs can enhance cell proliferation, effects of EMPs on differentiation of osteoblastic cell lines have been shown to be variable among different cell types and/or culture conditions (13, 14, 15, 16, 17, 18, 25, 26, 27, 28, 29). According to our findings, EMPs inhibited ALP activity and osteogenic differentiation of hAOBs, and the inhibitory effects on differentiation are consistent with further studies (16, 18, 26). We explored mechanisms of the inhibitory effects and found decrease in osteogenic differentiation markers regulated by EMPs.
Collagen I is the main protein in bone formed by osteogenic cell lineages. From our data, 200 μg/ml EMPs reduced mRNA expression of Col I, consistent with other research on human bone marrow stromal cells (26), but low concentration (50 and 100 μg/ml) had no significant inhibitory effects, in agreement with van den Dolder’s research on rat marrow stromal cells (25). Some studies have reported that EMD increased expression of Col I in MC3T3‐E1 cells (14) and primary human osteoblasts from femur and tibia (28). From the above mentioned studies, it is observed that inhibitory effects of EMPs on Col I depends on concentration and cell species.
As a membrane‐bound enzyme, alkaline phosphatase is involved in hydroxyapatitic crystal deposition during mineralization. From our results, it is observed that EMPs inhibited ALP activity in a concentration‐dependent manner, which confirmed previous research (16, 18, 26); however, our results did not agree with some others (14, 28, 29), in which EMD increased ALP activity or mRNA expression. Also, van den Dolder reported that there was no difference in ALP activity between EMD‐treated groups and EMD‐negative groups (25). Species of cell origin in these experiments were different from those in our study, which could result in such a discrepancy. As ALP is considered to be an early marker of osteoblast differentiation, we speculate that EMPs inhibit initial repopulation of osteoblasts.
Osteocalcin is expressed at late stages of osteoblastic differentiation and is associated with mineralization. In our findings, inhibition of OCN appeared in EMP‐treated groups, which is in agreement with some studies (16, 18, 26). However, it has been demonstrated that EMD (50 μg/ml) could increase OCN expression in human osteoblasts (28) but that EMD (100 μg/ml) had no effect on OCN expression on rat bone marrow cells (25). This discrepancy of results may be due to different culture systems and cell lines.
Osteopontin is a non‐collagenous protein of the extracellular matrix in calcified tissues. It has been reported that OPN expression has been found at margins of newly formed cementum and bone in periodontal regeneration research (30). According to our data, EMPs stimulated mRNA expression of OPN in a concentration‐dependent manner at day 7; however, they inhibited OPN expression at later stages, which is consistent with one piece of research (18), but inconsistent with another (15). As OPN is a multifunctional factor involved in bone modelling, immunity, infection and inflammation, its functions require further study in periodontal regeneration.
As a non‐collagenous extracellular matrix protein, bone sialoprotein is associated with mineralization of tissues and is involved in nucleation of hydroxyapatite during bone formation (30). It has been demonstrated that BSP could play a major role during formation and remodelling of bone and cementum (31, 32, 33). Weishaupt has reported that EMD increased mRNA expression of BSP (15), which is a result different from our data. Some work has demonstrated that EMD can regulate the promoter of BSP and increase BSP transcription in vitro (34, 35). According to Lamour’s research (36), Runx 2 has the capacity to bind to the promoter of BSP and increases its expression. In our findings, mRNA expression of Runx 2 was inhibited by EMPs, which could explain the decrease in mRNA expression of BSP in our experiments. Runx2 has dual regulatory activity, which represses and activates osteogenic gene expression. These effects depend on maturational stage of cells and cofactors involved at that stage (36). As the mechanism of EMP regulation on Runx 2 in human alveolar osteoblasts is still unclear, it needs to be further explored.
In tissue repair and regeneration, cell adhesion on extracellular matrix substrata is critical for organization of tissues. As Karring mentioned (37), ankylosis would not occur if cells derived from alveolar bone were to be excluded to reach and contact with root surfaces. Effects of EMPs on attachment of cells are still controversial. Lyngstadaas (11) found that attachment level of PDL cells over early hours increased nearly five times when the culture dish was coated with EMD, but Gestrelius (9) indicated that EMD had no significant effect on attachment of PDL cells. van der Pauw (38) noted that human periodontal ligament fibroblasts attached within 24 h, whereas human gingival fibroblasts barely attached on EMP‐coated substrata. Our previous studies have indicated that EMPs did not increase attachment levels of hBMSCs (27). Such conflicting results probably arose from different kinds of cells or EMPs being used by the various investigators. In our present study, EMPs at 50 μg/ml enhanced cell attachment at 1.5 h, but later, EMPs at this concentration inhibited cell attachment. We speculate that EMPs at low concentration only as particles provide a wider surface for cell adhesion. From our data, we observed that EMPs hindered hAOB attachment in vitro, which is possibly due to decrease in BSP. As a member of the SIBLING (small integrin binding ligand, N‐linked glycoproteins) family of proteins, BSP is commonly found in mineralized tissues. According to a study of Bernards et al., BSP enhance osteoblasts’ adhesion to cell culture polystyrene surface (39). Although the mechanism of inhibiting hAOB adhesion by EMPs needs to be further explored, this inhibitory effect is helpful for regenerating periodontal apparatus and preventing ankylosis formation.
In summary, EMPs significantly increased hAOB population growth, but decreased their osteoblastic differentiation and attachment. Inhibitory effects suggest that EMPs may function to allow other cell types to regenerate periodontal tissue by inhibiting osteoblastic differentiation and attachment, which could partly explain formation of periodontal apparatus but ankylosis after clinical and experimental application of EMPs for periodontal defects.
Acknowledgements
This study was funded by National Nature Science Foundation of China (81070838), Science and Technology Commission of Shanghai (08DZ2271100) and Shanghai Leading Academic Discipline Project (S30206).
References
- 1. Slavkin HC (1976) Towards a cellular and molecular understanding of periodontics. Cementogenesis revisited. J. Periodontol. 47, 249–255. [DOI] [PubMed] [Google Scholar]
- 2. Cortellinil P, Tonetti MS (2007) Minimally invasive surgical technique and enamel matrix derivative in intra‐bony defects. I: clinical outcomes and morbidity. J. Clin. Periodontol. 34, 1082–1088. [DOI] [PubMed] [Google Scholar]
- 3. Sculean A, Donos N, Windisch P, Brecx M, Gera I, Reich E et al. (1999) Healing of human infrabony defects following treatment with enamel matrix proteins or guided tissue regeneration. J. Periodontal. Res. 34, 310–322. [DOI] [PubMed] [Google Scholar]
- 4. Pontoriero R, Wennstrom J, Lindhe J (1998) The use of barrier membranes and enamel matrix proteins in the treatment of angular bone defects. A prospective controlled clinical study. J. Clin. Periodontol. 25, 524–530. [DOI] [PubMed] [Google Scholar]
- 5. Hammarstrom L (1997) Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 24, 658–668. [DOI] [PubMed] [Google Scholar]
- 6. Hammarstrom L, Heijl L, Gestrelius S (1997) Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J. Clin. Periodontol. 24, 669–677. [DOI] [PubMed] [Google Scholar]
- 7. Stenport V, Johansson C (2003) Enamel matrix derivative and titanium implants. J. Clin. Periodontol. 30, 359–363. [DOI] [PubMed] [Google Scholar]
- 8. Casati MZ, Sallum EA, Nociti FH Jr, Caffesse RG, Sallum AW (2002) Enamel matrix derivative and bone healing after guided bone regeneration in dehiscence‐type defects around implants. A histomorphometric study in dogs. J. Periodontol. 73, 789–796. [DOI] [PubMed] [Google Scholar]
- 9. Gestrelius S, Andersson C, Lindstrom D, Hammarstrom L, Somerman MJ (1997) In vitro studies on periodontal ligament cells and enamel matrix derivative. J. Clin. Periodontol. 24, 685–692. [DOI] [PubMed] [Google Scholar]
- 10. Hoang AM, Oates TW, Cocharn DL (2000) In vitro wound healing responses to enamel matrix derivative. J. Periodontol. 71, 1270–1277. [DOI] [PubMed] [Google Scholar]
- 11. Lyngstadaas S, Lundberg E, Ekadahl H, Gestrelius S (2001) Autocrine growth factors in periodontal ligament cells cultured on enamel matrix derivative. J. Clin. Periodontol. 28, 181–188. [DOI] [PubMed] [Google Scholar]
- 12. Cattaneo V, Rota C, Silverstri M, Piacentini C, Forlino A, Gallanti A et al. (2003) Effect of enamel matrix derivative on human periodontal fibroblasts: proliferation, morphology and root surface colonization: an in vitro study. J. Periodontal. Res. 38, 568–574. [DOI] [PubMed] [Google Scholar]
- 13. Schwartz Z, Carnes DL, Pulliam R, Lohmann CH, Sylvia VL, Liu Y et al. (2000) Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre‐osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast‐like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J. Periodontol. 71, 1287–1296. [DOI] [PubMed] [Google Scholar]
- 14. He J, Jiang J, Safavi KE, Spångberg LSW, Zhu Q (2004) Emdogain promotes osteoblast proliferation and differentiation and stimulates osteoprotegerin expression. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 97, 239–245. [DOI] [PubMed] [Google Scholar]
- 15. Weishaupt P, Bernimoulin JP, Trackman P, Hägewald S (2008) Stimulation of osteoblasts with Emdogain increases the expression of specific mineralization markers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 106, 304–308. [DOI] [PubMed] [Google Scholar]
- 16. Wada Y, Yamamoto H, Nanbu S, Mizuno M, Tamura M (2008) The suppressive effect of enamel matrix derivative on osteocalcin gene expression of osteoblasts is neutralized by an antibody against TGF‐β. J. Periodontal. 79, 341–347. [DOI] [PubMed] [Google Scholar]
- 17. Song AM, Shu R, Xie YF, Song ZC, Li HY, Liu XF et al. (2007) A study of enamel matrix proteins on differentiation of porcine bone marrow stromal cells into cementoblasts. Cell Prolif. 40, 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hama H, Azuma H, Seto H, Kido JI, Nagata T (2008) Inhibitory effect of enamel matrix derivative on osteoblastic differentiation of rat calvaria cells in culture. J. Periodontal. Res. 43, 179–185. [DOI] [PubMed] [Google Scholar]
- 19. Saffar JL, Lasfargues JJ, Cherruau M (1997) Alveolar bone and the alveolar process: the socket that is never stable. Periodontology 2000 13, 76–90. [DOI] [PubMed] [Google Scholar]
- 20. Sodek K, McKee MD (2000) Molecular and cellular biology of alveolar bone. Periodontology 2000 24, 99–126. [DOI] [PubMed] [Google Scholar]
- 21. Zernik JH, Nowroozi N, Liu YH, Maxson R Jr (1997) Development, maturation, and aging of the alveolar bone. New insights. Dent. Clin. North Am. 41, 1–15. [PubMed] [Google Scholar]
- 22. Jiang SY, Shu R, Xie YF, Zhang SY (2010) Age‐related changes in biological characteristics in human alveolar osteoblasts. Cell Prolif. 43, 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hebert C, Coletta RD, Norris K, Nikitakis N, Lopes M, Sauk JJ (2001) Nonnatural CBP2 binding peptides and peptomers modulate carcinoma cell adhesion and invasion. J. Cell. Biochem. 82, 145–154. [DOI] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- 25. van den Dolder J, Vloon AP, Jansen JA (2006) The effect of Emdogain® on the growth and differentiation of rat bone marrow cells. J. Periodontal. Res. 41, 471–476. [DOI] [PubMed] [Google Scholar]
- 26. Guida L, Annunziata M, Carinci F, Feo AD, Passaro I, Oliva A (2007) In vitro biologic response of human bone marrow stromal cells to enamel matrix derivative. J. Periodontol. 78, 2190–2196. [DOI] [PubMed] [Google Scholar]
- 27. Song ZC, Shu R, Zhang XL (2010) Cellular responses and expression profiling of human bone marrow stromal cells stimulated with enamel matrix proteins in vitro. Cell Prolif. 43, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reseland JE, Reppe S, Larsen AM, Berner HS, Reinholt FP, Gautvik KM et al. (2006) The effect of enamel matrix derivative on gene expression in osteoblasts. Eur. J. Oral Sci. 114, 205–211. [DOI] [PubMed] [Google Scholar]
- 29. Keila S, Nemcovsky CE, Moses O, Artzi Z, Weinreb M (2004) In vitro effects of enamel matrix proteins on rat bone marrow cells and gingival fibroblasts. J. Dent. Res. 83, 134–138. [DOI] [PubMed] [Google Scholar]
- 30. Sculean A, Junker R, Donos N, Windisch P, Brecx M, Dunker N (2003) Immunohistochemical evaluation of matrix molecules associated with wound healing following treatment with an enamel matrix protein derivative in humans. Clin. Oral Investig. 7, 167–174. [DOI] [PubMed] [Google Scholar]
- 31. Hunter GK, Goldberg HA (1993) Nucleation of hydroxyapatite by bone sialoprotein. Proc. Natl. Acad. Sci. USA 90, 8562–8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foster BL, Popowics TE, Fong HK, Somerman MJ (2007) Advances in defining regulators of cementum development and periodontal regeneration. Curr. Top. Dev. Biol. 78, 47–126. [DOI] [PubMed] [Google Scholar]
- 33. D’Errico JA, MacNeil RL, Takata T, Berry J, Strayhorn C, Somerman MJ (1997) Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone 20, 117–126. [DOI] [PubMed] [Google Scholar]
- 34. Shimizu E, Nakajima Y, Kato N, Nakayama Y, Saito R, Samoto H et al. (2004) Regulation of rat bone sialoprotein gene transcription by enamel matrix derivative. J. Periodontol. 75, 260–267. [DOI] [PubMed] [Google Scholar]
- 35. Shimizu E, Saito R, Nakayama Y, Nakajima Y, Kato N, Takai H et al. (2005) Amelogenin stimulates bone sialoprotein (BSP) expression through fibroblast growth factor 2 response element and transforming growth factor‐beta1 activation element in the promoter of the BSP gene. J. Periodontol. 76, 1482–1489. [DOI] [PubMed] [Google Scholar]
- 36. Lamour V, Detry C, Sanchez C, Henrotin Y, Castronovo V, Bellahcene A (2007) Runx2‐ and histone deacetylase 3‐mediated repression is relieved in differentiating human osteoblast cells to allow high bone sialoprotein expression. J. Biol. Chem. 282, 36240–36249. [DOI] [PubMed] [Google Scholar]
- 37. Karring T, Isidor F, Nyman S, Lindhe J (1985) New attachment formation on teeth with a reduced but healthy periodontal ligament. J. Clin. Periodontol. 12, 51–60. [DOI] [PubMed] [Google Scholar]
- 38. van der Pauw MTM, Everts V, Beertsen W (2002) Expression of integrins by human periodontal ligament and gingival fibroblasts and their involvement in fibroblast adhesion to enamel matrix‐derived proteins. J. Periodontal. Res. 37, 317–323. [DOI] [PubMed] [Google Scholar]
- 39. Bernards MT, Qin C, Ratner BD, Jiang S (2008) Adhesion of MC3T3‐E1 cells to bone sialoprotein and bone osteopontin specifically bound to collagen I. J. Biomed. Mater. Res. A 86, 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]


