Abstract
Objectives: Aqueous Viscum album L. extracts are widely used for anti‐cancer therapies. Due to their low solubility, triterpenes (which are known to act on cancers), do not occur in aqueous extracts in significant amounts. Using cyclodextrins, we have found it possible to solubilize mistletoe triterpene acids and to determine their effects on acute lymphoblastic leukaemia (ALL) in vitro and in vivo.
Materials and methods: A C.B‐17/SCID model of pre‐B ALL (NALM‐6) was used to test efficacy and mechanisms of treatment with lectin‐ and triterpene acid containing preparations in vivo. Cytotoxicity of increasing concentrations of V. album L. preparations was assessed in vitro. Apoptosis was determined using mitochondrial membrane potential measurements, annexin V/PI, western blot analyses and caspase inhibitor assays.
Results: Solubilized triterpene acid‐ or lectin‐containing V. album L. extracts inhibited cell proliferation and demonstrated cytotoxic properties in vitro. Annexin V/PI and mitochondrial membrane potential assays indicated that dose‐dependent induction of apoptosis was the main mechanism. Combination (viscumTT) of lectin‐ (viscum) and triterpene‐containing (TT) extracts resulted in greatest induction of apoptosis. Furthermore, caspase activity demonstrated that these extracts were able to induce apoptosis through both caspase‐8 and ‐9 dependent pathways. In vivo experimentation showed that treatment of mice with viscumTT combination prolonged mean survival to 50.5 days compared to 39.3 days in the phosphate‐buffered saline group.
Conclusion: Here for the first time, we have demonstrated that either solubilized triterpene acids or lectins and combinations thereof, induce dose‐dependent apoptosis in the ALL cell line NALM‐6 via caspase‐8 and ‐9 dependent pathways.
Introduction
Extracts produced from V. album L. extracts (Viscum album Loranthaceace, from mistletoe) have commonly been used in adjuvant cancer treatment since 1920 (1). The mistletoe plant has a variety of biologically active substances such as specific lectins (MLs), viscotoxins, polysaccharides, lipids, flavonoids, triterpene acids and alkaloids. Apart from their immunological and cytotoxic effects, aqueous mistletoe preparations activate NK cells, monocytes, granulocytes, dendritic cells and T‐helper cells, and induce cytokine release and apoptosis in vitro and in vivo (2, 3, 4, 5, 6). They are also able to stabilize DNA when in low concentration, whereas they exert cytotoxic activity at higher concentrations (7, 8, 9).
Most investigations on V. album (VAE) are based on aqueous mistletoe extracts, which contain cytotoxic and immunomodulatory proteins such as mistletoe lectins and viscotoxins (10, 11, 12). Best‐studied components are glycoproteins mistletoe lectin I–III (13, 14, 15, 16, 17, 18, 19), however, ML‐I has been shown to be the predominant cytotoxic ingredient (20). Cytotoxic effects of MLs are induced primarily via apoptosis, involving receptor‐independent activation of effector caspases (21, 22, 23, 24). Besides anti‐metastatic effects of MLs on different solid tumours in vivo, recent studies have for the first time shown therapeutic effects on acute leukaemia in vivo (2, 25). Due to their low solubility, triterpene acids [oleanolic acid (OA), betulinic acid (BA)], which are also known to possess cytotoxic and anti‐tumour properties (26, 27), do not reside in significant amounts in aqueous extracts (28). Pre‐clinical studies have indicated that pentacyclic triterpene acids OA and BA have anti‐inflammatory and anti‐carcinogenic properties (29, 30, 31, 32, 33). Moreover, it has been reported that OA and its derivatives are able to induce apoptosis in various malignant cells (32, 34, 35, 36, 37). Therapeutic properties of triterpene acids have been demonstrated by showing inhibition of cell population growth and induction of apoptotic cell death in leukaemia cells (38).
Here, our basic aim was to test the combination of mistletoe lectins and triterpene acids, as it occurs naturally in mistletoe as well as the single components. It is an old assumption of phytopharmacology that a naturally occurring combination is sometimes advantageous compared to single compounds, as illustrated for example, by pharmacological effects of St. John’s wort (Hypericum perforatum).
Triterpene acids and mistletoe lectins occur naturally in the mistletoe plant. However, because of the pharmacological characteristics of triterpene acids, they are difficult to extract and solubilize in aqueous extracts. Using cyclodextrins, it was possible to solubilize mistletoe triterpene acids (mainly oleanolic acid) and achieve a plant extract with high levels of OA and MLs in combination (39, 40). As composition of triterpene acids and MLs has not been analysed previously, the aim of our study was to characterize well‐defined compositions of VAE in acute lymphoblastic leukaemia (ALL). These experimental extracts contain either lectins and viscotoxins (viscum), or solubilized triterpene acids (OA + BA) and, more interestingly, a combination thereof (viscumTT). Induction of apoptosis was determined by annexin V and JC‐1 assay, caspase assays and further western blot analyses. In addition, in vivo anti‐cancer effects were examined using a human leukaemia mouse model.
Materials and methods
Viscum album L. extracts
Sprouts of V. album were harvested from apple trees (Malus domestica Borkh.) and identified by co‐author S. Jaeger. Two different extracts were prepared to obtain oleanolic acid‐containing ‘TT’ extract and mistletoe lectin‐containing ‘viscum’ extract. Combination of both extracts is further referred to as ‘viscumTT’.
Preparation of oleanolic acid‐containing TT extract. Oleanolic acid was extracted from dried plant material resulting in a dry substance containing 69.4% OA and 6.9% BA (41). A quantity of 100 mg of these triterpene acids was mixed with 2‐HP‐ß‐cyclodextrins (2‐hydroxypropyl‐β‐cyclodextrin) and was suspended in water. The dry (105 °C) mixture was pestled, the resulting powder was suspended in sodium dihydrogen phosphate buffer (30 mm, pH 8.0); the mixture was sonicated for 30 min. After adjusting pH to 7.5 (100 mm phosphoric acid), the volume was made up to 25 ml (30 mm sodium phosphate buffer, pH 7.5). After filtration, OA was quantified in triplicate in each solution, using GC‐FID, and external calibration with OA as reference substance (>97%; Extrasynthese, Genay Cedex, France) (28). Final triterpene content for triterpene extract (TT) was 6 mg/ml OA, 0.38 mg/ml BA and 230 mg/ml 2‐HP‐β‐cyclodextrins.
Preparation of mistletoe lectin‐containing ‘viscum’‐extract. For the aqueous extract (lectin and viscotoxin‐containing ‘viscum’), plant material was milled under liquid nitrogen using a cryo mill (Retsch, Germany) and was extracted using ascorbate phosphate buffer (30 mm sodium phosphate, 3.4 g/l ascorbic acid, pH 9.1), resulting in filtered (0.22 μm) extract pH 7.5. Mistletoe lectins were quantified using ELISA as previously described (42, 43). Final content of viscum was 470 ng/ml ML‐I and <1 μg/ml viscotoxin.
Preparation of ‘viscumTT’. viscum and TT were combined with ‘viscumTT’ by mixing both extracts as described above, containing 6 mg/ml OA, 0.38 mg/ml BA and 470 ng/ml ML‐I (Table 1). Required concentration was achieved by 1:1 v:v mixing of both extracts, lyophilization and reconstitution in ½ original volume with water/phosphate‐buffered saline (PBS).
Table 1.
Viscum album L. extracts. This table displays a scheme of concentrations of applied Viscum album L. extracts TT, viscum and viscumTT
| 2‐HP‐CD (mg/ml) | OA (mg/ml) | BA (mg/ml) | ML (μg/ml) | |
|---|---|---|---|---|
| TT | 230 | 6.0 | 0.38 | – |
| Viscum | 230 | – | – | 470 |
| ViscumTT | 230 | 6.0 | 0.38 | 470 |
| CD | 230 | – | – | – |
OA, oleanolic acid; ML, mistletoe lectin; BA, betulinic acid; CD, 2‐hydroxypropyl‐β‐cyclodextrin.
Materials and reagents
RPMI 1640, FCS, penicillin, streptomycin and PBS were purchased from PAA Laboratories (Coelbe, Germany). Protein inhibitors, molecular mass standards for SDS–PAGE, TX‐100, sodium dodecyl sulphate and propidium iodide were purchased from Sigma‐Aldrich (Munich, Germany). Acrylamide and dithiotreitol were obtained from Carl Roth GmbH (Karlsruhe, Germany) and ammonium persulphate and N,N,N,N‐tetramethylenediamine were purchased from Bio‐Rad (Munich, Germany).
Cell culture
Human acute lymphoblastic leukaemia NALM‐6 cell line, obtained from the DSMZ (Bonn, Germany), was used for all investigations in this study. Cells were cultured in RPMI 1640 medium supplemented with 10% heat‐inactivated FCS, 100 U/ml penicillin and 100 μg/ml streptomycin. For assays cells were diluted to concentration of 1 × 106 /ml, immediately before addition of distinct V. album L. extracts.
Transplantation of ALL (NALM‐6) into SCID mice
Eight week old female C.B‐17/SCID (CB17/Icr‐Prkdcscid/IcrCrl) mice were obtained from Charles River Laboratories (Sulzfeld, Germany). They were housed in our institution in a specific pathogen‐free (SPF) facility, maintained under pathogen‐free conditions, fed autoclaved standard diet purchased from Sniff (Soest, Germany) and provided with acidified drinking water ad libitum. Animal experiments were performed according to German legislation on care and use of laboratory animals (Tierschutzgesetz). Approval for the study was obtained from the institutional ethics review board (approval G0459/08 from 2009/10/07).
Determination of cell concentration and cell viability
Cytotoxicity of the different VAE was measured by LDH assay. Release of lactate dehydrogenase (LDH) was analysed using a Cytotoxicity Detection Kit (Roche, Grenzach‐Wyhlen, Germany). After incubation of NALM‐6 cells with the described VAE for 4 h in 96‐well plates, cells were centrifuged for 5 min at 360 g, 4 °C then 50 μl of supernatant was mixed with 50 μl reaction mixture. Time‐dependent results of the reaction were quantified photometrically at 490 nm. Maximum LDH release was induced with 1% Triton X‐100 in culture medium and set as 100% cell death. Cell viability was determined using a CASY® Cell Counter and Analyser System of Schaerfe System GmbH (Reutlingen, Germany). Settings were specifically defined for requirements of the cells used. Experimentation was performed as previously described (25).
PI and annexin V binding assay
The NALM‐6 cells were incubated for 24 h with the described VAE. After incubation, cells were washed twice in PBS, resuspended in 100 μl binding buffer (10 mm HEPES/NaOH, pH 7.4, 140 mm NaCl, 5 mm CaCl2) and stained with APC‐conjugated annexin V (Becton Dickinson, Heidelberg, Germany) for 20 min, at room temperature (RT) in the dark. After incubation, 300 μl binding buffer and 1 μl propidium iodide (PI; 1 mg/ml) were added and samples were analysed immediately using FACSCalibur (Becton Dickinson). Results were evaluated with FlowJo Software (TreeStar, Ashland, USA).
Analysis of mitochondrial membrane potential (ΔΨm)
Mitochondrial membrane potential was assessed using 5,5,6,6‐tetrachloro‐1,1,3,3‐tetraethylbenzimidazol‐carbocyanine iodide (JC‐1) and flow cytometric analysis. After incubation for 24 h with different concentrations of VAE, mitochondrial membrane potential was measured by JC‐1 (Sigma‐Aldrich). Cells were resuspended in 750 μl phenol red‐free RPMI 1640 with no further supplementation, and incubated in JC‐1 (2.5 μg/ml final concentration) for 30 min at 37 °C, 5% CO2. After incubation, cells were washed and resuspended in PBS followed by flow cytometric analysis, with excitation and emission settings of 484 and 500 nm, respectively (Becton Dickinson). Depolarizing carbonyl cyanide 3‐chlorophenylhydrazone (CCCP; Sigma‐Aldrich) was used as reference. Cells were treated with 50 μm CCCP (final concentration) for 30 min.
Protein extraction and western blot analyses
The cells were incubated for 24 h with concentrations of VAE as used previously. Then they were washed twice in PBS and lysed in buffer containing 10 mm Tris/HCl, pH 7.5, 300 mm NaCl, 1% Triton X‐100, 2 mm MgCl2, 5 μm EDTA and 1 mm PefaBloc. Protein concentration was determined using Bradford Reagent. Equal amounts of protein were separated by SDS–PAGE using the Bio‐Rad system and transferred to nitrocellulose membranes. Antibody incubations were performed overnight at 4 °C. Detection was performed by using HRP‐conjugated secondary antibodies, visualized using ECL (Thermo Fisher Scientific, Bonn, Germany) and Molecular Imager VersaDoc (Bio‐Rad). Polyclonal caspase‐3, PARP and Bcl‐2 antibodies were purchased from Cell Signaling Technology (Danvers, Maine, USA) and mAb β‐actin‐peroxidase antibody from Sigma‐Aldrich.
Preparation of mitochondrial proteins
Twenty‐four hours after incubation in VAE, cells were collected by centrifugation at 200 g for 10 min, 4 °C. After washing twice in ice‐cold PBS and centrifugation, pellets were lysed in ice‐cold extraction buffer (20 mm HEPES‐KOH, pH 7.5 10 mm KCl, 1.5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, 100 μm PMSF and protein inhibitor cocktail) and incubated for 30 min on ice. Lysates were homogenized with a 27G pestle (20 strokes) and centrifuged for 30 min at 15 000 g, 4 °C. Supernatant (cytosolic fraction) was collected and pellets (mitochondrial fraction) were resuspended in lysis buffer (44). Cell lysates were fractionated in SDS–PAGE and transferred to nitrocellulose membranes. Monoclonal cytochrome c antibody was purchased from Dianova (Hamburg, Germany).
Western blots were quantified using Quantity One software (Bio‐Rad) and level of cytochrome c release was expressed as Adjusted Density Value of cytochrome c over actin, normalized to control samples without VAE incubation (45). Relative density of Ctrl = 1.
Measurement of caspase activity
Activity of caspases‐8 and ‐9 was measured using a fluorescent caspase staining kit according to the manufacturer’s protocol (Promokine, Heidelberg, Germany). Control or induced cells were incubated for 0.5 h at 37 °C using FITC‐LEHD‐fmk or FITC‐IETD‐fmk. After incubation, cells were washed and analysed by flow cytometry. For caspase inhibitor experiments, cells were pre‐incubated with 100 μm Z‐VAD‐fmk, Z‐IETD‐fmk or Z‐LEDH‐fmk for 1 h (R&D Systems, Wiesbaden, Germany) (24, 46). After additional incubation with VAE for 24 h, apoptosis was determined by annexin V‐APC and propidium iodide and FACS analyses. DMSO was added to extracts as solvent control.
Animals and experimental procedures
Systemic leukaemia was induced by injection of 1 × 106 human NALM‐6 cells (DMSZ, Bonn, Germany) in CB17/Icr‐Prkdcscid/IcrCrl mice intravenously (i.v.). Mice were divided into four groups of eight and were medicated with 40 mg/kg oleanolic acid (TT), 3 μg/kg lectin (viscum), or a combination thereof (viscumTT). The control group received an equal volume of PBS. Injections were given intraperitoneally (i.p.) three times a week, for 21 days starting on day three after tumour cell injection. Body weight was measured twice a week and mice were carefully monitored for symptoms of toxicity. They were observed daily for onset of hind leg paralysis and were sacrificed when they developed paresis or paralysis. Kaplan–Meier plots were constructed from the data.
Statistical analyses
For animal experiments, Wilcoxon testing was carried out to identify statistical differences. Values of P ≤ 0.05 were considered statistically significant. Fractional product (Fp) of Webb was used to determine whether effects on apoptosis induced by viscumTT were additive, synergistic or antagonistic (47). The following formula was used, as shown previously (48):
E 1;2exp = (E 1 + E 2 − E 1 E 2), with E 1;2exp = expected (calculated) effect of combination viscumTT, where E 1 = observed effect of TT; E 2 = observed effect of vis‐cum; E 1;2obs = observed effect of combined mixture viscumTT.
Fp = E 1;2obs/E 1;2exp. Values Fp > 1 represent synergistic effects, whereas Fp = 1 is additive and Fp < 1 antagonistic.
Student′s t‐test was applied to determine differences between VAE‐treated cells and control for caspase inhibitor assay. Significance level was set at P ≤ 0.05 for all tests.
Results
Inhibition of proliferation of NALM‐6 cells in vitro by VAE
Release of LDH provides an accurate measure of cell membrane integrity and cell viability. To exclude any unwanted cytotoxic effect of necrosis, human leukaemia NALM‐6 cells were incubated with different concentrations of VAE (TT, viscum, viscumTT, Table 1). Solubilization agent CD (2‐hydroxypropyl‐β‐cyclodextrin) was used as medium control in all experiments. As shown in Fig. 1a, no significant release of LDH was measured for different doses of any V. album extracts, after 4 h. To examine ability of TT, viscum or viscumTT to inhibit cell proliferation, the cells were incubated for 24 h with doses of extracts as depicted, and analysed using a CASY® Cell Counter. As shown in Fig. 1b, proliferation was strongly inhibited in a concentration‐dependent manner, by up to 80% when treated with OA‐containing extract (TT, 60 μg/ml), by up to 47% after treatment with lectin‐containing extract (viscum, 8 ng/ml ML) and by 79% when incubated with a combination of both (viscumTT, Fig. 1b).
Figure 1.
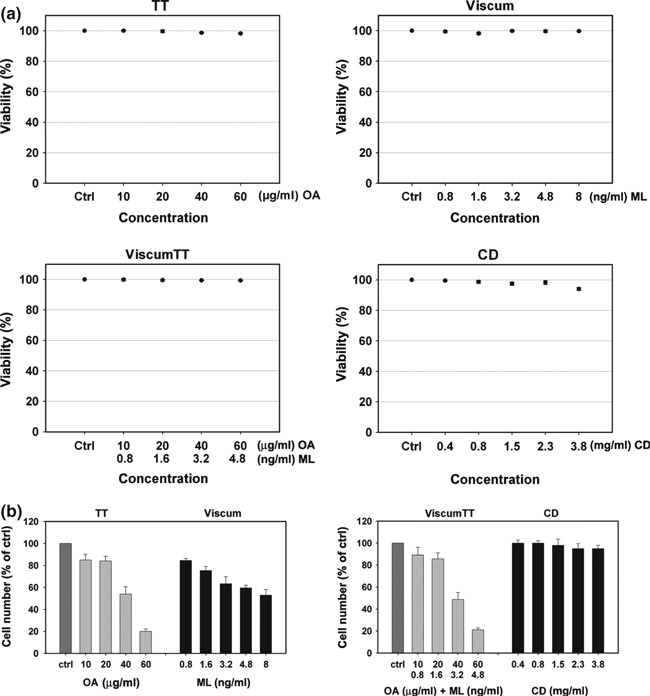
Viscum album L. extracts inhibited proliferation of NALM‐6 cells. (a) NALM‐6 cells were incubated with different doses of VAE (TT, viscum, viscumTT or CD) for 4 h. Cell viability was measured by LDH release assay. Values provided as percentage of control (±SD, n = 3). There was no relevant LDH release after 4 h (<10% compared to control). (b) NALM‐6 cells were treated with depicted concentrations of TT, viscum, viscumTT or CD and incubated for 24 h. Proliferation was measured using the CASY® Cell Counter System. VAE inhibited proliferation in a concentration‐dependent manner. TT and viscumTT had inhibition of up to 80%, whereas viscum inhibited proliferation by only up to 47% (n = 3).
Analyses of apoptosis induction by VAE
To determine whether inhibition of proliferation was associated with reduced cell viability, induction of apoptosis was analysed using annexin V. For this purpose, the cells were incubated with the same concentrations of TT, viscum, viscumTT and CD (as solubilization control) as before and stained with annexin V and propidium iodide (PI). As shown in Fig. 2a, viscum and TT showed clear dose‐dependent increase in annexin V positive cells. Nearly 50% apoptosis was observed in cells treated with TT at 40 μg/ml concentration OA and in cells treated with viscum containing 3.2 ng/ml ML. In addition, the first three combinations of both extracts (viscumTT) led to a synergistic cytotoxic effect, fractional product calculated by Webb, compared to the product of single‐agent treatments (*Fp > 1). We were not able to detect any effect of CD on the cells.
Figure 2.
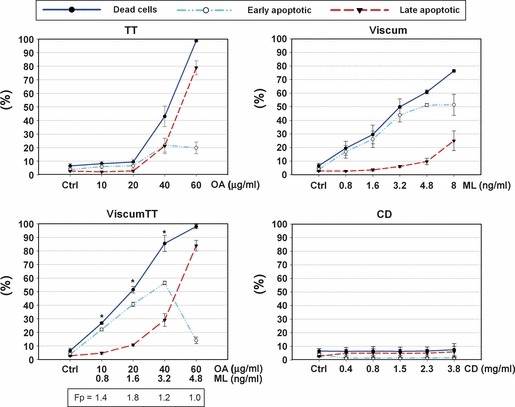
Triterpene acid‐ and lectin‐containing extracts induced apoptosis of NALM‐6 cells. To measure apoptotic induction by VAE, NALM‐6 cells were treated with distinct concentrations of the described extracts. Twenty‐four hours after incubation, early and late apoptosis were determined using annexin V‐APC and propidium iodide, by flow cytometry. Values given as percentages of annexin V‐positive/PI‐negative (early apoptotic) or annexin V‐positive/PI positive (late apoptotic) cells (±SD, n = 3). Results indicate dose‐dependent induction of apoptosis as the main mechanism of cell death. Webbs fractional product was used to analyse additive, antagonistic or synergistic effects of the combination viscumTT. Single asterisk indicates Fp > 1 and reveals a synergistic effect using viscumTT compared to the product of single agents TT and viscum. Fp, fractional product.
Synergistic induction of mitochondrial depolarization and cytochrome c release
To measure involvement of mitochondria in induction of apoptosis, mitochondrial depolarization induced by TT, viscum, viscumTT and CD‐treated cells was evaluated. Dissipation of mitochondrial inner membrane potential (Δψm) was measured using the cationic dye JC‐1. There was significant dose‐dependent loss of mitochondrial membrane polarization after treatment with TT, viscum or viscumTT (Fig. 3a). Δψm depolarization increased to 94 % when cells were treated with 60 μg/ml OA (TT) and to 77% for viscum (8 ng/ml ML). Interestingly, viscumTT caused significantly enhanced Δψm depolarization and confirmed the synergistic effect, fractional product calculated by Webb, observed in the annexin V assay (*Fp > 1). Cells incubated with cyclodextrins showed no depolarization of mitochondrial membrane potential. Next, we analysed cytochrome c release from mitochondria into the cytosol after treatment with TT, viscum and viscumTT (Fig. 3b). Treatment with viscum and TT resulted in dose‐dependent cytochrome c release after 24 h, indicating that both triterpene acids and lectins were able to induce apoptosis with mitochondria and cytochrome c release involvement. Moreover, cells incubated with combination viscumTT, led to enhanced increase in cytosolic cytochrome c compared to single‐agent treatment. These results suggest that VAE induced apoptosis by the intrinsic signalling pathway.
Figure 3.
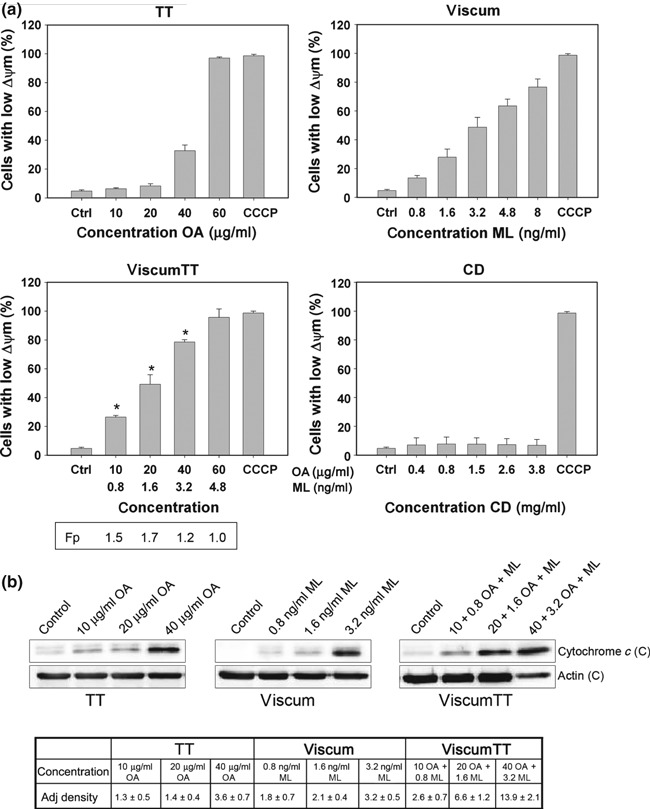
VAE induced mitochondria permeability and cytochrome c release. (a) NALM‐6 cells were incubated with TT, viscum, viscumTT or CD for 24 h. After incubation, mitochondrial permeability transition was measured by JC‐1 and flow cytometry at single‐cell level. Values of mitochondrial permeability transition are given in percentages of cells with low mitochondrial potential (ΔΨm) (±SD, n = 3). Webbs fractional product was used to analyse additive, antagonistic or synergistic effect of the combination viscumTT. Single asterisk represents synergistic effect of viscumTT compared to the product of the single agents (*Fp > 1). Mitochondria permeability transition did not change in negative controls. CCCP (carbonyl cyanide 3‐chlorophenylhydrazone) was used as mitochondrial membrane potential disrupter. (b) Cytochrome c release was analysed after 24 h VAE incubation. Western blots showing dose‐dependent release of cytochrome c from mitochondria after treatment with TT, viscum or viscumTT. Adjusted density value cytochrome c from three independent experiments was quantified using Quantity One software (Density Ctrl = 1). Blots are representative of three independent experiments. C, Cytosol.
Apoptosis was mediated by caspase‐3 activation
To analyse mistletoe‐induced cell death in more detail, involvement of different proteins was investigated by western blot analyses. NALM‐6 cells were treated with VAE, and samples were harvested 24 h post‐exposure. Caspase‐3 activation was detected in western blots probed with a specific polyclonal antibody that recognized procaspase‐3 (35 kDa), and 17 kDa cleaved form of caspase‐3 (Fig. 4). Here, we observed dose‐dependent increase in cleaved caspase‐3. Furthermore, we detected decrease in Bcl‐2 expression and demonstrated cleavage of PARP‐1 in TT, viscum and viscumTT. Equal loading was verified by probing samples with anti‐β‐actin antibody. These data indicated that VAE‐induced apoptosis proceeded through caspase activation, and pointed to apoptosis by the intrinsic pathway.
Figure 4.
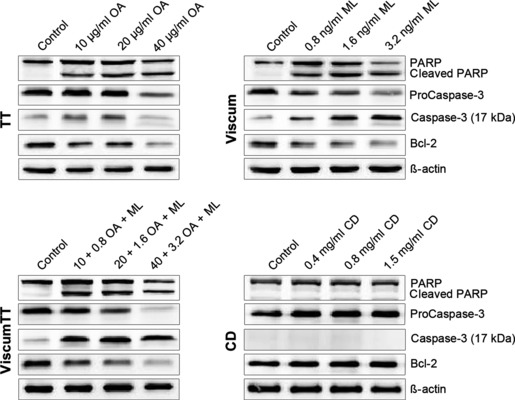
VAE induced apoptosis by caspase activation. Western blots showing different proteins involved in apoptosis. NALM‐6 cells were exposed to depicted concentrations of VAE and analysed 24 h later. Active caspase‐3 was recognized using specific polyclonal antibody, which reacts with the 17 kDa cleaved form of the enzyme and procaspase‐3 form, 37 kDa. Bcl‐2 expression and PARP cleavage were detected by polyclonal antibodies from Cell signaling. Equal loading was verified using β‐actin as sample internal protein control. Blots are representative of three independent experiments.
Oleanolic acid and mistletoe lectin induced caspase‐8‐ and ‐9‐dependent apoptosis
To dissect roles of caspases in VAE‐induced apoptosis, cells were treated in presence of caspase inhibitors Z‐VAD‐fmk, Z‐IETD‐fmk and Z‐LEHD‐fmk. Incubation with caspase‐8 and‐9 inhibitors did not or only in part prevent apoptosis induction by TT, viscum and viscumTT, whereas general caspase inhibitor Z‐VAD‐fmk prevented loss of cell viability induced by VAE. Here, we observed highly significant inhibition for viscum and viscumTT‐treated cells (viscum 75% inhibition, viscumTT 73%) and approximately 58% reduction in apoptosis induction for TT‐treated cells (Fig. 5a). To further analyse involvement of caspases, activation of caspase‐8 and‐9 in VAE‐treated cells was monitored. For this, cells were incubated with TT, viscum or viscumTT and fluorescence caspase activity assays were performed. As shown in Fig. 5b, viscum and TT induced concentration‐dependent cleavage of both caspase‐8 and ‐9. Interestingly, we were able to detect similar activation of caspase‐8 and ‐9 in VAE‐treated cells. In addition, viscumTT‐treated cells activated caspase‐8 and ‐9 in a synergistic manner as shown previously (*Fp > 1). These results indicate that both extrinsic and intrinsic pathways play a role in VAE‐induced apoptosis.
Figure 5.
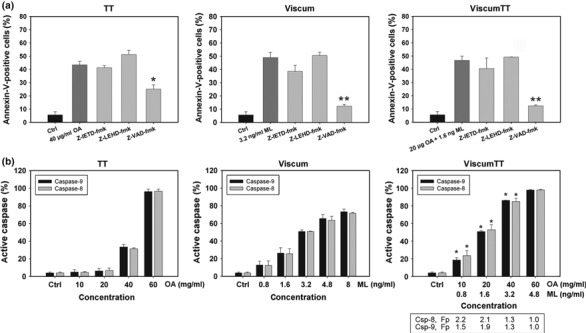
Activation of caspase‐8 and ‐9 in oleanolic‐ and lectin‐induced apoptosis. (a) Cells were treated with TT, viscum or viscumTT for 24 h in presence or absence of 100 μm Z‐VAD‐fmk, Z‐IETD‐fmk or Z‐LEHD‐fmk. Effects of caspase inhibitors were analysed by annexin V/propidium iodide staining and flow cytometry (±SD, n = 3). Asterisks represent statistically significant differences compared to control groups (*P < 0.05, **P < 0.005). (b) NALM‐6 cells were incubated for 24 h with indicated doses of VAE. Caspase‐8 and‐9 activity was measured by FITC‐LEHD and FITC‐IETD and flow cytometry (±SD, n = 3). Synergism was calculated via fractional product of Webb; *Fp > 1, displays synergistic effect of viscumTT.
Effect of VAE on survival of C.B‐17/SCID mice with human ALL
For in vivo assay, concentrations of 40 mg/kg OA (TT), 3 μg/kg MLs (viscum) and combination of both (viscumTT) were administered 3 days after tumour inoculation. Results, shown in Fig. 6, demonstrated that administered concentrations of viscum and TT produced no significant effect on body weight of the mice, whereas viscumTT was more toxic. Kaplan–Meier survival curves of C.B‐17/SCID mice carrying the human acute lymphoblastic leukaemia cells and treated with viscumTT showed it had a remarkable therapeutic effect. Mice receiving PBS had mean survival of 39.3 days (Fig. 6); viscum prolonged mean survival time to 43.2 days and TT to 45.8 days. In contrast, viscumTT extended life span of these animals to mean survival of 50.5 days (P = 0.009).
Figure 6.
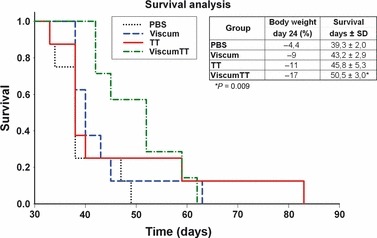
C.B‐17/SCID in vivo efficacy of Viscum album L. extracts. Kaplan–Meier survival curve of C.B‐17/SCID mice carrying systemic human acute lymphoblastic leukaemia (NALM‐6) treated with 40 mg/kg oleanolic acid (TT extract), 3 μg/kg lectins (viscum extract) or a combination thereof (viscumTT), three times a week for 21 days post‐transplantation of cells. Control mice received PBS only. Treatment with viscumTT had a significant therapeutic advantage on survival time (p) compared to TT and viscum. ViscumTT was more toxic than TT or viscum as indicated by body weight change in the viscumTT group.
Discussion
Mistletoe extracts and their components exert cytotoxic effects on diverse cancer cell lines (7, 11). However, cytotoxicity varies greatly depending on host tree, preparation of extracts and cell type (25, 49). Furthermore, many in vitro studies have examined cytotoxic and anti‐cancer properties of aqueous V. album L. extracts, but there are few published studies using triterpene acid‐containing extracts (38, 50). Thus, we used defined compositions of either aqueous extracts (viscum), triterpene acids‐containing extracts (TT) or a combination thereof (viscumTT) to analyse their cytotoxic effects in vitro and in vivo. Data presented in this study indicate that all compositions were significantly toxic to NALM‐6 cells in a dose‐dependent manner. We were able to show that inhibition of proliferation could be attributed to effective induction of apoptosis by TT, viscum and viscumTT. Annexin V detection of phosphatidylserine on outer plasma membranes indicated dose‐dependent increase in cell death when cells were treated with TT, viscum or viscumTT. Furthermore, we were able to show synergistic effects on cell death induction with the combination agent viscumTT. It is known that cytotoxic effects of V. album varies on different cancer cell lines (49). The triterpene acid‐containing extract we used consists mainly of oleanolic and betulinic acids, and these have partly been characterized for their apoptosis‐inducing properties in vitro (32, 34, 51). Here, we have been able to confirm apoptotic effects for these triterpene acids, isolated from V. album, in an ALL cell line, by annexin V/PI assays, in vitro. For the lectin‐containing extract ‘viscum’, we were able to show that the cells were sensitive to low doses of lectins, in contrast to triterpene acids. In addition, we confirmed VAE‐mediated induction of apoptosis by significant loss of mitochondria potential, PARP cleavage and activation of procaspase‐3. For betulinic acid, it has been reported that there is a direct effect on mitochondria leading to cytochrome c release and subsequent apoptosis, without involvement of the extrinsic death receptor pathway (32, 52, 53). Release of cytochrome c regulates downstream caspase activation by binding to Apaf‐1 and procaspase‐3, the process being determined by actual amounts of apoptosis‐inhibiting (Bcl‐2, Bcl‐X1, Mcl‐1) and apoptosis‐promoting (Bax, Bid, Bak) proteins. Also, we were able to observe dose‐dependent cytochrome c release for both triterpene acid‐containing and lectin‐containing extracts. Moreover, we were able to show that TT, viscum and viscumTT altered expression of mitochondrial protein Bcl‐2. These results suggest that VAE induces apoptosis by the intrinsic pathway as described, for example, for betulinic acid and mistletoe lectins (24). However, caspase inhibitor and caspase activation analyses demonstrated that triterpene acids and mistletoe lectins activated both extrinsic, caspase‐8‐dependent, and intrinsic, caspase‐9‐dependent, signalling pathways. The extrinsic pathway of apoptosis is activated by pro‐apoptotic members of the tumour necrosis factor family of cytokines (TNF, Fas, TRAIL) to activate either caspase‐10 or caspase‐8 with further activation of downstream caspases‐3 and ‐7. In addition, caspase‐8 can cleave Bid followed by cross‐talk with intrinsic pathway events (54). For OA and its derivatives such as CDOO, CDOO‐Im and CDDO‐Me, it has been reported that they are able to induce TRAIL‐dependent apoptosis (55, 56, 57, 58, 59). However, they are also able to induce apoptosis by the intrinsic pathway (60, 61, 62). Moreover, for synthetic OA derivatives, caspase‐dependent and ‐independent apoptosis induction has been reported in myeloid leukaemia cell lines (61, 63). Interestingly, in our experiments, it was not possible to inhibit apoptosis by Z‐LEHD‐fmk or Z‐IETD‐fmk alone, whereas we observed significant inhibition of apoptosis by Z‐VAD‐fmk in VAE‐treated cells. However, caspase activity assay showed caspase‐8 and ‐9 activation by TT, viscum and viscumTT treatment. For ML‐I, it has been reported that it is able to induce apoptosis via mitochondrial death receptor‐independent signalling pathway, with involvement of caspase‐8 and ‐9 (24). To analyse whether viscum induced apoptosis by a similar pathway, further experiments are required.
As Z‐VAD‐fmk only partly inhibited apoptosis induction by TT, we suggest activation of a further caspase‐independent apoptosis mechanism as described for OA derivatives (56, 63). This is consistent with other results on ovarian cancer cells, which showed that z‐VAD‐fmk only partly prevented CDDO‐induced apoptosis (56). However, the precise mechanism of apoptosis induced by Viscum album components remains unclear, but seems to be worth further investigation.
Regarding in vivo studies with V. album L., anti‐metastatic effects have been described for aqueous extracts (2). In addition, an anti‐cancer effect of aqueous V. album L. has been reported in an ALL model by our group (25). Significantly improved animal survival in an in vivo SCID mouse model of pre‐B ALL (NALM‐6) was found when the mice were treated with aqueous mistletoe extract. The difference between these findings and previous data from Seifert et al. (25) and our in vivo data concerning lectins is fully explained by use of different mistletoe host trees, preparation procedures and natural diversity of mistletoe. For our experiments, we used ML‐I containing aqueous mistletoe extracts from plant material harvested from apple trees, whereas in former investigations, ML‐II/III (Helixor Heilmittel GmbH & Co. KG, Rosenfeld, Germany)‐containing mistletoe extracts harvested from pine and fir trees were used. It is known that ML‐I is significantly less toxic compared to ML‐III (64, 65). Moreover, we detected marked difference between extracts concerning tolerance, in vivo. This may be due to further differences in constituents of the different extracts. Tolerance of triterpene acids was significantly higher than for mistletoe lectins.
Triterpene acids, including ursolic acid, betulinic acid and oleanolic acid, are known to be phytochemicals that exist widely in nature, and can be used as supplements (41). It has been shown that synthetic triterpenoid CDDO and its derivatives are able to induce cell population growth arrest and apoptosis in solid tumours and murine leukaemic cell lines in vivo (35, 66, 67). Furthermore, Yamai et al. (68) have reported anti‐metastatic effects in vivo of a combination of 5‐fluorouracil (5‐FU) and Japanese apricot extract (containing triterpene acids, JAE). Additional use of JAE potentiates anti‐neoplastic activity of 5‐FU. In addition, it was shown that Dex‐OA inhibited growth of osteosarcomas and decreased rates of lung metastasis in vivo (69). However, there were no in vivo data concerning efficacy of naturally occurring triterpene acids in ALL to date. Here, we have been able to show for the first time, significant efficacy of a combination extract with triterpene acids (viscumTT) in an ALL‐model in vivo, whereas we were only able to detect non‐significant anti‐leukaemic effects of both viscum and TT. These results, combined with our in vitro data, suggest that overall therapeutic effect and impressive induction of apoptosis by viscumTT derived from several compounds acting together synergistically. Treatment with a single agent induced apoptosis alone, but treatment with viscumTT enhanced apoptosis induction more effectively, as supported by our in vivo experimentation. It is noteworthy that our studies suggest that substance combination occurring in the mistletoe plant represents a kind of phytotherapeutic ‘polychemotherapy’. This matches clinical data showing, for example, that ALL can only be lastingly treated by combination treatments, not by using a single substance alone. Our experiments using the example of the ALL‐model indicate that individual plants may already contain synergistic combinations of substances, which have a better effect in certain illnesses than an isolated single substance, as we know from the clinical use of St. John’s Wort, for example. This interesting hypothesis warrants further investigation as, although the use of isolated and possibly synthetic substances is easier to handle experimentally and in pharmacological manufacture, their superiority in clinical use has never been investigated.
Conclusion
In summary, we were able to show that this new formulation “viscumTT” of aqueous mistletoe extracts and triterpene acids can induce apoptosis in NALM‐6 cells via intrinsic and extrinsic signalling pathways. Furthermore, we were able to show synergistic effects in combination viscumTT, in apoptosis induction. Although we propose involvement of both extrinsic and intrinsic signalling pathways in apoptosis induction by triterpene acids, it remains unclear whether additional caspase‐independent induction of apoptosis exists. In vivo studies displayed significant extension of life span after i.p. administration of viscumTT over a period of 3 weeks. However, further studies with these components from mistletoe in other tumour models seem warranted.
Competing interests
The authors have no conflict of interest to disclose.
Authors’ contributions
CID performed the majority of the experimental work, contributed to its design and coordination and drafted the manuscript. KKH assisted with the experiments. SJ provided Viscum album L. extracts in collaboration, and GS designed and supervised experimentation, assisted with writing of and proof read the manuscript. HNL and GH contributed to study design and were involved in drafting the manuscript. All authors read and approved the final version.
Acknowledgement
This work was supported by the ‘Software AG‐Stiftung’.
References
- 1. Lenartz D, Dott U, Menzel J, Schierholz JM, Beuth J (2000) Survival of glioma patients after complementary treatment with galactoside‐specific lectin from mistletoe. Anticancer Res. 20, 2073–2076. [PubMed] [Google Scholar]
- 2. Duong Van Huyen JP, Delignat S, Bayry J, Kazatchkine MD, Bruneval P, Nicoletti A et al. (2006) Interleukin‐12 is associated with the in vivo anti‐tumor effect of mistletoe extracts in B16 mouse melanoma. Cancer Lett. 243, 32–37. [DOI] [PubMed] [Google Scholar]
- 3. Hajto T (1986) Immunomodulatory effects of iscador: a Viscum album preparation. Oncology 43(Suppl. 1), 51–65. [DOI] [PubMed] [Google Scholar]
- 4. Thies A, Dautel P, Meyer A, Pfuller U, Schumacher U (2008) Low‐dose mistletoe lectin‐I reduces melanoma growth and spread in a SCID mouse xenograft model. Br. J. Cancer 98, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elluru SR, Van Huyen JP, Delignat S, Kazatchkine MD, Friboulet A, Kaveri SV et al. (2008) Induction of maturation and activation of human dendritic cells: a mechanism underlying the beneficial effect of Viscum album as complimentary therapy in cancer. BMC Cancer 8, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huber R, Rostock M, Goedl R, Ludtke R, Urech K, Buck S et al. (2005) Mistletoe treatment induces GM‐CSF‐ and IL‐5 production by PBMC and increases blood granulocyte‐ and eosinophil counts: a placebo controlled randomized study in healthy subjects. Eur. J. Med. Res. 10, 411–418. [PubMed] [Google Scholar]
- 7. Park JH, Hyun CK, Shin HK (1999) Cytotoxic effects of the components in heat‐treated mistletoe (Viscum album). Cancer Lett. 139, 207–213. [DOI] [PubMed] [Google Scholar]
- 8. Bussing A, Azhari T, Ostendorp H, Lehnert A, Schweizer K (1994) Viscum album L. extracts reduce sister chromatid exchanges in cultured peripheral blood mononuclear cells. Eur. J. Cancer 30A, 1836–1841. [DOI] [PubMed] [Google Scholar]
- 9. Ribereau‐Gayon G, Jung ML, Baudino S, Salle G, Beck JP (1986) Effects of mistletoe (Viscum album L.) extracts on cultured tumor cells. Experientia 42, 594–599. [DOI] [PubMed] [Google Scholar]
- 10. Bussing A (2000) Biological and pharmacological properties of Viscum album L. Mistletoe‐The Genus Viscum , p. 123–182. Amsterdam: Harwood Academic Publishers.
- 11. Schaller G, Urech K, Grazi G, Giannattasio M (1998) Viscotoxin composition of the three European subspecies of Viscum album. Planta Med. 64, 677–678. [DOI] [PubMed] [Google Scholar]
- 12. Urech K, Schaller G, Jaggy C (2006) Viscotoxins, mistletoe lectins and their isoforms in mistletoe (Viscum album L.) extracts Iscador. Arzneimittelforschung 56, 428–434. [DOI] [PubMed] [Google Scholar]
- 13. Elsasser‐Beile U, Voss M, Schuhle R, Wetterauer U (2000) Biological effects of natural and recombinant mistletoe lectin and an aqueous mistletoe extract on human monocytes and lymphocytes in vitro. J. Clin. Lab. Anal. 14, 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elsasser‐Beile U, Lusebrink S, Grussenmeyer T, Wetterauer U, Schultze‐Seemann W (1998) Comparison of the effects of various clinically applied mistletoe preparations on peripheral blood leukocytes. Arzneimittelforschung 48, 1185–1189. [PubMed] [Google Scholar]
- 15. Gabius HJ, Andre S, Kaltner H, Siebert HC, von der Lieth CW, Gabius S (1996) The mistletoe myth – claims, reality and provable perspectives. Z. Arztl. Fortbild. (Jena) 90, 103–110. [PubMed] [Google Scholar]
- 16. Franz H (1986) Mistletoe lectins and their A and B chains. Oncology 43(Suppl. 1), 23–34. [DOI] [PubMed] [Google Scholar]
- 17. Franz H, Ziska P, Kindt A (1981) Isolation and properties of three lectins from mistletoe (Viscum album L.). Biochem. J. 195, 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valentiner U, Pfuller U, Baum C, Schumacher U (2002) The cytotoxic effect of mistletoe lectins I, II and III on sensitive and multidrug resistant human colon cancer cell lines in vitro. Toxicology 171, 187–199. [DOI] [PubMed] [Google Scholar]
- 19. Hajto T, Hostanska K, Berki T, Palinkas L, Boldizsar F, Nemeth P (2005) Oncopharmacological perspectives of a Plant Lectin (Viscum album Agglutinin‐I): overview of recent results from in vitro experiments and in vivo animal models, and their possible relevance for clinical applications. Evid. Based. Complement Alternat. Med. 2, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jung ML, Baudino S, Ribereau‐Gayon G, Beck JP (1990) Characterization of cytotoxic proteins from mistletoe (Viscum album L.). Cancer Lett. 51, 103–108. [DOI] [PubMed] [Google Scholar]
- 21. Bussing A, Vervecken W, Wagner M, Wagner B, Pfuller U, Schietzel M (1999) Expression of mitochondrial Apo2.7 molecules and caspase‐3 activation in human lymphocytes treated with the ribosome‐inhibiting mistletoe lectins and the cell membrane permeabilizing viscotoxins. Cytometry 37, 133–139. [PubMed] [Google Scholar]
- 22. Bussing A, Suzart K, Bergmann J, Pfuller U, Schietzel M, Schweizer K (1996) Induction of apoptosis in human lymphocytes treated with Viscum album L. is mediated by the mistletoe lectins. Cancer Lett. 99, 59–72. [DOI] [PubMed] [Google Scholar]
- 23. Lyu SY, Park WB, Choi KH, Kim WH (2001) Involvement of caspase‐3 in apoptosis induced by Viscum album var. coloratum agglutinin in HL‐60 cells. Biosci. Biotechnol. Biochem. 65, 534–541. [DOI] [PubMed] [Google Scholar]
- 24. Bantel H, Engels IH, Voelter W, Schulze‐Osthoff K, Wesselborg S (1999) Mistletoe lectin activates caspase‐8/FLICE independently of death receptor signaling and enhances anticancer drug‐induced apoptosis. Cancer Res. 59, 2083–2090. [PubMed] [Google Scholar]
- 25. Seifert G, Jesse P, Laengler A, Reindl T, Luth M, Lobitz S et al. (2008) Molecular mechanisms of mistletoe plant extract‐induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro. Cancer Lett. 264, 218–228. [DOI] [PubMed] [Google Scholar]
- 26. Fulda S, Jeremias I, Steiner HH, Pietsch T, Debatin KM (1999) Betulinic acid: a new cytotoxic agent against malignant brain‐tumor cells. Int. J. Cancer 82, 435–441. [DOI] [PubMed] [Google Scholar]
- 27. Laszczyk MN (2009) Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 75, 1549–1560. [DOI] [PubMed] [Google Scholar]
- 28. Jager S, Winkler K, Pfuller U, Scheffler A (2007) Solubility studies of oleanolic acid and betulinic acid in aqueous solutions and plant extracts of Viscum album L. Planta Med. 73, 157–162. [DOI] [PubMed] [Google Scholar]
- 29. Ovesna Z, Vachalkova A, Horvathova K, Tothova D (2004) Pentacyclic triterpenoic acids: new chemoprotective compounds. Minireview. Neoplasma 51, 327–333. [PubMed] [Google Scholar]
- 30. Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K et al. (1994) Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 54, 701–708. [PubMed] [Google Scholar]
- 31. Safayhi H, Sailer ER (1997) Anti‐inflammatory actions of pentacyclic triterpenes. Planta Med. 63, 487–493. [DOI] [PubMed] [Google Scholar]
- 32. Fulda S, Debatin KM (2000) Betulinic acid induces apoptosis through a direct effect on mitochondria in neuroectodermal tumors. Med. Pediatr. Oncol. 35, 616–618. [DOI] [PubMed] [Google Scholar]
- 33. Wollenweber E, Wieland A, Haas K (2000) Epicuticular waxes and flavonol aglycones of the European mistletoe, Viscum album L. Z. Naturforsch. C. 55, 314–317. [DOI] [PubMed] [Google Scholar]
- 34. Assefa H, Nimrod A, Walker L, Sindelar R (1999) Synthesis and evaluation of potential complement inhibitory semisynthetic analogs of oleanolic acid. Bioorg. Med. Chem. Lett. 9, 1889–1894. [DOI] [PubMed] [Google Scholar]
- 35. Deeb D, Gao X, Jiang H, Dulchavsky SA, Gautam SC (2009) Oleanane triterpenoid CDDO‐Me inhibits growth and induces apoptosis in prostate cancer cells by independently targeting pro‐survival Akt and mTOR. Prostate 69, 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deeb D, Gao X, Jiang H, Janic B, Arbab AS, Rojanasakul Y et al. (2010) Oleanane triterpenoid CDDO‐Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS‐dependent mechanism. Biochem. Pharmacol. 79, 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao X, Deeb D, Jiang H, Liu Y, Dulchavsky SA, Gautam SC (2007) Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF‐kappaB and Notch1 signaling. J. Neurooncol. 84, 147–157. [DOI] [PubMed] [Google Scholar]
- 38. Urech K, Scher JM, Hostanska K, Becker H (2005) Apoptosis inducing activity of viscin, a lipophilic extract from Viscum album L. J. Pharm. Pharmacol. 57, 101–109. [DOI] [PubMed] [Google Scholar]
- 39. Li R, Quan P, Liu DF, Wei FD, Zhang Q, Xu QW (2009) The influence of cosolvent on the complexation of HP‐beta‐cyclodextrins with oleanolic acid and ursolic acid. AAPS PharmSciTech. 10, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo M, Zhang S, Song F, Wang D, Liu Z, Liu S (2003) Studies on the non‐covalent complexes between oleanolic acid and cyclodextrins using electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 38, 723–731. [DOI] [PubMed] [Google Scholar]
- 41. Jager S, Trojan H, Kopp T, Laszczyk MN, Scheffler A (2009) Pentacyclic triterpene distribution in various plants – rich sources for a new group of multi‐potent plant extracts. Molecules 14, 2016–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manojlovic V, Winkler K, Bunjes V, Neub A, Schubert R, Bugarski B et al. (2008) Membrane interactions of ternary phospholipid/cholesterol bilayers and encapsulation efficiencies of a RIP II protein. Colloids Surf. B Biointerfaces 64, 284–296. [DOI] [PubMed] [Google Scholar]
- 43. Jager S, Beffert M, Hoppe K, Nadberezny D, Frank B, Scheffler A (2011) Preparation of herbal tea as infusion or by maceration at room temperature using mistletoe tea as an example. Scientia Pharmaceutica 79, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cho SH, Chung KS, Choi JH, Kim DH, Lee KT (2009) Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase‐8‐dependent pathway in HL‐60 human leukemia cells. BMC Cancer 9, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gassmann M, Grenacher B, Rohde B, Vogel J (2009) Quantifying Western blots: pitfalls of densitometry. Electrophoresis 30, 1845–1855. [DOI] [PubMed] [Google Scholar]
- 46. Kim MS, So HS, Lee KM, Park JS, Lee JH, Moon SK et al. (2000) Activation of caspase cascades in Korean mistletoe (Viscum album var. coloratum) lectin‐II‐induced apoptosis of human myeloleukemic U937 cells. Gen. Pharmacol. 34, 349–355. [DOI] [PubMed] [Google Scholar]
- 47. Webb RJ (1963) Effect of more than one inhibitor. In: Enzyme and Metabolic Inhibitors, Vol. 1, 66–79, 488–512. New York: Academic Press. [Google Scholar]
- 48. Jesse P, Mottke G, Eberle J, Seifert G, Henze G, Prokop A (2009) Apoptosis‐inducing activity of Helleborus niger in ALL and AML. Pediatr. Blood Cancer 52, 464–469. [DOI] [PubMed] [Google Scholar]
- 49. Duong Van Huyen JP, Delignat S, Kazatchkine MD, Kaveri SV (2003) Comparative study of the sensitivity of lymphoblastoid and transformed monocytic cell lines to the cytotoxic effects of Viscum album extracts of different origin. Chemotherapy 49, 298–302. [DOI] [PubMed] [Google Scholar]
- 50. Kim MS, Lee J, Lee KM, Yang SH, Choi S, Chung SY et al. (2003) Involvement of hydrogen peroxide in mistletoe lectin‐II‐induced apoptosis of myeloleukemic U937 cells. Life Sci. 73, 1231–1243. [DOI] [PubMed] [Google Scholar]
- 51. Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti‐Passerini C et al. (2002) Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 175, 17–25. [DOI] [PubMed] [Google Scholar]
- 52. Ehrhardt H, Fulda S, Fuhrer M, Debatin KM, Jeremias I (2004) Betulinic acid‐induced apoptosis in leukemia cells. Leukemia 18, 1406–1412. [DOI] [PubMed] [Google Scholar]
- 53. Fulda S, Scaffidi C, Susin SA, Krammer PH, Kroemer G, Peter ME et al. (1998) Activation of mitochondria and release of mitochondrial apoptogenic factors by betulinic acid. J. Biol. Chem. 273, 33942–33948. [DOI] [PubMed] [Google Scholar]
- 54. Hengartner MO (2000) The biochemistry of apoptosis. Nature 407, 770–776. [DOI] [PubMed] [Google Scholar]
- 55. Zou W, Liu X, Yue P, Zhou Z, Sporn MB, Lotan R et al. (2004) c‐Jun NH2‐terminal kinase‐mediated up‐regulation of death receptor 5 contributes to induction of apoptosis by the novel synthetic triterpenoid methyl‐2‐cyano‐3,12‐dioxooleana‐1, 9‐dien‐28‐oate in human lung cancer cells. Cancer Res. 64, 7570–7578. [DOI] [PubMed] [Google Scholar]
- 56. Petronelli A, Saulle E, Pasquini L, Petrucci E, Mariani G, Biffoni M et al. (2009) High sensitivity of ovarian cancer cells to the synthetic triterpenoid CDDO‐Imidazolide. Cancer Lett. 282, 214–228. [DOI] [PubMed] [Google Scholar]
- 57. Zou W, Chen S, Liu X, Yue P, Sporn MB, Khuri FR et al. (2007) c‐FLIP downregulation contributes to apoptosis induction by the novel synthetic triterpenoid methyl‐2‐cyano‐3, 12‐dioxooleana‐1, 9‐dien‐28‐oate (CDDO‐Me) in human lung cancer cells. Cancer Biol. Ther. 6, 1614–1620. [DOI] [PubMed] [Google Scholar]
- 58. Yue P, Zhou Z, Khuri FR, Sun SY (2006) Depletion of intracellular glutathione contributes to JNK‐mediated death receptor 5 upregulation and apoptosis induction by the novel synthetic triterpenoid methyl‐2‐cyano‐3, 12‐dioxooleana‐1, 9‐dien‐28‐oate (CDDO‐Me). Cancer Biol. Ther. 5, 492–497. [DOI] [PubMed] [Google Scholar]
- 59. Zhang P, Li H, Chen D, Ni J, Kang Y, Wang S (2007) Oleanolic acid induces apoptosis in human leukemia cells through caspase activation and poly(ADP‐ribose) polymerase cleavage. Acta Biochim. Biophys. Sin. (Shanghai) 39, 803–809. [DOI] [PubMed] [Google Scholar]
- 60. Ikeda T, Nakata Y, Kimura F, Sato K, Anderson K, Motoyoshi K et al. (2004) Induction of redox imbalance and apoptosis in multiple myeloma cells by the novel triterpenoid 2‐cyano‐3,12‐dioxoolean‐1,9‐dien‐28‐oic acid. Mol. Cancer Ther. 3, 39–45. [PubMed] [Google Scholar]
- 61. Konopleva M, Tsao T, Estrov Z, Lee RM, Wang RY, Jackson CE et al. (2004) The synthetic triterpenoid 2‐cyano‐3,12‐dioxooleana‐1,9‐dien‐28‐oic acid induces caspase‐dependent and ‐independent apoptosis in acute myelogenous leukemia. Cancer Res. 64, 7927–7935. [DOI] [PubMed] [Google Scholar]
- 62. Vene R, Larghero P, Arena G, Sporn MB, Albini A, Tosetti F (2008) Glycogen synthase kinase 3beta regulates cell death induced by synthetic triterpenoids. Cancer Res. 68, 6987–6996. [DOI] [PubMed] [Google Scholar]
- 63. Samudio I, Kurinna S, Ruvolo P, Korchin B, Kantarjian H, Beran M et al. (2008) Inhibition of mitochondrial metabolism by methyl‐2‐cyano‐3,12‐dioxooleana‐1,9‐diene‐28‐oate induces apoptotic or autophagic cell death in chronic myeloid leukemia cells. Mol. Cancer Ther. 7, 1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Frantz M, Jung ML, Ribereau‐Gayon G, Anton R (2000) Modulation of mistletoe (Viscum album L.) lectins cytotoxicity by carbohydrates and serum glycoproteins. Arzneimittelforschung 50, 471–478. [DOI] [PubMed] [Google Scholar]
- 65. Ribereau‐Gayon G, Jung ML, Frantz M, Anton R (1997) Modulation of cytotoxicity and enhancement of cytokine release induced by Viscum album L. extracts or mistletoe lectins. Anticancer Drugs 8(Suppl. 1), S3–S8. [DOI] [PubMed] [Google Scholar]
- 66. Place AE, Suh N, Williams CR, Risingsong R, Honda T, Honda Y et al. (2003) The novel synthetic triterpenoid, CDDO‐imidazolide, inhibits inflammatory response and tumor growth in vivo. Clin. Cancer Res. 9, 2798–2806. [PubMed] [Google Scholar]
- 67. Liby K, Risingsong R, Royce DB, Williams CR, Ma T, Yore MM et al. (2009) Triterpenoids CDDO‐methyl ester or CDDO‐ethyl amide and rexinoids LG100268 or NRX194204 for prevention and treatment of lung cancer in mice. Cancer Prev. Res. (Phila) 2, 1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yamai H, Sawada N, Yoshida T, Seike J, Takizawa H, Kenzaki K et al. (2009) Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int. J. Cancer 125, 952–960. [DOI] [PubMed] [Google Scholar]
- 69. Hua Y, Zhang Z, Li J, Li Q, Hu S, Li J et al. (2009) Oleanolic acid derivative Dex‐OA has potent anti‐tumor and anti‐metastatic activity on osteosarcoma cells in vitro and in vivo. Invest. New Drugs 29, 258–265. [DOI] [PubMed] [Google Scholar]


