Abstract
Igf2 (insulin‐like growth factor 2) and H19 genes are imprinted in mammals; they are expressed unevenly from the two parental alleles. Igf2 is a growth factor expressed in most normal tissues, solely from the paternal allele. H19 gene is transcribed (but not translated to a protein) from the maternal allele. Igf2 protein is a growth factor particularly important during pregnancy, where it promotes both foetal and placental growth and also nutrient transfer from mother to offspring via the placenta. This article reviews epigenetic regulation of the Igf2/H19 gene‐cluster that leads to parent‐specific expression, with current models including parental allele‐specific DNA methylation and chromatin modifications, DNA‐binding of insulator proteins (CTCFs) and three‐dimensional partitioning of DNA in the nucleus. It is emphasized that key genomic features are conserved among mammals and have been functionally tested in mouse. ‘The enhancer competition model’, ‘the boundary model’ and ‘the chromatin‐loop model’ are three models based on differential methylation as the epigenetic mark responsible for the imprinted expression pattern. Pathways are discussed that can account for allelic methylation differences; there is a recent study that contradicts the previously accepted fact that biallelic expression is accompanied with loss of differential methylation pattern.
Introduction
The aim of this article has been to review and discuss the imprinted gene complex Igf2/H19. An imprinted gene differs from non‐imprinted counterparts since it is not expressed equally from each of the two parental alleles. Instead, one allele is preferentially expressed, some imprinted genes being expressed from the paternally inherited allele, such as Igf2 and Dlk‐1, and others characteristically expressed from the maternally inherited allele, such as H19, Igf2r and Snrpn.
In the 1970s, a theory appeared that proposed complex interplay of different interests between parents and offspring within the endosperm of flowering plants, a tissue analogous to the mammalian placenta. The theory postulated that offspring, especially when derived from different fathers, compete with each other for maternal resources, while the mother attempts to provide resources equally to all offspring since they are equally related to her 1, 2. A decade later, the discovery that for correct development a mammalian one‐cell embryo needs one pro‐nucleus from a parent of each sex made it clear that there is functional distinction between paternal and maternal chromosomes. The zygote cannot function with two maternal or two paternal sets of chromosomes 3. This was contradictory to the Mendelian law of inheritance which assumes maternal and paternal gene copies are essentially equivalent.
Haig and Westoby 4 summarized these ideas concerning conflict of different interests in mammals, marsupials and flowering plants and presented a model describing how evolution could favour an allele that, when derived from the paternal genome, promotes acquisition of maternal resources. This is now widely accepted as a likely explanation for evolution of imprinted gene expression, at least at some loci, and is known as the parent–offspring conflict theory (or conflict theory). They also hypothesized that evolutionary response in the maternal allele would be to use silencing in an attempt to forestall foetal overgrowth. It is applicable to mammals and flowering plants since both start life as dependent on their mother for sustenance.
Insulin‐like growth factor 2 protein (Igf2) was well characterized and known to induce cell proliferation in vitro 5, 6. DeChiara et al. 7, 8 studied mice with Igf2 gene deletions and found that if the gene deletion was inherited via the egg, the offspring was phenotypically normal, but when it came via the sperm offspring were growth deficient with birth weight approximately only 60% of a normal mouse. Paternal expression of Igf2 was demonstrated in the majority of tissues where the gene is active and it was thus the first imprinted gene to be identified 8. Paternal expression of a growth‐promoting gene also validated the genetic conflict theory. Remarkably, this finding was soon followed by the discovery of Igf2r, a gene expressed from the maternally inherited allele with a growth‐inhibitory function, which was again consistent with the conflict theory. Disruption of the maternal copy of Igf type 2 receptor (Igf2r) gene in mice was associated with foetal overgrowth 9. Since then, many more imprinted genes have been identified and in mouse over 100 are currently known (http://www.mousebook.org/catalog.php?catalog=imprinting). Many imprinted genes have roles in foetal growth and development, but there are also those with functions in energy homoeostasis 11, 12 and also brain function and behaviour 13. Whether all of these genes fit with the parental conflict theory has been a matter of debate, as described in 14.
It is now known that Igf2 affects the size of the placenta, and transfer of nutrients from mother to offspring, as well as foetal growth and resulting birth weight. Growth‐promoting effects of this paternally expressed gene are directly countered by maternal expression of Igf2r, a fact established unequivocally in further mouse genetic studies 10. Igf2r is also known as cation‐independent mannose‐6‐phosphate receptor and functions to sequester its ligands for lysosomal degradation. While mammalian Igf2r/mannose‐6‐phosphate receptor has a binding site specific for Igf2, the orthologous receptor in bird does not. All of these facts elegantly match the parent–offspring conflict hypothesis, in the mouse at least, and interaction between paternally derived Igf2 and maternally derived Igf2r has been described as a ‘parental tug‐of‐war’ 15, 16.
Igf2 gene structure
Igf2 gene in mammals, is comprised of a varying number of exons and promoters. It is transcribed and translated into a precursor hormone. After a number of processing steps, the end result is Igf2, in most species a 67 amino acid protein, although a number of variants arising from alternative splicing also occur. Different promoters and splicing patterns contribute to the complex regulation of Igf2 expression and its effects in both foetal and adult tissues (Fig. 1). Igf2 promoters and exons vary in number and length in mammals. Its various transcriptional isoforms emerge unevenly in different tissues and developmental stages and there are also considerable species differences. Promoter 0 (P0) (Fig. 1) is uniquely activated from the paternal allele in mouse placenta and also is crucial for normal growth in the womb 17. Human P0 is expressed in placenta, but also in foetal skeletal muscle and also in the adult, albeit at a lower level 18.
Figure 1.
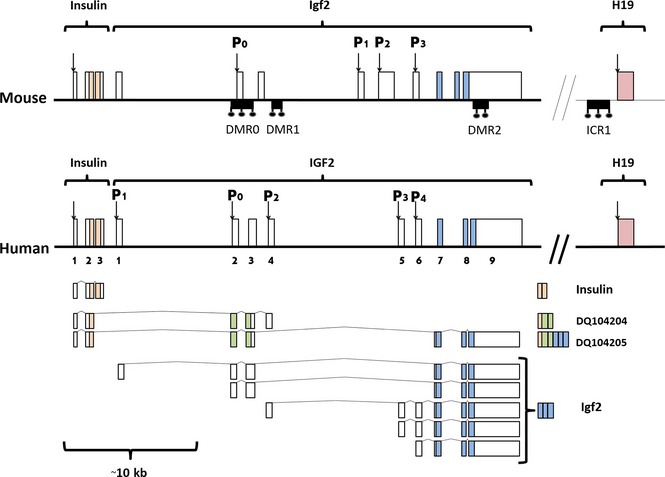
Mouse and human Ins2/Igf2/H19 locus (top) and differently spliced human transcripts (bottom). Boxes above the line indicate exons; coloured exons are protein coding. Small, black boxes below the line represent differently methylated regions and the H19 imprinting control region. Pink checked H19 boxes represent a simplified scheme of a gene organized in several variable exons (so‐called cassette exons) with intervening introns that differ between different species. Modified from Monk et al. 18.
Igf2 protein: function and receptors
Translated Igf2 protein is, in most species, a 67 amino acid protein that is related in sequence and structure to Igf1, insulin and relaxin [see 19 for review]. Igf2 is a growth factor of particular importance for placental and embryonic growth. In mice, Igf2 expression terminates in almost all tissues after birth. Mice have no adult‐specific promoters, and knockout of Igf2 P0‐transcript leads to reduced placental size and subsequent foetal growth inhibition 17. An exception is the choroid plexus and leptomeninges of the brain, where Igf2 is expressed biallelically and persists into adult life 8, 20, 21. In other mammalian species, including humans 22, pigs 23 and horses 24, Igf2 protein is present in adult tissues as well. In addition, in sheep there is now evidence for a role for Igf2 in foetal and placental development 25.
Igf type 1 receptor functions as a dimer similar to the two isoforms of the insulin receptor, Insr‐A and Insr‐B. These three receptors and the heterodimer Insr‐A/Igf2r mediate proliferative and growth‐inducing effects of both Igf2 and Igf1, while binding of Igf2 to Igf2r results in degradation. In mice Igf2r, is also imprinted, expressed only from the maternally inherited chromosome (Fig. 2), which has an important bearing on overall biological effects 16. However, whereas Igf2 (and IGF2) is maternally imprinted (silenced) in both mouse 8 and humans 20, IGF2R appears to be non‐imprinted in humans 26 and some other mammals.
Figure 2.
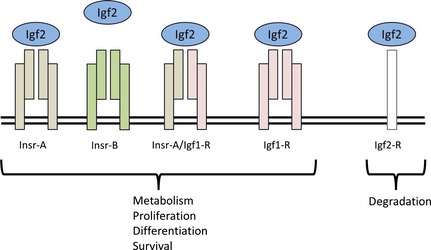
Igf2 functions mainly through binding to Igf1r but can also bind, although with lower affinity, to both isoforms of the insulin receptor (Insr‐A and Insr‐B) and to the Insr‐A/Igf1r heterodimer. Binding to these receptors induces various cellular responses, particularly proliferation and survival, mediated by Igf1r, while binding to Igf2r mediates internalization and lysosomal degradation. Adapted from Chao and D'Amore 27.
Igf2 is produced locally in tissues in an autocrine or paracrine fashion and in liver, from where it is distributed via the blood in the endocrine manner. In vitro studies have shown that Igf1 receptors are often expressed by the same cells that express Igf2, making an autocrine loop possible. Igf2 expression studies in several species show transcriptional activity during development in several tissues, that support an autocrine or paracrine function 6. For example, human corneal cells exposed to recombinant IGF2 enter S‐phase preparing to divide, but do not express IGF2 themselves. Cells in the posterior eye express IGF2, thereby illustrating paracrine mode of function 28. Moreover, studies in transgenic mice overexpressing Igf2 suggest that growth effects are local to the sites of expression 29, 30.
Igf2/H19 cluster and the role of H19
H19 gene is located immediately downstream of Igf2 (Fig. 1). H19 is transcribed, but not translated into protein. Both Igf2 and H19 are imprinted in a reciprocal manner in most somatic cells, where the paternal chromosome expresses Igf2 but not H19, and the maternal chromosome transcribes H19 but not Igf2. The H19 gene encodes a 2.3 kb non‐coding mRNA which is strongly expressed during embryogenesis. This gene belongs to an imprinted cluster, conserved on mouse chromosome 7 and human chromosome 11p15. H19 and Igf2 are oppositely imprinted and co‐expressed in endoderm‐ and mesoderm‐derived tissues during embryonic development, which suggests a common mechanism of regulation. The role of H19, which lacks a conserved open reading frame, yet is abundantly expressed during mammalian development in both embryonic and extraembryonic tissues, is intriguing. Lack of an open reading frame infers that H19 functions as an RNA 31, 32, however, apart from involvement of H19 locus in imprinted regulation of Igf2, the physiological role of H19 RNA has until recently been unclear. Evidence has been presented that H19 can act as either an oncogene 33 or tumour suppressor 34, 35, 36 and that it can influence mouse growth independently of the mechanism co‐regulating imprinted expression of Igf2 and H19 37. It appears that H19 RNA may influence growth regulation through at least two distinct mechanisms involving microRNAs (microRNAs consist of 19–25 non‐coding nucleotides that have the ability to repress translation or promote RNA degradation). First, it has been shown that sequences within the first exon of H19 are the source of microRNA miR‐675, which is expressed exclusively in placenta and serves to suppress genes that promote placental growth, including Igf type‐1 receptor gene (Igf1r) 38. More recently, H19 RNA has been shown to be a sink for let‐7 family microRNAs 38. By sequestering let‐7 microRNAs, H19 was found to influence expression of let‐7 target genes and promote differentiation of myoblasts 39.
Epigenetic modifications control gene expression
DNA methylation comprises addition of a methyl group (CH3) to certain residues on the DNA, usually to a cytosine in a CpG dinucleotide. CpGs often occur in ‘islands’, with many CpGs congregated in a DNA sequence. DNA methyltransferases (Dnmts) are the enzymes that carry out DNA methylation. Some Dnmts use hemi‐methylated DNA (newly replicated DNA where only one strand is methylated) as a substrate, adding a methyl group to C‐residues. The old DNA methylation pattern is reproduced. Other Dnmts are responsible for de novo methylation, where unmethylated DNA is the substrate, and these Dnmts can create new DNA methylation patterns (Fig. 3). As a rule, genes with a methylated promoter cannot be expressed, albeit there are notable exceptions 40. The H19 promoter is methylated on the paternal chromosome, inhibiting transcription of paternal H19.
Figure 3.
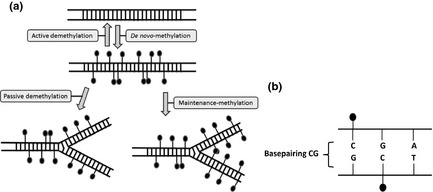
Some DNA methyltransferases (Dnmts) use hemi‐methylated DNA (newly replicated DNA where only one strand is methylated) as a substrate, adding a methyl group to C‐residues. Other Dnmts are responsible for de novo‐methylation. Adapted from Reik and Walter 41.
Chromatin, by definition, comprises DNA associated with histone octamers that can be packed as dense heterochromatin or more loosely in euchromatin, depending on modifications that alter local structures and, as a consequence, influence gene expression. These modifications are exerted by enzymes that acetylate, deacetylate, methylate or demethylate specific histone amino‐acid residues.
Igf2 and H19 share enhancers
During development, Igf2 and H19 are concomitantly expressed in a variety of tissues 20. This has led to the idea of common enhancer elements that mechanistically link the genes together. Enhancers are short sequences of DNA that can bind transcription factors and enhance transcription level of genes. Leighton et al. 42 made a targeted deletion of two endoderm‐specific enhancers, in mice, that lie 3′ of H19 as well as H19 itself 43. Subsequent breeding showed that a maternally inherited enhancer deletion resulted in dramatic decline of H19 in endoderm‐derived tissues, including liver. Identical deletion on the paternally inherited allele resulted in an equivalent reduction of Igf2 expression that was associated with growth impairment, so that mice were, at birth, about only 80% of normal weight, reflecting partial loss of Igf2 expression. This elegant deletion experiment showed that these enhancers work on both alleles to promote either maternal H19 expression or paternal Igf2 expression.
Enhancer competition model
Differences in allelic methylation at Igf2/H19 locus shown by Bartolomei et al. 44 revealed important information that was tested functionally in further transgenic mouse studies. The paternal H19 allele was found to be methylated and inactive, while the maternal allele remained unmethylated from 900 bp upstream of the H19 coding region to 700 bp downstream. Bartolomei et al. 44 ruled out the possibility that it was expression of H19 that gave this parental‐specific pattern by comparing different tissues, including foetal liver, where H19 is maternally expressed, adult liver where maternal H19 expression has ceased, and adult brain where H19 is not expressed, as well as embryonic stem cells, that do not express H19. In all tissues, the maternal allele was unmethylated and had open chromatin structure in the region of the H19 promoter, independent of expression pattern. Two downstream enhancers where open in chromatin structure on both parental alleles. Bartolomei et al. 44 combined these findings with data from Sasaki et al. 45 concerning allele‐specific methylation in a DMR sequence 5′ to Igf2 and presented an ‘enhancer competition model’ that could explain reciprocal imprint of both Igf2 and H19. The hypothesis was that enhancers could work on either Igf2 or H19 and suggested that H19 methylation on the paternal allele was the key determinant of H19 silencing and Igf2 activation.
Two transgenic mouse lines containing the H19 domain including 4 kb 5′ and 8 kb 3′ DNA were examined regarding parental‐specific methylation and H19 expression. Homozygous male and female mice were bred to non‐transgenic mice. Liver cells from progeny typically exhibited methylation and expression patterns similar to endogenous H19, were hypermethylated and silent when inherited from the father and unmethylated and expressed when maternally derived. A few maternally inherited transgenes exhibited a hypermethylated and unexpressed pattern, that typically found in paternally inherited H19 transgenes, possibly due to insufficient sequence upstreams of H19, or perhaps due to influence of endogenous sequences at the transgene insertion site, or some other unknown influence on the imprinting process. Even so, these results showed that information contained within the H19 domain transgene, including a region of 2 kb located 4–6 kb upstream of H19 transcription, was enough to confer parental‐specific methylation and expression patterns after passage through the germ cells. The result indicates that it is not the methylation itself that is the parental mark, but that the passage through the germline cells somehow establishes these differences. It also showed that enough information for parental‐specific expression of H19 is localized in the vicinity of the gene itself.
The enhancer competition model attempted to explain reciprocal imprinting of both H19 and Igf2 (Fig. 4). It predicted that enhancers could work on either Igf2 or H19 and suggested that methylation of the paternal H19 inhibits expression and thus gives enhancers a chance to work on the Igf2 gene. However, it could not explain subsequent findings concerning differently methylated domain 5′ to H19, called imprint control region 1 (ICR1).
Figure 4.
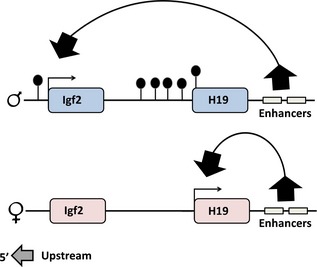
The enhancer competition model to explain imprinted regulation of Igf2 and H19. Big boxes represent genes on the paternal (top) and maternal chromosome, with arrows indicating gene expression. CH3‐lollipops represent regions containing methylated cytosine residues. Small boxes show position of downstream enhancers.
Igf2/H19 imprint control region
Human chromosomal region 11p15 contains two independent imprinted regions controlled by two ICRs, ICR1 and ICR2. One region, located approximately 2–4 kb upstream of H19 transcription start site has been shown to be important to the imprinting state of both H19 and Igf2 and is referred to as ICR1. ICR1 corresponds to a region of differential methylation, also known as the H19 DMD since it is rich in CpG residues that differ in state of methylation on the two alleles, with the paternal allele being methylated (Fig. 5). The unmethylated maternal allele enables binding of an insulator protein CTCF, which prevents common enhancers acting on Igf2. ICR1 is essential for imprinting of H19 in vivo, since mice with deleted H19 ICR1 lose their imprint 46. Paternal H19 alleles were expressed to a level of approximately half that of wild‐type maternal H19 expression. Parental‐specific DNA methylation was lost in a short, remaining sequence of ICR1 and in the sequence between the DMD and the H19 promoter. This and subsequent studies showed that H19 ICR1 is required for H19 transcription and to prevent downstream enhancers from accessing Igf2 promoters on the maternal chromosome, and that ICR1‐dependent transcription of H19 is required for maintenance of key epigenetic marks 46, 47.
Figure 5.
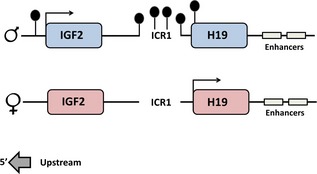
Parental‐specific DNA methylation of ICR 1 is required for the imprinted status of both H19 and Igf2.
Adapted from Chao and D'Amore 27.
Light has also been shed on this issue by studying two rare disorders caused by alterations in the 11p15 region in humans, Beckwith–Wiedemann Syndrome (BWS) 48, 49 and Silver–Russell Syndrome (SRS) 50. BWS was one of the first syndromes in which overexpression of IGF2 was coupled to a growth disorder. BWS also provided the first link between overgrowth and tumour development 48, 49. BWS can occur as a result of alterations in ICR1 as well as ICR2. First, simple methylation of maternal ICR1 has been found to account for 5% of BWS cases. Secondly, ICR2 is normally paternally imprinted and thus regulates expression of maternally expressed CDKNIC, a growth regulatory gene. ICR2 resides adjacent to a non‐imprinted gene, KCNQ1 and is the promoter for KCNQ1OT1, a paternally expressed RNA whose expression regulates CDKNIC in cis 51. Hypomethylation of ICR2 is the most frequent genetic aberration in BWS. In parallel, it has been shown that SRS patients display aberrations including hypomethylation of ICR1 as well as ICR2 50. Opposing epigenetic lesions in BWS and SRS are consistent with their defining features as disorders of overgrowth and growth restriction, respectively.
CTCFs and the boundary model
The ICR1 sequence contains direct and indirect repeats, including several CAGCCC motifs, discovered in human H19 ICR as early as 1987 52. CTCFs are specific zinc‐finger proteins that bind to CAGCCC and related sequences to influence gene expression at numerous loci, both imprinted and non‐imprinted. Wang et al. 53, using ChIP‐seq, identified 77811 separate CTCF‐binding sites in the human genome. The 19 cell types examined exhibited marked variability, with average number of bound CTCF sites being in the order of 55 000. DNA methylation is one factor that can prevent CTCF binding, with 41% variation of CTCF binding found to be due to differences in DNA methylation.
Dixon et al. 54 used the Hi‐C method to reveal information concerning three‐dimensional architecture of chromosomes. CTCFs were concentrated on boundaries of topological domains, along with transfer RNAs, short interspersed elements and housekeeping genes, indicating that these are necessary for establishment of the three‐dimensional architecture of genome domains in cell nuclei. An updated genome‐wide CTCF‐binding site database includes calculations of distance from CTCF sites to nearest domain boundary, thus allowing further research on how CTCF‐binding sites act to organize the three‐dimensional structure of DNA 55. Following the demonstration that CTCF‐mediated chromatin organization at the Igf2/H19 locus is dependent on cohesin 56, it has become clear that CTCF and cohesin‐mediated chromatin interactions are important for gene regulation at many loci 57.
Bell and Felsenfeld 58 and Hark et al. 59 suggested that CTCF binding on maternal ICR1 blocks downstream enhancer sequences from accessing Igf2 promoters and thereby silences maternal Igf2 expression, while paternal‐specific ICR1 methylation prevents CTCF binding and is permissive for paternal Igf2 expression (Fig. 6). This enhancer‐blocking, or boundary function of ICR1 was confirmed by different approaches, making clear that ICR1‐bound CTCF functions as a genomic insulator and that ICR1 position between H19 and Igf2 made this possible. The enhancer competition model was ruled out, since it could not explain why ICR1 position between the two genes was essential. Conservation of the cluster was to some part explained since the cluster could not be regulated if genes, insulator binding domains or enhancers were not in the correct order. Hark et al. 59 were more specific when analysing CTCF binding on methylated DNA; they analysed hemi‐methylated DNA and found that only hemi‐methylation on the top strand of DNA inhibits CTCF binding, while bottom‐strand methylation was unimportant. In replicating cells, this means that the paternal allele transiently has one hemi‐methylated bottom strand where CTCF proteins could in theory bind, but (at least in normal cells) does not. Involvement of CTCF in the boundary model added components to the imprinting puzzle, but it was still not clear whether methylation at ICR1 was fundamental for establishing imprinted expression.
Figure 6.

The boundary model of imprinted regulation of Igf2 and H19 states that binding of CTCF proteins on maternal ICR 1 works as an insulator preventing enhancer elements from acting on the Igf2 maternal allele.
Adapted from Chao and D'Amore 27.
Histone acetylation and DNA methylation affect gene expression
Pedone et al. 60 immunoprecipitated cells with antibodies against acetylated histone 3 (H3) and H4 tails to detect hypoacetylated histone tails, commonly found in dense chromatin and hyperacetylated tails, associated with chromatin open for transcription (Fig. 7). The silent paternal H19 allele was hypoacetylated compared to the maternal allele, but Igf2 alleles were equally acetylated. These authors 60 also cultured cells either in the presence of DNA methylation inhibitors (leading to reduced DNA methylation), or inhibitors of histone deacetylases (leading to more open chromatin), or in a medium with both, and these treatments were found to affect the imprints. H19 imprint was lost only when both inhibitors were added, whilst Igf2 was biallelically expressed when either or both inhibitors were present. These findings suggest that both DNA methylation and histone modification are important for maintenance of the Igf2 imprint.
Figure 7.
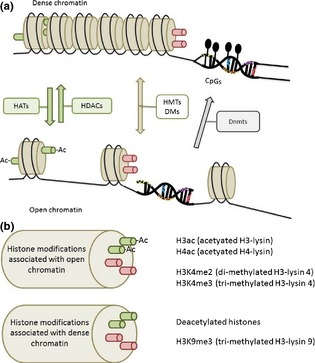
Chromatin regulation mediated by histone modifications. (a) Histone acetyltransferases (HATs), histone deacetyltransferases (HDACs), histone methyltransferases (HMTs), demethylases (DMs) are enzymes involved in forming open or dense chromatin. (b) Some key histone modifications associated with open or dense chromatin states. Model created with data from Verona et al. 61.
Are chromatin differences a consequence of transcriptional status?
Verona et al. 61 characterized histone modification in imprinted regions, including the H19 ICR1 region on both parental alleles. Allele‐specific histone acetylation and histone methylation were observed and active maternal H19 allele was associated with active chromatin modifications (described in detail in Fig. 7b). The paternal H19 allele was in a dense chromatin state. Igf2 was reciprocally in a state of low chromatin density while enhancers were open (Fig. 8). Highest level of ‘active histones’ was found at the maternal H19 promoter. Differences in ICR1 raised a question: do specific chromatin modifications in ICR1 allow transcription or are they a consequence of transcription? To answer this, Verona et al. 61 compared an H19 gene with deleted ICR1 in tissues where H19 was not expressed, to neonatal liver where it was expressed (even without the ICR). The result was ‘active’ chromatin at H19 in neonatal liver, but not in other tissues, suggesting that it is transcription level that provides the allele‐specific chromatin pattern. Thus, differences in chromatin are not an effect of some imprinted mark but an effect of transcriptional level. These authors did not rule out that observed allele‐specific DNA methylation and chromatin differences could be interconnected and reinforcing each other.
Figure 8.
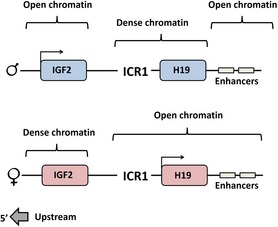
Summary of chromatin differences at the Ig2/H19 paternal and maternal alleles, according to Verona et al. (61).
Parent‐specific loops and the chromatin loop‐model in mice
Murrell et al. 62 made a targeted insertion of Igf2/H19‐genes to generate a mouse where the genes and promoters showed activity, ascribable to a normal cell. They used a chromosome conformation capture technique that enabled analysis of physical organization of specific chromosomal regions in the nucleus. Murrell et al. 62 reported parental allele‐specific differences that involved differentially methylated region 1 (DMR1) and DMR2 within the Igf2 gene (Fig. 9), showing that maternal ICR1 interacts with Igf2 DMR1, and paternal ICR1 meshes with Igf2 DMR2. This generated a three‐dimensional model that provided a simple epigenetic explanation for imprinted expression of Igf2 and H19: DMRs and ICRs contain insulators, silencers and activators and are turned on with differential methylation that enables or inhibits expression of the genes.
Figure 9.
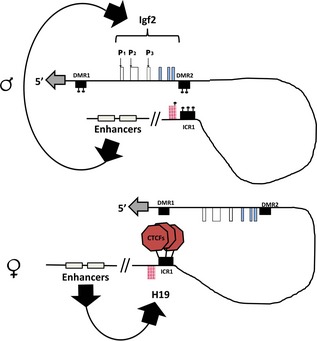
Chromatin looping at paternal and maternal Igf2/H19 alleles. DMR1, DMR2 and ICR1 are methylated on the paternal chromosome, and putative proteins bind methylated ICR1 to DMR2, bringing downstream enhancers into close proximity with the paternal Igf2 promoters. On the maternal chromosome, ICR1 binds CTCFs (also cohesion and likely other proteins) and this promotes interaction with DMR1, creating an inactive domain where maternal Igf2 has no access to enhancers. Pink checked H19 boxes represent a simplified scheme of a gene organized in several variable exons (so‐called cassette exons) with intervening introns that differ between species. Modified from Murrell et al. 62.
Germ cells carry parental information
Methylation prevents CTCF binding on the paternal Igf2 allele 63, 64, but how is methylation established? According to earlier findings 44, correct parental methylation pattern is an effect of passage through either the female or the male germline cells in an embryo, and thus probably not to the imprinted mark itself.
In a developing embryo, some cells become primordial germ cells (PGCs), still diploid but destined to migrate to the gonads, proliferate and differentiate into haploid sperm or oocytes. Epigenetic status of these cells changes dramatically before meiosis. PGCs undergo demethylation, where most DNA methylation is erased, including imprinted areas. Parental differences in modification of histones and chromatin are also removed before meiosis. So far, there is no definitive explanation for the observed demethylation and removal of chromatin differences. It is not known if demethylation causes removal of chromatin modifications, or alternatively if chromatin modifications are a necessary prerequisite for demethylation 65, 66, 67, 68. Demethylation of haploid PGC is a necessary step, since all old parental methylation needs to be erased, so that all alleles can acquire new methylation patterns appropriate to the sex of the embryo in which they are developing. Alignment and recombination of homologous chromosomes during meiosis is not possible without the demethylation 63.
In mouse development, between 10.5 and 12.5 dpc, patterns of DNA methylation in imprinted and testis‐specific genes are erased in PGCs 63, 64. In the male, DNA methylation begins to be reestablished around 15.5 dpc for imprinted genes 65, 66, 67.
Concluding remarks
Imprinted expression of Igf2 has evolved in mammals, but why?
The silent maternal Igf2 copy has no effect on foetal growth; however, in mice, maternal Igf2r acts as a direct antagonist of Igf2 by targeting it for lysosomal destruction. It is in the mother's interest to provide her offspring with sufficient resources for their growth and subsequent survival, with resources allocated evenly to the offspring throughout her reproductive span. In species where one female will typically reproduce with more than one male, it is in the interest of the male to promote resource allocation to his offspring in a more opportunistic manner. This difference in parental interests was recognized by Haig and Westoby 4 and is known as the ‘parent–offspring conflict theory’ to explain evolution of imprinted genes. It is accepted that for imprinted gene expression to evolve there must be benefits that outweigh cost, or risks, associated with silencing one of the two parental alleles of a gene, notably that a single mutation in the active copy may abolish all the gene's function. The conflict theory appears to hold true for at least a subset of imprinted genes that function to regulate foetal growth and can explain why genomic imprinting is essentially confined to mammals and flowering plants, in which the placenta and endosperm serve similar functions in nutrient provision from mother to offspring during foetal growth. The theory has been extended, for instance, to consider behaviour of imprinted genes in populations but does not obviously accommodate all the 100 or so genes that are now known to be imprinted and have diverse functions, including in adult physiology and behaviour 11, 13, 69.
In whose interest is this paternal‐specific expression?
It is unclear how imprinted parental‐specific gene expression arose, and the question remains who benefits from it. Paternal‐specific Igf2 expression is explainable only if there are differences in Igf2‐allele efficacy. Assuming some Igf2 alleles are more demanding of maternal resources and others less, then selective pressure supports the more resource‐demanding paternal‐specific expression of Igf2. Likewise, it is assumed that, at least in mice, selective pressure also supports Igf2r alleles that are more effective at neutralizing Igf2 and expression from the maternal Igf2r allele. Ultimately, these antagonistic control overgrowth must be in balance to achieve an optimum for offspring survival and fitness. The balance must work at population level, as well as at the level of individuals, and this is recognized by extension of the parent–offspring conflict hypothesis to include the idea of kinship, or relatedness within populations 69.
It is commonly assumed that there exists interbrood competition for resources in utero. In theory, this could be countered by females reallocating resources within a litter or conserving resources for investment in future broods. Mouse manipulation experiments have shown that females that had invested lightly into the first brood did not invest more heavily into the second brood than females that invested more heavily into the first brood 70. It was therefore concluded that females are somehow ‘primed’ by their experience in their first brood so that number in the second brood is determined in part by number in the first 70. In addition, there may also exist a trade‐off between litter size and size of individual offspring, so that reproductive success may benefit under some circumstances from larger litters with smaller offspring, and in other instances from smaller litters of larger offspring. There is evidence of this from manipulation in mice of another imprinted gene, Grb10. Grb10 encodes a signalling adapter protein from the maternal allele, expressed widely throughout foetal development, that normally acts to limit placental as well as embryonic growth. Although Grb10 knockout mice are at birth significantly larger than their wild‐type littermates, mothers carrying disrupted maternal Grb10 allele had larger litters and smaller offspring than those inheriting disrupted Grb10 allele from the father. It was therefore concluded that there is a grandparental effect whereby Grb10 can affect reproductive strategy by allocating maternal resources, so as to influence the relationship between offspring number and size 71.
The operational role of Igf2 protein is only partly known. Even though we have gathered considerable information concerning structure and function of the gene, the time is ripe to link this information to how the peptide participates in both normal growth development and pathogenesis of key diseases 19, 72, 73. There are clearly one or more epigenetic marks that distinguish parental alleles, and there are key element(s) that make parental‐specific expression possible. Imprinting of the H19/Igf2 gene cluster is important in many different cancers and syndromes including growth disorders, and deeper knowledge of imprinting mechanisms could enable development of diagnostic tests and possible future therapies.
References
- 1. Trivers RL (1974) Parent‐offspring conflict. Am. Zool. 14, 249–264. [Google Scholar]
- 2. Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am. Nat. 108, 499–506. [Google Scholar]
- 3. Surani MAH, Barton SC, Norris ML (1984) Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 308, 548–550. [DOI] [PubMed] [Google Scholar]
- 4. Haig D, Westoby M (1989) Parent‐specific gene expression and the triploid endosperm. Am. Nat. 134, 147–155. [Google Scholar]
- 5. Engström W, Rees AR, Heath JK (1985) Proliferation of a human embryonal carcinoma‐derived cell line in serum‐free medium: inter‐relationship between growth factor requirements and membrane receptor expression. J. Cell Sci. 73, 361–373. [DOI] [PubMed] [Google Scholar]
- 6. Biddle C, Li CH, Schofield PN, Tate VE, Hopkins B, Engström W et al (1988) Insulin‐like growth factors and the multiplication of Tera‐2, a human teratoma‐derived cell line. J. Cell Sci. 90, 475–484. [DOI] [PubMed] [Google Scholar]
- 7. DeChiara TM, Efstratiadis A, Robertson EJ (1990) A growth‐deficiency phenotype in heterozygous mice carrying an insulin‐like growth factor II gene disrupted by targeting. Nature 345, 78–80. [DOI] [PubMed] [Google Scholar]
- 8. DeChiara TM, Robertson EJ, Efstratiadis A (1991) Parental imprinting of the mouse insulin‐like growth factor II gene. Cell 64, 849–859. [DOI] [PubMed] [Google Scholar]
- 9. Lau MM, Stewart CE, Liu Z, Bhatt H, Rotwein P, Stewart CL (1994) Loss of the imprinted IGF2/cation‐independent mannose 6‐phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 8, 2953–2963. [DOI] [PubMed] [Google Scholar]
- 10. Ludwig T, Eggenschwieler J, Fisher P, D'Ercole AJ, Davenport ML, Efstratiadis A (1996) Mouse mutants lacking the type 2 IGF receptor are rescued from perinatal lethality in Igf2 and Igf1r null backgrounds. Dev. Biol. 177, 517–535. [DOI] [PubMed] [Google Scholar]
- 11. Smith FM, Garfield AS, Ward A (2006) Regulation of growth and metabolism by imprinted genes. Cytogenet. Genomics Res. 113, 279–291. [DOI] [PubMed] [Google Scholar]
- 12. Turnster SJ, Jensen AB, John RM (2013) Imprinted genes in mouse placental development and the regulation of fetal energy stores. Reproduction 145, R117–R137. [DOI] [PubMed] [Google Scholar]
- 13. Isles AR, Davies W, Wilkinson LS (2006) Genomic imprinting and the social brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 2229–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hurst L, McVean GT (1998) Do we understand the evolution of genomic imprinting? Curr. Opin. Genet. Dev. 8, 701–708. [DOI] [PubMed] [Google Scholar]
- 15. Graham CF (1993) Strife in the germ line. Int. J. Dev. Biol. 37, 25–31. [PubMed] [Google Scholar]
- 16. Haig D, Graham C (1991) Genomic imprinting and the strange case of the insulin‐like growth factor II receptor. Cell 64, 1045–1046. [DOI] [PubMed] [Google Scholar]
- 17. Constância M, Hemberger M, Hughes J, Dean W, Ferguson‐Smith A, Fundele R et al (2002) Placental‐specific IGF‐II is a major modulator of placental and fetal growth. Nature 417, 945–948. [DOI] [PubMed] [Google Scholar]
- 18. Monk D, Sanches R, Arnaud P, Apostolidou S, Hills FA, Abu‐Amero S et al (2006) Imprinting of IGF2 P0 transcript and novel alternatively spliced INS‐IGF2 isoforms show differences between mouse and human. Hum. Mol. Genet. 15, 1259–1269. [DOI] [PubMed] [Google Scholar]
- 19. Bergman D, Halje M, Nordin M, Engström W (2013) Insulin like growth factor 2 in development and disease. Gerontology 59, 240–249. [DOI] [PubMed] [Google Scholar]
- 20. Olsson R, Hedborg F, Holmgren L, Walsh C, Ekström TJ (1994) Overlapping patterns of IGF2 and H19 expression during human development: biallelic IGF2 expression correlates with a lack of H19 expression. Development 120, 361–368. [DOI] [PubMed] [Google Scholar]
- 21. Charalambous M, Menheniott TR, Bennett WR, Kelly SM, Dell G, Dandolo L et al (2004) An enhancer element at the Igf2/H19 locus drives gene expression in both imprinted and non‐imprinted tissues. Dev. Biol. 271, 488–497. [DOI] [PubMed] [Google Scholar]
- 22. Davies SM (1994) Developmental regulation of genomic imprinting of the IGF2 gene in human liver. Cancer Res. 54, 2560–2562. [PubMed] [Google Scholar]
- 23. Braunschweig MH, Owczarek‐Lipska M, Stahlberger‐Saitbekova N (2011) Relationship of porcine IGF2 imprinting status to DNA methylation at the H19 DMD and the IGF2 DMRs 1 and 2. BMC Genet. 12, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otte K, Engström W (1994) Insulin like growth factor II in the horse: determination of a cDNA nucleotide sequence and expression in fetal and adult tissue. Gen. Comp. Endocrinol. 96, 270–275. [DOI] [PubMed] [Google Scholar]
- 25. Young LE, Schnieke AE, McCreath KJ, Wieckowski S, Konfortova G, Fernandes K et al (2003) Conservation of IGF2‐H19 and IGF2R imprinting in sheep: effects of somatic cell nuclear transfer. Mech. Dev. 120, 1433–1442. [DOI] [PubMed] [Google Scholar]
- 26. Kalscheuer VM, Mariman EC, Schepens MT, Rehder H, Ropers HH (1993) The insulin like growth factor type 2 receptor gene is imprinted in the mouse but not in humans. Nat. Genet. 5, 74–78. [DOI] [PubMed] [Google Scholar]
- 27. Chao W, D'Amore P (2008) IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev. 19, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hyldahl L, Engström W, Schofield PN (1986) Stimulatory effects of insulin‐like growth factors on DNA synthesis in the human embryonic cornea. J. Embryol. Exp. Morphol. 98, 71–83. [PubMed] [Google Scholar]
- 29. Ward A, Bates P, Fisher R, Richardson L, Graham CF (1994) Disproportionate growth in mice with Igf‐2 transgenes. Proc. Natl Acad. Sci. USA 91, 10365–10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bates P, Fischer R, Ward A, Richardson L, Hill DJ, Graham CF (1995) Mammary cancer in transgenic mice expressing insulin like growth factor II. Br. J. Cancer 72, 1189–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brannan CI, Dees EC, Ingram RS, Tilghman SM (1990) The product of the H19 gene may function as an RNA. Mol. Cell. Biol. 10, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gabory A, Ripoche MA, Yoshimizu T, Dandolo L (2006) The H19 gene: regulation and function of a non‐coding RNA. Cytogenet. Genome Res. 113, 188–193. [DOI] [PubMed] [Google Scholar]
- 33. Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu‐lail R, Hochberg A et al (2007) The H19 non‐coding RNA is essential for human tumorgrowth. PLoS ONE 2, e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hao Y, Crenshaw T, Moulton T, Newcomb E, Tycko B (1993) Tumour‐suppressor activity of H19 RNA. Nature 365, 764–767. [DOI] [PubMed] [Google Scholar]
- 35. Yoshimizu T, Moroglio A, Ripoche MA, Gabory A, Vernucci M, Riccio A et al (2008) The H19 locus acts in vivo as a tumor suppressor. Proc. Natl Acad. Sci. USA 105, 12417–12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsang WP, Ng EKO, Ng SSM, Jin H, Yu J, Sung JJY et al (2010) Oncofetal H19‐derived miR‐675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis 31, 350–358. [DOI] [PubMed] [Google Scholar]
- 37. Gabory A, Ripoche MA, Le Digarcher A, Watrin F, Ziyyat A, Forne T et al (2009) H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. Development 136, 3413–3421. [DOI] [PubMed] [Google Scholar]
- 38. Keniry A, Oxley D, Monnier P, Kyba M, Dandolo L, Smits G et al (2012) The H19 lincRNA is a developmental reservoir of miR‐675 that suppresses growth and Igf1r. Nat. Cell Biol. 14, 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kallen AN, Zhou XB, Xu J, Qiao C, Ma J, Yan L et al (2013) The imprinted H19 lncRNA antagonizes Let‐7 microRNAs. Mol. Cell 52, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jones MJ, Feies AP, Kobor MS (2013) DNA methylation, genotype and gene expression: who is driving and who is along for the ride? Genome Biol. 14, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reik W, Walter J (2001) Genomic imprinting: parental influence on the genome. Nat. Rev. Genet. 2, 21–32. [DOI] [PubMed] [Google Scholar]
- 42. Leighton PA, Ingram RS, Eggenschwiler J, Efstradiatis A, Tilghman SM (1995) Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature 375, 34–39. [DOI] [PubMed] [Google Scholar]
- 43. Leighton PA, Saam JR, Ingram RS, Stewart CL, Tilghman SM (1995) An enhancer deletion affects both H19 and Igf2 expression. Genes Dev. 9, 2079–2089. [DOI] [PubMed] [Google Scholar]
- 44. Bartolomei MS, Webber AL, Brunkow ME, Tilghman SM (1993) Epigenetic mechanisms underlying the imprinting of the mouse H19 gene. Genes Dev. 7, 1663–1673. [DOI] [PubMed] [Google Scholar]
- 45. Sasaki H, Jones PA, Chaillet JR, Ferguson‐Smith AC, Barton SC, Reik W et al (1992) Parental imprinting: potentially active chromatin of the repressed maternal allele of the mouse insulin‐like growth factor II (Igf2) gene. Genes Dev. 6, 1843–1856. [DOI] [PubMed] [Google Scholar]
- 46. Thorvaldsen JL, Duran KL, Bartolomei MS (1998) Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2 . Genes Dev. 12, 3693–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thorvaldsen JL, Fedoriw AM, Nguyen S, Bartolomei MS (2006) Developmental profile of H19 differentially methylated domain (DMD) deletion alleles reveals multiple roles of the DMD in regulating allelic expression and DNA methylation at the imprinted H19/Igf2 locus. Mol. Cell. Biol. 26, 1245–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Engström W, Lindham S, Schofield PN (1987) Wiedemann‐Beckwith syndrome. Eur. J. Pediatr. 148, 320–324. [DOI] [PubMed] [Google Scholar]
- 49. Ward A (1997) Beckwith‐Wiedemann syndrome and Wilms tumour. Mol. Hum. Reprod. 3, 157–168. [DOI] [PubMed] [Google Scholar]
- 50. Begemann M, Spengler S, Kanber D, Haake A, Baudis M, Leisten I et al (2011) Silver‐Russell patients showing a broad range of ICR1 and ICR2 hypomethylation in different tissues. Clin. Genet. 80, 83–88. [DOI] [PubMed] [Google Scholar]
- 51. Mancini‐DiNardo D, Steele SJ, Ingram RS, Tilghman SM (2003) A differentially methylated region within the gene Kcnq1 functions as an imprinted promoter and silencer. Hum. Mol. Genet. 12, 283–294. [DOI] [PubMed] [Google Scholar]
- 52. de Pagter‐Holthuizen P, Jansen M, van Schaik FM, van der Kammen R, Oosterwijk C, Van den Brande JL et al (1987) The human insulin‐like growth factor II gene contains two development‐specific promoters. FEBS Lett. 214, 259–264. [DOI] [PubMed] [Google Scholar]
- 53. Wang H, Maurano MT, Qu H, Varley KE, Gertz J, Pauli F et al (2012) Widespread plasticity in CTCF occupancy linked to DNA methylation. Genome Res. 22, 1680–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y et al (2012) Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ziebarth JD, Bhattacharya A, Cui Y (2013) CTCFBSDB 2.0: a database for CTCF‐binding sites and genome organization. Nucleic Acids Res. 41, D188–D194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nativio R, Wendt KS, Ito Y, Huddleston JE, Uribe‐Lewis S, Woodfine K et al (2009) Cohesin is required for higher‐order chromatin conformation at the imprinted IGF2‐H19 locus. PLoS Genet. 5, e1000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Merkenschlager M, Odom DT (2013) CTCF and cohesin. Linking gene regulatory elements with their targets. Cell 152, 1285–1297. [DOI] [PubMed] [Google Scholar]
- 58. Bell AC, Felsenfeld G (2000) Methylation of a CTCF‐dependent boundary controls imprinted expression of the Igf2 gene. Nature 405, 482–485. [DOI] [PubMed] [Google Scholar]
- 59. Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM (2000) CTCF mediates methylation‐sensitive enhancer‐blocking activity at the H19/Igf2 locus. Nature 405, 486–489. [DOI] [PubMed] [Google Scholar]
- 60. Pedone PV, Pikaart MJ, Cerrato F, Vernucci M, Ungaro P, Bruni CB et al (1999) Role of histone acetylation and DNA methylation in the maintenance of the imprinted expression of the H19 and Igf2 genes. FEBS Lett. 458, 45–50. [DOI] [PubMed] [Google Scholar]
- 61. Verona RI, Thorvaldsen JL, Reese KJ, Bartolomei MS (2008) The transcriptional status but not the imprinting control region determines allele‐specific histone modifications at the imprinted H19 locus. Mol. Cell. Biol. 28, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Murrell A, Heeson S, Reik W (2004) Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent‐specific chromatin loops. Nat. Genet. 36, 889–893. [DOI] [PubMed] [Google Scholar]
- 63. Kota SK, Feil R (2010) Epigenetic transitions in germ cell development and meiosis. Dev. Cell 19, 675–686. [DOI] [PubMed] [Google Scholar]
- 64. Hajkova P, Erhardt S, Lane N, Haaf T, El‐Maarri O, Reik W et al (2002) Epigenetic reprogramming in mouse priordial germ cells. Mech. Dev. 117, 15–23. [DOI] [PubMed] [Google Scholar]
- 65. Maatouk DM, Kellam LD, Mann MR, Lei H, Li E, Bartolomei MS et al (2006) DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development 133, 3411–3418. [DOI] [PubMed] [Google Scholar]
- 66. Davis CD, Uthus EO, Finley JW (2000) The H19 methylation imprint is erased and reestablished differentially on the parental alleles during male germ cell development. Hum. Mol. Genet. 9, 141–148. [DOI] [PubMed] [Google Scholar]
- 67. Ueda T, Abe K, Miura A, Yuzuriha M, Zubair M, Noguchi M et al (2000) The paternal methylation imprint of the mouse H19 locus is acquired in the gonocyte stage suring foetal testis development. Genes Cells 5, 649–659. [DOI] [PubMed] [Google Scholar]
- 68. Li JY, Lees Murdoch DJ, Xu GL, Walsh CP (2004) Timing of establishment of imprints in the mouse. Genomics 84, 952–960. [DOI] [PubMed] [Google Scholar]
- 69. Haig D (2000) Genomic imprinting, sex‐biased dispersal, and social behavior. Ann. N. Y. Acad. Sci. 907, 149–163. [DOI] [PubMed] [Google Scholar]
- 70. Charalambous M, Ward A, Hurst LD (2003) Evidence for a priming effect on maternal resource allocation. Implications for interbrood competition. Proc. Biol. Sci. 270, S100–S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Charalambous M, Cowley M, Geoghegan F, Smith FM, Radford EJ, Marlow BP et al (2010) Maternally inherited Gb10 reduces placental size and efficiency. Dev. Biol. 337, 1–8. [DOI] [PubMed] [Google Scholar]
- 72. Halje M, Nordin M, Bergman D, Engström W (2012) The effect of insulin like growth factor II in the regulation of tumour cell growth in vitro and tumourigenesis in vivo. In Vivo 26, 519–526. [PubMed] [Google Scholar]
- 73. Engström W, Shokrai A, Otte K, Granerus M, Gessbo Å, Bierke P et al (1998) Transcriptional regulation and biological significance of the insulin like growth factor gene. Cell Prolif. 31, 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]


