Abstract
Objectives
Periodontitis is one of the most widespread inflammatory diseases; it causes tooth loss and is also associated with a variety of systemic diseases. Mesenchymal stem cells (MSCs) have been used to treat periodontitis. However, it is unknown whether bacterial toxins in the periodontal environment affect MSC‐mediated periodontal regeneration. Porphyromonas gingivalis lipopolysaccharides (Pg‐LPS) are key toxins for development of periodontitis. The purpose of the present study was to investigate effects of P. gingivalis LPS on biological properties of MSCs.
Materials and methods
Mesenchymal stem cells from bone marrow (BMMSCs) were treated with different concentrations of P. gingivalis LPS (0.1–10 μg/ml), then its effects were evaluated on biological properties of BMMSCs including proliferation, apoptosis, osteogenic differentiation and capacities to inhibit activated T cells.
Results
Low concentration of P. gingivalis LPS (0.1 μg/ml) accelerated MSC proliferation, osteogenic differentiation and capacities to inhibit activated T cells via up‐regulation of nitric oxide. However, high concentration of P. gingivalis LPS (10 μg/ml) reduced MSC proliferation, osteogenic differentiation and capacities to inhibit activated T cells.
Conclusions
Mesenchymal stem cells were functionally different following exposure to P. gingivalis LPS at the investigated concentrations. These findings suggest that MSC‐mediated periodontal regeneration may be regulated by P. gingivalis LPS.
Introduction
Periodontitis is one of the most widespread inflammatory diseases; it is also associated with a variety of systemic diseases such as diabetes and coronary artery disease 1. It is characterized by progressive destruction of the periodontal tooth supporting structure, due to inadequate host inflammatory immune response to bacteria from oral microbial biofilms 2, ultimately leading to tooth loss. Although conventional periodontal therapies (such as guided tissue regeneration, use of enamel matrix derivative and treatment with various growth factors), may improve clinical outcomes, it is still difficult to achieve functional and predictable periodontal tissue regeneration 3, 4, 5, 6, 7. Recently, mesenchymal stem cell (MSC)‐based tissue regeneration has been considered as a prospective approach to treat periodontitis. Our previous studies have demonstrated that local administration of both autologous and allogeneic periodontal ligament stem cells (PDLSCs) ameliorated periodontitis with appropriate periodontal tissue regeneration, in miniature pigs 8, 9. In addition, bone marrow MSCs (BMMSCs) are also capable of differentiating into numerous kinds of cell type including osteocytes. They also possess immunomodulatory properties in terms of interplay with multiple subsets of immune cells, by secreting a number of soluble factors, or by direct cell–cell contact to regulate crucial biological functions. Based on these properties, BMMSCs may serve as an alternative cell source for periodontal regeneration 10, 11, 12. Thus, MSC‐based therapies may offer a promising approach to periodontal regeneration.
Better understanding of effects of recipient periodontal microenvironment, on MSC functions, may help clinical use of MSCs to regenerate periodontal tissue. Potential influence of oral bacterial toxins on MSC‐mediated periodontal regeneration has up to now, largely been unknown. Porphyromonas gingivalis lipopolysaccharides (Pg‐LPS) have been reported as a key factor in development of periodontitis 13. Recent study has shown that it could be a negative regulator, for such as stimulation of secretion of inflammatory cytokines and inhibition of PDLSC osteogenic differentiation 14. Specifically, Pg‐LPS at 1 and 10 μg/ml promoteds proliferation and inhibits osteogenic differentiation of PDLSCs as well as induces production of IL‐1β, IL‐6 and IL‐8. However, whether Pg‐LPS could affect immunomodulatory properties of MSCs has, up to now, remained unknown. In this work, we show that Pg‐LPS regulated MSC proliferation, osteogenic differentiation and immunomodulation in a dose‐dependent manner.
Materials and methods
Animals
Female Sprague–Dawley (SD) rats and immunocompromised nude mice (Vital River, Beijing, China) were housed in a specific pathogen‐free animal facility under controlled temperature (25 °C) and photoperiod (12:12‐h light–dark cycle). They were and allowed free access to standard diet and water. Animals were acclimatised to these conditions for 7 days before inclusion in the investigation. All animal experiments were performed in accordance with protocols approved by the Animal Care and Use Committee of Capital Medical University School of Stomatology, Beijing, China.
Antibodies and chemicals
Unconjugated antibody to iNOS was purchased from Cell Signaling Technology (Danvers, MA, USA). Unconjugated antibodies to ALP and Runx2 were purchased from Abcam (Cambridge, MA, USA). Anti‐rat‐CD3ε, anti‐rat‐CD28 and anti‐rat‐APC‐CD3 antibodies were bought from Biolegends (San Diego, CA, USA) and ultrapure lipopolysaccharides from Porphyromonas gingivalis (LPS) were obtained from InvivoGen (San Diego, CA, USA). Anti‐β‐actin antibody, NG‐monomethyl‐l‐arginine (l‐NMMA), dexamethasone, l‐ascorbic acid, β‐Glycerophosphate were purchased from Sigma‐Aldrich (St. Louis, MO, USA).
Assessment kits
Cell Counting Kit‐8 (CCK‐8) was purchased from Dojindo Molecular Technologies (Gaithersburg, MD, USA) and annexin V‐PI Apoptosis Detection kit was purchased from BD Bioscience (Franklin Lakes, NJ, USA). Total nitric oxide (NO) and Nitrate/Nitrite Parameter Assay Kit was bought from R&D Systems (Minneapolis, MN, USA) and PGE2 EIA kits were obtained from Enzo Life Sciences (Farmingdale, NY, USA). Rat IL‐10 and TGF‐β1 ELISA kits were purchased from Dakewei Biotech (Shenzhen, China).
Isolation and culture of rat BMMSCs
Bone marrow MSCs were isolated from 4‐week‐old SD rats as previously described 12. In brief, rat bone marrow cells were flushed from cavities of femurs and tibias, with 2% heat‐inactivated foetal bovine serum (FBS; Equitech‐Bio, Kerrville, TX, USA) in PBS. Single‐cell suspensions of all nucleate cells were obtained by passing all bone marrow cells through a 70 m cell strainer (BD Bioscience). 10–15 million cells were seeded on to 10 cm culture dishes (Corning, Corning, NY, USA) and initially incubated for 48 h with alpha minimum essential medium (α‐MEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat‐inactivated FBS, 2 mm l‐glutamine (Invitrogen), 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen) at 37 °C and 5% CO2, in a humidified environment. Medium was changed every 2–3 days. Cells were passaged when they became 70–80% confluent and were used at passage 3–5. To confirm MSC character, flow cytometric analysis was used to prove that they were positive for CD90 and STRO‐1, and did not express CD31 or CD34 (data not shown).
Cell survival assay
Cell proliferation rate was assayed by using CCK‐8 kit or trypan blue exclusion assay. For CCK‐8 assay, BMMSCs were seeded in 96‐well culture plates (Corning), 2 × 103 cells/well. After 12‐h culture for cell adherence, medium was exchanged for serum‐free medium, for 24 h, to synchronize cell cycles. Then, serum‐supplemented medium was replaced with complete medium containing Pg‐LPS, and cells were incubated for 12, 24, 48 or 72 h. Numbers of viable cells at each time point were determined by measuring OD value at 450 nm in six wells per group, using an ELISA Reader (Promega, Madison, WI, USA) following the manufacturer's instructions. OD value in culture medium without cells was set up as blank control, and we subtracted average absorbance of blank control wells from that of the other wells.
For trypan blue exclusion assay, BMMSCs were seeded in six‐well culture plates (Corning), 1 × 105 cells/well. After 12‐h culture for cell adherence, medium was exchanged for serum‐free medium for 24 h, to synchronize cell cycles. Then, serum‐supplemented medium was replaced with complete medium containing Pg‐LPS (0, 0.1, 1 and 10 μg/ml) and cells were incubated for 12, 24, 48, 72 and 168 h. Numbers of viable cells were counted using a Cell Counter (Bio‐Rad, Hercules, CA, USA).
Osteogenic differentiation assay
The BMMSCs were cultured in osteogenic culture medium, α‐MEM supplemented with 10% FBS, dexamethasone (10 nm), l‐ascorbic acid (50 μm) and β‐glycerophosphate (10 mm). A range of doses of LPS (0, 0.1, 1 and 10 μg/ml) was added to osteogenic culture medium every 3 days. After osteogenic induction for 2 weeks, cultures were stained with alizarin red, or total protein was extracted from the cells to estimate expression of ALP and Runx2 by western blot analysis. Size of calcified nodules and percentage of alizarin red‐positive areas were analysed using NIH Image J software (Bethesda, MD, USA). Five fields were selected and red‐positive areas in each field were calculated and shown as percentage of total area. After 7 days osteogenic induction, mRNA expressions of Runx2 and ALP were assayed by quantitative real‐time PCR (qRT‐PCR).
Quantitative real time PCR analysis
Total RNA was extracted using Trizol reagent (Sigma) and was transcribed using the PrimeScript RT reagent kit (Takara, Kyoto, Japan). cDNA amplification and detection were performed using Bio‐Rad iQ5 real‐time PCR system with SYBR Premix Ex Taq kit (Invitrogen). Primers used in the experiments were as follows: GAPDH, 5′‐AGACAGCCGCATCTTCTTGT‐3′ (forward) and 5′‐CTTGCCGTGGGTAGAGTCAT‐3′ (reverse); ALP, 5′‐CCTTGAAAAATGCCCTGAAA‐3′ (forward) and 5′‐CTTGGAGAGAGCCACAAAGG‐3′ (reverse); Runx2, 5′‐GAGCACAAACATGGCTGAGA‐3′ (forward) and 5′‐TGGAGATGTTGCTCTGTTCG‐3′ (reverse). Relative gene expression level was normalized to the internal control (GAPDH) based on the 2−ΔΔCt method.
BMMSC‐mediated bone formation
Approximately, 4 × 106 BMMSCs pre‐treated with a range of concentrations of Pg‐LPS (0, 0.1, 1 and 10 μg/ml) for 12 h were mixed with 40 mg hydroxyapatite tricalcium phosphate (HA‐TCP) ceramic particles (Biomedical Materials and Engineering Center of Wuhan University of Technology, Wuhan, China). These were subcutaneously implanted beneath dorsal skin of 8‐ to 10‐week‐old nude mice. Each mouse was implanted with four different groups randomly. 8 weeks after implantation, the results were harvested. Haematoxylin and eosin (H&E) stained histological sections were analysed using NIH Image J software. Five fields were selected, and newly formed areas of mineralized tissue in each field were calculated and shown as percentage of total tissue area.
Co‐culture of BMMSCs and splenocytes
Rat splenocytes (SP) cells were activated with plate‐bound antibody to CD3ε, at 2 μg/ml, and soluble antibody to CD28, 2 μg/ml, in complete 1640 medium (Invitrogen) supplemented with 10% FBS, for 48 h. BMMSCs at passage 3 were seeded in culture plates and incubated overnight before being pre‐treated with Pg‐LPS for 12 h. Activated SP cells were loaded on to the BMMSCs at 1:1 ratio, and then were co‐cultured for 48 h. Cells in suspension were first stained with anti‐rat‐APC‐CD3 (1:100), then positively stained cells were analysed using flow cytometry (FACSCalibur; BD Bioscience), to examine apoptosis of activated T cells. BMMSCs were analysed using western blotting, to assay expression of iNOS. Subsequently, cells in suspension and in culture supernatant were collected. Supernatant was collected to analyse levels of soluble factors, following the manufacturer's instructions.
Transwell cultures
Transwell chambers, membrane pore size 0.4 μm (Costar, Cambridge, MA, USA) were used to physically separate SP cells from BMMSCs. SP cells were seeded in the upper chamber, while BMMSCs were placed in the bottom chamber.
Detection of nitric oxide
Levels of NO were evaluated by assay for nitrates using modified Griess reagents following the manufacturer's instructions.
Flow cytometric analysis
To detect ratio of apoptotic T cells, floating cells were stained with anti‐rat CD3‐APC antibodies for 30 min, then were resuspended in 100 μl binding buffer with 5 μl annexin V‐FITC and 5 μl propidium iodide solution, and incubated for 15 min in the dark. Cell preparations were immediately analysed using flow cytometry. These experiments were performed at 4 °C in the dark.
Inhibition of NO production
To inhibit the production of NO, a selective inhibitor of inducible NO synthase (iNOS) activity, l‐NMMA was added at 100 mm 6 h before pre‐treating BMMSCs with Pg‐LPS (0.1 μg/ml).
Western blot analysis
Total protein was extracted using M‐PER mammalian protein extraction reagent (Thermo, Waltham, MA, USA). 30 μg were separated on 10% polyacrylamide‐SDS gels (Applygen, Beijing, China) and transferred to Immobilon™‐P membranes. After blocking with TBS/5% non‐fat dry milk for 1 h, the membrane was incubated with antibodies against ALP (1:1000), Runx2 (1:400), iNOS (1:200) and β‐actin (1:400), overnight at 4 °C. This was followed by incubation with HRP‐conjugated secondary antibodies (1:1000) (Pierce, Malibu, CA, USA) for 1 h at room temperature. Antibody binding was visualized using an enhanced chemiluminescence kit according to the manufacturer's protocols (Pierce).
Statistical analysis
Statistical analysis was carried out using spss 13.0 software (Chicago, IL, USA). Results were expressed as mean ± SD. Statistical significance of (*) P < 0.05 was determined using independent two‐tailed Student's t‐test, or analysis of a one‐way variance (ANOVA).
Results
0.1 μg/ml Pg‐LPS improved and 10 μg/ml Pg‐LPS reduced survival of BMMSCs
To determine effects of Pg‐LPS on survival of BMMSCs, a range of doses of Pg‐LPS (0.1–10 μg/ml) was used to treat the cells. Both CCK‐8 assay (Fig. 1a) and trypan blue exclusion assay (Fig. 1b) were used to evaluate cell proliferation. Ratio of apoptosis was shown by percentage of the annexin V‐positive population, in flow cytometry (Fig. 1c). Pg‐LPS had no effect on BMMSC proliferation at 12 h following Pg‐LPS treatment (Fig. 1a,b). Pg‐LPS at 0.1 μg/ml significantly promoted proliferation of BMMSCs both in 24 h and in 48 h following Pg‐LPS treatment (P < 0.05) (Fig. 1a,b). Pg‐LPS at 0.1 μg/ml increased the cells proliferation, while Pg‐LPS at 10 μg/ml reduced it (P < 0.05) (Fig. 1a,b) and induced apoptosis (P < 0.05) (Fig. 1c) 72 h following Pg‐LPS treatment. Thus, Pg‐LPS at concentrations as low as 0.1 μg/ml enhanced cell proliferation, while Pg‐LPS at 10 μg/ml inhibited it as well as induced BMMSC apoptosis.
Figure 1.
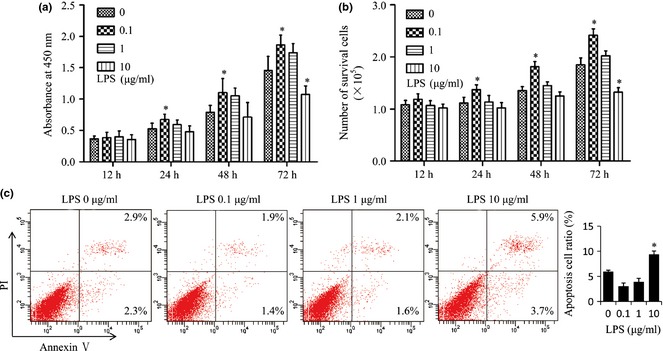
Porphyromonas gingivalis lipopolysaccharides ( Pg‐ LPS) at 0.1 μg/ml accelerated and Pg‐ LPS at 10 μg/ml inhibited survival of bone marrow mesenchymal stem cells ( BMMSC s). (a, b) BMMSCs were treated with different concentrations (0, 0.1, 1 and 10 μg/ml) of Pg‐LPS. At 12, 24, 48 or 72 h after Pg‐LPS treatment, proliferation of BMMSCs was evaluated by Cell Counting Kit‐8 (a) or by using the trypan blue exclusion assay (b). (c) Apoptotic assay of BMMSCs was assessed by percentage of annexin V‐positive cells, by flow cytometry, 72 h after Pg‐LPS treatment. n = 6 in (a), n = 5 in (b) and n = 4 in (c). Results are representative of three independent experiments and are expressed as mean ± SD; statistical significance is shown as (*) P < 0.05, compared to BMMSCs without Pg‐LPS treatment. LPS, lipopolysaccharides from Porphyromonas gingivalis.
0.1 μg/ml Pg‐LPS promoted and 10 μg/ml Pg‐LPS inhibited osteogenic differentiation of BMMSCs
Next, we investigated possible influence of Pg‐LPS on osteogenic differentiation of BMMSCs. We found that 0.1 μg/ml Pg‐LPS promoted and 10 μg/ml Pg‐LPS suppressed calcium deposition of BMMSCs in vitro, by using alizarin red staining (P < 0.05) (Fig. 2a,b). However, differences in alizarin red staining may solely have been due to increased cell numbers following low Pg‐LPS (0.1 μg/ml) treatment and increased cell death following high Pg‐LPS (10 μg/ml) treatment (Fig. 1a,b). To exclude effects of difference in cell survival on osteogenic differentiation, we used qRT‐PCR assay to analyse expression of osteogenic genes (ALP and Runx2); this was based on the same amount of total RNA 15, 16. Notably, qRT‐PCR showed that both ALP and Runx2 were significantly promoted in BMMSCs following Pg‐LPS (0.1 μg/ml) treatment while they were markedly inhibited following Pg‐LPS (10 μg/ml) treatment (P < 0.05) (Fig. 2c,d). Furthermore, western blotting confirmed that Pg‐LPS (0.1 μg/ml) up‐regulated expression of ALP and Runx2, while Pg‐LPS (10 μg/ml) down‐regulated expression of ALP (Fig. 2e).
Figure 2.
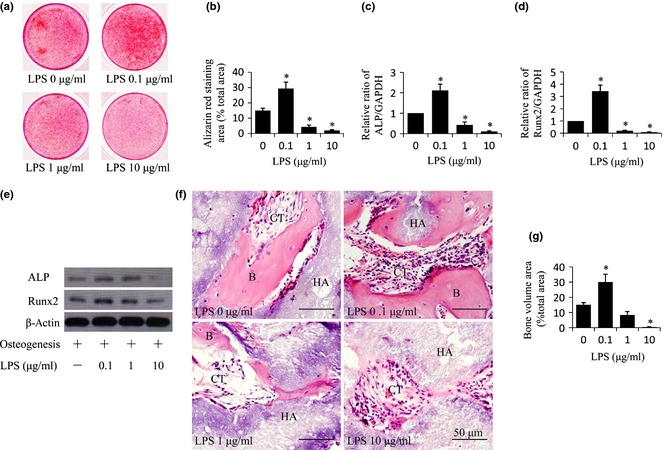
Porphyromonas gingivalis lipopolysaccharides ( Pg‐ LPS) at 0.1 μg/ml promoted and Pg‐ LPS at 10 μg/ml inhibited osteogenic differentiation of bone marrow mesenchymal stem cells ( BMMSC s). (a) Alizarin red staining indicating extracellular calcium deposition of BMMSCs after treatment with osteogenic media plus different concentrations (0, 0.1, 1 and 10 μg/ml) of Pg‐LPS, for 2 weeks. (b) Quantitative analysis of amounts of alizarin staining area as described in (a). (c, d) Relative mRNA levels of ALP (c) and Runx2 (d) in BMMSCs were measured by qRT‐PCR after treatment with osteogenic media plus different concentrations (0, 0.1, 1 and 10 μg/ml) of Pg‐LPS, for 7 days. (e) Western blot analysis of groups indicated in (a). (f) H&E staining of tissue samples from nude mice 8 weeks after subcutaneous implantation with BMMSCs pre‐treated with different concentrations (0, 0.1, 1 and 10 μg/ml) of Pg‐LPS for 12 h. Formation of bone (B) and connective tissue (CT) around HA‐TCP (HA) were indicated. Scale bar = 50 μm. (g) Quantitative analysis of amount of bone formation as described in (f). Results are representative of three independent experiments and are expressed as mean ± SD, n = 3 in (a–d), n = 5 in (e); statistical significance is shown as (*) P < 0.05, compared to BMMSCs without Pg‐LPS treatment. LPS, lipopolysaccharides from Porphyromonas gingivalis.
Furthermore, we explored the effects of different concentration of Pg‐LPS (0.1–10 μg/ml) on osteogenic differentiation of BMMSCs in vivo. First, we pre‐treated the cells with Pg‐LPS for 12 h as we had already verified that Pg‐LPS had no effect on their proliferation at 12 h following Pg‐LPS treatment (Fig. 1a,b). Then [using an established BMMSC implantation system containing approximately 4 × 106 cells and carrier hydroxyapatite tricalcium phosphate (HA‐TCP) particles] we subcutaneously implanted cells and particles into the dorsal surface of nude mice. We found that BMMSCs pre‐treated with Pg‐LPS at 0.1 μg/ml for 12 h generated bone, and cells with Pg‐LPS at 10 μg/ml did not generate any (P < 0.05) (Fig. 2f,g), compared to BMMSCs without Pg‐LPS pre‐treatment. These data together illustrated that Pg‐LPS at 0.1 μg/ml improved and Pg‐LPS at 10 μg/ml impaired osteogenic differentiation.
0.1 μg/ml Pg‐LPS up‐regulated and 10 μg/ml Pg‐LPS down‐regulated immunomodulatory properties of BMMSCs
Next, we investigated whether Pg‐LPS treatment would influence immunomodulatory properties of BMMSCs. We found that Pg‐LPS (0.1 μg/ml) pre‐treatment for 12 h improved the stem cells ability to induce apoptosis of activated T cells, while 10 μg/ml Pg‐LPS inhibited immunoregulatory properties of BMMSCs (P < 0.05) (Fig. 3).
Figure 3.
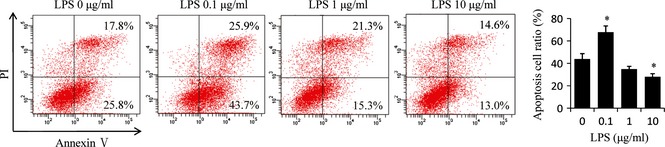
Porphyromonas gingivalis lipopolysaccharides ( Pg‐ LPS) at 0.1 μg/ml promoted and Pg‐ LPS at 10 μg/ml inhibited capacity of bone marrow mesenchymal stem cells ( BMMSC s) to inhibit activated T cells. BMMSCs pre‐treated with different concentrations (0, 0.1, 1 and 10 μg/ml) of Pg‐LPS for 12 h, were co‐cultured with activated splenocytes (SP) cells at 1:1 ratio for 48 h; then apoptosis of T cells was assessed by percentage annexin V‐positive cells. Results are representative of three independent experiments expressed as mean ± SD, n = 4; statistical significance is shown as (*) P < 0.05, compared to BMMSCs without Pg‐LPS treatment. LPS, lipopolysaccharides from Porphyromonas gingivalis.
0.1 μg/ml Pg‐LPS promoted immunomodulatory properties of BMMSCs by production of NO
To explore the mechanisms of promoted immunoregulatory properties of BMMSCs induced by 0.1 μg/ml Pg‐LPS, we analysed expression levels of some soluble molecules associated with immunomodulatory function of BMMSCs, including NO, TGF‐β1, IL‐10 and PGE2, in supernatants of Pg‐LPS‐pre‐treated cells. We found that Pg‐LPS treatment did not influence production of TGF‐β1, IL‐10 and PGE2 (data not shown). On the other hand, 0.1 μg/ml Pg‐LPS significantly increased levels of NO in BMMSC culture supernatants (P < 0.05) (Fig. 4a). We also found that expression of iNOS was up‐regulated with 0.1 μg/ml Pg‐LPS treated BMMSCs (Fig. 4b). Supporting this, presence of the selective inhibitor of iNOS activity, NG‐monomethyl‐l‐arginine (l‐NMMA) abolished the ability of Pg‐LPS to elevate immunomodulatory potential of BMMSCs (P < 0.05) (Fig. 4c,d) as well as inhibit expression of iNOS (Fig. 4e) and production of NO (P < 0.05) (Fig. 4f).
Figure 4.
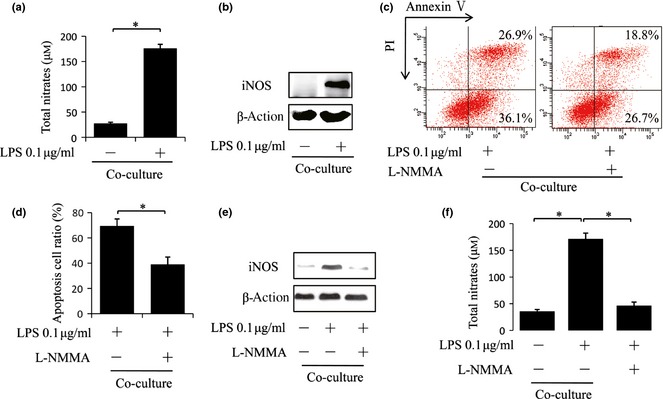
Porphyromonas gingivalis lipopolysaccharides ( Pg‐ LPS) at 0.1 μg/ml improved immunomodulatory properties of bone marrow mesenchymal stem cells ( BMMSC s) via promoting their nitric oxide ( NO ) production. (a) BMMSCs pre‐treated with 0.1 μg/ml Pg‐LPS for 12 h were co‐cultured with activated splenocytes (SP) cells at 1:1 ratio for 48 h. Supernatants from the co‐culture system were assayed for nitrates using a modified Griess reagent. (b) Treated as described in (a), total protein of BMMSCs was collected and iNOS expression was assessed by western blot analysis. (c–f) BMMSC pre‐treated with 0.1 μg/ml LPS for 12 h co‐cultured with activated splenocytes (SP) cells at 1:1 ratio for 48 h with or without iNOS inhibitor, l‐NMMA (100 μm), then assay for apoptosis of activated T cells (c, d), iNOS expression of BMMSCs (e) and nitrate production (f) was performed. Results are representative of three independent experiments expressed as mean ± SD, n = 4; statistical significance is shown as (*) P < 0.05. LPS, lipopolysaccharides from Porphyromonas gingivalis.
To identify whether cell–cell contact was required for Pg‐LPS (0.1 μg/ml)‐mediated promotion of the immunomodulatory properties of BMMSCs, a transwell culture system was used to separate anti‐CD3‐activated SP from BMMSCs. We found that transwell culture reversed Pg‐LPS‐mediated elevation of BMMSC immunomodulatory functions (P < 0.05) (Fig. 5a), as well as inhibited production of NO (P < 0.05) (Fig. 5b) and expression of iNOS (Fig. 5c). These above data suggest that Pg‐LPS at 0.1 μg/ml promoted immunomodulatory properties of BMMSCs through NO dependent cell–cell contact.
Figure 5.
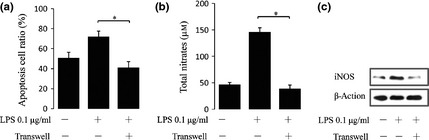
Cell–cell contact was required for improved immunomodulatory properties of bone marrow mesenchymal stem cells ( BMMSC s) by Porphyromonas gingivalis lipopolysaccharide ( Pg‐ LPS ) pre‐treatment. BMMSCs pre‐treated with Pg‐LPS (0.1 μg/ml) for 12 h were cultured with activated splenocytes (SP) cells at 1:1 ratio for 48 h with or without transwells, then assayed for apoptosis of activated T cells (a), nitrate production (b) and iNOS expression of BMMSCs (c). Results are representative of three independent experiments expressed as mean ± SD, n = 4; statistical significance is shown as (*) P < 0.05. LPS, lipopolysaccharides from Porphyromonas gingivalis.
Discussion
By their multipotency, BMMSCs hold great promise for clinical applications of achieving tissue regeneration. Recent studies have shown that they contribute to formation of all periodontal tissues, including periodontal ligament, cementum, and alveolar bone, thus might be an alternative cell source to achieve periodontal regeneration 10, 11, 12. However, precise mechanisms underlying these effects had remained poorly understood, which limited advanced application of BMMSCs for treating periodontitis. As we know, Pg‐LPS is one of the critical pathogenic factors strongly involved in initiation and development of periodontitis 13, to which BMMSCs are known to respond 17. Meanwhile, concentrations of Pg‐LPS on the local periodontal microenvironment are always positively related to severity of periodontitis 18, thus a better understanding of the effects of different concentrations of Pg‐LPS on biological properties of BMMSCs involved in tissue regeneration is pivotal to their successful application. Most previous studies have focused on negative impacts of high concentration of Pg‐LPS on properties of progenitor cells 14, 17, but no studies have reported effects of lower concentration Pg‐LPS on MSCs. Our preliminary investigation showed that Pg‐LPS concentration lower than 0.1 μg/ml had almost no effect on BMMSC proliferation and apoptosis (data not shown); thus we selected 0.1 μg/ml Pg‐LPS to represent low concentration. Here, we report for the first time that function of BMMSCs varied depending on Pg‐LPS concentration. Specifically, Pg‐LPS stimulation at concentrations as low as 0.1 μg/ml improved functions of BMMSCs, including proliferation, osteogenic differentiation and immunomodulatory properties. In contrast, high LPS concentration (10 μg/ml) harmed their functions as well as induced BMMSC apoptosis. These data together indicate that recipient local concentration of Pg‐LPS determines the outcome of BMMSC‐mediated periodontal tissue regeneration.
Capacities of BMMSCs to self‐renew, undergo extensive proliferation and differentiate into osteocytes, contribute to their potential to achieve bone regeneration 19. In addition, their immunomodulatory properties may also function importantly in BMMSCs‐mediated bone regeneration, as sites of tissue damage are always accompanied by inadequate immune response. We have recently shown that the local inflammatory microenvironment plays a key role in determining success of BMMSC‐based bone regeneration 20. Recipient proinflammatory T cells inhibit the ability of local implanted BMMSCs to generate bone via interferon γ and tumour necrosis factor α signalling, to induce BMMSC apoptosis. Recent studies have shown that T cell‐mediated immunity is involved in regulation of host immune response to subgingival pathogens, leading to local inflammatory reactions in the progression of periodontitis 21, 22, 23. For instance, proinflammatory cytokines, including IL‐17, osteoclastogenic factor of activated T cells (SOFAT), produced by activated T cells promotes inflammatory responses and stimulates activity of osteoclasts in development of periodontitis 23. Periodontitis is caused by inadequate host inflammatory immune response to bacteria from oral microbial biofilms 2, so periodontal regeneration‐related characteristics of BMMSCs, specially immunomodulatory properties, are very important to periodontal regeneration. In this study, we found that low Pg‐LPS stimulation (0.1 μg/ml) promoted BMMSC immunomodulatory properties to induce apoptosis of activated T cells, which in turn protects local or implanted BMMSCs from recipient inflammatory response. This thus reduces BMMSC apoptosis and contributes to bone regeneration. Supporting this, our previous studies revealed that systemic infusion of CD4+ CD25+ Foxp3+ Tregs was able to inhibit activated T cells to induce immune tolerance, which, in turn, promoted cell‐based bone formation in vivo 20. Meanwhile, our latest investigation showed that systemic infusion of BMMSCs promoted cell‐based bone formation in critical‐sized calvarial defects, in a murine model, via immunoregulatory capacity of BMMSCs 24. On the other hand, our results showed that the capacity of BMMSCs to induce apoptosis of activated T cells was impaired under high Pg‐LPS treatment (10 μg/ml), which might in turn result in apoptosis of BMMSCs and inhibited bone regeneration.
Previous studies have shown that BMMSCs have immunoregulatory properties as a result of secreting soluble molecules including NO, TGF‐β1, IL‐10 and PGE2 25, 26, 27, 28. In the present study, we confirmed that secretion level of NO was critical in Pg‐LPS‐associated BMMSC immunomodulation. 0.1 μg/ml Pg‐LPS promoted immunomodulatory properties of BMMSCs via NO. We also found that BMMSC‐activated T cell contact was required for Pg‐LPS‐mediated promotion of immunomodulatory properties of BMMSCs. Recent studies have revealed that systemic administration of BMMSCs results in recruitment of T cells to initiate T cells apoptosis, and apoptotic T cells trigger macrophages to produce high levels of transforming growth factor‐β (TGF‐β), leading, in turn, to up‐regulation of regulatory T cells (Tregs) and, subsequently, immune tolerance 29. Our findings suggest that implanted BMMSCs stimulated by low Pg‐LPS concentration regulate the local immune microenviroment by producing NO and are beneficial for periodontal regeneration. However, these data could also indicate a need for increased local concentration of NO (which has a short half‐life), or poor diffusion characteristics, thus further experiments need to be performed in the future.
Recent studies have revealed that 10 μg/ml LPS reduced osteogenic differentiation of human PDLSCs but not BMMSCs, this is in contrast to our findings 30. This diversity may be attributed to differences in kinds of LPS and species investigated. Specifically, the source of LPS in our study was extraction from P. gingivalis, while in the previous study, they were from Escherichia coli. In addition, BMMSCs in our experiments were isolated from rats, while others were from humans. Thus, further comparison needs to be performed. As proliferation, apoptosis, differentiation and immunoregulatory properties of BMMSCs were all influenced by Pg‐LPS stimulation here, the exact property that plays a critical role in periodontal regeneration needs to be further investigated. In addition, further explorations need to be performed to verify effects and mechanisms of periodontal microenvironment on therapeutic effects of BMMSCs in vivo.
In conclusion, we have demonstrated that BMMSCs had different responses to various local concentrations of Pg‐LPS; this seemed to determine the outcome of BMMSC‐mediated periodontal tissue regeneration. In addition, we showed that low LPS concentration stimulation promoted immunomodulatory properties of BMMSCs via NO. Thus, according to our results, we hypothesize that BMMSCs could be used as biological grafting in treating slight periodontitis. On the other hand, effective measures should be taken before application of BMMSCs to treat severe periodontitis such as improving the local inflammatory environment or taking action to promoting functions of BMMSCs.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Fig. S1 Schematic diagram of co‐culture/separate culture of BMMSCs and activated T cells.
Acknowledgements
This work was supported by grants from Beijing Municipal Committee for Science and Technology(Z121100005212004; to S.W.), the National Basic Research Program of China (2010CB944801; to S.W.), the Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality (PHR20090510; to S.W.), the Funding Project to Science Facility in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality (PXM 2009‐014226‐074691; to S.W.), the National Natural Science Foundation of China (81222011 to Y.L.), Science and Technology Activities of Beijing Overseas Students Preferred Foundation (to Y.L.), Beijing Key Laboratory Foundation of Science and Technology Special Work (Z121107002812034 to Z.S.), and the National Natural Science Foundation of China (81300892 to J.X.)
Tang ZG and Liu Y contributed to this study equally.
References
- 1. Kinane DF, Marshall GJ (2001) Periodontal manifestations of systemic disease. Aust. Dent. J. 46, 2–12. [DOI] [PubMed] [Google Scholar]
- 2. Van Dyke TE (2008) The management of inflammation in periodontal disease. J. Periodontol. 79, 1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang HL, Greenwell H, Fiorellini J, Giannobile W, Offenbacher S, Salkin L et al (2005) Periodontal regeneration. J. Periodontol. 76, 1601–1622. [DOI] [PubMed] [Google Scholar]
- 4. Hoffmann T, Richter S, Meyle J, Gonzales JR, Heinz B, Arjomand M et al (2006) A randomized clinical multicentre trial comparing enamel matrix derivative and membrane treatment of buccal class II furcation involvement in mandibular molars. Part III: patient factors and treatment outcome. J. Clin. Periodontol. 33, 575–583. [DOI] [PubMed] [Google Scholar]
- 5. Kitamura M, Nakashima K, Kowashi Y, Fujii T, Shimauchi H, Furuuchi T et al (2008) Periodontal tissue regeneration using fibroblast growth factor‐2: randomized controlled phase II clinical trial. PLoS ONE 3, e2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nygaard‐Ostby P, Bakke V, Nesdal O, Susin C, Wikesjo UM (2010) Periodontal healing following reconstructive surgery: effect of guided tissue regeneration using a bioresorbable barrier device when combined with autogenous bone grafting. A randomized‐controlled trial 10‐year follow‐up. J. Clin. Periodontol. 37, 366–373. [DOI] [PubMed] [Google Scholar]
- 7. Maeda H, Wada N, Tomokiyo A, Monnouchi S, Akamine A (2013) Prospective potency of TGF‐beta1 on maintenance and regeneration of periodontal tissue. Int. Rev. Cel. Mol. Biol. 304, 283–367. [DOI] [PubMed] [Google Scholar]
- 8. Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM et al (2008) Periodontal ligament stem cell‐mediated treatment for periodontitis in miniature swine. Stem Cells 26, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z et al (2010) Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells 28, 1829–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H et al (2010) Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J. Periodontol. 75, 1281–1287. [DOI] [PubMed] [Google Scholar]
- 11. Shen Y, Feng Z, Lin C, Hou X, Wang X, Wang J et al (2012) An oligodeoxynucleotide that induces differentiation of bone marrow mesenchymal stem cells to osteoblasts in vitro and reduces alveolar bone loss in rats with periodontitis. Int. J. Mol. Sci. 13, 2877–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Du J, Shan Z, Ma P, Wang S, Fan Z (2014) Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration. J. Dent. Res. 93, 183–188. [DOI] [PubMed] [Google Scholar]
- 13. Bostanci N, Belibasakis GN (2012) Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS. Microbio. Lett. 333, 1–9. [DOI] [PubMed] [Google Scholar]
- 14. Kato H, Taguchi Y, Tominaga K, Umeda M, Tanaka A (2014) Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro‐inflammatory cytokine production in human periodontal ligament stem cells. Arch. Oral Biol. 59, 167–175. [DOI] [PubMed] [Google Scholar]
- 15. Wang Y, Zheng Y, Wang Z, Li J, Wang Z, Zhang G et al (2013) 10(‐7) m 17β‐oestradiol enhances odonto/osteogenic potency of human dental pulp stem cells by activation of the NF‐κB pathway. Cell Prolif. 46, 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou C, Lin Y (2014) Osteogenic differentiation of adipose‐derived stem cells promoted by quercetin. Cell Prolif. 47, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chatzivasileiou K, Lux CA, Steinhoff G, Lang H (2013) Dental follicle progenitor cells responses to Porphyromonas gingivalis LPS. J. Cell Mol. Med. 17, 766–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Socransky SS, Haffajee AD (2013) Periodontal microbial ecology. Periodontol. 2000 38, 135–187. [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, Wang S, Shi S (2012) The role of recipient T cells in mesenchymal stem cell‐based tissue regeneration. Int. Biochem. Cell Biol. 44, 2044–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X et al (2011) Mesenchymal stem cell‐based tissue regeneration is governed by recipient T lymphocytes via IFN‐gamma and TNF‐alpha. Nat. Med. 17, 1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S (2005) The potential role of interleukin‐17 in the immunopathology of periodontal disease. J. Clin. Periodontol. 32, 369–374. [DOI] [PubMed] [Google Scholar]
- 22. Gaffen SL, Hajishengallis G (2008) A new inflammatory cytokine on the block: re‐thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL‐17. J. Dent. Res. 87, 817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jarry CR, Duarte PM, Freitas FF, de Macedo CG, Clemente‐Napimoga JT, Saba‐Chujfi E et al (2013) Secreted osteoclastogenic factor of activated T cells (SOFAT), a novel osteoclast activator, in chronic periodontitis. Hum. Immunol. 74, 861–866. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Yang R, Shi S (2014) Systemic infusion of mesenchymal stem cells improves cell‐based bone regeneration via upregulation of regulatory T cells. Tissue Eng. Part A 2014 Aug 26. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T et al (2007) Nitric oxide plays a critical role in suppression of T‐cell proliferation by mesenchymal stem cells. Blood 109, 228–234. [DOI] [PubMed] [Google Scholar]
- 26. Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI et al (2008) Mesenchymal stem cell‐mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150. [DOI] [PubMed] [Google Scholar]
- 27. Hsu W‐T, Lin C‐H, Chiang B‐L, Jui H‐Y, Wu KK‐Y, Lee C‐M (2007) Prostaglandin E2 potentiates mesenchymal stem cell‐induced IL‐10 + IFN‐gamma+CD4 + regulatory T cells to control transplant arteriosclerosis. J. Immunol. 190, 2372–2380. [DOI] [PubMed] [Google Scholar]
- 28. Yang R, Liu Y, Kelk P, Qu C, Akiyama K, Chen C et al (2013) A subset of IL‐17(+) mesenchymal stem cells possesses anti‐Candida albicans effect. Cell Res. 23, 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T et al (2012) Mesenchymal‐stem‐cell‐induced immunoregulation involves FAS‐ligand‐/FAS‐mediated T cell apoptosis. Cell Stem Cell 10, 544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li C, Li B, Dong Z, Gao L, He X, Liao L et al (2014) Lipopolysaccharide differentially affects the osteogenic differentiation of periodontal ligament stem cells and bone marrow mesenchymal stem cells through Toll‐like receptor 4 mediated nuclear factor κB pathway. Stem Cell Res. Ther. 5, 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Schematic diagram of co‐culture/separate culture of BMMSCs and activated T cells.


